Performance of the Triglyceride-Glucose (TyG) Index for Early Detection of Insulin Resistance in Young Adults: Comparison with HOMA-IR and QUICKI in Western Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Clinical and Anthropometric Determinations
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
3.1. Descriptive Analysis
3.2. Correlation Analysis
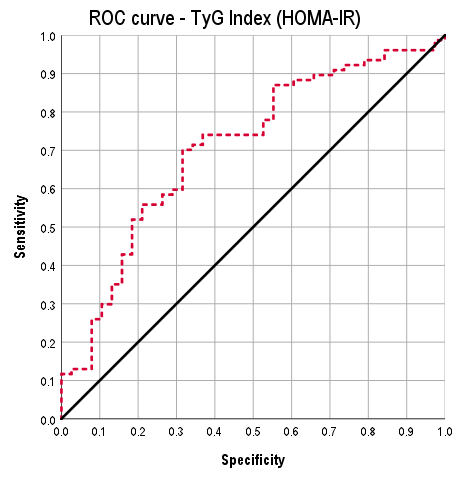
3.3. ROC Analysis
3.4. Test Predictors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| AUC | Area under the curve |
| BMI | Body mass index |
| CI | Confidence interval |
| ELISA | Enzyme-linked immunosorbent assay |
| HDL | High-density lipoprotein |
| HOMA-IR | Homeostatic model assessment of insulin resistance |
| IR | Insulin resistance |
| LDL | Low-density lipoprotein |
| NPV | Negative predictive value |
| PPV | Positive predictive value |
| QUICKI | Quantitative insulin sensitivity check index |
| ROC | Receiver operating characteristic |
| SD | Standard deviation |
| SE | Standard error |
| TyG | Triglyceride–glucose index |
| VLDL | Very-low density lipoprotein |
References
- Basto-Abreu, A.; López-Olmedo, N.; Rojas-Martínez, R.; Aguilar-Salinas, C.A.; Moreno-Banda, G.L.; Carnalla, M.; Barrientos-Gutiérrez, T.; De la Cruz-Góngora, V.; Durán-Acevedo, M.E.; Hernández-Ávila, M.; et al. Prevalence of prediabetes and diabetes in Mexico: ENSANUT 2022. Salud Publica Mex. 2023, 65 (Suppl. S1), s163–s168. [Google Scholar] [CrossRef]
- James, D.E.; Stöckli, J.; Birnbaum, M.J. The aetiology and molecular landscape of insulin resistance. Nat. Rev. Mol. Cell Biol. 2021, 22, 751–771. [Google Scholar] [CrossRef]
- Khalid, M.; Alkaabi, J.; Khan, M.A.B.; Adem, A. Insulin Signal Transduction Perturbations in Insulin Resistance. Int. J. Mol. Sci. 2021, 22, 8590. [Google Scholar] [CrossRef]
- Placzkowska, S.; Pawlik-Sobecka, L.; Kokot, I.; Piwowar, A. Indirect insulin resistance detection: Current clinical trends and laboratory limitations. Biomed. Pap. 2019, 163, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Tahapary, D.L.; Pratisthita, L.B.; Fitri, N.A.; Marcella, C.; Wafa, S.; Kurniawan, F.; Rizka, A.; Tarigan, T.J.E.; Harbuwono, D.S.; Purnamasari, D.; et al. Challenges in the diagnosis of insulin resistance: Focusing on the role of HOMA-IR and triglyceride/glucose index. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102581. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; González-Ortiz, M.; Martínez-Abundis, E.; Ramos-Zavala, M.G.; Hernández-González, S.O.; Jacques-Camarena, O.; Rodríguez-Morán, M.; Villalobos-Molina, R.; Méndez-Cruz, R.; et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity: Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef]
- Sodero, G.; Rigante, D.; Pane, L.C.; Sessa, L.; Quarta, L.; Candelli, M.; Cipolla, C.; Zanza, C.; Pignataro, G.; Franceschi, F.; et al. Cardiometabolic risk assessment in a cohort of children and adolescents diagnosed with hyperinsulinemia. Diseases 2024, 12, 119. [Google Scholar] [CrossRef]
- Felipe Pollak, C. Resistencia a la insulina: Verdades y controversias. Rev. Médica Clínica Las Condes 2016, 27, 171–178. [Google Scholar] [CrossRef]
- Santos Lozano, E. Resistencia a Insulina: Revisión de literatura. Rev. Med. Hondur. 2022, 90, 63–70. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef]
- NOM-012-SSA3-2012; Mexican Official Standard for the Ethical Conduct of Health Research Involving Human Subjects. Secretaría de Salud: Mexico City, Mexico, 2012.
- Vega-Cárdenas, M.; Flores-Sánchez, J.; Torres-Rodríguez, M.L.; Sánchez-Armáss, O.; Vargas-Morales, J.M.; Cossío-Torres, P.E.; Terán-García, M.; Aradillas-García, C.; UP-AMIGOS Team. Distribution of triglycerides and glucose (TyG) index and homeostasis model assessment insulin resistance for the evaluation of insulin sensitivity on late adolescence in Mexicans. Nutr. Hosp. 2022, 39, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jaramillo, P.; Gomez-Arbelaez, D.; Martinez-Bello, D.; Abat, M.E.M.; Alhabib, K.F.; Avezum, Á.; Barbarash, O.; Chifamba, J.; Diaz, M.L.; Gulec, S.; et al. Association of the triglyceride-glucose index as a measure of insulin resistance with mortality and cardiovascular disease in populations from five continents (PURE study): A prospective cohort study. Lancet Healthy Longev. 2023, 4, e23–e33. [Google Scholar] [CrossRef]
- Molavizadeh, D.; Cheraghloo, N.; Tohidi, M.; Azizi, F.; Hadaegh, F. The association between index-year, average, and variability of the triglyceride-glucose index with health outcomes: More than a decade of follow-up in the Tehran Lipid and Glucose Study. Cardiovasc. Diabetol. 2024, 23, 321. [Google Scholar] [CrossRef]
- Unger, G.; Benozzi, S.F.; Perruzza, F.; Pennacchiotti, G.L. Índice triglicéridos y glucosa: Un indicador útil de insulinorresistencia. Endocrinol. Nutr. 2014, 61, 533–540. [Google Scholar] [CrossRef]
- Paramanathan, T.; Sandrasegarampillai, B.; Arasaratnam, V.; Thirunavukarasu, K.; Gunasekaran, M.; Sivananthan, S.; Kumar, S.; Rajendra, S.; Hemachandra, N.; Sinnadurai, K.; et al. The discriminative ability of the triglyceride-glucose index to identify metabolic syndrome among adults of the northern Sri Lankan population. BMC Endocr. Disord. 2024, 24, 101. [Google Scholar] [CrossRef]
- Lelis, D.F.; Baldo, T.O.F.; Andrade, J.M.O.; Griep, R.H.; Bensenor, I.M.; Lotufo, P.A.; Santos, R.D.; Oliveira, P.P.; Silva, J.F.; Almeida, M.B.; et al. High triglyceride-glucose index and HOMA-IR are associated with different cardiometabolic profile in adults from the ELSA-Brasil study. Clin. Biochem. 2024, 131–132, 110793. [Google Scholar] [CrossRef]
- Cho, W.; Seo, M.-W.; Rosenberg, J.; Kim, J.Y. Single point insulin sensitivity estimator index for identifying metabolic syndrome in US adults: NHANES 2017-March 2020. Obes. Res. Clin. Pract. 2024, 18, 280–285. [Google Scholar] [CrossRef]
- Wan, H.; Cao, H.; Ning, P. Superiority of the triglyceride-glucose index over the homeostasis model in predicting metabolic syndrome based on NHANES data analysis. Sci. Rep. 2024, 14, 15499. [Google Scholar] [CrossRef]
- Alizargar, J.; Hsieh, N.-C.; Wu, S.-F.V. The correct formula to calculate triglyceride-glucose index (TyG). J. Pediatr. Endocrinol. Metab. 2020, 33, 945–946. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, K. Mini-review on insulin resistance assessment: Advances in surrogate indices and clinical applications. World J. Clin. Cases 2025, 13, 108380. [Google Scholar] [CrossRef] [PubMed]
- Almeda-Valdés, P.; Bello-Chavolla, O.Y.; Caballeros-Barragán, C.R.; Gómez-Velasco, D.V.; Viveros-Ruiz, T.; Vargas-Vázquez, A.; Aguilar-Salinas, C.A. Índices para la evaluación de la resistencia a la insulina en individuos mexicanos sin diabetes. Gac. Med. Mex. 2018, 154 (Suppl. S2), S50–S55. [Google Scholar] [CrossRef] [PubMed]


| (N (%) or Mean ± SD) | |
|---|---|
| Number of participants | 115 |
| Demographic variables | |
| Age (years) | 21.13 ± 4.58 |
| Sex | |
| Male | 37 (32.2) |
| Female | 78 (67.8) |
| Anthropometric characteristics | |
| Height (m) | 1.64 ± 0.08 |
| Weight (kg) | 69.13 ± 18.25 |
| Body mass index (BMI) | 25.38 ± 5.92 |
| Underweight | 7 (6.1) |
| Normal weight | 62 (53.9) |
| Overweight | 25 (21.7) |
| Obesity class I | 11 (9.6) |
| Obesity class II | 8 (7.0) |
| Obesity class III | 2 (1.7) |
| Waist circumference (cm) | 83.06 ± 13.38 |
| Hip circumference (cm) | 100.89 ± 11.92 |
| Waist-to-hip ratio | 0.82 ± 0.09 |
| Body fat percentage | 29.98 ± 27.90 |
| Muscle mass percentage | 46.65 ± 10.91 |
| Visceral fat | 3.70 ± 3.23 |
| (N (%) or Mean ± SD) | |
|---|---|
| Number of participants | 115 |
| Biochemical variables | |
| Glucose (mg/dL) | 88.43 ± 18.79 |
| Lipoproteins (mg/dL) | |
| Total cholesterol | 169.77 ± 54.15 |
| Triglycerides | 97.49 ± 50.68 |
| Insulin (µU/mL) | 21.97 ± 20.88 |
| Calculated indices | |
| HOMA-IR | 5.09 ± 6.91 |
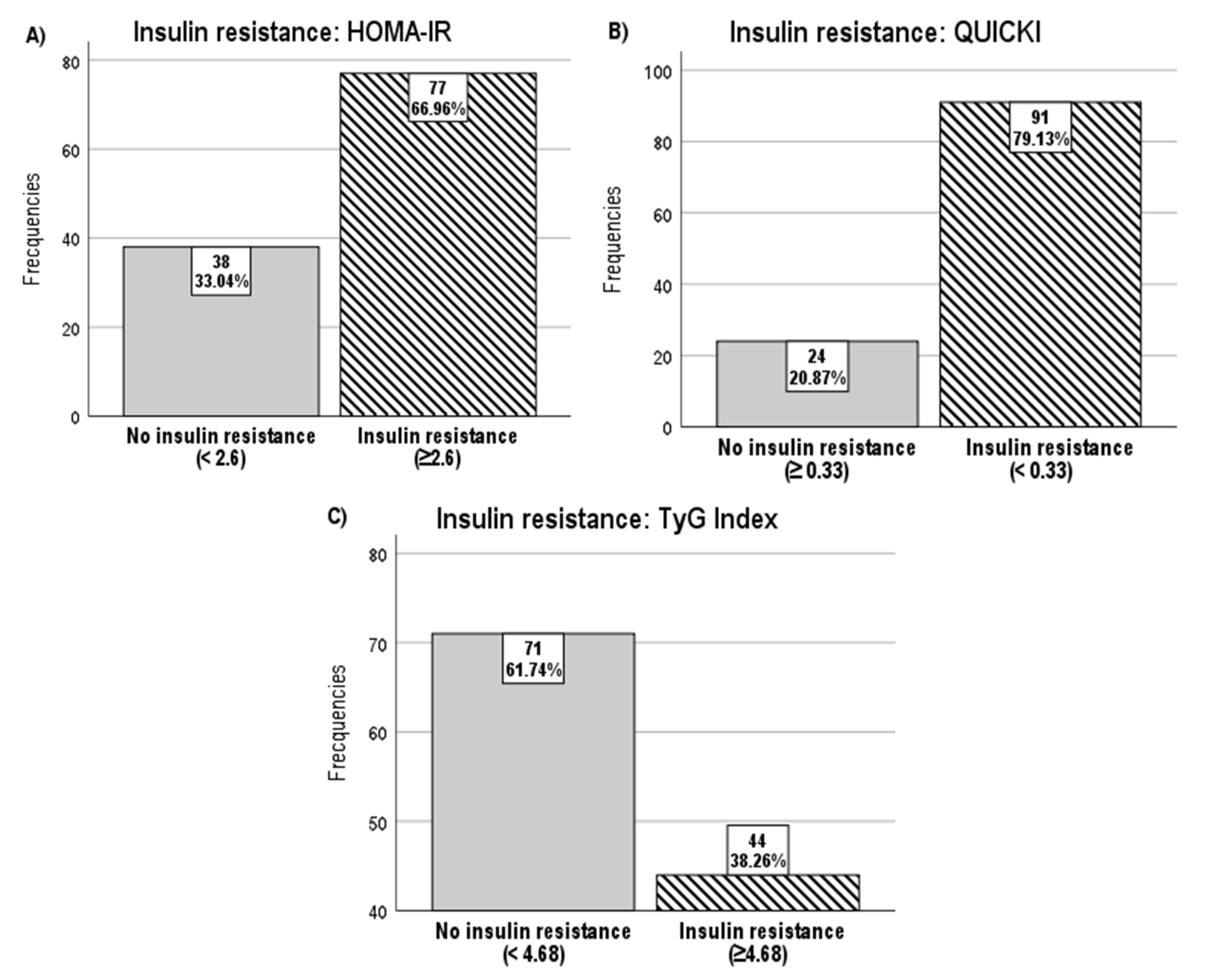
| With IR | 77 (66.96) |
| Without IR | 38 (33.04) |
| TyG index | 4.46 ± 0.26 |
| With IR | 25 (21.7) |
| Without IR | 90 (78.3) |
| QUICKI index | 0.31 ± 0.03 |
| With IR | 91 (79.13) |
| Without IR | 24 (20.87) |
| Variable | TyG Index (ρ, p) | QUICKI Index (ρ, p) | HOMA-IR Index (ρ, p) |
|---|---|---|---|
| Weight | 0.375, p < 0.001 * | −0.292, p = 0.002 * | 0.292, p = 0.002 * |
| BMI | 0.363, p < 0.001 * | −0.305, p = 0.001 * | 0.305, p = 0.001 * |
| Total cholesterol | 0.525, p < 0.001 * | −0.307, p = 0.001 * | 0.308, p = 0.001 * |
| Waist circumference | 0.473, p < 0.001 * | −0.231, p = 0.044 * | 0.231, p = 0.044 * |
| Hip circumference | 0.341, p = 0.002 * | −0.220, p = 0.053 | 0.220, p = 0.053 |
| Body fat percentage | 0.194, p = 0.042 * | −0.174, p = 0.069 | 0.174, p = 0.069 |
| Muscle mass percentage | 0.317, p = 0.001 * | −0.204, p = 0.033 * | 0.204, p = 0.033 * |
| Visceral fat | 0.337, p < 0.001 * | −0.282, p = 0.003 * | 0.282, p = 0.003 * |
| Age | 0.118, p = 0.209 | −0.018, p = 0.850 | 0.019, p = 0.843 |
| Height | 0.148, p = 0.115 | −0.070, p = 0.459 | 0.069, p = 0.462 |
| Comparator Index | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| HOMA-IR | 70.1 | 68.4 | 81.8 | 53.1 |
| QUICKI | 67.0 | 79.2 | 92.4 | 38.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reynoso-Roa, A.S.; Gutiérrez-Rubio, S.A.; Castillo-Romero, A.; García-Iglesias, T.; Suárez-Rico, D.O.; Becerra-Orduñez, K.M.; Temblador-Dominguez, C.A.; García-Cobián, T.A. Performance of the Triglyceride-Glucose (TyG) Index for Early Detection of Insulin Resistance in Young Adults: Comparison with HOMA-IR and QUICKI in Western Mexico. Diabetology 2025, 6, 141. https://doi.org/10.3390/diabetology6110141
Reynoso-Roa AS, Gutiérrez-Rubio SA, Castillo-Romero A, García-Iglesias T, Suárez-Rico DO, Becerra-Orduñez KM, Temblador-Dominguez CA, García-Cobián TA. Performance of the Triglyceride-Glucose (TyG) Index for Early Detection of Insulin Resistance in Young Adults: Comparison with HOMA-IR and QUICKI in Western Mexico. Diabetology. 2025; 6(11):141. https://doi.org/10.3390/diabetology6110141
Chicago/Turabian StyleReynoso-Roa, Africa Samantha, Susan Andrea Gutiérrez-Rubio, Araceli Castillo-Romero, Trinidad García-Iglesias, Daniel Osmar Suárez-Rico, Karen Marcela Becerra-Orduñez, Cynthia Areli Temblador-Dominguez, and Teresa Arcelia García-Cobián. 2025. "Performance of the Triglyceride-Glucose (TyG) Index for Early Detection of Insulin Resistance in Young Adults: Comparison with HOMA-IR and QUICKI in Western Mexico" Diabetology 6, no. 11: 141. https://doi.org/10.3390/diabetology6110141
APA StyleReynoso-Roa, A. S., Gutiérrez-Rubio, S. A., Castillo-Romero, A., García-Iglesias, T., Suárez-Rico, D. O., Becerra-Orduñez, K. M., Temblador-Dominguez, C. A., & García-Cobián, T. A. (2025). Performance of the Triglyceride-Glucose (TyG) Index for Early Detection of Insulin Resistance in Young Adults: Comparison with HOMA-IR and QUICKI in Western Mexico. Diabetology, 6(11), 141. https://doi.org/10.3390/diabetology6110141







