Efficacy of Hyperbaric Oxygen Therapy in Diabetic Retinopathy and Macular Edema: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
1.1. Rationale
1.2. Background
1.3. Objectives
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Data Extraction
2.4. Certainty of Evidence Assessment
2.5. Statistical Analysis
2.5.1. Data Transformation and Standardization
- logMAR = 1.0 − (ETDRS Letters/50).
- From Snellen Equivalents [21]:logMAR = −log10(Snellen Fraction).
- From IQR:SD = IQR/1.35.
- From Range:SD = Range/4.
2.5.2. Meta-Analytic Methodology
- Model: Random Effects Model, selected to address clinical and methodological heterogeneity across studies.
- Method: Inverse Variance, appropriate for continuous data such as BCVA.
- Summary Measure: Mean Difference (MD), summarizing the difference in logMAR values between HBOT and control groups after standardization.
2.5.3. Heterogeneity Assessment
- Studies comparing HBOT versus placebo/control were analyzed as one group.
- Studies combining HBOT with pharmacological treatments (e.g., aflibercept) were treated as a distinct subgroup.
- Low heterogeneity: I2 < 25%;
- Moderate heterogeneity: 25% ≤ I2 < 75%;
- High heterogeneity: I2 ≥ 75%;
- Cochran’s Q test was used to assess the statistical significance of heterogeneity.
2.5.4. Handling Missing Standard Deviations for CMT
2.5.5. Software and Statistical Rationale
3. Results
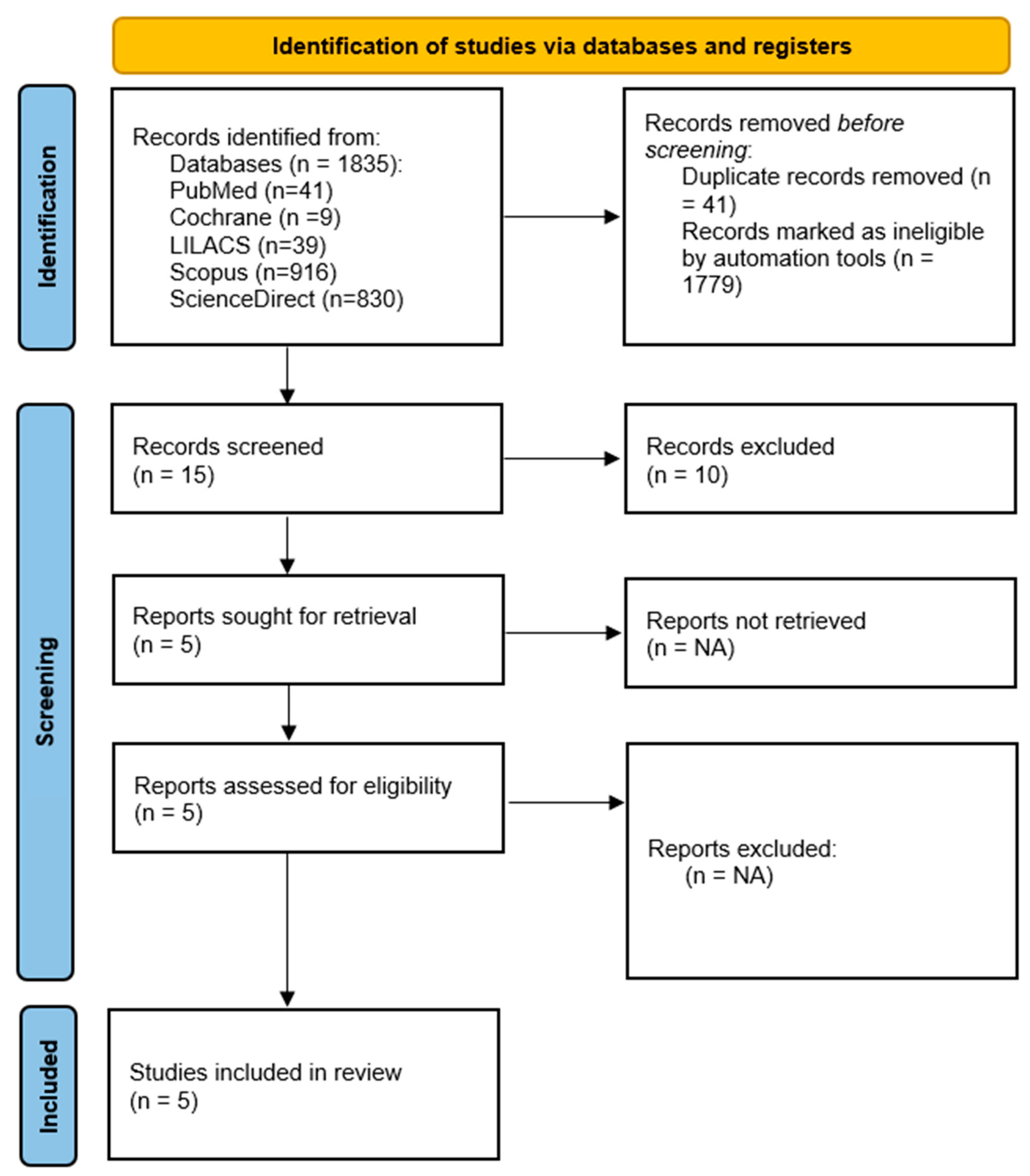
3.1. Study Selection
3.2. Study Characteristics
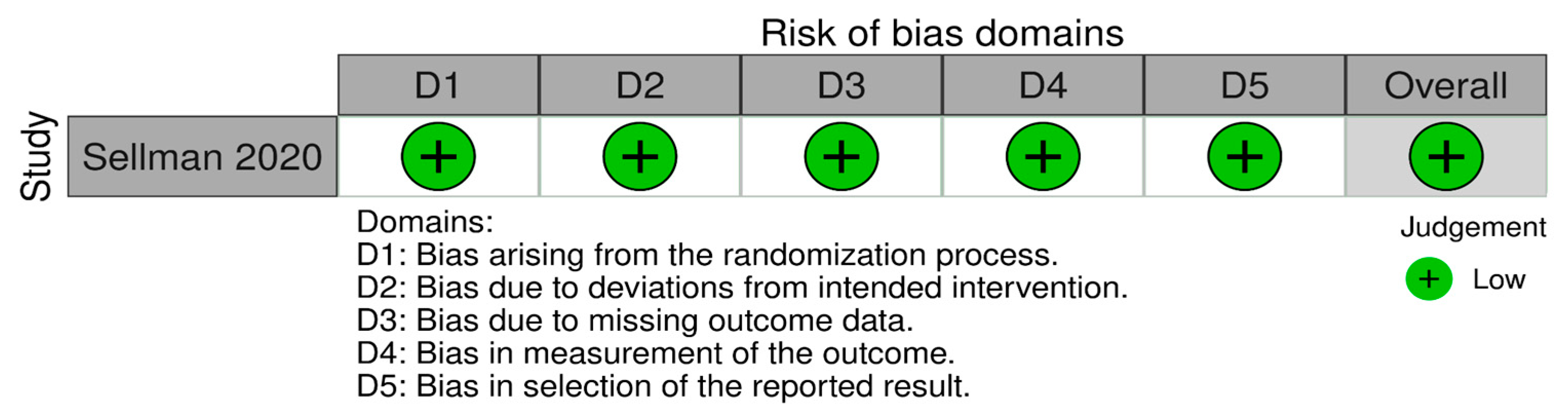
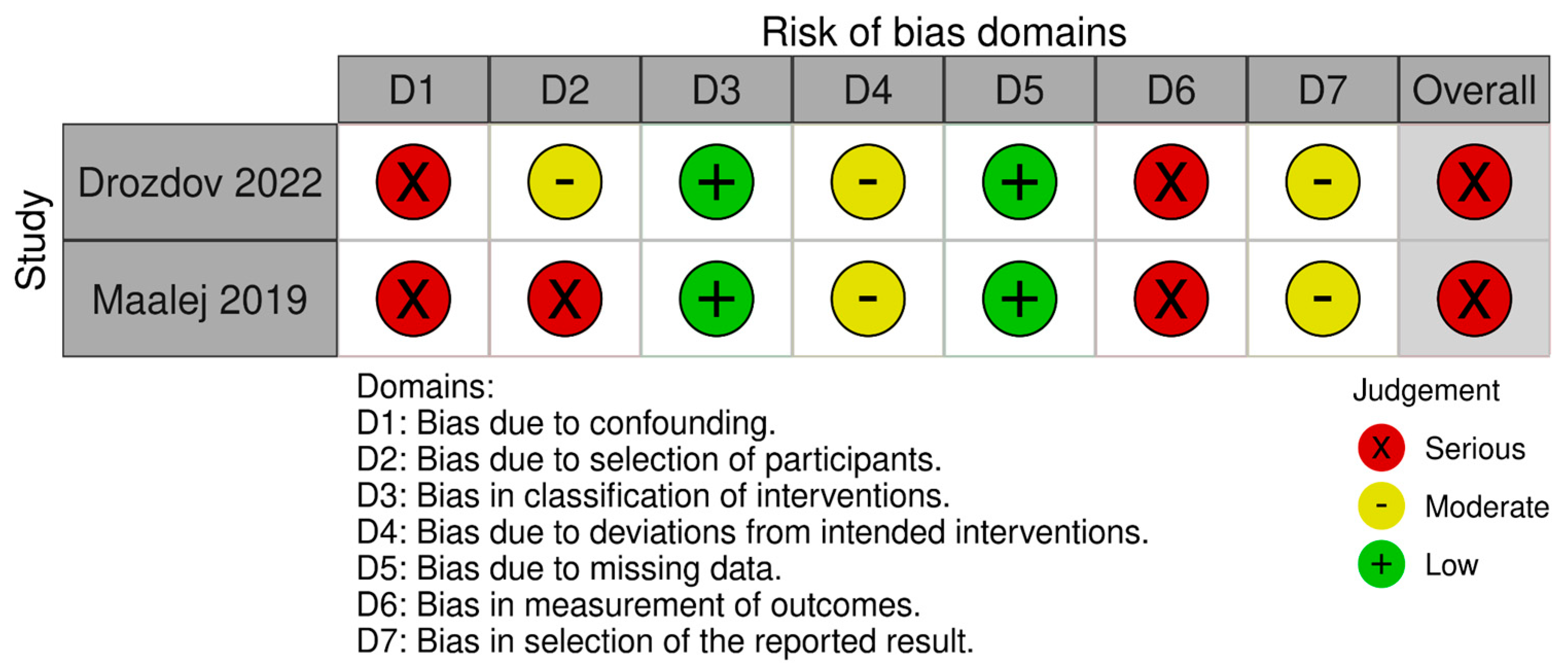
3.3. Risk of Bias
3.3.1. Risk of Bias in Randomized Studies
3.3.2. Risk of Bias in Non-Randomized Studies
3.3.3. Risk of Bias in Before–After Studies
3.4. Subgroup Analyses and Synthetic Control Group Estimation
3.4.1. Pre-Specified Sub-Groups
- To minimize clinical heterogeneity, two meta-analytic strata were defined as a priori:HBOT + pharmacologic agent—studies delivering hyperbaric oxygen therapy (HBOT) together with an intravitreal drug (anti-VEGF or implant).
- HBOT without an internal control—prospective cohorts lacking a true comparator arm; here a synthetic control arm was generated, as detailed below.
3.4.2. Source of the Synthetic Baseline
3.4.3. Ethnicity Rationale
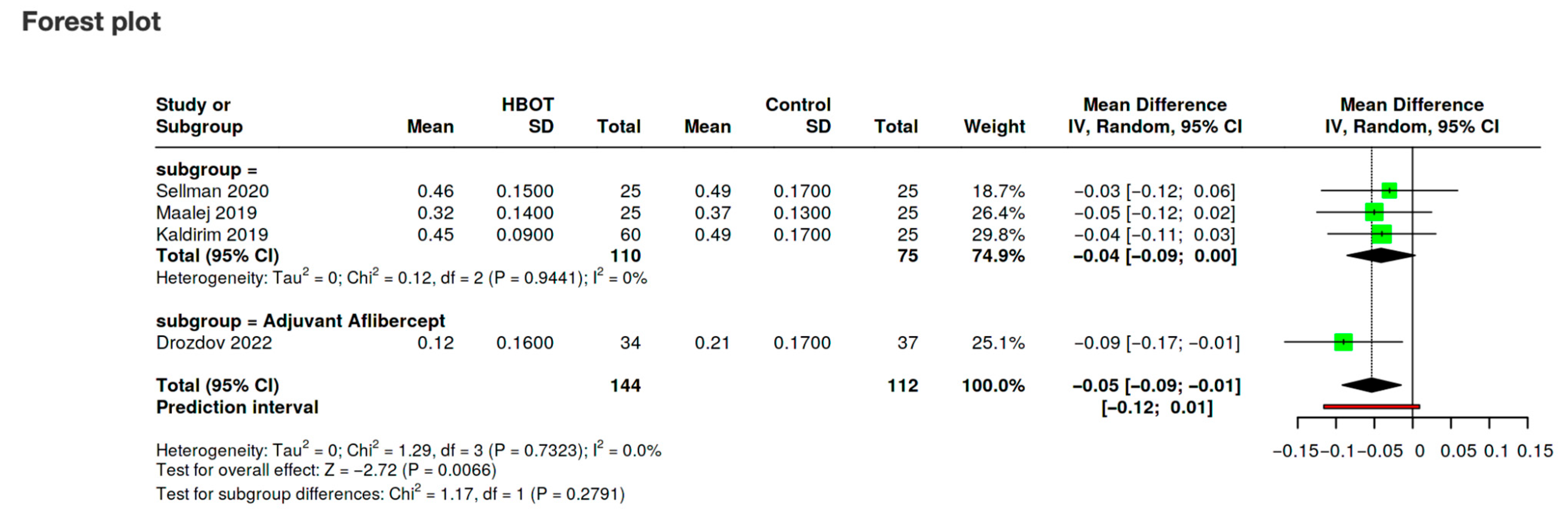
3.5. Synthesis of Results
Certainty of the Evidence (GRADE)
3.6. Effects of Interventions
3.6.1. Visual Acuity
- HBOT vs. Control: Studies directly comparing HBOT to placebo or control interventions.
- HBOT + Pharmacological Therapy vs. Pharmacological Therapy Alone: Studies evaluating HBOT as an adjunctive treatment with drugs like aflibercept.
3.6.2. Central Macular Thickness
4. Discussion
4.1. Summary of Evidence
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DR | Diabetic retinopathy |
| DME | Diabetic macular edema |
| DM | Diabetes mellitus |
| HBOT | Hyperbaric oxygen therapy |
| BCVA | Best corrected visual acuity |
| CMT | Central macular thickness |
| NPDR | Non-proliferative diabetic retinopathy |
| PDR | Proliferative diabetic retinopathy |
| ERM | Epiretinal membrane |
| PKC | protein kinase C |
| AGE | Advanced glycation end-product |
| ROS | Reactive oxygen species |
| BRB | Blood–retinal barrier |
| VEGF | Vascular endothelial growth factor |
| HIF | Hypoxia inducible factor |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GSR | Glutathione reductase |
| ATA | Atmospheres absolute |
| GRADE | Grading of Recommendations Assessment, Development and Evaluation |
| IQRs | Interquartile ranges |
| SDs | Standard deviations |
| MD | Mean difference |
| I2 | Heterogeneity |
| CSME | Clinically significant macular edema |
| kPA | Kilopascal |
| ETDRS | Early Treatment of Diabetic Retinopathy Study |
| CT | Choroidal thickness |
| CI | Confidence interval |
References
- Al-Kharashi, A.S. Role of oxidative stress, inflammation, hypoxia and angiogenesis in the development of diabetic retinopathy. Saudi J. Ophthalmol. 2018, 32, 318–323. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Barber, A.J.; Bronson, S.K.; Freeman, W.M.; Gardner, T.W.; Jefferson, L.S.; Kester, M.; Kimball, S.R.; Krady, J.K.; LaNoue, K.F.; et al. Diabetic Retinopathy. Diabetes 2006, 55, 2401–2411. [Google Scholar] [CrossRef]
- Chatziralli, I.; Touhami, S.; Cicinelli, M.V.; Agapitou, C.; Dimitriou, E.; Theodossiadis, G.; Theodossiadis, P. Disentangling the association between retinal non-perfusion and anti-VEGF agents in diabetic retinopathy. Eye 2022, 36, 692–703. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martinez, O.; García-Montero, C.; Callejón-Peláez, E.; Sáez, M.A.; Álvarez-Mon, M.A.; García-Honduvilla, N.; Monserrat, J.; Álvarez-Mon, M.; Bujan, J.; et al. A General Overview on the Hyperbaric Oxygen Therapy: Applications, Mechanisms and Translational Opportunities. Medicina 2021, 57, 864. [Google Scholar] [CrossRef]
- Gnanasambandam, B.; Prince, J.; Limaye, S.; Moran, E.; Lee, B.; Huynh, J.; Irudayaraj, J.; Tsipursky, M. Addressing retinal hypoxia: Pathophysiology, therapeutic innovations, and future prospects. Ther. Adv. Ophthalmol. 2024, 26, 16. [Google Scholar] [CrossRef]
- Okamoto, N. Effect of Hyperbaric Oxygen on Ophthalmic Artery Blood Velocity in Patients With Diabetic Neuropathy. Jpn. J. Ophthalmol. 1998, 42, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J. A new view of diabetic retinopathy: A neurodegenerative disease of the eye. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 283–290. [Google Scholar] [CrossRef]
- Drozdov, V.O.; Sakovych, V.M. Changes in clinical and biochemical characteristics in combination treatment for diabetic macular edema. Oftalmol. Zh. 2022, 98, 18–23. [Google Scholar] [CrossRef]
- Santos, J.M.; Mohammad, G.; Zhong, Q.A.; Kowluru, R. Diabetic Retinopathy, Superoxide Damage and Antioxidants. Curr. Pharm. Biotechnol. 2011, 12, 352–361. [Google Scholar] [CrossRef]
- Mohammad, G.; Alam, K.; Nawaz, M.I.; Siddiquei, M.M.; Mousa, A.; Abu El-Asrar, A.M. Mutual enhancement between high-mobility group box-1 and NADPH oxidase-derived reactive oxygen species mediates diabetes-induced upregulation of retinal apoptotic markers. J. Physiol. Biochem. 2015, 71, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Poulaki, V.; Le, M.L.; Koizumi, K.; Esser, C.; Janicki, H.; Schraermeyer, U.; Kociok, N.; Fauser, S.; Kirchhof, B.; et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004, 18, 1450–1452. [Google Scholar] [CrossRef]
- Romeo, G.; Liu, W.-H.; Asnaghi, V.; Kern, T.S.; Lorenzi, M. Activation of Nuclear Factor-κB Induced by Diabetes and High Glucose Regulates a Proapoptotic Program in Retinal Pericytes. Diabetes 2002, 51, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Curtis, T.M.; Gardiner, T.A.; Stitt, A.W. Microvascular lesions of diabetic retinopathy: Clues towards understanding pathogenesis? Eye 2009, 23, 1496–1508. [Google Scholar] [CrossRef]
- Zhang, S.X.; Ma, J.X. Ocular neovascularization: Implication of endogenous angiogenic inhibitors and potential therapy. Prog. Retin. Eye Res. 2007, 26, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, J.F.; Wu, L.; Sanchez, J.G.; Maia, M.; Saravia, M.J.; Fernandez, C.F.; Evans, T. Intravitreal bevacizumab (avastin) for proliferative diabetic retinopathy: 6-months follow-up. Eye 2009, 23, 117–123. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fisher, Y.L. Intravitreal bevacizumab (avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina 2006, 26, 275–278. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Piggott, T.; Morgan, R.L.; Cuello-Garcia, C.A.; Santesso, N.; Mustafa, R.A.; Meerpohl, J.J.; Schünemann, H.J. Grading of Recommendations Assessment, Development, and Evaluations (GRADE) notes: Extremely serious, GRADE’s terminology for rating down by three levels. J. Clin. Epidemiol. 2020, 120, 116–120. [Google Scholar] [CrossRef]
- Bailey, I.L.; Lovie-Kitchin, J.E. Visual acuity testing. From the laboratory to the clinic. Vis. Res. 2013, 90, 2–9. [Google Scholar] [CrossRef]
- Ferris, F.L.; Kassoff, A.; Bresnick, G.H.; Bailey, I. New Visual Acuity Charts for Clinical Research. Am. J. Ophthalmol. 1982, 94, 91–96. [Google Scholar] [CrossRef]
- Raasch, T.W.; Bailey, I.L.; Bullimore, M.A. Repeatability of Visual Acuity Measurement. Optom. Vis. Sci. 1998, 75, 342–348. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar]
- Higgins, J.P.T. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Sellman, A.; Katzman, P.; Andreasson, S.; Löndahl, M. Long-term effects of hyperbaric oxygen therapy on visual acuity and retinopathy. Undersea Hyperb. Med. 2020, 47, 423–430. [Google Scholar] [CrossRef]
- Maalej, A.; Khallouli, A.; Choura, R.; Ben Sassi, R.; Rannen, R.; Gharsallah, H. The effects of hyperbaric oxygen therapy on diabetic retinopathy: A preliminary study. J. Fr. Ophtalmol. 2020, 43, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Kaldırım, H.; Yazgan, S.; Ceylan, B.; Atalay, K. The effect of hyperbaric oxygen therapy on retinal thickness and progression of retinopathy in patients with Type 2 diabetes: A prospective cohort study. Cutan. Ocul. Toxicol. 2019, 38, 233–239. [Google Scholar] [CrossRef]
- Gün, R.D.; Gümüş, T.; Kardaş, A.S.Y.; Kardaş, G. Acute effect of hyperbaric oxygen therapy on macular and choroidal thickness in patients with type 2 diabetes and diabetic foot ulcers: Optical coherence tomography based study. Photodiagnosis Photodyn. Ther. 2022, 39, 102926. [Google Scholar] [CrossRef] [PubMed]
- Querques, G.; Lattanzio, R.; Querques, L.; Del Turco, C.; Forte, R.; Pierro, L.; Souied, E.H.; Bandello, F. Enhanced Depth Imaging Optical Coherence Tomography in Type 2 Diabetes. Investig. Opthalmology Vis. Sci. 2012, 53, 6017. [Google Scholar] [CrossRef]
- Gopalakrishnan, M.; Appukuttan, B.; Giridhar, A.; Sivaprasad, S. Normative spectral domain optical coherence tomography data on macular and retinal nerve fiber layer thickness in Indians. Indian J. Ophthalmol. 2014, 62, 316. [Google Scholar] [CrossRef]
- Shen, L.; Gao, F.; Xu, X.; Lin, Z.; Zhang, Z.; Zhao, B.; Zhang, X.; Li, B.; Jonas, J.B. Macular thickness in Chinese. Acta Ophthalmol. 2013, 91, e77–e79. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Bhisitkul, R.B.; Shapiro, H.; Rubio, R.G. Vascular Endothelial Growth Factor Promotes Progressive Retinal Nonperfusion in Patients with Retinal Vein Occlusion. Ophthalmology 2013, 120, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Yeo, J.H.; Son, G.; Kang, H.; Sung, Y.S.; Lee, J.Y.; Kim, J.-G.; Yoon, Y.H. Efficacy of intravitreal AFlibercept injection for Improvement of retinal Nonperfusion in diabeTic retinopathY (AFFINITY study). BMJ Open Diabetes Res. Care. 2020, 8, e001616. [Google Scholar] [CrossRef] [PubMed]
- Terui, T.; Kondo, M.; Sugita, T.; Ito, Y.; Kondo, N.; Ota, I.; Miyake, K.; Terasaki, H. Changes in areas of capillary nonperfusion after intravitreal injection of bevacizumab in eyes with branch retinal vein occlusion. Retina 2011, 31, 1068–1074. [Google Scholar] [CrossRef]
- Chung, E.J.; Roh, M.I.; Kwon, O.W.; Koh, H.J. Effects of Macular Ischemia on the Outcome of Intravitreal Bevacizumab Therapy for Diabetic Macular Edema. Retina 2008, 28, 957–963. [Google Scholar] [CrossRef]
- Karst, S.G.; Deak, G.G.; Gerendas, B.S.; Waldstein, S.M.; Lammer, J.; Simader, C.; Guerin, T.; Schmidt-Erfurth, U.M. Association of Changes in Macular Perfusion with Ranibizumab Treatment for Diabetic Macular Edema. JAMA Ophthalmol. 2018, 136, 315. [Google Scholar] [CrossRef]
- Michaelides, M.; Fraser-Bell, S.; Hamilton, R.; Kaines, A.; Egan, C.; Bunce, C.; Peto, T.; Hykin, P. Macular Perfusion determined by Fundus Fluorescein Angiography at the 4-month time point in a prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT Study). Retina 2010, 30, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Micun, Z.; Dobrzyńska, W.; Sieśkiewicz, M.; Zawadzka, I.; Dmuchowska, D.A.; Wojewodzka-Zelezniakowicz, M.; Konopińska, J. Hyperbaric Oxygen Therapy in Ophthalmology: A Narrative Review. J. Clin. Med. 2023, 13, 29. [Google Scholar] [CrossRef]
- Malik, A.; Golnik, K. Hyperbaric Oxygen Therapy in the Treatment of Radiation Optic Neuropathy. J. Neuro-Ophthalmol. 2012, 32, 128–131. [Google Scholar] [CrossRef]
- Mcmonnies, C.W. Hyperbaric oxygen therapy and the possibility of ocular complications or contraindications. Clin. Exp. Optom. 2015, 98, 122–125. [Google Scholar] [CrossRef]
- Yonekawa, Y.; Hypes, S.M.; Abbey, A.M.; A Williams, G.; Wolfe, J.D. Exacerbation of macular oedema associated with hyperbaric oxygen therapy. Clin. Exp. Ophthalmol. 2016, 44, 625–626. [Google Scholar] [CrossRef]
- International Diabetes Federation. Clinical Practice Recommendations for Managing Diabetic Macular Edema; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Wong, T.Y.; Sun, J.; Kawasaki, R.; Ruamviboonsuk, P.; Gupta, N.; Lansingh, V.C.; Maia, M.; Mathenge, W.; Moreker, S.; Muqit, M.M.; et al. Guidelines on Diabetic Eye Care. Ophthalmology 2018, 125, 1608–1622. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Bandello, F.; Berg, K.; Chakravarthy, U.; Gerendas, B.S.; Jonas, J.; Larsen, M.; Tadayoni, R.; Loewenstein, A. Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2017, 237, 185–222. [Google Scholar] [CrossRef]
- Lim, J.I.; Kim, S.J.; Bailey, S.T.; Kovach, J.L.; Vemulakonda, G.A.; Ying, G.-S.; Flaxel, C.J. Diabetic Retinopathy Preferred Practice Pattern®. Ophthalmology 2025, 132, P75–P162. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee; ElSayed, N.A.; McCoy, R.G.; Aleppo, G.; Balapattabi, K.; Beverly, E.A.; Early, K.B.; Bruemmer, D.; Callaghan, B.C.; Echouffo-Tcheugui, J.B.; et al. 12. Retinopathy, Neuropathy, and Foot Care: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48 (Suppl. 1), S252–S265. [Google Scholar]
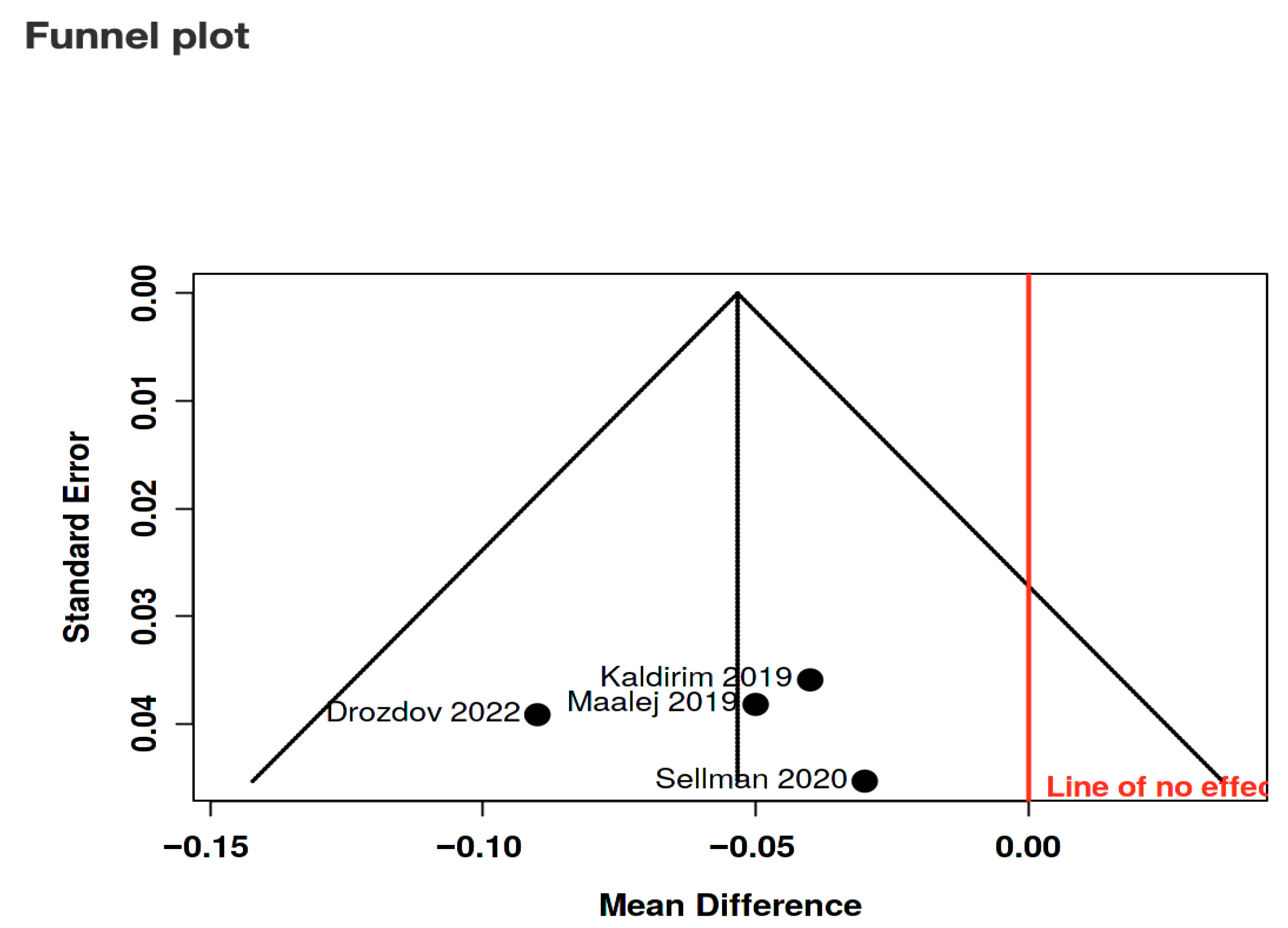
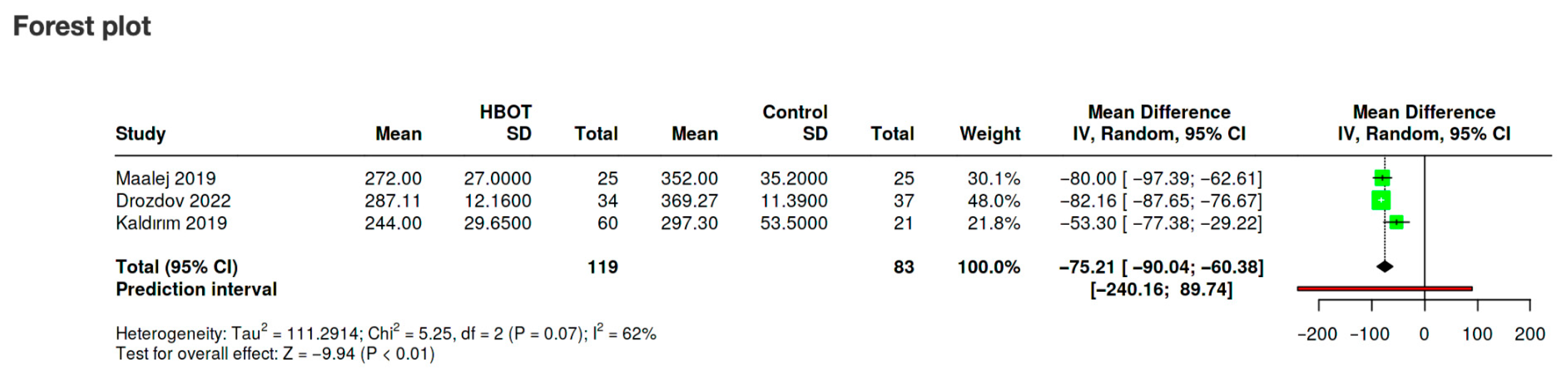
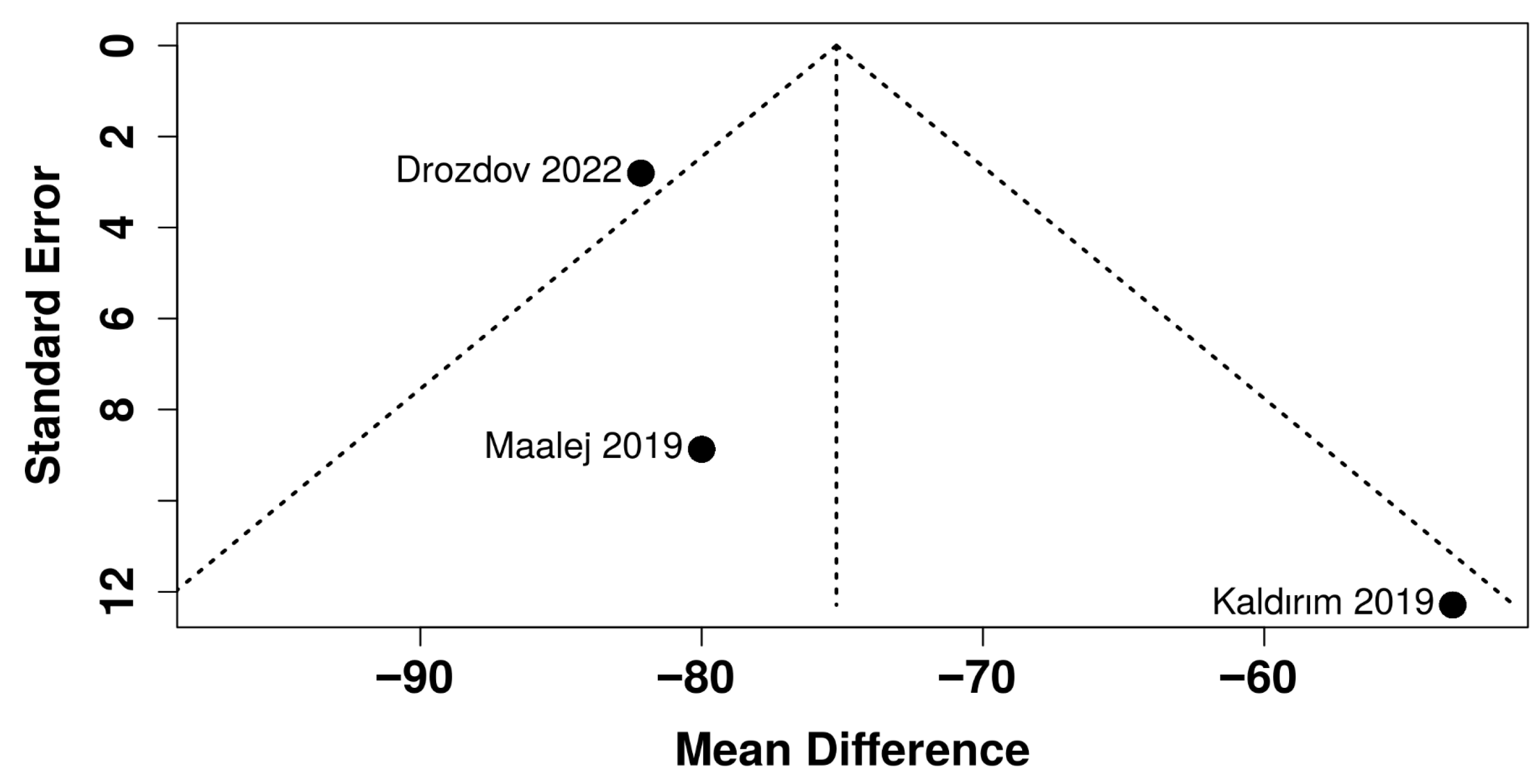
| Study, Ref | Purpose | Population & Baseline DR/DME Severity | Comparison Groups | Number of HBOT Treatments | HBOT Treatment |
|---|---|---|---|---|---|
| Sellman A, 2020, Sweden [24] | Effects of HBOT on visual acuity and retinopathy in patients with chronic diabetic foot ulcers | 62 adults with chronic diabetic foot ulcers (median age ≈ 64–70 y). DR graded with the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) scale, ranging from none/slight (levels 1–5) to proliferative (≥10). CSME assessed at baseline according to ETDRS criteria. | HBOT Group vs. Placebo Group | Max 40 treatments | Compression at 2.5 ATA (253.31 kPa) with 100% oxygen or air |
| Maalej A, 2019, Tunisia [25] | Effects of HBOT on diabetic retinopathy lesions and macular edema | 50 patients receiving HBOT for foot ulcers; all eyes showed non-proliferative DR (ETDRS). Stages recorded as mild, moderate, or severe; no proliferative lesions reported at inclusion. OCT used to document macular thickness, with limited DME prevalence. | HBOT+ (diabetic ulcers) vs. HBOT− (no ulcers) | 30 sessions | Compression at 2.5 ATA (253.31 kPa) for 90 min |
| Kaldrim H, 2019, Turkey [26] | Effect of HBOT on retinopathy progression and retinal/choroidal thickness | Prospective cohort of 30 type 2 DM patients (60 eyes) divided into three groups: mild-to-moderate NPDR (14 eyes), severe NPDR (20 eyes), and inactive post-laser PDR (26 eyes). Only isolated DME cases noted at baseline. | Mild/moderate DR, Severe DR, Inactive proliferative | 30 sessions | Inhalation of 100% oxygen at 2–2.5 ATA (202.65–253.31 kPa) |
| Gun, 2022, Turkey [27] | Acute effect of HBOT on macular and choroidal thickness in patients with type 2 diabetes | Cross-sectional series of 26 patients (49 eyes). Baseline grading: 10 eyes mild NPDR, 10 moderate NPDR, 13 severe NPDR; remaining eyes with no DR. PDR, recent anti-VEGF/laser, and clinical DME were exclusion criteria. | Insulin Group vs. Insulin+ Oral Antidiabetics Group | 1 session | Compression at 2.4 ATA (243.18 kPa) with ‘air breaks’ |
| Drozdov, 2022, Ukraine [8] | Efficacy of the combination treatment for DME in type 2 diabetic patients with non-proliferative diabetic retinopathy | 71 type 2 DM patients with both NPDR and center-involved DME. Inclusion required NPDR (PDR excluded) and ETDRS-confirmed DME; mean age 62 y, diabetes duration ~12 y, moderate glycemic control. | Aflibercept + HBOT vs. Aflibercept-only (as control) | 10 sessions | Monthly aflibercept for 3 months with 10 HBOT sessions |
| Study, Ref | Ophthalmological Evaluation | Measured Outcomes | Results |
|---|---|---|---|
| Sellman A, 2020 [24] | Visual acuity (ETDRS), fundus photo | Diabetic retinopathy grading and visual acuity | No significant difference in VA or retinopathy levels between groups over 2 years |
| Maalej A, 2019 [25] | OCT, fundus photography, BCVA | Central macular thickness, fundus lesions | Reduction in macular thickness and stabilization of lesions in the HBOT+ group |
| Kaldrim H, 2019 [26] | OCT, EDI-OCT, fluorescein angiography | Macular and choroidal thickness | Significant reduction in CT, no significant change in DR |
| Gun, 2022 [27] | OCT | Macular and choroidal thickness | Significant increase in nasal CT after HBOT in eyes with DR |
| Drozdov, 2022 [8] | OCT, BCVA, Clinical and biochemical exams | Retinal light sensitivity, serum glucose levels, serum activities of enzymes | Improvements in visual acuity, retinal light sensitivity and in antioxidant protection |
| Criterion | Gun 2022 [27] | Kaldirim 2019 [26] |
|---|---|---|
| 1. Was the study question or objective clearly stated? | Yes | Yes |
| 2. Were eligibility/selection criteria for the study population prespecified and clearly described? | Yes | NR |
| 3. Were the participants representative of those who would be eligible for the intervention in the general or clinical population? | Yes | NR |
| 4. Were all eligible participants that met the prespecified entry criteria enrolled? | NR | NR |
| 5. Was the sample size sufficiently large to provide confidence in the findings? | No | No |
| 6. Was the intervention clearly described and consistently delivered across the study population? | Yes | Yes |
| 7. Were the outcome measures prespecified, clearly defined, valid, reliable, and assessed consistently across all study participants? | Yes | Yes |
| 8. Were the people assessing the outcomes blinded to the participants’ exposures/interventions? | No | No |
| 9. Was the loss to follow-up after baseline 20% or less? | Yes | Yes |
| 10. Did the statistical methods examine changes in outcome measures from before to after the intervention? | Yes | Yes |
| 11. Were outcome measures taken multiple times before and after the intervention? | No | No |
| 12. If the intervention was conducted at a group level, did the analysis account for the use of individual-level data to determine effects at the group level? | NA | NA |
| Certainty Assessment | № of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Hyperbaric Oxygen Therapy | standard Therapy | Relative (95% CI) | Absolute (95% CI) | ||
| Visual acuity (assessed with: logMAR) | ||||||||||||
| 5 a | non-randomised studies | Serious b | not serious | not serious | not serious | none | 121 | 35 | - | see comment | ⨁⨁⨁◯ Moderate b | IMPORTANT |
| Central Macular Thickness (assessed with: OCT (Optical Coherence Tomography)) | ||||||||||||
| 4 c | non-randomised studies | Serious d | Serious e | not serious | Serious f | none | 112 | 0 | - | see comment | ⨁◯◯◯ Very low d,e,f | IMPORTANT |
| Diabetic Retinopathy Progression (assessed with: Retinal grading/DR stage) | ||||||||||||
| 2 g | non-randomised studies | Serious h | not serious | serious i | serious j | none | 35/35 (100.0%) | 11/35 (31.4%) | not pooled | see comment | ⨁◯◯◯ Very low h,i,j | CRITICAL |
| Need for Rescue Treatment (assessed with: Intravitreal injection requirement) | ||||||||||||
| 2 k | non-randomised studies | Serious l | not serious | not serious | Serious m | none | 11/51 (21.6%) | 0/0 | not pooled | see comment | ⨁⨁◯◯ Low l,m | CRITICAL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moccia, E.; Rizzuto, V.; Longobardi, P.; Ferrone, A.; Laurino, M.; Zemītis, A.; Covello, G. Efficacy of Hyperbaric Oxygen Therapy in Diabetic Retinopathy and Macular Edema: A Systematic Review and Meta-Analysis. Diabetology 2025, 6, 133. https://doi.org/10.3390/diabetology6110133
Moccia E, Rizzuto V, Longobardi P, Ferrone A, Laurino M, Zemītis A, Covello G. Efficacy of Hyperbaric Oxygen Therapy in Diabetic Retinopathy and Macular Edema: A Systematic Review and Meta-Analysis. Diabetology. 2025; 6(11):133. https://doi.org/10.3390/diabetology6110133
Chicago/Turabian StyleMoccia, Enrico, Vincenzo Rizzuto, Pasquale Longobardi, Anita Ferrone, Marco Laurino, Artūrs Zemītis, and Giuseppe Covello. 2025. "Efficacy of Hyperbaric Oxygen Therapy in Diabetic Retinopathy and Macular Edema: A Systematic Review and Meta-Analysis" Diabetology 6, no. 11: 133. https://doi.org/10.3390/diabetology6110133
APA StyleMoccia, E., Rizzuto, V., Longobardi, P., Ferrone, A., Laurino, M., Zemītis, A., & Covello, G. (2025). Efficacy of Hyperbaric Oxygen Therapy in Diabetic Retinopathy and Macular Edema: A Systematic Review and Meta-Analysis. Diabetology, 6(11), 133. https://doi.org/10.3390/diabetology6110133








