Abstract
Background/Objectives: Diabetes mellitus, a prevalent metabolic disorder, causes severe complications and economic burden, requiring effective therapeutic strategies. While triglycerides and low-density lipoproteins (LDLs) have been widely studied in type 2 diabetes mellitus (T2DM) and related cardiovascular risks, the roles of other lipids, such as sphingolipids and glycerophospholipids, remain unclear. This study aimed to investigate their involvement in T2DM and its complications. Methods: We examined sphingolipid and glycerophospholipid profiles in T2DM patients to assess alterations associated with diabetes mellitus and its related complications. Results: Patients with T2DM showed significant modulations in sphingolipid and glycerophospholipid levels, suggesting these lipids contribute to metabolic dysregulation and progression of diabetes-related complications. Conclusions: Alterations in sphingolipids and glycerophospholipids play a critical role in T2DM, indicating their potential as novel targets for therapeutic intervention and risk mitigation in patients with diabetes and its complications.
1. Introduction
Type 2 diabetes mellitus (T2DM) is alarmingly prevalent, contributing to increased mortality and morbidity rates worldwide [1]. The prevalence of T2DM, particularly among older adults and patients with DM, is currently increasing, exceeding 463 million worldwide [1]. T2DM is notorious for inducing macrovascular and microvascular complications, including coronary diseases and diabetic nephropathy (DN), exacerbating mortality rates and physical morbidity among affected individuals. Consequently, T2DM poses a dual challenge to both public health and economic prosperity, thereby warranting urgent attention on a global scale [2]. Low-density lipoprotein cholesterol (LDL-C) is essential for preventing macrovascular complications, particularly the control of dyslipidemia [3]. Currently, LDL-C is the most recognized marker for predicting diabetes-associated cardiovascular risks; various sophisticated clinical studies have underscored the effectiveness of statins and ezetimibe in reducing LDL-C levels, thereby mitigating cardiovascular complications in T2DM. Nonetheless, despite LDL reduction, cardiovascular diseases persist to some extent, thereby leading to residual risk [4]. Efforts for addressing this residual risk have focused on high-density lipoprotein cholesterol (HDL-C) and triglycerides (TGs); however, there remains a pressing need to uncover novel biomarkers and therapeutic targets through laboratory investigations [2,3,5,6]. Furthermore, while certain agents such as renin–angiotensin system inhibitors and sodium–glucose cotransporter-2 inhibitors have shown promise in curbing the advancement of DN, a substantial gap in the development of new biomarkers and treatment targets within the scope of diabetes mellitus persists [3,7].
Two major classes of lipids that play integral roles in cell membrane structure and function are glycerophospholipids and sphingolipids (Supplemental Figure S1) [8]. Their involvement in the pathogenesis of diabetic complications is intricate and can vary, depending on the specific complication. However, several proposed mechanisms elucidate the impact of diabetes on glycerophospholipid and sphingolipid production and structural modification. These mechanisms encompass alterations in membrane structure and function [9,10,11], inflammation and oxidative stress [8,12], endothelial dysfunction and vascular complications [10,11], advanced glycation end product (AGE) formation [13,14], and intracellular signaling pathway activation [15,16].
Glycerophospholipids and sphingolipids play crucial roles as cell membrane constituents. In diabetes, persistent hyperglycemia and related metabolic disruptions can induce modifications in cellular membrane composition and characteristics. These alterations may influence membrane fluidity, permeability, and signaling pathways, ultimately contributing to the cellular dysfunction and pathological damage observed in diabetic complications [9,10,11].
In diabetes, dysregulated lipid metabolism can result in the accumulation of specific lipid intermediates, notably sphingolipid-derived ceramides (CERs). These CERs have been implicated in promoting inflammation and oxidative stress, both of which are pivotal in the pathogenesis and advancement of diabetic complications, including nephropathy, retinopathy, and neuropathy [8,12].
Glycerophospholipids and sphingolipids play crucial roles in endothelial cell function and vascular homeostasis. In diabetes, endothelial function can be adversely affected by perturbed lipid metabolism, thereby leading to impaired vasodilation, increased vasoconstriction, and heightened vascular permeability. These alterations significantly contribute to the pathogenesis of microvascular complications, including DN and retinopathy, as well as macrovascular complications, including cardiovascular diseases [10,11].
Glycerophospholipids and sphingolipids can undergo nonenzymatic glycation reactions with reducing sugars. These reactions result in AGE formation. Over time, AGEs accumulate in various tissues and contribute to tissue damage through several mechanisms, including inflammation, oxidative stress, and protein cross-linking. AGE accumulation has been closely associated with the pathogenesis of diabetic complications, including nephropathy, retinopathy, and neuropathy [13,14].
Specific lipid intermediates including diacylglycerol and CERs play a pivotal role in intracellular signaling pathway activation. These pathways are closely associated with insulin resistance and cell survival. In diabetes, disrupted lipid metabolism can result in the accumulation of these lipid intermediates. Consequently, this dysregulation contributes to insulin resistance and cellular dysfunction in tissues, such as skeletal muscle, liver, and adipose tissues [15,16]. Overall, glycerophospholipid and sphingolipid dysregulation in diabetes contributes to cellular dysfunction, inflammation, oxidative stress, and vascular complications, ultimately leading to diabetic complication development and progression. Targeting lipid metabolism and signaling pathways may offer potential therapeutic strategies for preventing or managing diabetic complications.
Aim
Considering these backgrounds, this study aimed to establish an association between the modulations of these lipids and diabetes and diabetic complications to contribute to facilitating early diagnosis and identifying potential treatment intervention targets for patients with T2DM and associated complications [5].
This study aimed to explore the associations of sphingolipids and glycerophospholipids, with T2DM, particularly regarding the crucial biomarker glycated hemoglobin (HbA1c) levels among patients with diabetes, and understand their potential impact on diabetic complications. Notably, previous studies have not extensively explored these relationships to date.
2. Participants and Methods
2.1. Study Participants
We screened 95 patients from the University of Tokyo Hospital, Tokyo, Japan, with T2DM as diagnosed according to the criteria stated by the World Health Organization. The participants’ characteristics are shown in Table 1. Personal backgrounds and current and past medical histories were recorded for all participants. A total of 184 participants were initially recruited for this study. After applying exclusion criteria, including incomplete clinical or biochemical data, poor sample quality, and failure to meet diagnostic criteria for either T2DM or healthy control status, a final cohort of 180 participants was included in the analysis. These comprised 85 healthy controls and 95 T2DM patients, as detailed in Table 1. Personal background information and detailed current and past medical histories were obtained for all T2DM and healthy participants. In addition, participants with Type 2 Diabetes Mellitus were further stratified into clinical subgroups based on documented complications (see Supplementary Data). Coronary Artery Disease (CAD) was present in 17 patients, defined by a confirmed history of cardiologist-diagnosed angina, myocardial infarction, coronary interventions (e.g., stenting or bypass), or diagnostic imaging evidence. Diabetic Nephropathy (DN; stages 1–4) was identified in 84 patients, based on established staging criteria using albuminuria levels, estimated glomerular filtration rate (eGFR), and other markers of diabetic kidney damage. Cerebral Infarction (CI) was observed in 10 patients, confirmed by neurologist diagnosis and brain imaging (MRI or CT). These subgroup classifications facilitated tailored analyses of complication-specific outcomes in the T2DM cohort. Some participants were included in multiple subgroups if they exhibited more than one complication. Full details of the complication-based stratification are provided in the Supplementary Data.

Table 1.
Participant characteristics.
2.2. Ethics
The Institutional Research Ethics Committee of the Faculty of Medicine, The University of Tokyo approved the study protocol (approval numbers: 10266 and 11158 and approval date: 3 October 2013 and 19 May 2016, respectively). All patients with T2DM signed an informed consent stating approval for the use of their intravenous blood serum specimens in this study. Moreover, this study was conducted in accordance with the written ethical guidelines laid down in the Declaration of Helsinki.
2.3. Measurement of Serum Glycerophospholipids and Sphingolipids and Other Clinical Parameters in T2DM
Using liquid chromatography–mass spectrometry (LC–MS), we assessed sphingolipid and glycerophospholipid concentrations in serum samples collected from 95 and 85 patients with and without diabetes, respectively. The measurements were performed using the Nexera system HPLC (SHIMADZU, Kyoto, Japan) and LC-8060 (SHIMADZU, Kyoto, Japan), equipped with a quantum ultra-triple quadrupole mass spectrometer, and analyzed using the LC–MS/MS system solution software (SHIMADZU, Kyoto, Japan) to conduct simultaneous analysis of 368 compounds, utilizing 14 deuterium-labeled internal standards. These standards encompassed a range of lipid classes, including Cer, S1P, dhSph, LPA, lysophosphatidylcholine (LPC), LPS, lysophosphatidylinositol (LPI), lysophosphatidylglycerol (LPG), lysophosphatidylethanolamine (LPE), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), Phosphatidylglycerol (PG), and Phosphatidylserine (PS) (Avanti Polar Lipids, Inc., Alabaster, AL, USA). Glycerophospholipids and sphingolipids were identified using multiple reactions monitoring and subsequently subjected to analysis using the LabSolutions software system (SHIMADZU, Kyoto, Japan). Comprehensive details of the analysis and methodology were described in previous studies [2,17]. CER species (Cer d18:1/16:0 [C16:0], Cer d18:1/18:0 [C18:0], Cer d18:1/18:1 [C18:1], Cer d18:1/20:0 [C20:0], Cer d18:1/22:0 [C22:0], and Cer d18:1/24:0 [C24:0]), Sph, and dhSph were simultaneously quantified, as previously described. Additionally, S1P and dhS1P were measured, following established protocols [18,19]. Moreover, LPA, LPC, LPS, LPI, LPG, and LPE were assessed, as described in previous studies [18,19,20]. Acyl chains (14:0, 16:0, 16:1, 18:0, 18:1, 18:2, 18:3, 20:3, 20:4, 20:5, and 22:6) for lysophospholipids and 22:5 LPI were monitored. Sphingomyelin (SM) and diacyl phospholipids including PC, PE, PI, PG, and PS were measured, with 17 diacyl chains for SM and 64 diacyl chains for PC, PE, PI, PG, and PS. Intra- and inter-day coefficients of variation for all metabolites, except for SM and diacyl phospholipids, were below 20%, as previously validated [21,22,23].
2.4. Statistical Analyses
Categorical variables were presented as proportions, whereas continuous variables were expressed as means ± Standard Error of the Means (SEM). The Kolmogorov–Smirnov test was used to assess the normality of continuous variable distributions. Categorical data were compared using the Chi-square test, and continuous data were compared using Student’s t-test. Using a multivariate cumulative logistic model, the effects of risk factors on MCI in patients with T2DM were quantified. Spearman rank correlation analysis was employed for non-normally distributed variables, whereas Pearson correlation analysis was used for normally distributed variables. To assess the diagnostic potential of plasma phospholipids, receiver operating characteristic (ROC) analysis was conducted. p < 0.05 was considered statistically significant.
3. Results
3.1. Participants’ Clinical Demographics
The characteristics of the participants are presented in Table 1. The mean patient age was 65.41 ± 10.98 years, and all patients were hospitalized at The University of Tokyo Hospital affiliated with The University of Tokyo. Patients with T2DM (59 males and 36 females) were diagnosed on the basis of the percentage value of their high HbA1c scores of 7.56% ± 1.21%, which was based on the criteria established by the Japan Diabetes Society to National Glycohemoglobin Standardization Program (NGSP). The patient’s HbA1c values are standardized according to NGSP [24]. The other 85 healthy participants (67 males and 18 females) who attained the expected range of HbA1c scores of 5.14% ± 0.79% were categorized as patients without T2DM (control). Participants with T2DM had higher total cholesterol and LDL-C levels than the control. Additionally, participants with T2DM had lower estimated glomerular filtration rate levels and higher urinary albumin and total protein levels than the control, suggesting the presence of progressing DN in some of the participants with T2DM. The estimated glomerular filtration rate (eGFR) in our study was calculated based on serum creatinine levels [25].
Biochemical and molecular analysis was conducted on biomarker specimens obtained from 184 participants, including 95 and 85 with and without diabetes, respectively. Comparative assessment of participant demographics and medication profiles showed no disparities across age groups, cardiovascular risk factors, or prevalence of symptomatic carotid artery stenosis. There were notable differences in medication use between the two groups, particularly regarding statin therapy: statin use was significantly more common in the T2DM group, consistent with their older age and elevated metabolic risk factors such as higher BMI, fasting glucose, and hypertension. As anticipated, individuals with diabetes were more likely to be clinically obese (body mass index [BMI] > 30 kg/m2; p < 0.001) and taking medications, such as β-blockers, anti-platelets, and insulin (p < 0.05; Table 1).
- Urinary TP/Cre ratio and Urinary Alb/Cre ratio related measures:
These indices are derived from urine samples, not from blood serum. The TP/Cre ratio reflects the amount of total urinary protein (TP) normalized to urinary creatinine (Cre), and the Alb/Cre ratio reflects the amount of urinary albumin (Alb) normalized to urinary creatinine. These ratios are conventionally expressed as mg/g·Cr (or mg/mmol·Cr) and are internationally recognized markers for the evaluation of diabetic nephropathy. Differences observed between groups may reflect physiological variations in creatinine excretion, protein metabolism, renal function, and the effects of diabetic medications, particularly in individuals with diabetes. Such differences are biologically plausible and consistent with known metabolic and renal alterations in diabetic patients. This clarification ensures that the reported values are correctly understood as being derived from urinary, not serum, measurements.
- Definitions of Therapeutic Categories in Table 1:
Other anti-diabetic agents 1: This category encompasses the major classes used in Type 2 diabetes management, including biguanides (e.g., metformin), sulfonylureas, meglitinides, thiazolidinediones (TZDs), α-glucosidase inhibitors, DPP-4 inhibitors, bile acid sequestrants, dopamine agonists, SGLT-2 inhibitors, and oral GLP-1 receptor agonists prescribed to our T2DM participants.
Antihypertensives 2: This category encompasses the principal classes of drugs employed to control blood pressure and manage cardiovascular risk in our T2DM participants, such as diuretics (e.g., thiazide, loop, potassium-sparing), ACE inhibitors, angiotensin II receptor blockers (ARBs), calcium channel blockers, beta-blockers, as well as alpha-blockers, alpha-2 agonists, vasodilators, direct renin inhibitors, and aldosterone receptor antagonists.
Dyslipidemia medications (non-statin) 3: Lipid-lowering medications that used to manage dyslipidemia beyond statins. Examples include fibrates, niacin, bile acid sequestrants, ezetimibe, bempedoic acid, PCSK9 inhibitors, and other newer non-statin agent therapies.
3.2. Total PE, PG, and PI Serum Concentrations Were Elevated, Whereas Total PC Serum Levels Were Reduced in Individuals with T2DM
Total PC, PE, PG, and PI levels, along with those of PG 32:0 and PG 34:1, are shown in Figure 1. The T2DM group had lower total PC levels and higher total PG, PI, and PE levels (Figure 1a–d). Upon separate analysis of molecular species, significant differences were observed among PG species, particularly with PG 32:0 and PG 34:1, which were the only species detected (Figure 1e,f). Other PG species did not yield measurable results. Conversely, several PC species (30:1, 32:0, 32:2, 32:4, 34:1, 34:3, 36:0, 36:2, 36:4, 38:0, 38:2, 38:4, 38:6, 38:8, 40:1, 40:5, 40:7, 40:9, 42:0, and 42:2) exhibited lower serum levels, whereas PI (42:0, 42:1, 42:2, 42:3, 42:4, 42:5, 42:6, 42:7, 42:9, 42:10, and 44:12) and PE (34:1, 36:0, 36:3, 36:6, 38:4, 38:7, and 40:7) showed higher levels in the T2DM group than those in the HI group (Supplemental Figure S2a–c). However, certain PE species (28:0, 30:0, 32:0, 32:3, 34:4, 38:1, 40:1, 40:4, 40:10, 42:2, 42:5, 42:8, 42:11, and 44:2) displayed lower levels in the T2DM group; however, the differences did not reach statistical significance.
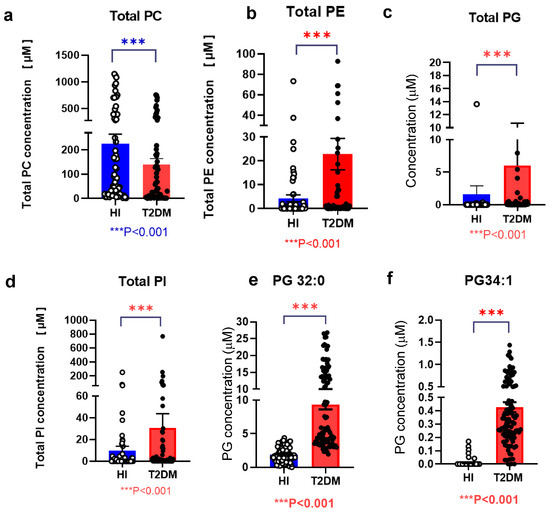
Figure 1.
Modulations of diacyl-glycerophospholipid serum levels of in patients with type 2 diabetes mellitus (T2DM) Total PC (a), PE (b), PG (c), PI (d), PG 32:0 (e), and PG 34:1 (f) serum levels are shown. Error bars represent the mean ± standard error of the mean (SEM). Statistical significance is indicated by *** p < 0.001, denoting comparison between patients with T2DM (T2DM, n = 95) and healthy control individuals (HI, n = 85). Healthy individuals are represented in blue; T2DM patients are represented in red.
3.3. Total LPG and LPI Serum Levels Were Higher, Whereas Total LPC and LPE Serum Levels Were Lower in Individuals with T2DM
The T2DM group had lower LPC and LPE levels (Figure 2a,b), consistent with previous findings [26,27]. Conversely, total LPG and LPI levels were higher in the T2DM group (Figure 2c,d). Upon analyzing individual molecular species, various alterations were noted among lipid species. Specifically, LPG 16:0, LPG 16:1, LPG 18:0, LPG 18:1, and LPG 18:2 serum levels were higher, whereas LPG 20:4 levels were lower in the T2DM group (Figure 2e). Similarly, LPI 16:0, LPI 18:0, LPI 18:1, LPI 18:2, LPI 20:3, and LPI 20:4 serum levels were higher in the T2DM group (Figure 2f). Conversely, LPC 16:0, LPC 18:0, LPC 18:1, and LPC 20:4 serum levels were lower in the T2DM group (Figure 2g). Furthermore, in the T2DM group, LPE 18:0, LPE 18:1, LPE 18:2, LPE 18:3, LPE 20:3, and LPE 20:4 serum levels were lower, whereas LPE 16:0 and LPE 16:1 levels were higher (Figure 2h).
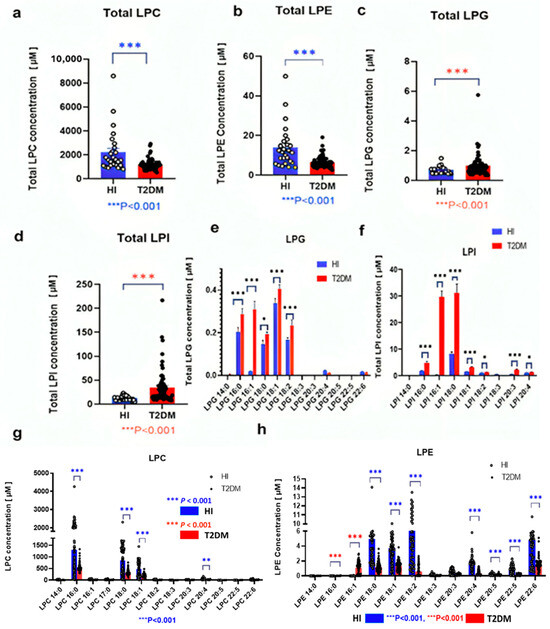
Figure 2.
Modulations of glycerolysophospholipid serum levels in patients with T2DM The total serum levels of LPC (a), LPE (b), LPG (c), and LPI (d), along with the levels of individual molecular species of LPG (e), LPI (f), LPC (g), and LPE (h) are shown. Bars depict the mean ± SEM. Statistical significance is indicated by *** p < 0.001, ** p < 0.01, and * p < 0.05, denoting comparison between patients with T2DM (T2DM, n = 95) and healthy control individuals (HI, n = 85). The red and blue p-values were intentionally used to distinguish the relative significance of different group comparisons, blue for the Healthy Individual group and red for the T2DM group. Specifically, the asterisk annotations: *** p < 0.001, ** p < 0.01, and * p < 0.05, with blue representing the Healthy Individual group and red representing the T2DM group. For example, in Figure 2(g,h), significance levels for the Healthy group are shown in blue (e.g., *** p < 0.001 [blue], ** p < 0.01 [blue]), while those for the T2DM group are shown in red (e.g., *** p < 0.001 [red], ** p < 0.01 [red]).
3.4. CER Serum Concentrations Were Higher in Individuals with T2DM
Variations in serum sphingolipid species SM 36:3, SM 36:4, SM 38:3, and SM 38:4 were lower in the T2DM group, whereas a few species such as SM40:2 and SM38:1 showed higher levels in the same group (Figure 3a–e). Conversely, total CER levels were higher in the T2DM group, particularly C16:0 Cer, C18:0 Cer, and C22:0 Cer (Figure 3g,h), and total SM levels showed no significant associations with T2DM complications (Supplemental Figure S3). Among SM species, only SM 34:1, SM 38:1, SM 40:1, and SM 40:2 levels were higher in participants with T2DM with coronary artery disease (CAD), whereas SM 38:1, SM 40:1, and SM 40:2 levels were lower in those with advanced DN (Figure 4a,b). Conversely, serum CER levels notably C16:0 Cer and C18:0 Cer were higher in participants with CAD (Figure 4c,d) and were positively correlated with DN stages (Figure 4e,f). Overall, sphingolipids especially CER may play a significant role in T2DM and its complications.
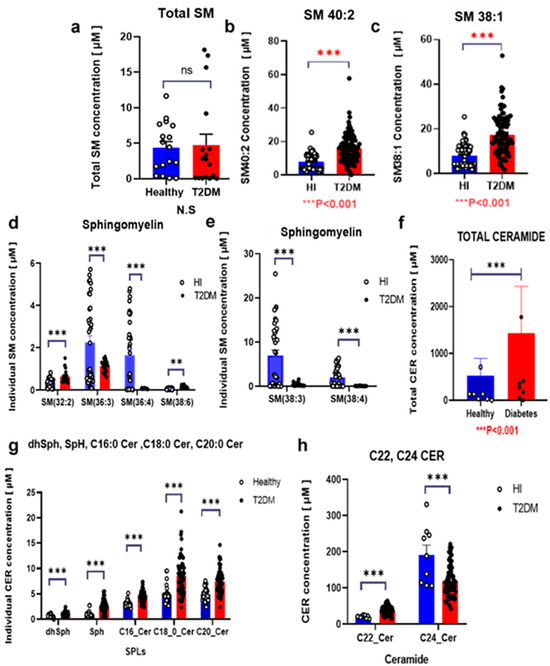
Figure 3.
Modulations of serum sphingolipid levels in patients with T2DM The serum levels of sphingomyelins (SM) (a–e) and ceramides (CER) (f–h) are shown. Bars depict the mean ± SEM. Statistical significance is indicated by *** p < 0.001, ** p < 0.01, and, denoting comparison between patients with T2DM (T2DM, n = 95) and healthy control individuals (HI, n = 85). Healthy individuals are represented in blue; T2DM patients are represented in red.
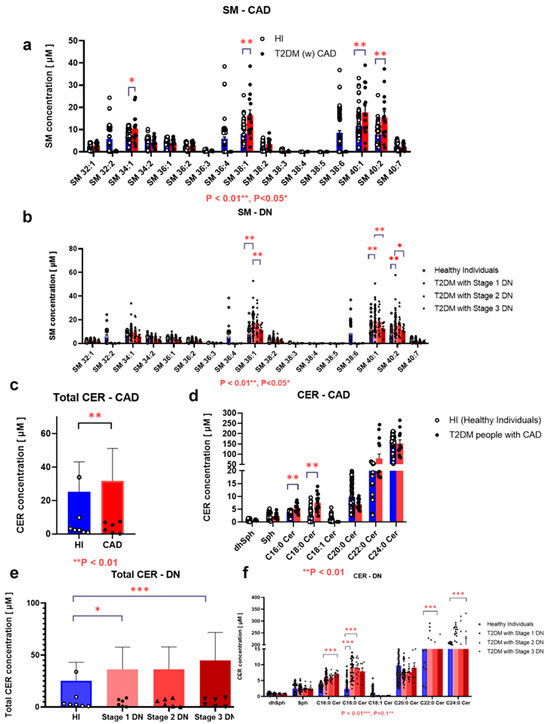
Figure 4.
Associations between serum sphingolipid levels and diabetic complications in patients with T2DM Associations of serum levels of Sphingomyelins (SM) (a,b) and Ceramides (CER) (c–f) with Coronary Artery Disease (CAD) (a,c,d) or Diabetic Nephropathy (DN) stages 1–4 (b,e,f) are shown. Bars depict the mean ± SEM. *** p < 0.001, ** p < 0.01, and * p < 0.05. While the plotted means for C22:0 Cer and C24:0 Cer may appear similar across the groups due to axis scaling and threshold adjustments by GraphPad Prism, version 10.0.3, the underlying statistical analysis was performed using raw, unrounded data and confirmed significant differences. Healthy individuals are represented in blue; T2DM patients are represented in red.
3.5. LPG Serum Levels Were Associated with T2DM-Associated Coronary, Cerebral, and Nephropathy Complications
Regarding the relationship between LPG, LPI, PG, and PI levels with T2DM complications, of the 95 patients with T2DM, total PG levels were increased in 55 individuals with CAD (Figure 5a). Conversely, individuals with a history of CAD exhibited significantly lower total LPG levels and levels of specific LPG species, including LPG 16:0, LPG 18:0, LPG 20:4, and LPG 22:6, except for LPG 16:1 and LPG 18:1 (Figure 5c). Conversely, patients with T2DM with a history of cerebral infarctions (CIs) showed higher total LPG levels and levels of specific LPG species, including LPG 16:0, LPG 16:1, LPG 18:0, LPG 18:1, LPG 18:2, LPG 20:4, and LPG 22:6 (Figure 5e). Moreover, among participants with DN stages 1 and 4, higher LPG 16:0, LPG 16:1, LPG 18:0, LPG 18:1, LPG 18:2, LPG 20:4, and LPG 22:6 levels were observed in those with DN stage 4 (Figure 5d).
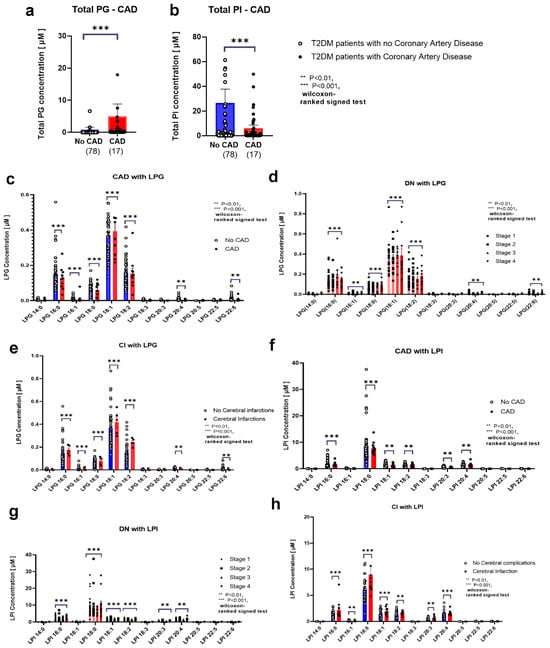
Figure 5.
Associations between serum glycerophospholipid levels and diabetic complications in patients with T2DM Associations of serum levels of phosphatidylglycerol (PG) (a), phosphatidylinositol (PI) (b), lysophosphatidylglycerol (LPG) (c–e), and lysophosphatidylinositol (LPI) (f–h) with coronary artery disease (a–c,f), cerebral infarction (e,h), or diabetic nephropathy stages 1–4 (d,g) are shown. Bars depict the mean ± SEM.*** p < 0.001, ** p < 0.01. Healthy individuals are represented in blue; T2DM patients are represented in red.
3.6. LPI Levels Were Associated with T2DM-Associated Coronary, Cerebral, and Nephropathy Complications
In 55 of the 95 patients with T2DM who had experienced CAD, total PI levels were lower (Figure 5b). In individuals with CAD, LPI 18:0, LPI 18:1, LPI 18:2, and LPI 20:3 serum levels were markedly lower among patients with T2DM (Figure 5f). Conversely, patients with T2DM with CIs showed higher LPI 18:0, LPI 18:1, LPI 18:2, LPI 20:3, and LPI 20:4 serum levels (Figure 5h). Patients with DN stage 4 had higher LPI 18:0 serum levels than those with stage 1 (Figure 5g).
3.7. Association of Lipids with Laboratory Data in Participants with T2DM
Furthermore, our findings showed the intricate relationships between glycerophospholipids or sphingolipids and key clinical biomarkers associated with diabetes (Table 2 and Table 3 and Figure 6), including HbA1c, LDL-C, and the LDL/HDL ratio. These findings highlighted the potential impact of highly involved glycerophospholipids and sphingolipids (PE 28:0, PG 32:0, PG 34:1, LPE 16:0, LPE 16:1, LPG 16:0, LPG 16:1, LPG 18:1, LPI 18:0, C16:0 Cer, C18:0 Cer, and SM 38:1) on lipid profiles and overall metabolic health, specifically in individuals with T2DM. The overall findings of our study suggested that glycerophospholipids including PE 28:0, PG 32:0, and PG 34:1 played a significant role in glucose metabolism, and these lipids were strongly associated with HbA1c levels. As shown in Table 2 and Table 3, significant correlations are observed across various clinical parameters, particularly that of LDL-C and HDL-C with glycerophospholipids and sphingolipids, including PE 28:0, PG 32:0, PG 34:1, LPE 16:0, LPE 16:1, LPG 16:0, LPG 16:1, LPG 18:1, LPI 18:0, C16:0 Cer, C18:0 Cer, SM 38:1, and SM 40:2. Glucose exhibited strong positive associations with specific glycerophospholipids, particularly PE 28:0, PG 32:0, and PG 34:1, emphasizing a strong role in glucose metabolism. Similarly, HbA1c levels were significantly correlated with these PG/LPG and PI/LPI glycerophospholipids. As depicted in Figure 6a–d, phosphatidylglycerol (PG), LPG, PI, and LPI plasma concentrations exhibit an upward trend with respect to HbA1c levels, even within the lower range (5.7–6.4% vs. 6.5% and above). Notably, PG and PI levels were particularly elevated in patients with uncontrolled and higher HbA1c values in T2DM. Conversely, as observed in Figure 6e–h, PE, LPE, PC, and LPC concentrations also increase with increasing HbA1c levels, even within the lower HbA1c ranges (5.7–6.4%).

Table 2.
Correlations between the top specific molecular species of glycerophospholipids including PE 28:0, PG 32:0, PG 34:1, LPE 16:0, LPE 16:1, LPG 16:0, LPG 16:1, LPG 18:1, and LPI 18:0 in patients with diabetes and clinical biomarkers.

Table 3.
Correlations between glycerophospholipids or sphingolipids and key clinical biomarkers associated with diabetes.
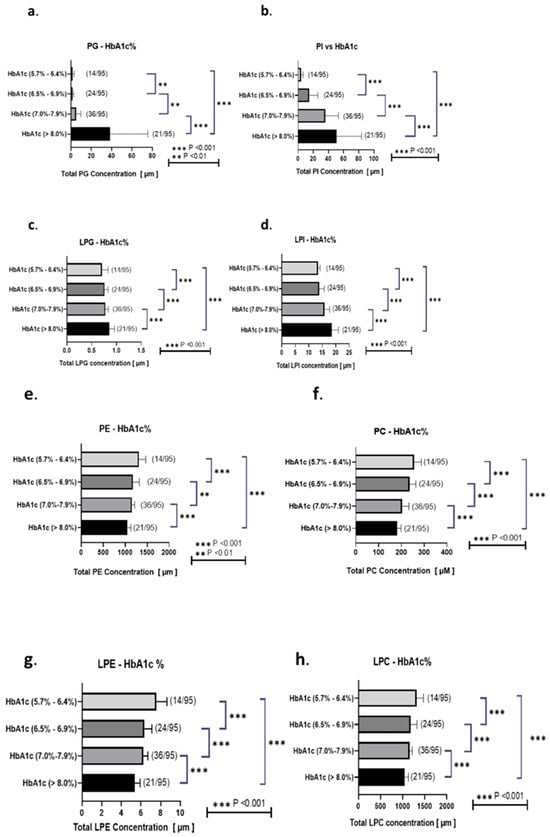
Figure 6.
Associations of total PG, PI, LPG, LPI, PE, PC, LPE, and LPC levels with HbA1c Associations of total PG (a), PI (b), LPG (c), LPI (d), PE (e), PC (f), LPE (g), and LPC (h) levels with the ranks according to HbA1c levels (5.7–6.4%, 6.5–6.9%, 7.0–7.9%, and >8.0%). This study categorized participants on the basis of their HbA1c levels and noted 14, 24, 36, and 21 participants with diabetes having HbA1c levels of 5.7–6.4%, 6.5–6.9%, 7.0–7.9%, and >8.0%, respectively. Correlation ranking analyses were performed, and data were expressed as r2 correlation factors. Statistical significance was denoted as *** p < 0.001, ** p < 0.01. Healthy individuals are represented in blue; T2DM patients are represented in red.
Furthermore, our study revealed that HDL-C was closely linked to PE 28:0, whereas LDL-C showed strong associations with both PE 28:0 and PG 32:0 (Table 2 and Table 3). TG levels were markedly influenced by PG 34:1. Furthermore, total cholesterol was closely related to PE 28:0, PG 32:0, and PG 34:1. Therefore, the presence and levels of specific glycerophospholipids such as PE 28:0, PG 32:0, and PG 34:1 were significantly correlated with key clinical biomarkers associated with cardiovascular risk, including LDL-C, HDL-C, glucose, and HbA1c levels. These findings highlighted the potential significance of glycerophospholipids as biomarkers for assessing cardiovascular risk and monitoring metabolic health in individuals with T2DM, particularly concerning their lipid profiles and overall metabolic health.
3.8. Principal Component Analysis (PCA) and Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) Effectively Differentiated the Presence of T2DM in Sphingolipids and Glycerophospholipids, Particularly Emphasizing the Contributions of PE 28:0, PG 32:0, and PI 40:1 to T2DM
To further explore the lipid characteristics of T2DM, PCA and OPLS-DA were conducted using the lipidomics data of T2DM to assess whether lipids could distinguish the presence of T2DM and identify significant lipid contributors to T2DM. The loading plots of PCA and OPLS-DA demonstrated effective separation between the T2DM and control groups (Figure 7a), with a PC1 value of 75.4% and a T score of 19.7 (Figure 7b), respectively. These findings suggested that glycerophospholipids and sphingolipids serum levels significantly contributed to discriminating T2DM.
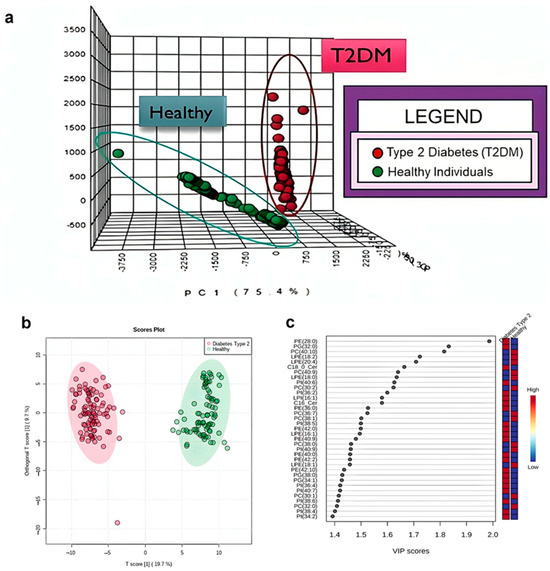
Figure 7.
Comprehensive analysis of the associations between glycerophospholipids, sphingolipids, and T2DM Orthogonal partial least squares discriminant analysis was performed on the top metabolites identified in the variable importance of projection scores from the OPLS-DA model (a) of glycerophospholipids and sphingolipids present in both healthy control individuals and participants with T2DM. The response ranges of the participants with T2DM and healthy control individuals are depicted in red (T2DM) and blue (healthy), respectively. The T Score plot of the participants with T2DM and healthy control individuals (b) used the first two principal components obtained from patients with T2DM (n = 95) compared with healthy control individuals (n = 85), with T2DM represented in red and healthy control individuals in green. Principal component analysis (PCA) of glycerophospholipids and sphingolipids was performed to compare healthy control individuals and those with T2DM. The lipids with the top 40 highest VIP scores are shown (c).
To rank the contributions of glycerophospholipids, sphingolipids, and CER metabolites in distinguishing between patients with T2DM and healthy control individuals, variable importance in projection (VIP) score analysis was used. PE 28:0 (VIP score: 2.00), PG 32:0 (VIP score: 1.86), and PI 40.1 (VIP score: 1.83) were the primary discriminating lipids (Figure 7c). These results suggested that elevated diacyl-glycerophospholipids serum levels, particularly PE 28:0, PG 32:0, and PI 40:1, can significantly contribute to T2DM pathogenesis. VIP (Variable Importance in Projection) scores indicate how much each variable of a metabolite contributes to the PLS-DA model in T2DM patients: A VIP score > 1.0 typically marks a variable as significant in explaining variance in the response. Scores around 0.8 or below are often considered unimportant and candidates for exclusion. VIP scores are computed as a weighted sum across all PLS components, reflecting both the variable’s weight in each component and the amount of variance each component explains. Variables with VIP > 1 (highlighted or bolded in the legend) are the most influential in distinguishing groups. Lower scorers (< 1) contribute less and may be of secondary interest.
3.9. ROC Analysis Revealed Elevated Area Under the Curve (AUC) Values for PG 32:0, PG 34:1, PE 28:0, and C16:0 Cer
To discriminate between the T2DM and control groups, ROC analyses were conducted. Of note, the results showed high AUC values for PG 32:0 (AUC: 0.998) (Figure 8a), PG 34:1 (AUC: 0.984) (Figure 8b), PE 28:0 (AUC: 0.989) (Figure 8c), and C16:0 Cer (AUC: 0.980) (Figure 8d). Collectively, these findings suggested that glycerophospholipids including PG 32:0, PG 34:1, and PE 28:0, along with C16:0 Cer, could serve as highly suitable potential biomarkers for T2DM.
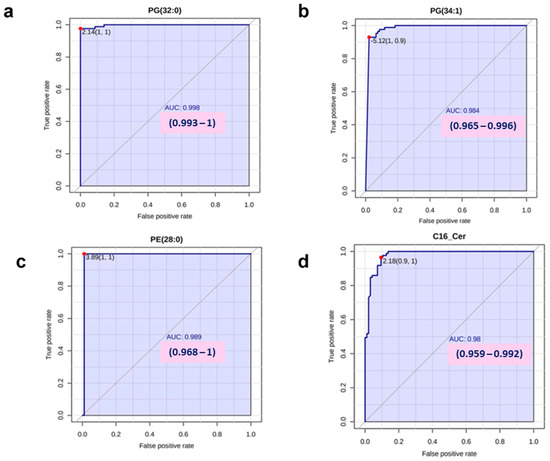
Figure 8.
Receiver operating characteristic (ROC) curves of lipids with the top four highest area under the curve (AUC) levels for discriminating T2DM ROC curves demonstrating the top four highest AUC levels for lipid discrimination in T2DM are presented (a–d).
4. Discussion
Here, we investigated the modulations of glycerophospholipids and sphingolipids in the serum of participants with T2DM; particularly, we observed that the PC/LPC and PE/LPE axes were suppressed, whereas the PG/LPG and PI/LPI axes were enhanced in T2DM among glycerophospholipids. Among sphingolipids, CER levels, particularly C16:0 Cer, were prominently elevated in T2DM; however, SMs were also positively modulated. Currently, LPC, LPE, PC, PE, and CER have been extensively investigated for their associations with metabolic diseases and complications [15,28,29]. However, the involvement of LPG and PG, as well as LPI and PI, in metabolic diseases, particularly in human samples has not been reported yet. Moreover, the levels of several specific lipid species increased in participants with T2DM, including PC (30:1, 32:0, 32:2, 32:4, 34:1, 34:3, 36:0, 36:2, 36:4, 38:0, 38:2, 38:4, 38:6, 38:8, 40:1, 40:5, 40:7, 40:9, 42:0, and 42:2), PI (42:0, 42:1, 42:2, 42:3, 42:4, 42:5, 42:6, 42:7, 42:9, 42:10, and 44:12), PE (34:1, 36:0, 36:3, 36:6, 38:4, 38:7, and 40:7), PG (32:0 and 34:0), LPE (16:0 and 16:1), LPI (16:0, 18:0, 18:1, 18:2, and 20:5), LPG (16:0, 16:1, 18:0, 18:1, and 18:2), SM (32:2, 40:2, and 38:1), dhSph, Sph, C16:0 Cer, C18:0 Cer, and C20:0 Cer. Conversely, LPC (16:0, 18:0, 18:1, and 20:4), LPE (18:0, 18:1, 18:2, 20:4, 20:5, 22:5, and 22:6), SM (32:2, 36:3, 36:4, 38:6, 38:3, and 38:4), C22:0 Cer, and C24:0 Cer levels were lower in the T2DM group.
Regarding the PG and LPG axis in T2DM, an elevation in the PG and LPG levels in the serum of patients with T2DM was observed, and their levels were positively associated with the disturbance in glucose metabolism, represented by HbA1c (Figure 7). PG is produced by the aminoacylation reaction of lipid ionic pairing to LPG, displaying an association attribute with PG [30,31,32]. To date, only serum LPG 12:0 has been reported to predict T2DM onset [33]. In the present study, for the first time, substantial LPG species were observed to be higher in T2DM. Regarding PG modulation, PG levels were positively associated with T2DM, which was concordant with this study [34]. PG is the second most abundant phospholipid in different areas of the human body [35,36,37]. It is reportedly involved in the pathogenesis of several diseases, inducing protein kinase activation in cancer and human spleen inflammatory diseases [26,27]. Moreover, recent studies have suggested that PG plays a significant role in stabilizing membrane proteins in cells to ensure effective ionic channel signaling and in protecting cells from mitochondrial apoptotic death [38,39]. Regarding the pathological conditions observed in T2DM, PG may be particularly involved in mitochondrial functions, as PG contents in the mitochondria help maintain mitochondrial activity to perform its energy-providing functions and survivability LPG and PG homeostasis through LPGAT1 possesses significant roles in maintaining the PG/CL regulation by the mitochondria function to prevent oxidative stress, which causes insulin resistance and other metabolic diseases [38,39,40,41,42]. Among the monitored lipids in this study, PG seemed to be the most strongly associated with HbA1c. Interestingly, patients with T2DM with HbA1c values of approximately 6.5–6.9% had higher PG 32:0 and PG 34:1 levels than those with HbA1c values below 6.5%, suggesting that disturbances in PG dynamics could be observed even in the early stage of diabetes, where glucose homeostasis should be only mildly disturbed. To better understand the disturbance in lipid metabolism observed in diabetes including the PG/LPG axis, further investigations are required. However, from the perspective of clinical laboratory testing, PG could directly affect the association between HbA1c and glucose homeostasis rather than the disturbed PG metabolism being associated with the pathogenesis of diabetes; HbA1c levels could be affected by red blood cell kinetics. Considering that PG 16:0/18:1 and PG 18:1/18:1 have potent pro-proliferative properties [42], higher red blood cell production due to PG 34:1 level elevation may underestimate the modulations of the associations between PG and HbA1c levels. In addition to the potential association with mitochondrial functions, LPG has been proposed to possess strong agonistic activity for G-protein–coupled receptor 55 (GPR55), which LPI also possesses as a potent innate agonist for this receptor [43]. The potential involvement of GPR55 in T2DM pathogenesis is discussed further in the following section of the PI and LPI axis. Conversely, PG reportedly promotes hemoglobin glycation through its physical and chemical properties [44]. Although the extent of this physical effect on HbA1c is unclear in vivo, these properties may overestimate the association between PG and HbA1c levels.
Regarding the PI and LPI axis, PI and LPI serum levels were higher in T2DM and positively associated with HbA1c. The biological actions of LPI compared with those of PI were associated and mediated by a function of PI degradation to form LPI on the GPR55 receptor in response to changes in human health [44]. To the best of our knowledge, six LPI species (16:1, 18:1, 18:2, 20:3, 20:4, and 22:6) are higher in T2DM. PI is positively associated with T2DM. These results are consistent with the results of the present study; however, their associations with the clinical phenotypes of T2DM have not been investigated in detail.
PI is a precursor synthesis of LPI and plays a significant role in several diseases [45,46]. PI is mainly observed in the minority component of cellular membranes in eukaryotic cells. Its structure is relatively similar to other glycerophospholipids, with the head group being the cyclic myo-inositol. This PI head group has free hydroxyl groups at positions D2–D6, and the hydroxyl groups at D3, D4, and D5 are available for phosphorylation by lipid kinases. The seven combinatorically inositol head group phosphorylated forms are involved in intracellular signal transduction and are frequently detected in the endoplasmic reticulum, early and late endosomes, trans-Golgi, secretory granules, and plasma membrane. LPI has been suggested to play significant roles in several diseases. GPR55 is a potential LPI receptor [47]. GPR55 activation stimulates p38 signal in macrophages and malignant cells and induces inflammation and cell proliferation [48]. Considering these properties of GPR55, treatment with a GPR55 antagonist improves insulin resistance in mouse models through attenuating inflammation, which was by us [48]. The elevation of LPI, as well as LPG, a partial agonist for GPR55, may aggravate insulin resistance, while it may reportedly promote insulin secretion [49]. Moreover, LPI reportedly induces insulin secretion from pancreatic β-cells [50] and induces glucagon-like peptide 1, which also promotes insulin secretion [51]. Considering these previous studies together with the characteristics of participants with T2DM enrolled in the present study, the average BMI was >24 kg/m2 (Table 1), and the inverse association between LPI and insulin resistance may largely contribute to the positive association between serum LPI and HbA1c levels in the present study. Conversely, total PI and LPI levels were strongly correlated with HbA1c levels. Moreover, in this study, total PI and LPI levels were inversely related with CAD. Furthermore, no significant associations were observed with HbA1c levels in T2DM-associated CAD. These associations may result from the fact that the lipid levels of the participants with CAD were strictly controlled with intensive pharmacological drug treatments, including statins, to maintain LDL-C levels and subsequently prevent CAD recurrence; [52] these drug treatments may also affect glycerophospholipid levels. Therefore, the possibility of some uncontrolled confounding factors including T2DM disease duration and drug treatments, which could affect these apparently contradictory results, cannot be ruled out. However, we believe that our results will contribute valuable insights for future research and clinical management of patients with T2DM.
In addition, we acknowledge the limitation of multiple testing in a small sample size. To reduce false positives, results with p < 0.001 were considered robust, while all unadjusted p-values are reported for transparency. Strict corrections (e.g., Bonferroni, FDR) lower type I error but may obscure biologically relevant signals in exploratory lipidomic studies. Thus, associations with higher p-values are interpreted as exploratory and require future validation in larger cohorts. Moreover, current lipidomic assays are more complex and costly and are not proposed currently as diagnostic tools for diabetes or CAD. Rather, this study characterizes lipid alterations associated with these conditions, providing mechanistic insights to inform future biomarker discovery, risk stratification, and therapeutic targeting. Accordingly, the observed differences should be viewed as hypothesis-generating, with directions of associations in complications of diabetes mellitus, particularly in CAD that requires confirmation in future studies.
5. Conclusions
This study delineates distinct distributions of glycerophospholipids and sphingolipids in T2DM: PG, LPG, PI, LPI, PE, and CER serum levels were elevated, whereas those of LPE, PC, and LPC were reduced in T2DM. Notably, the molecular species of PG 34:1, PG 32:0, PE 28:0, C16:0 Cer, and C18:0 Cer may hold significance as these lipids were associated with clinical parameters. Regarding their association with complications, PG 34:1, PG 32:0, C16:0 Cer, and C18:0 Cer exhibited strong associations with coronary and nephropathic complications of T2DM. Diabetes is a contributing factor to DN, CI, and particularly CAD. Our current findings indicate that glycerophospholipid and sphingolipid fluctuations were associated with T2DM complications, emphasizing the potential use of these lipids as diagnostic biomarkers for early T2DM diagnosis, treatment, and prevention of further onset of T2DM complications. These results will provide valuable insights for lipidomic researchers to comprehend the pathological conditions accompanying T2DM and encourage the development of novel clinical laboratory biomarkers and targeted lipid drugs for T2DM treatment, including PG 32:0, PG 34:1, C16:0 Cer, and C18:0 Cer, as well as investigate their therapeutic applications for T2DM prevention.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diabetology6100112/s1. Figure S1: [Structure and metabolic pathway of lipids analyzed in the present study]. Figure S2: [Changes in diacyl-glycerophospholipids species in patients with T2DM]. Figure S3: [Associations of serum SM levels and their relationship with diabetic complications in patients with T2DM].
Author Contributions
H.T. and M.K. designed the research and analyzed the data; H.T. and B.U. performed the research; H.T., M.K. and Y.Y. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI Grant Number 16H06236, 20H03573, and 24K02358 (M.K.).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of The University of Tokyo Medical Research Centre Ethics Committee (Approval Number 10266 and 11158).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Acknowledgments
H.T. gratefully acknowledges the Moritani Foundation of Japan for the generous scholarship that supported this research.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
Alb: Albumin; Alb/Cre: Albumin/Creatinine ratio; AGEs: Advanced glycation end products; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; BSA: Body Surface Area; BMI: Body Mass Index; CAD: Coronary artery disease; CER: Ceramide; CL: Cardiolipin; CRE: Creatinine; DN: Diabetic nephropathy; eGFR: Estimated Glomerular Filtration Rate; EDTA: Ethylenediaminetetraacetic acid; GPR55: G-protein–coupled receptor 55; HbA1c: Hemoglobin A1C; HDL-C: High-density lipoprotein cholesterol; HPLC: High-performance liquid chromatography; LC–MS: Liquid chromatography–mass spectrometry; LDL-C: Low-density lipoprotein cholesterol; LPA: Lysophosphatidic acid; LPC: Lysophosphatidylcholine; LPE: Lysophosphatidylethanolamine; LPG: Lysophosphatidylglycerol; LPGAT1: Lysophosphatidylglycerol acyltransferase 1; LPI: Lysophosphatidylinositol; LPS: Lysophosphatidylserine; MRM: Multiple reaction monitoring; OPLS-DA: Orthogonal partial least squares discriminant analysis; PC: Phosphatidylcholine; PCA: Principal component analysis; PE: Phosphatidylethanolamine; PG: Phosphatidylglycerol; PI: Phosphatidylinositol; PS: Phosphatidylserine; SEM: Standard Error of the Mean; SGLT2 inhibitors: Sodium–glucose cotransporter-2 inhibitors; SM: Sphingomyelin; T2DM: Type 2 diabetes mellitus; TP: Total Protein; TP/Cre: Total Protein/Creatinine ratio; VIP score: Variable Importance in Projection score.
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Morita, Y.; Kurano, M.; Sakai, E.; Sawabe, M.; Aoki, J.; Yatomi, Y. Simultaneous analyses of urinary eicosanoids and related mediators identified tetranor-prostaglandin E metabolite as a novel biomarker of diabetic nephropathy. J. Lipid Res. 2021, 62, 100120. [Google Scholar] [CrossRef]
- Wang, C.; Kong, H.; Guan, Y.; Yang, J.; Gu, J.; Yang, S.; Xu, G. Plasma phospholipid metabolic profiling and biomarkers of type 2 diabetes mellitus based on high-performance liquid chromatography/electrospray mass spectrometry and multivariate statistical analysis. Anal. Chem. 2005, 77, 4108–4116. [Google Scholar] [CrossRef] [PubMed]
- Packard, C.J. Remnants, LDL, and the quantification of lipoprotein-associated risk in atherosclerotic cardiovascular disease. Curr. Atheroscler. Rep. 2022, 24, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Dal Canto, E.; Ceriello, A.; Rydén, L.; Ferrini, M.; Hansen, T.B.; Schnell, O.; Standl, E.; Beulens, J.W. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur. J. Prev. Cardiol. 2019, 26 (Suppl. S2), 25–32. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E.; Berezin, A.A. Circulating cardiac biomarkers in diabetes mellitus: A new dawn for risk Stratification—A narrative review. Diabetes Ther. 2020, 11, 1271–1291. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Cannon, C.P.; Blazing, M.A.; Nicolau, J.C.; Corbalán, R.; Špinar, J.; Park, J.-G.; White, J.A.; Bohula, E.A.; Braunwald, E.; et al. Benefit of adding ezetimibe to statin therapy on cardiovascular outcomes and safety in patients with versus without diabetes mellitus: Results from IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation 2018, 137, 1571–1582. [Google Scholar] [CrossRef]
- Mandal, N.; Grambergs, R.; Mondal, K.; Basu, S.K.; Tahia, F.; Dagogo-Jack, S. Role of ceramides in the pathogenesis of diabetes mellitus and its complications. J. Diabetes Complicat. 2021, 35, 107734. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, S.Y.; Bae, Y. Functional roles of sphingolipids in immunity and their implication in disease. Molecules 2023, 55, 1110–1130. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Kusminski, C.M.; Scherer, P.E. Lowering ceramides to overcome diabetes. Science 2019, 365, 319–320. [Google Scholar] [CrossRef]
- Basta, G.; Schmidt, A.M.; De Caterina, R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. J. Intern. Med. 2004, 63, 582–592. [Google Scholar]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef]
- Rodriguez Cuenca, S.; Pellegrinelli, V.; Campbell, M.; Oresic, M.; Vidal Puig, A. Sphingolipids and glycerophospholipids—The “ying and yang” of lipotoxicity in metabolic diseases. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 66, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Meikle, P.J.; Summers, S.A. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Prog. Lipid Res. 2017, 13, 79–91. [Google Scholar] [CrossRef]
- Yamada, M.; Kita, Y.; Kohira, T.; Yoshida, K.; Hamano, F.; Tokuoka, S.M.; Shimizu, T. A comprehensive quantification method for eicosanoids and related compounds by using liquid chromatography/mass spectrometry with high-speed continuous ionization polarity switching. J. Chromatogr. B 2015, 995–996, 74–84. [Google Scholar] [CrossRef]
- Masukawa, Y.; Narita, H.; Sato, H.; Naoe, A.; Kondo, N.; Sugai, Y.; Oba, T.; Homma, R.; Ishikawa, J.; Takagi, Y.; et al. Comprehensive quantification of ceramide species in human stratum corneum. J. Lipid Res. 2009, 50, 1708–1719. [Google Scholar] [CrossRef]
- Jiang, X.; Han, X. Characterization and direct quantitation of sphingoid base-1-phosphates from lipid extracts: A shotgun lipidomics approach. J. Lipid Res. 2006, 47, 1865–1873. [Google Scholar] [CrossRef][Green Version]
- Wang, C.; Wang, M.; Han, X. Comprehensive and quantitative analysis of lysophospholipid molecular species present in obese mouse liver by shotgun lipidomics. Anal. Chem. 2015, 87, 4879–4887. [Google Scholar] [CrossRef]
- Borges-Araújo, L.; Domingues, M.M.; Fedorov, A.; Santos, N.C.; Melo, M.N.; Fernandes, F. Acyl-chain saturation regulates the order of phosphatidylinositol 4,5-bisphosphate nanodomains. Commun. Chem. 2021, 4, 164. [Google Scholar] [CrossRef]
- Rabinovich, A.L.; Lyubartsev, A.P.; Zhurkin, D.V. Unperturbed hydrocarbon chains and liquid phase bilayer lipid chains: A computer simulation study. Eur. Biophys. J. 2018, 47, 109–130. [Google Scholar]
- Angala, S.K.; Carreras-Gonzalez, A.; Huc-Claustre, E.; Anso, I.; Kaur, D.; Jones, V.; Palčeková, Z.; Belardinelli, J.M.; de Sousa-D’aUria, C.; Shi, L.; et al. Acylation of glycerolipids in mycobacteria. Nat. Commun. 2023, 14, 6694. [Google Scholar] [CrossRef]
- Kashiwagi, A.; Kasuga, M.; Araki, E.; Oka, Y.; Hanafusa, T.; Ito, H.; Tominaga, M.; Oikawa, S.; Noda, M.; Kawamura, T.; et al. International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J. Diabetes Investig. 2012, 3, 39–40. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Verheij, H.M.; Smith, P.F.; Bonsen, P.P.M.; Van Deenen, L.L.M. The chemical synthesis of a phosphatidylglucose. Biochim. Biophys. Acta 1970, 218, 97–101. [Google Scholar] [CrossRef]
- Klemm, D.J.; Elias, L. Phosphatidylglycerol-modulated protein kinase activity from human spleen: II. Interaction with phospholipid vesicles. Arch. Biochem. Biophys. 1988, 265, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Green, C.D.; Maceyka, M.; Cowart, L.A.; Spiegel, S. Sphingolipids in metabolic disease: The good, the bad, and the unknown. Cell Metab. 2021, 33, 1293–1306. [Google Scholar] [CrossRef]
- Hernández-Bello, F.; Franco, M.; Pérez-Méndez, Ó.; Donis-Maturano, L.; Zarco-Olvera, G.; Bautista-Pérez, R. Sphingolipid metabolism and its relationship with cardiovascular, renal and metabolic diseases. Arch. Med. Res. 2023, 93, 88–95. [Google Scholar]
- Tan, S.T.; Ramesh, T.; Toh, X.R.; Nguyen, L.N. Emerging roles of lysophospholipids in health and disease. Prog. Lipid Res. 2020, 80, 101068. [Google Scholar] [CrossRef] [PubMed]
- Wölk, C.; Youssef, H.; Guttenberg, T.; Marbach, H.; Vizcay-Barrena, G.; Shen, C.; Brezesinski, G.; Harvey, R.D. Phase diagram for a Lysyl-Phosphatidylglycerol analogue in biomimetic mixed monolayers with phosphatidylglycerol: Insights into the tunable properties of bacterial membranes. ChemPhysChem 2020, 21, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Khatib, T.O.; Stevenson, H.; Yeaman, M.R.; Bayer, A.S.; Pokorny, A. Binding of daptomycin to anionic lipid vesicles is reduced in the presence of lysyl-phosphatidylglycerol. Antimicrob. Agents Chemother. 2016, 60, 5051–5053. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Y.; Ong, C.; Subramaniam, T.; Choi, H.W.; Yuan, J.-M.; Koh, W.-P.; Pan, A. Metabolic signatures and risk of type 2 diabetes in a Chinese population: An untargeted metabolomics study using both LC-MS and GC-MS. Diabetologia 2016, 59, 2349–2359. [Google Scholar] [CrossRef]
- Meikle, P.J.; Wong, G.; Barlow, C.K.; Weir, J.M.; Greeve, M.A.; MacIntosh, G.L.; Almasy, L.; Comuzzie, A.G.; Mahaney, M.C.; Kowalczyk, A.; et al. Plasma lipid profiling shows similar associations with prediabetes and type 2 diabetes. PLoS ONE 2013, 8, e74341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Potting, C.; Tatsuta, T.; König, T.; Haag, M.; Wai, T.; Aaltonen, M.J.; Langer, T. TRIAP1/PRELI complexes prevent apoptosis by mediating intramitochondrial transport of phosphatidic acid. Cell Metab. 2013, 18, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Hallman, M.; Gluck, L. Phosphatidylglycerol in lung surfactant II. Subcellular distribution and mechanism of biosynthesis in vitro. Biochim. Biophys. Acta 1975, 409, 172–191. [Google Scholar] [CrossRef]
- Chen, W.; Chao, Y.; Chang, W.; Chan, J.; Hsu, Y.H. Phosphatidylglycerol incorporates into cardiolipin to improve mitochondrial activity and inhibits inflammation. Sci. Rep. 2018, 8, 4919. [Google Scholar] [CrossRef]
- Klemm, D.J.; Elias, L. Purification and assay of a phosphatidylglycerol-stimulated protein kinase from murine leukemic cells and its perturbation in response to IL-3 and PMA treatment. Exp. Hematol. 1988, 16, 855–860. [Google Scholar]
- Laganowsky, A.; Reading, E.; Allison, T.M.; Ulmschneider, M.B.; Degiacomi, M.T.; Baldwin, A.J.; Robinson, C.V. Membrane proteins bind lipids selectively to modulate their structure and function. Nature 2014, 510, 172–175. [Google Scholar] [CrossRef]
- Marty, N.J.; Holman, C.L.; Abdullah, N.; Johnson, C.P. The C2 domains of otoferlin, dysferlin, and myoferlin alter the packing of lipid bilayers. Biochemistry 2013, 52, 5585–5592. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Sun, H.; Liu, X.; Zheng, Y.; Xu, D.; Wang, J.; Jia, D.; Han, X.; Liu, F.; et al. Defective phosphatidylglycerol remodeling causes hepatopathy, linking mitochondrial dysfunction to hepatosteatosis. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 763–781. [Google Scholar] [CrossRef]
- Veldhuizen, R.; Nag, K.; Orgeig, S.; Possmayer, F. The role of lipids in pulmonary surfactant. Biochim. Biophys. Acta 1998, 1408, 90–108. [Google Scholar] [CrossRef]
- Uranbileg, B.; Kurano, M.; Kano, K.; Sakai, E.; Arita, J.; Hasegawa, K.; Nishikawa, T.; Ishihara, S.; Yamashita, H.; Seto, Y.; et al. Sphingosine 1-phosphate lyase facilitates cancer progression through converting sphingolipids to glycerophospholipids. Clin. Transl. Med. 2022, 12, e1056. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark. Insights 2016, 2016, 95–104. [Google Scholar] [CrossRef]
- Helsley, R.N.; Varadharajan, V.; Brown, A.L.; Gromovsky, A.D.; Schugar, R.C.; Ramachandiran, I.; Fung, K.; Kabbany, M.N.; Banerjee, R.; Neumann, C.K.; et al. Obesity-linked suppression of membrane-bound O-acyltransferase 7 (MBOAT7) drives non-alcoholic fatty liver disease. eLife 2019, 8, e49882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shimizu, K.; Ono, M.; Mikamoto, T.; Urayama, Y.; Yoshida, S.; Hase, T.; Michinaga, S.; Nakanishi, H.; Iwasaki, M.; Terada, T.; et al. Overexpression of lysophospholipid acyltransferase, LPLAT10/LPCAT4/LPEAT2, in the mouse liver increases glucose-stimulated insulin secretion. FASEB J. 2024, 38, e23425. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, P.; Leidl, K.; Boettcher, A.; Schmitz, G.; Liebisch, G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J. Lipid Res. 2009, 50, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Kurano, M.; Kobayashi, T.; Sakai, E.; Tsukamoto, K.; Yatomi, Y. Lysophosphatidylinositol, especially albumin-bound form, induces inflammatory cytokines in macrophages. FASEB J. 2021, 35, e21673. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Song, S.; Ruz-Maldonado, I.; Pingitore, A.; Huang, G.C.; Baker, D.; Jones, P.M.; Persaud, S.J. GPR55-dependent stimulation of insulin secretion from isolated mouse and human islets of langerhans. Diabetes 2016, 18, 1263–1273. [Google Scholar] [CrossRef]
- Razquin, C.; Toledo, E.; Clish, C.B.; Ruiz-Canela, M.; Dennis, C.; Corella, D.; Papandreou, C.; Ros, E.; Estruch, R.; Guasch-Ferré, M.; et al. Plasma lipidomic profiling and risk of type 2 diabetes in the PREDIMED trial. Diabetes Care 2018, 41, 2617–2624. [Google Scholar] [CrossRef]
- Arifin, S.A.; Paternoster, S.; Carlessi, R.; Casari, I.; Ekberg, J.H.; Maffucci, T.; Newsholme, P.; Rosenkilde, M.M.; Falasca, M. Oleoyl-lysophosphatidylinositol enhances glucagon-like peptide-1 secretion from enteroendocrine L-cells through GPR119. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Eldor, R.; Raz, I. American diabetes association indications for statins in diabetes. Diabetes Care 2009, 32, S384–S391. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).