Semaglutide Therapy and Cardiorenal Risk Management in Type 2 Diabetes: hsCRP as a Biomarker of Risk
Abstract
1. Introduction
2. Material and Applied Methodology
3. Tracking Design
- Glycated hemoglobin (HbA1c);
- Serum creatinine and estimated glomerular filtration rate (eGFR) through the CKD-EPI formula;
- High-sensitivity C-reactive protein (hsCRP);
- Microalbuminuria (MALB);
- Anthropometric measurements: height, body weight, body mass index (BMI), and waist circumference.
3.1. Data Collection Procedure
- Structured individual survey;
- Semi-standardized clinical interview;
- Direct clinical observation.
- Medical Center “Doctors for Us”—Burgas;
- Individual Practice of Specialized Medical Care “Dr. Nikolay Kostadinov”—city of Burgas.
3.2. Patient Characteristics
- Age over 18 years;
- Confirmed diagnosis of type 2 diabetes mellitus for at least 6 months;
- Undergone prior treatment with metformin and/or a sulfonylurea;
- Signed informed consent for study participation.
- Type 1 diabetes mellitus;
- Gestational diabetes;
- Age under 18 years;
- Presence of cognitive deficits and/or mental illnesses limiting the validity of informed consent and/or access to objective information.
4. Results
4.1. Demographic and Anthropometric Profile of the Studied Population
4.2. Clinical and Anthropometric Profile
4.3. Monitored Laboratory Parameters and Their Dynamics During the Course of Administered Treatment
4.4. Inflammatory Marker hsCRP in Patients Treated with GLP-1 RA
4.5. Dynamics of Changes in the Blood Glucose Profile
4.6. Descriptive and Comparative Analysis of the Therapeutic Effect of the Therapy with GLP-1 RA
4.7. Correlation Analysis
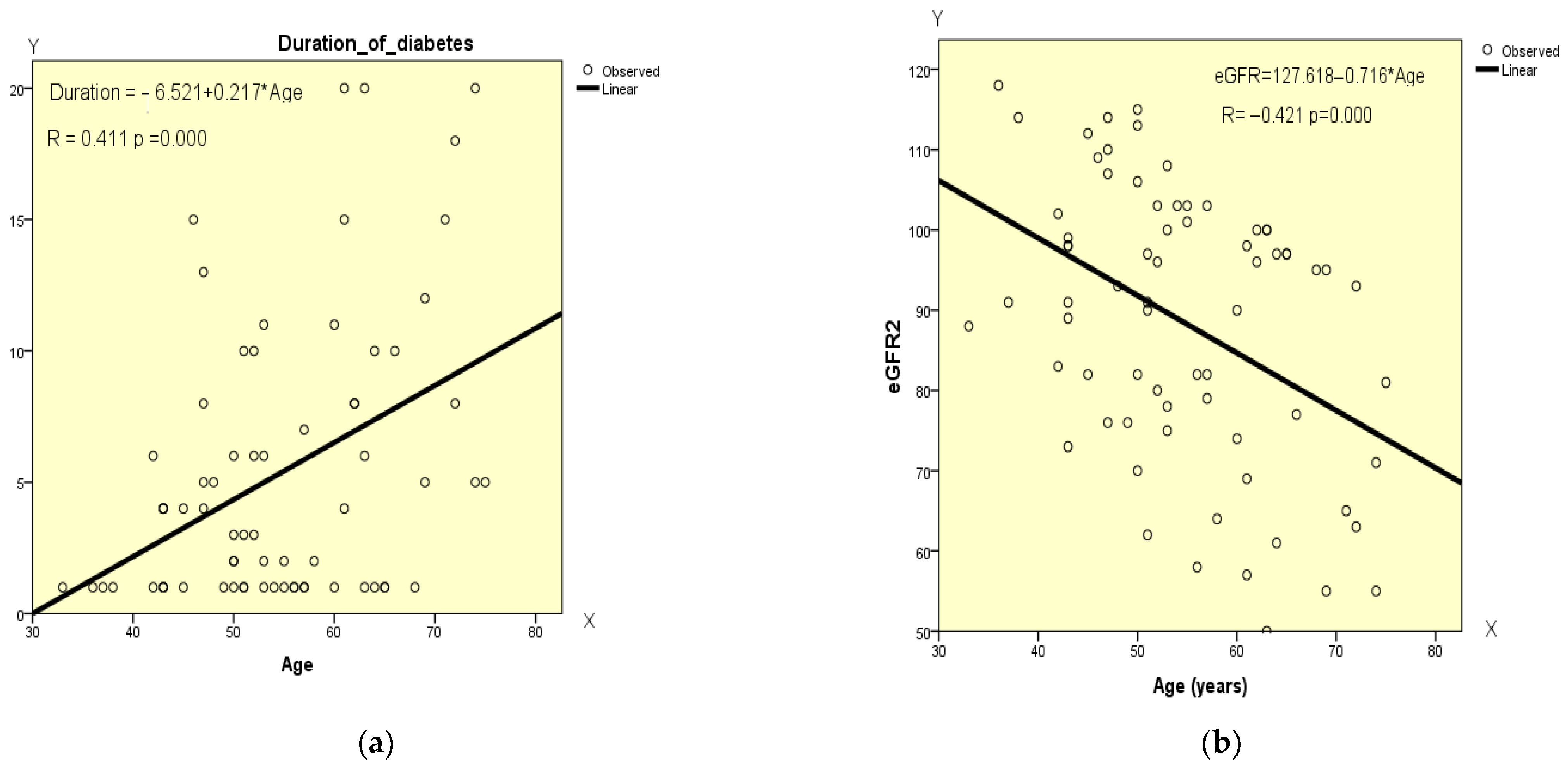
4.8. Correlation Analysis of the Data at the Beginning of GLP-1 RA Therapy
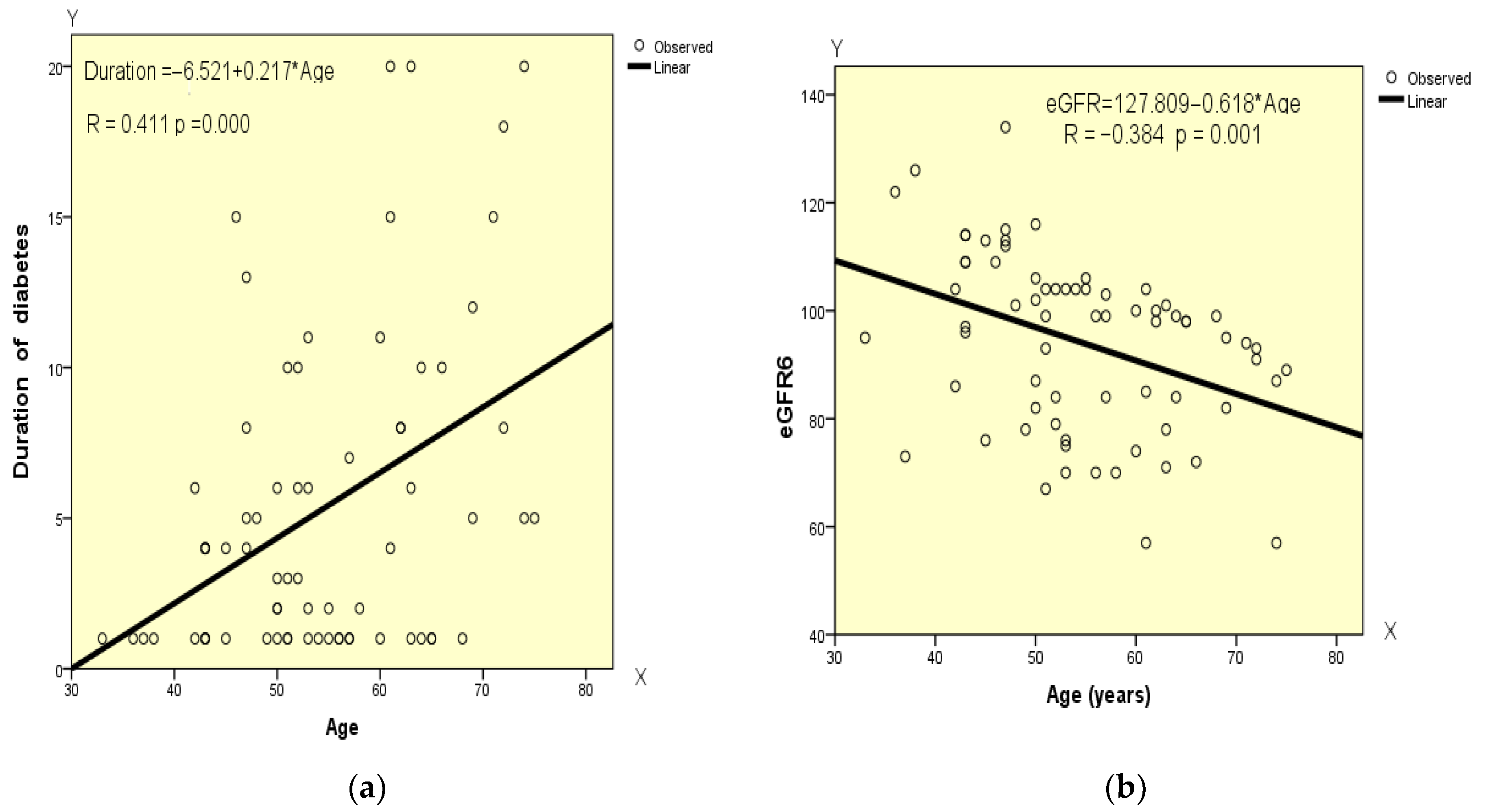
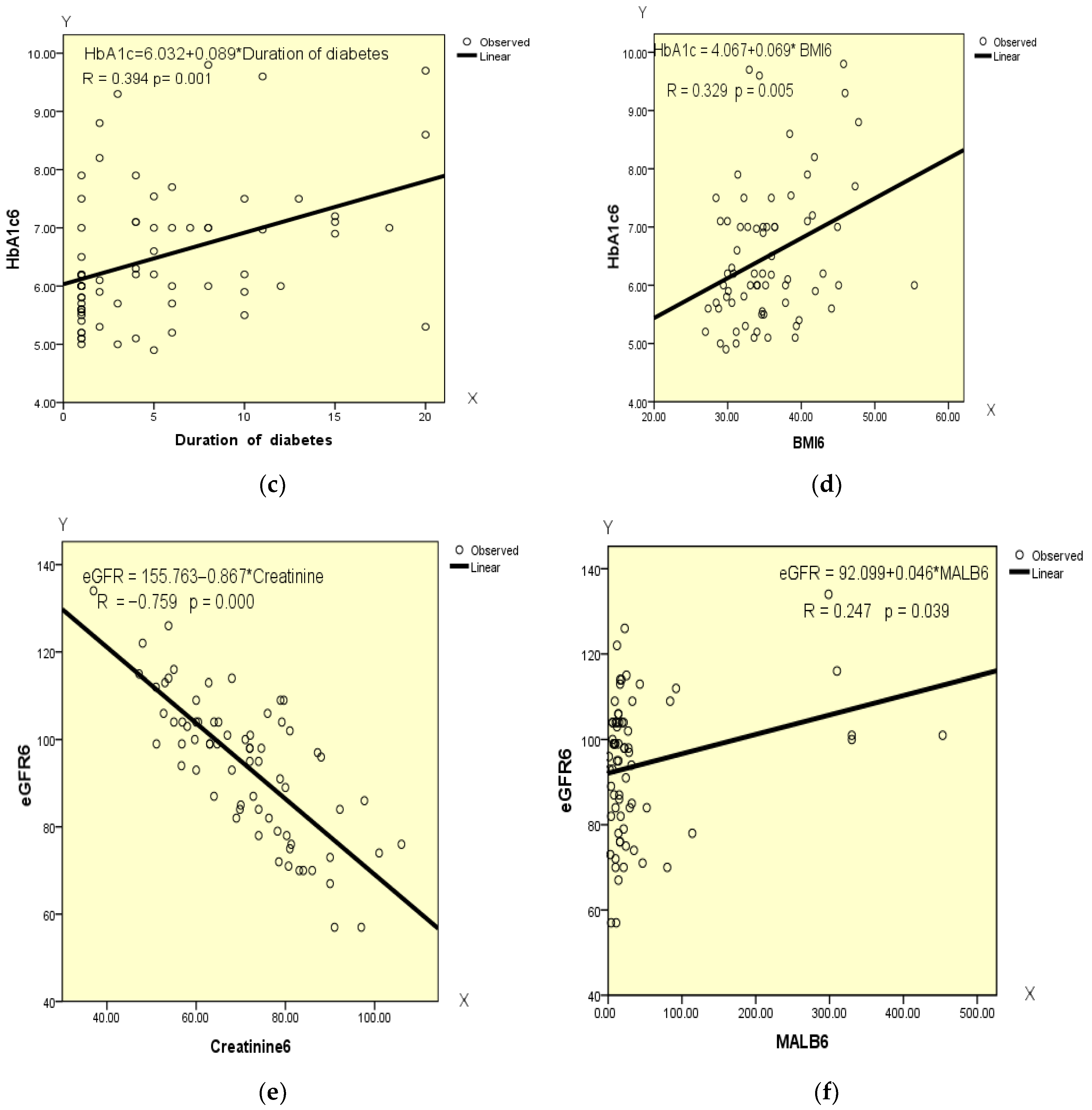
4.9. Correlation Analysis of the Data at the End of GLP-1 RA Therapy
5. Discussion
5.1. GLP-1 RA in the Context of the Cardiorenal Continuum
5.2. Renoprotection and Cardiovascular Risk—The Two Faces of the Therapy with GLP-1 RA
5.3. Author Contribution and Clinical Significance
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banait, T.; Wanjari, A.; Danade, V.; Banait, S.; Jain, J. Role of High-Sensitivity C-reactive Protein (Hs-CRP) in Non-communicable Diseases: A Review. Cureus 2022, 14, e30225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clyne, B.; Olshaker, J.S. The C-reactive protein. J. Emerg. Med. 1999, 17, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Cushman, M.; Stampfer, M.J.; Tracy, R.P.; Hennekens, C.H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N. Engl. J. Med. 1997, 336, 973–979. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Rose, L.; Buring, J.E.; Cook, N.R. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med. 2002, 347, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Franko, M.A.; Obergfell, A.; Gabrielsen, A.; Jernberg, T. hsCRP level and the risk of death or recurrent cardiovascular events in patients with myocardial infarction: A healthcare-based study. J. Am. Heart Assoc. 2019, 8, e012638. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Liu, K.; Tian, L.; Greenland, P. Narrative review: Assessment of C-reactive protein in risk prediction for cardiovascular disease. Ann. Intern. Med. 2006, 145, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tao, S.; Peng, J.; Zhao, J.; Li, S.; Wu, N.; Wen, Y.; Xue, Q.; Yang, C.; Pan, X. High-sensitivity C-reactive protein and risk of type 2 diabetes: A nationwide cohort study and updated meta-analysis. Diabetes Metab. Res. Rev. 2021, 37, e3446. [Google Scholar] [CrossRef]
- Ragy, M.M.; Kamal, N.N. Linking senile dementia to type 2 diabetes: Role of oxidative stress markers, C-reactive protein and tumor necrosis factor-α. Neurol. Res. 2017, 39, 587–595. [Google Scholar] [CrossRef]
- Ridker, P.M.; Buring, J.E.; Shih, J.; Matias, M.; Hennekens, C.H. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 1998, 98, 731–733. [Google Scholar] [CrossRef]
- Aroda, V.; Ahmann, A.; Cariou, B.; Chow, F.; Davies, M.; Jódar, E.; Mehta, R.; Woo, V.; Lingvay, I. Comparative efficacy, safety, and cardiovascular outcomes with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: Insights from the SUSTAIN 1–7 trials. Diabetes Metab. 2019, 45, 409–418. [Google Scholar] [CrossRef]
- Lingvay, I.; Catarig, A.-M.; Frias, J.P.; Kumar, H.; Lausvig, N.L.; le Roux, C.W.; Thielke, D.; Viljoen, A.; McCrimmon, R.J. Efficacy and safety of once-weekly semaglutide versus daily canagliflozin as add-on to metformin in patients with type 2 diabetes (SUSTAIN 8): A double-blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, B.; Wang, J.; Yang, C.; Wu, S.; Wu, Y.; Chen, S.; Li, Q.; Zhang, H.; Wang, G.; et al. Reduction in serum high-sensitivity C-reactive protein favors kidney outcomes in patients with impaired fasting glucose or diabetes. J. Diabetes Res. 2020, 2020, 2720905. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O., III; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.; Martínez, M.S.; Chávez-Castillo, M.; Núñez, V.; Añez, R.; Torres, Y.; Toledo, A.; Chacín, M.; Silva, C.; Pacheco, E.; et al. C-reactive protein: An in-depth look into structure, function, and regulation. Int. Sch. Res. Not. 2014, 2014, 653045. [Google Scholar] [CrossRef]
- Luan, Y.-Y.; Yao, Y.-M. The clinical significance and potential role of c-reactive protein in chronic inflammatory and neurodegenerative diseases. Front. Immunol. 2018, 9, 1302. [Google Scholar] [CrossRef]
- Kandelouei, T.; Abbasifard, M.; Imani, D.; Aslani, S.; Razi, B.; Fasihi, M.; Shafiekhani, S.; Mohammadi, K.; Jamialahmadi, T.; Reiner, Ž.; et al. Effect of statins on serum level of hs-CRP and CRP in patients with cardiovascular diseases: A systematic review and meta-analysis of randomized controlled trials. Mediat. Inflamm. 2022, 2022, 8732360. [Google Scholar] [CrossRef]
- Alharbi, S.H. Anti-inflammatory role of glucagon-like peptide 1 receptor agonists and its clinical implications. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188231222367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jara, M.; Norlin, J.; Kjær, M.S.; Almholt, K.; Bendtsen, K.M.; Bugianesi, E.; Cusi, K.; Galsgaard, E.D.; Geybels, M.; Gluud, L.L.; et al. Modulation of metabolic, inflammatory and fibrotic pathways by semaglutide in metabolic dysfunction-associated steatohepatitis. Nat. Med. 2025, 31, 3128–3140. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.M.; Coleman, R.L.; Holman, R.R. Prognostic significance of silent myocardial infarction in newly diagnosed type 2 diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS) 79. Circulation 2013, 127, 980–987. [Google Scholar] [CrossRef]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated c-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef]
- Kim, J.; Pyo, S.; Yoon, D.W.; Lee, S.; Lim, J.-Y.; Heo, J.S.; Lee, S.; Shin, C. The co-existence of elevated high sensitivity C-reactive protein and homocysteine levels is associated with increased risk of metabolic syndrome: A 6-year follow-up study. PLoS ONE 2018, 13, e0206157. [Google Scholar] [CrossRef]
- Mugabo, Y.; Li, L.; Renier, G. The connection between C-reactive protein (CRP) and diabetic vasculopathy. Focus on preclinical findings. Curr. Diabetes Rev. 2010, 6, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ghule, A.; Kamble, T.K.; Talwar, D.; Kumar, S.; Acharya, S.; Wanjari, A.; Gaidhane, S.A.; Agrawal, S. Association of serum high sensitivity C-reactive protein with pre-diabetes in rural population: A two-year cross-sectional study. Cureus 2021, 13, e19088. [Google Scholar] [CrossRef]
- Rasheed, A.; Acharya, S.; Shukla, S.; Kumar, S.; Yarappa, R.; Gupte, Y.; Hulkoti, V. High-sensitivity C-reactive protein in met-abolic healthy obesity (MHO). J. Evol. Med. Dent. Sci. 2020, 9, 443–447. Available online: https://www.jemds.com/data_pdf/sourya%20acharya-feb-17-.pdf (accessed on 6 April 2020). [CrossRef]
- Stanimirovic, J.; Radovanovic, J.; Banjac, K.; Obradovic, M.; Essack, M.; Zafirovic, S.; Gluvic, Z.; Gojobori, T.; Isenovic, E.R. Role of C-Reactive Protein in Diabetic Inflammation. Mediat. Inflamm. 2022, 2022, 3706508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, J.; Chen, J.; Lan, H.-Y.; Tang, Y. Role of C-Reactive Protein in Kidney Diseases. Kidney Dis. 2022, 9, 73–81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verma, S.; McGuire, D.K.; Bain, S.C.; Bhatt, D.L.; Leiter, L.A.; Mazer, C.D.; Fries, T.M.; Pratley, R.E.; Rasmussen, S.; Vrazic, H.; et al. Effects of glucagon-like peptide-1 receptor agonists liraglutide and semaglutide on cardiovascular and renal outcomes across body mass index categories in type 2 diabetes: Results of the LEADER and SUSTAIN 6 trials. Diabetes Obes. Metab. 2020, 22, 2487–2492. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Webb, J.; Mount, J.; von Arx, L.; Rachman, J.; Spanopoulos, D.; Wood, R.; Tritton, T.; Massey, O.; Idris, I. Cardiovascular risk profiles: A cross-sectional study evaluating the generalizability of the glucagon-like peptide-1 receptor agonist cardiovascular outcome trials REWIND, LEADER and SUSTAIN-6 to the real-world type 2 diabetes population in the United Kingdom. Diabetes Obes. Metab. 2021, 24, 289–295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mosenzon, O.; Capehorn, M.S.; De Remigis, A.; Rasmussen, S.; Weimers, P.; Rosenstock, J. Impact of semaglutide on high-sensitivity C-reactive protein: Exploratory patient-level analyses of SUSTAIN and PIONEER randomized clinical trials. Cardiovasc. Diabetol. 2022, 21, 172. [Google Scholar] [CrossRef]


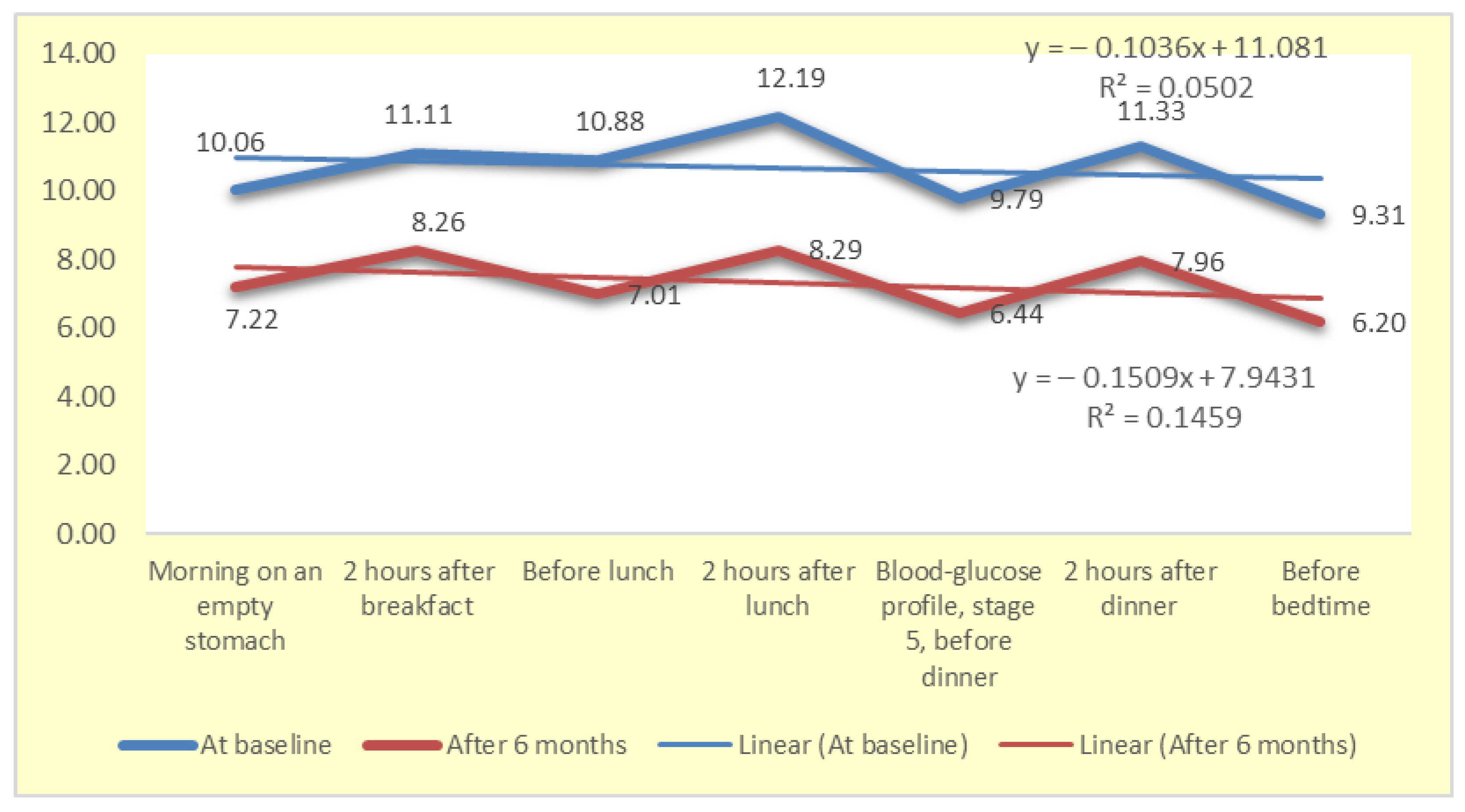

| MALB (mg/L) | Creat (mkmol/L) | eGFR (mL/min) | HbA1c (%) | HsCRP (mg/L) | |
|---|---|---|---|---|---|
| Baseline value | 43.54 | 77.00 | 88.35 | 8.18 | 4.90 |
| Value after 6 months | 44.55 | 71.08 | 94.13 | 6.50 | 1.21 |
| Beginning of Therapy | |||||||
| hsCRP (mg/L) | eGFR (mL/min 1.73 m2) | Creatinine (µmol/L) | MALB (mg/L) | HbA1c (%) | BMI | ||
| N | Valid | 70 | 70 | 70 | 70 | 70 | 70 |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mean value | 4.9025 | 4.9025 | 88.57 | 77.0033 | 43.5409 | 8.1897 | |
| Std. Deviation | 5.2119 | 5.2119 | 17.077 | 14.56903 | 73.62230 | 0.97865 | |
| Range | 34.39 | 34.39 | 68 | 56.00 | 399.00 | 4.00 | |
| Minimum | 0.44 | 0.44 | 50 | 55.00 | 1.00 | 7.50 | |
| Maximum | 34.83 | 34.83 | 118 | 111.00 | 400.00 | 11.50 | |
| Results After 6 Months | |||||||
| hsCRP (mg/L) | eGFR (mL/min 1.73 m2) | Creatinine (µmol/L) | MALB (mg/L) | HbA1c (%) | BMI | ||
| N | Valid | 70 | 70 | 70 | 70 | 70 | 70 |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mean | 2.2304 | 94.13 | 71.0876 | 44.5559 | 6.5036 | 35.5551 | |
| Std. Deviation | 2.2172 | 16.154 | 14.14804 | 87.54986 | 1.19244 | 5.72462 | |
| Range | 12.35 | 77 | 69.00 | 452.17 | 4.90 | 28.40 | |
| Minimum | 0.45 | 57 | 37.00 | 1.00 | 4.90 | 26.97 | |
| Maximum | 12.80 | 134 | 106.00 | 453.17 | 9.80 | 55.37 | |
| Correlations | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Duration of Diabetes | BMI | hsCRP (mg/L) | eGFR (mL/min 1.73 m2) | Creatinine (µmol/L) | MALB (mg/B) | HbA1c (%) | ||
| Age | Pearson’s Correlation | 1 | 0.411 ** | −0.222 | −0.020 | −0.421 ** | 0.072 | −0.043 | −0.187 |
| Sig. (2-tailed) | 0.000 | 0.065 | 0.872 | 0.000 | 0.554 | 0.725 | 0.122 | ||
| Duration of diabetes | Pearson’s Correlation | 0.411 ** | 1 | −0.157 | −0.118 | −0.058 | −0.041 | −0.023 | 0.172 |
| Sig. (2-tailed) | 0.000 | 0.194 | 0.335 | 0.635 | 0.736 | 0.852 | 0.154 | ||
| BMI | Pearson’s Correlation | −0.222 | −0.157 | 1 | 0.045 | −0.058 | 0.170 | −0.011 | −0.021 |
| Sig. (2-tailed) | 0.065 | 0.194 | 0.711 | 0.636 | 0.159 | 0.927 | 0.866 | ||
| hsCRP (mg/L) | Pearson’s Correlation | −0.020 | −0.118 | 0.045 | 1 | −0.294 * | 0.354 ** | 0.035 | 0.091 |
| Sig. (2-tailed) | 0.872 | 0.335 | 0.711 | 0.014 | 0.003 | 0.774 | 0.456 | ||
| eGFR (mL/min 1.73 m2) | Pearson’s Correlation | −0.421 ** | −0.058 | −0.058 | −0.294 * | 1 | −0.776 ** | 0.193 | 0.088 |
| Sig. (2-tailed) | 0.000 | 0.635 | 0.636 | 0.014 | 0.000 | 0.109 | 0.467 | ||
| Creatinine (µmol/L) | Pearson’s Correlation | 0.072 | −0.041 | 0.170 | 0.354 ** | −0.776 ** | 1 | −0.078 | 0.136 |
| Sig. (2-tailed) | 0.554 | 0.736 | 0.159 | 0.003 | 0.000 | 0.520 | 0.263 | ||
| MALB (mg/L) | Pearson’s Correlation | −0.043 | −0.023 | −0.011 | 0.035 | 0.193 | −0.078 | 1 | 0.000 |
| Sig. (2-tailed) | 0.725 | 0.852 | 0.927 | 0.774 | 0.109 | 0.520 | 0.998 | ||
| HbA1c (%) | Pearson’s Correlation | −0.187 | 0.172 | −0.021 | 0.091 | 0.088 | 0.136 | 0.000 | 1 |
| Sig. (2-tailed) | 0.122 | 0.154 | 0.866 | 0.456 | 0.467 | 0.263 | 0.998 | ||
| N | 70 | 70 | 70 | 69 | 70 | 70 | 70 | 70 | |
| Parameters | Linear Correlation Dependence | R | R2 | Std. Error of the Estimate | F | Sig. (p-Value) |
|---|---|---|---|---|---|---|
| X—Age Y—Duration of diabetes | Y = −6.521 + 0.217 × X | 0.411 | 0.169 | 4.876 | 13.842 | 0.000 |
| X—Age Y—eGFR | Y = 127.618 − 0.716 marked with yellow color × X | −0.421 | 0.178 | 15.600 | 14.681 | 0.000 |
| X—hsCRP Y—eGFR | Y = 92.236 − 0.803 × X | −0.246 | 0.061 | 16.632 | 3.994 | 0.050 |
| X—hsCRP Y—Creatinine | Y = 72.099 + 0.971 × X | 0.354 | 0.125 | 14.224 | 9.596 | 0.003 |
| X—eGFR Y—Creatinine | Y = 137.453 − 0.681 × X | −0.776 | 0.602 | 9.528 | 102.796 | 0.000 |
| Correlations | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Duration of Diabetes | BMI | hsCRP (mg/L) | eGFR (mL/min 1.73 m2) | Creatinine (µmol/L) | MALB (mg/L) | HbA1c (%) | ||
| Age | Pearson’s Correlation | 1 | 0.411 ** | −0.045 | 0.058 | −0.384 ** | 0.030 | −0.027 | 0.044 |
| Sig. (2-tailed) | 0.000 | 0.713 | 0.632 | 0.001 | 0.805 | 0.821 | 0.717 | ||
| Duration of diabetes | Pearson’s Correlation | 0.411 ** | 1 | −0.044 | 0.084 | −0.144 | 0.017 | 0.031 | 0.394 ** |
| Sig. (2-tailed) | 0.000 | 0.720 | 0.490 | 0.233 | 0.889 | 0.798 | 0.001 | ||
| BMI | Pearson’s Correlation | −0.045 | −0.044 | 1 | 0.179 | −0.044 | 0.108 | 0.082 | 0.329 ** |
| Sig. (2-tailed) | 0.713 | 0.720 | 0.139 | 0.716 | 0.373 | 0.499 | 0.005 | ||
| hsCRP (mg/L) | Pearson’s Correlation | 0.058 | 0.084 | 0.179 | 1 | −0.137 | 0.082 | 0.211 | 0.233 |
| Sig. (2-tailed) | 0.632 | 0.490 | 0.139 | 0.259 | 0.499 | 0.080 | 0.052 | ||
| eGFR (mL/min 1.73 m2) | Pearson’s Correlation | −0.384 ** | −0.144 | −0.044 | −0.137 | 1 | −0.759 ** | 0.247 * | 0.060 |
| Sig. (2-tailed) | 0.001 | 0.233 | 0.716 | 0.259 | 0.000 | 0.039 | 0.619 | ||
| Creatinine (µmol/L) | Pearson’s Correlation | 0.030 | 0.017 | 0.108 | 0.082 | −0.759 ** | 1 | −0.174 | −0.146 |
| Sig. (2-tailed) | 0.805 | 0.889 | 0.373 | 0.499 | 0.000 | 0.150 | 0.227 | ||
| MALB (mg/L) | Pearson’s Correlation | −0.027 | 0.031 | 0.082 | 0.211 | 0.247 * | −0.174 | 1 | 0.205 |
| Sig. (2-tailed) | 0.821 | 0.798 | 0.499 | 0.080 | 0.039 | 0.150 | 0.088 | ||
| HbA1c (%) | Pearson’s Correlation | 0.044 | 0.394 ** | 0.329 ** | 0.233 | 0.060 | −0.146 | 0.205 | 1 |
| Sig. (2-tailed) | 0.717 | 0.001 | 0.005 | 0.052 | 0.619 | 0.227 | 0.088 | ||
| N | 70 | 70 | 70 | 70 | 70 | 70 | 70 | 70 | |
| Parameters | Linear Correlation Dependence | R | R2 | Std. Error of the Estimate | F | Sig. (p-Value) |
|---|---|---|---|---|---|---|
| X—Age Y—Duration of diabetes | Y = −6.521 + 0.217 × X | 0.411 | 0.169 | 4.876 | 13.842 | 0.000 |
| X—Age Y—eGFR | Y = 127.809 − 0.618 × X | −0.384 | 0.148 | 15.023 | 11.778 | 0.001 |
| X—Duration of diabetes Y—HbA1c | Y = 6.032 + 0.089 × X | 0.394 | 0.155 | 1.104 | 12.503 | 0.001 |
| X—BMI Y—HbA1c | Y = 4.067 + 0.069 × X | 0.329 | 0.108 | 1.134 | 8.257 | 0.005 |
| X—Creatinine Y—eGFR | Y = 155.763 − 0.867 × X | −0.759 | 0.557 | 10.588 | 92.614 | 0.000 |
| X—MALB Y—eGFR | Y = 92.099 + 0.046 × X | 0.247 | 0.061 | 15.769 | 4.411 | 0.039 |
| Coefficients a | ||||||
|---|---|---|---|---|---|---|
| Model | Unstandardized Coefficients | Standardized Coefficients | t | Sig. | ||
| B | Std. Error | Beta | ||||
| 1 | (Constant) | −0.125 | 0.873 | −0.143 | 0.887 | |
| BMI | 0.068 | 0.026 | 0.198 | 2.607 | 0.010 | |
| 2 | (Constant) | 1.359 | 1.041 | 1.305 | 0.194 | |
| BMI | 0.075 | 0.026 | 0.217 | 2.896 | 0.004 | |
| eGFR | −0.020 | 0.008 | −0.189 | −2.524 | 0.013 | |
| Model Summary | ||||
|---|---|---|---|---|
| Model | R | R Square | Adjusted R Square | Std. Error of the Estimate |
| 1 | 0.198 a | 0.039 | 0.033 | 1.99348 |
| 2 | 0.273 b | 0.075 | 0.063 | 1.96219 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostadinov, N.K.; Totomirova, T.; Nonchev, B.I. Semaglutide Therapy and Cardiorenal Risk Management in Type 2 Diabetes: hsCRP as a Biomarker of Risk. Diabetology 2025, 6, 142. https://doi.org/10.3390/diabetology6120142
Kostadinov NK, Totomirova T, Nonchev BI. Semaglutide Therapy and Cardiorenal Risk Management in Type 2 Diabetes: hsCRP as a Biomarker of Risk. Diabetology. 2025; 6(12):142. https://doi.org/10.3390/diabetology6120142
Chicago/Turabian StyleKostadinov, Nikolay Krasimirov, Tcvetelina Totomirova, and Boyan Ivanov Nonchev. 2025. "Semaglutide Therapy and Cardiorenal Risk Management in Type 2 Diabetes: hsCRP as a Biomarker of Risk" Diabetology 6, no. 12: 142. https://doi.org/10.3390/diabetology6120142
APA StyleKostadinov, N. K., Totomirova, T., & Nonchev, B. I. (2025). Semaglutide Therapy and Cardiorenal Risk Management in Type 2 Diabetes: hsCRP as a Biomarker of Risk. Diabetology, 6(12), 142. https://doi.org/10.3390/diabetology6120142





