1. Introduction
Type 2 diabetes mellitus (T2DM) is now recognized as being driven not only by persistent hyperglycemia but also by chronic oxidative stress and low-grade inflammation [
1]. These interrelated processes accelerate cardiovascular, renal, and hepatic complications, underscoring the necessity for therapeutic approaches that modulate redox balance and inflammatory pathways [
2,
3,
4].
Excess reactive oxygen species (ROS) activate redox-sensitive transcription factors, such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), leading to the upregulation of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-6 [
5]. These cytokines subsequently impair mitochondrial function and promote sustained ROS production, creating a self-perpetuating vicious cycle that contributes to endothelial dysfunction, insulin resistance, and tissue fibrosis. Recent evidence also highlights that lysosomal dysfunction amplifies oxidative stress and inflammation, further promoting cardiovascular and metabolic complications [
6]. At low levels, however, ROS can act as signaling molecules that activate protective antioxidant pathways—a concept termed mitochondrial hormesis or mitohormesis [
7,
8]. These mechanisms involve nuclear factor erythroid 2-related factor 2 (Nrf2) and related transcription factors, helping to maintain tissue homeostasis and potentially extending lifespan [
9]. As a physiological example, regular moderate physical activity generates low-level oxidative stress, which induces an anti-inflammatory environment by upregulating antioxidant and anti-inflammatory defense systems [
10]. Nutritional states such as caloric restriction and nutritional ketosis also engage mitohormetic pathways [
11].
Oxidative stress-induced molecular and organelle damage—including endoplasmic reticulum and mitochondrial dysfunction as well as cellular senescence—can cause chronic inflammation in patients with diabetes. This can further exacerbate metabolic and vascular injury [
12]. This vicious cycle has been implicated in the pathogenesis of cardiovascular disease, sarcopenia, osteoarthritis, and cancer.
Direct antioxidant supplementation has not consistently shown benefits and sometimes even increases disease risk [
13,
14]. However, therapeutic strategies that modulate rather than abolish ROS signaling have garnered attention. This principle aligns with the hormesis framework, which has long been discussed in biogerontology and drug development [
15,
16].
Sodium–glucose cotransporter 2 (SGLT2) inhibitors, originally developed as glucose-lowering agents, have demonstrated remarkable cardiorenal protective effects in large outcome trials. Recent experimental and clinical findings suggest that these benefits may involve the modulation of oxidative stress, inflammation, and engagement of mitohormetic pathways. In this review, we summarize emerging data on how SGLT2 inhibitors may attenuate organ stress through adaptive redox responses and compare these insights with those from other hormetic models such as caloric restriction, exercise, aging, and inter-organ ROS signaling.
2. Mechanisms of Mitohormesis
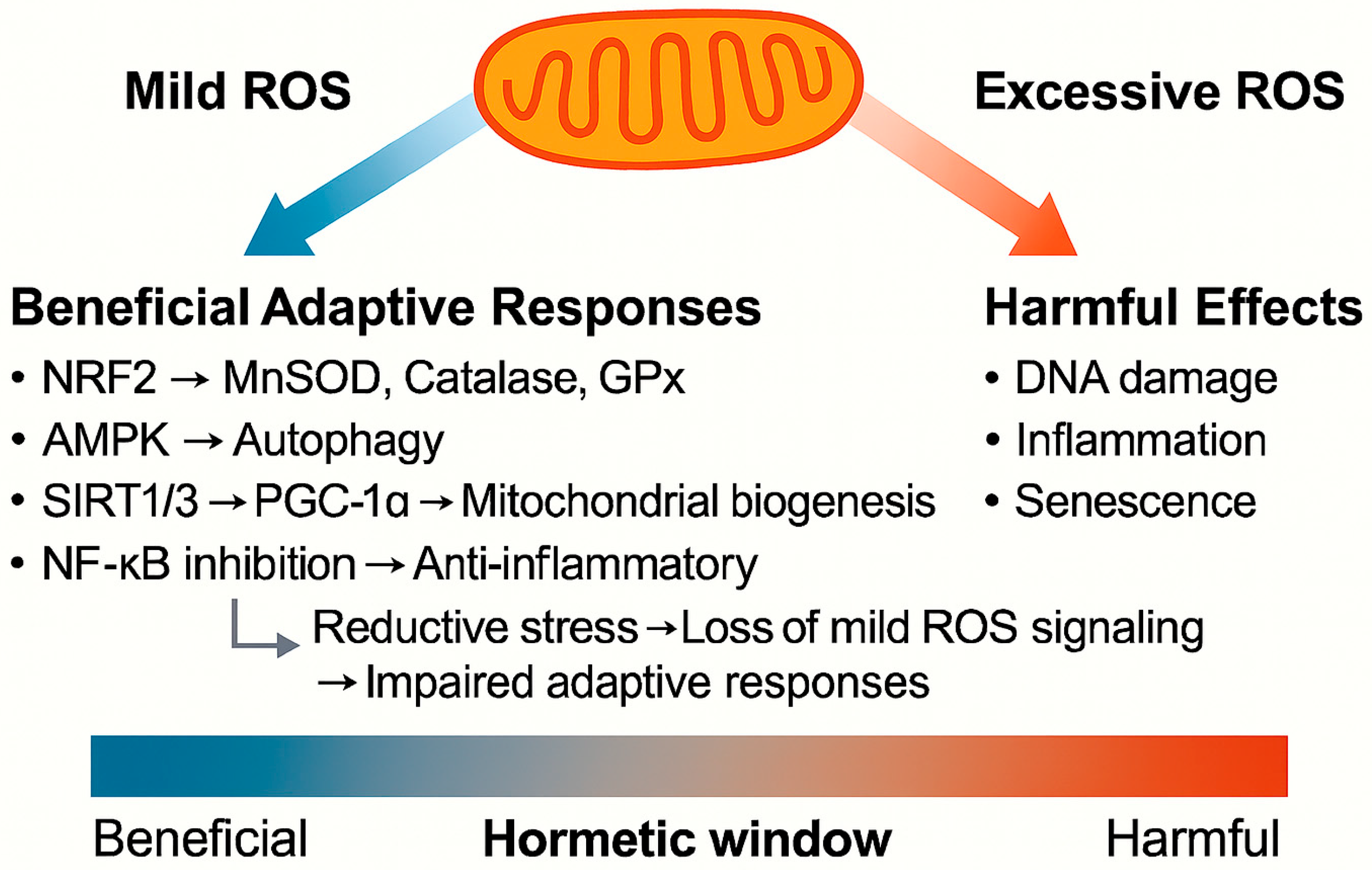
Mitohormesis refers to the adaptive biological response triggered by a transient and moderate increase in mitochondrial ROS levels. Unlike pathological oxidative stress, which causes irreversible molecular damage, mild elevations of ROS levels serve as signaling cues that activate protective stress response pathways.
Key molecular sensors of mitohormesis include the following:
Nrf2 activates the transcription of antioxidant enzymes, such as manganese superoxide dismutase (MnSOD), catalase, and glutathione peroxidase [
17].
AMP-activated protein kinase (AMPK) senses energy depletion, promotes autophagy, and enhances metabolic flexibility [
18].
Sirtuin (SIRT)1 and SIRT3: nicotinamide adenine dinucleotide-dependent deacetylases that regulate mitochondrial protein acetylation and energy metabolism. SIRT3 is localized in mitochondria and has been implicated in promoting mitochondrial function and oxidative stress resistance, whereas SIRT1 interacts with peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC-1α) to enhance mitochondrial biogenesis and antioxidant responses [
19].
NF-κB: its suppression mitigates chronic inflammation by downregulating pro-inflammatory cytokines, such as TNF-α and IL-6 [
5].
As illustrated in
Figure 1, these pathways collectively improve mitochondrial quality control, enhance antioxidant capacity, reduce chronic inflammation, and promote cellular resilience. Physiological stimuli, such as caloric restriction [
20], exercise [
21], and nutritional ketosis [
22], and pharmacological interventions, such as SGLT2 inhibition, are all thought to engage these hormetic processes. The net effect is the preservation of tissue homeostasis and the potential delay of age-related complications, provided ROS levels remain within a “hormetic window.”
Preclinical data also support the involvement of SGLT2 inhibitors in mitohormetic pathways. For instance, Tomita et al. demonstrated in murine models of diabetic kidney disease that SGLT2 inhibition promoted ketone body-induced suppression of mechanistic target of rapamycin complex 1 (mTORC1), leading to enhanced autophagic flux, improved mitochondrial quality control, and adaptive redox balance [
23]. These findings suggest that SGLT2 inhibitors may indirectly engage mitohormetic signaling by modulating mitochondrial function and stress adaptation, thereby providing a mechanistic bridge between experimental findings and clinical observations.
3. Caloric Restriction and Mitohormesis
Caloric restriction is widely recognized as a potent nongenetic intervention that extends lifespan and delays the onset of age-related diseases across species [
24]. One of the key mechanisms underlying the benefits of caloric restriction is the induction of mitohormesis, which enhances antioxidant defenses and mitochondrial biogenesis [
9,
20]. This hormetic process, mediated through pathways such as AMPK, SIRT1/3, and PGC-1α, ultimately contributes to improved metabolic health, reduced chronic inflammation, and protection against T2DM and cardiovascular complications [
20,
25,
26].
Caloric restriction exerts its effects not solely through the reduction of energy intake but also by activating beneficial biological pathways. For example, under conditions of caloric restriction, increased mitochondrial activity leads to mild ROS production, which stimulates the endogenous antioxidant system without overwhelming it. The occurrence of this phenomenon is corroborated by human and animal studies demonstrating improvements in insulin sensitivity and reductions in pro-inflammatory signaling, including NF-κB suppression, as well as maintenance of genomic stability [
24,
27]. Notably, findings from the CALERIE randomized controlled trials consistently demonstrated that 6–24 months of moderate caloric restriction in healthy, nonobese adults lowered insulin levels and body temperature, reduced DNA damage, and improved oxidative stress and metabolic markers, supporting the role of moderate caloric restriction in slowing biological aging [
27,
28,
29,
30,
31].
In model organisms, such as
Caenorhabditis elegans, impaired insulin/insulin-like growth factor 1 signaling extends lifespan by promoting mild mitochondrial ROS production. Zarse et al. reported that this effect involves mitochondrial L-proline catabolism, which transiently increases ROS levels and activates protective pathways, providing a clear example of mitohormesis in the context of extended longevity [
32].
Insights from starvation biology further suggest that mild caloric restriction may protect renal function by enhancing metabolic resilience [
33]. Additionally, nutritional ketosis has been proposed as a complementary pathway that can induce mitohormesis and further enhance mitochondrial function, thereby reinforcing the protective metabolic adaptations observed during caloric restriction [
11].
Collectively, these findings support the development of nutritional and pharmacological interventions that leverage mitohormesis for organ protection in T2DM.
4. Exercise Physiology and Mitohormesis
Regular physical activity is another well-established physiological trigger of mitohormesis. Moderate exercise transiently increases ROS production in skeletal muscles, thereby activating redox-sensitive transcription factors and endogenous antioxidant defenses through pathways involving Nrf2, MnSOD, catalase, and glutathione peroxidase [
10]. This mild oxidative stress also induces an anti-inflammatory environment by upregulating cytokines such as IL-10 and suppressing pro-inflammatory mediators. Interestingly, IL-6, although widely recognized as a pro-inflammatory cytokine, can also act as a myokine during exercise, exerting context-dependent anti-inflammatory and metabolic effects [
34].
Clinical and experimental studies consistently support that regular exercise improves insulin sensitivity, endothelial function, and organ stress markers, partly through adaptive redox modulation. Recent redox proteomics studies further emphasize that mild, localized ROS production is essential for optimal organ function. For example, mild ROS production can fine-tune proteins involved in energy metabolism. In brown fat, the ROS-dependent modification of uncoupling protein 1 (UCP1) enhances its heat-producing activity, showing how ROS signaling contributes to beneficial adaptations [
35].
Importantly, activity-dependent hormetic effects depend strongly on the type, intensity, and duration of exercise. Although moderate, regular exercise promotes metabolic resilience and reduces the risk of cardiovascular and neurodegenerative diseases, excessive or unaccustomed inessential exercise can overwhelm antioxidant systems, leading to cellular damage and impaired recovery [
36,
37]. This duality highlights the importance of achieving an optimal redox balance to harness the protective effects of exercise-induced ROS.
Different exercise modalities may also influence the extent and quality of the hormetic response. For example, moderate aerobic training is recognized for its ability to increase mitochondrial biogenesis and MnSOD activity [
38,
39], whereas resistance training supports mitochondrial turnover and muscle quality in aging populations [
40,
41]. High-intensity interval training also induces transient oxidative stress, which, when appropriately dosed, further enhances endogenous antioxidant systems [
42,
43,
44].
However, it is crucial to acknowledge that the excessive suppression of physiological ROS production can also be detrimental. For example, selenoprotein P, a liver-derived antioxidant protein, induces reductive stress in skeletal muscle, thereby inhibiting beneficial hormetic ROS signaling and reducing exercise endurance [
45]. In brown adipose tissue, selenoprotein P-mediated ROS scavenging suppresses UCP1 activation by limiting Cys254 sulfenylation, consequently impairing thermogenesis [
46]. These observations caution against the oversimplified view that indiscriminate antioxidant supplementation is always beneficial. As an example, a randomized human study demonstrated that the supplementation with vitamins C and E negated the exercise-induced improvements in insulin sensitivity and endogenous antioxidant defense [
47]. These findings illustrate that oxidative and reductive stress can both disrupt the fragile hormetic window that is necessary for organ resilience.
5. Cellular Senescence, Senescence-Associated Secretory Phenotype, and Redox Balance
Cellular senescence is increasingly recognized as a substantial source of chronic oxidative stress and inflammation in aging and metabolic diseases [
48,
49]. Senescent cells maintain elevated levels of ROS production due to persistent DNA damage responses and mitochondrial dysfunction, which further sustain the senescence program through p53/p21 and p16INK4a pathways [
50,
51]. Lysosomal dysfunction is also a key hallmark of senescent cells, driving chronic senescence-associated secretory phenotype (SASP) activity and sustaining tissue inflammation. Our recent review summarized how impaired lysosomal integrity, NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome activation, and defective autophagic flux collectively promote cellular senescence in cardiovascular tissues [
6]. Through the SASP, senescent cells release pro-inflammatory cytokines, chemokines, and matrix-remodeling enzymes, thereby creating a pro-inflammatory microenvironment that promotes fibrosis and multi-organ dysfunction. Key SASP components include interleukins such as IL-6 and IL-8, chemokines like monocyte chemoattractant protein-1 (MCP-1), and matrix metalloproteinases [
49,
52]. In metabolic organs such as adipose tissue and the kidney, the accumulation of senescent cells impairs regenerative capacity and exacerbates insulin resistance [
53,
54].
Recent evidence suggests that SGLT2 inhibitors may exert indirect senolytic effects. Katsuumi et al. demonstrated that SGLT2 inhibition reduces senescent cell burden and alleviates pathological aging in murine models [
55]. Furthermore, SGLT2 inhibition may elevate ketone body levels, leading to mTORC1 suppression and reduced cellular senescence [
23]. Ketone bodies may act as a double-edged sword, with protective and potentially deleterious effects depending on the context [
23]. This potential senescence-modulating effect, combined with redox balance adaptation, may partially explain the multi-organ protective effects of SGLT2 inhibition that have been observed in clinical trials and real-world practice.
These findings highlight the complex interplay between mitohormesis and cellular senescence. Although mild ROS levels can activate protective pathways that delay senescence, excessive oxidative stress promotes senescent cell accumulation and chronic inflammation [
9]. A comprehensive understanding of this duality is key to optimizing therapeutic strategies that target the Redox–Senescence–Organ Stress Axis in T2DM. Emerging strategies that combine senolytic agents with metabolic therapies aim to eliminate dysfunctional senescent cells while preserving beneficial adaptive responses such as mitohormesis [
56,
57]. Such integrative approaches might aid in disrupting the vicious cycle of oxidative stress, cellular senescence, and organ stress in T2DM, ultimately improving long-term cardiometabolic outcomes.
6. Inter-Organ Mitohormesis and Extracellular Vesicle Signaling
Recent advancements suggest that mitohormesis may extend beyond single tissues to encompass inter-organ stress signaling [
9]. Crewe et al. demonstrated that energetically stressed adipocytes can release small extracellular vesicles (EVs) containing damaged mitochondria [
58]. These EVs are taken up by distant cardiomyocytes, transiently elevating mitochondrial ROS levels and preconditioning the heart against ischemia–reperfusion injury. As illustrated in
Figure 2, mildly elevated ROS levels in adipocytes promote the release of extracellular vesicles carrying mitochondrial components that can precondition the heart, whereas hypertrophic adipocytes with excessively elevated ROS levels release vesicles enriched in damaged mitochondria that exacerbate cardiac injury. This duality underscores the role of adipose tissue health in shaping inter-organ mitohormesis. This remarkable example of “inter-organ mitohormesis” broadens the concept of hormesis into a systemic adaptive response. At present, the clinical relevance of EV-mediated inter-organ signaling in T2DM remains conceptual, with evidence largely derived from preclinical models and limited human observational data; prospective, controlled clinical studies are needed to establish causality and therapeutic relevance.
Notably, 3-hydroxybutyrate enhances antioxidative gene expression in adipocytes, suggesting that ketone body metabolism may modulate inter-organ stress signaling through EV-mediated pathways [
59]. Thus, metabolic interventions that alter ketone levels may influence the systemic redox balance by affecting EV cargo.
In the context of T2DM, adipose tissue dysfunction might dysregulate this EV-mediated crosstalk, shifting the balance toward chronic oxidative stress and inflammation across organs [
58,
60]. Conversely, therapeutic interventions that restore healthy adipose function, such as SGLT2 inhibitors, may help normalize EV signaling, potentially enhancing resilience through controlled inter-organ ROS signaling. Although direct evidence for SGLT2 inhibitors engaging this pathway remains limited, the conceptual parallel highlights the need for future research exploring whether these agents indirectly promote inter-organ mitohormesis, inherent to their multi-organ protective effects.
Beyond EVs, other mediators are also involved in inter-organ crosstalk. Although EVs offer a compelling mechanism for stress relay, inter-organ communication across the adipose–heart–liver–kidney–brain axis is not limited to EVs. Soluble factors—including adipokines (e.g., adiponectin), myokines (e.g., IL-6 in its exercise-induced anti-inflammatory role), hepatokines (e.g., fibroblast growth factor 21 [FGF21]), and cardiokines (e.g., growth differentiation factor 15)—as well as metabolites (e.g., ketone bodies, lactate, bile acids) and autonomic/neuroendocrine pathways can also modulate the redox and inflammatory status across organs. Conceptually, SGLT2 inhibitors may influence several of these channels indirectly via weight and adipose remodeling, natriuresis/hemoconcentration, blood pressure reduction, and ketone metabolism, thereby shaping systemic redox homeostasis without implying a single dominant route of action. For instance, adiponectin promotes mitochondrial biogenesis via p38 mitogen-activated protein kinase-mediated activation of PGC-1α in skeletal muscle [
61]. Similarly, FGF21—primarily characterized as a hepatokine but also inducibly secreted from skeletal muscle (myokine) and adipose tissue (adipokine) under metabolic stress—enhances mitochondrial oxidative capacity by activating AMPK and SIRT1, leading to PGC-1α-mediated mitochondrial adaptation [
62,
63,
64].
Emerging evidence also indicates that, apart from mitochondria, EVs transport microRNAs, proteins, and lipids that modulate redox balance in recipient tissues [
65]. Such inter-organ vesicle-mediated crosstalk may have context-dependent effects: facilitating protective preconditioning in healthy states, whereas in metabolic disorders, it might exacerbate systemic oxidative stress and inflammation. Understanding how physiological or pharmacological interventions, including SGLT2 inhibitors and nutritional ketosis, modulate the cargo and destination of these vesicles might reveal new avenues for systemically targeting the Redox–Inflammation–Organ Stress Axis [
11].
Future work should standardize EV isolation and cargo profiling, pair circulating signals with tissue-level readouts, and use stratified or crossover designs (e.g., SGLT2 inhibitor vs. glucagon-like peptide-1 receptor agonist [GLP-1RA] vs. their combination) to test whether EV cargo or soluble mediators track with redox biomarkers and organ-stress indices in humans.
As summarized in
Table 1, various therapeutic approaches, such as exercise, caloric restriction, and senolytics, may exert beneficial effects on redox homeostasis by enhancing adaptive stress responses via hormetic mechanisms. In contrast, traditional antioxidant supplementation has shown limited clinical efficacy, likely due to the indiscriminate suppression of physiologic ROS signaling. EV-mediated inter-organ signaling represents a novel conceptual axis that may help explain systemic redox adaptation. However, its potential as a therapeutic target has yet to be validated.
7. Our Clinical Data: SGLT2 Inhibitors and Redox Adaptation
SGLT2 inhibitors are hypothesized to exert organ-protective effects through multiple mechanisms, including low-level mitochondrial ROS signaling that activates adaptive pathways, such as AMPK and NRF2, and suppresses the NLRP3 inflammasome (
Figure 3). These processes may reduce inflammation, attenuate cellular senescence, and contribute to systemic resilience.
In our recently published investigator-initiated prospective study [
67], treatment with an SGLT2 inhibitor significantly reduced albuminuria in patients with T2DM, while simultaneously lowering the serum levels of C-reactive protein and tumor necrosis factor receptors 1 and 2 (TNFR1/2). The reduction in albuminuria was positively correlated with the decrease in serum TNFR1 levels, suggesting that the anti-proteinuric effect may be partly mediated by anti-inflammatory mechanisms. These findings are consistent with emerging evidence that SGLT2 inhibitors may modulate lysosomal pathways, enhance autophagic flux, and suppress NLRP3 inflammasome activation [
6].
Specifically, empagliflozin treatment was also associated with significant reductions in markers of subclinical organ stress, including cardio–ankle vascular index (CAVI), fibrosis-4 index (FIB-4), and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels [
68]. In our multivariate analysis, the reduction in CAVI was independently associated with empagliflozin use; the decrease in FIB-4 was related to changes in HbA1c, derivatives of reactive oxygen metabolites (d-ROMs), and TNFR2 levels, whereas NT-proBNP concentrations showed a trend-level association with urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) concentrations. These results suggest that, in addition to glycemic control, anti-inflammatory and redox-adaptive mechanisms may contribute to the organ-protective effects observed with SGLT2 inhibition.
As summarized in
Table 2, SGLT2 inhibitors modulate multiple redox- and senescence-related pathways. In our study, we observed that while the levels of oxidative stress markers such as urinary 8-OHdG and d-ROMs increased following treatment, the biological antioxidant potential (BAP; a marker of antioxidant capacity) rose concurrently. This pattern may suggest an adaptive redox response consistent with the concept of mitohormesis; however, it cannot be excluded that elevated 8-OHdG levels also reflect unresolved oxidative DNA damage. Future studies incorporating complementary biomarkers, such as mitochondrial DNA deletions, will be needed to clarify the balance between adaptive signaling and persistent oxidative injury. The simultaneous increase in BAP indicates that the mild increase in ROS levels may have triggered endogenous antioxidant defense systems rather than causing net oxidative damage.
Interestingly, not only the use of SGLT2 inhibitors but also the concomitant use of a GLP-1RA emerged as an independent explanatory factor for the increase in urinary 8-OHdG levels in our multivariate analysis [
24]. Although GLP-1RAs are known for their anti-inflammatory and modest antioxidant properties, they can paradoxically enhance mitochondrial respiration and mild ROS generation in certain metabolic contexts, potentially activating adaptive redox pathways consistent with the mitohormesis concept. Although GLP-1RA use emerged as an independent explanatory factor for elevated urine levels of 8-OHdG in our multivariate analysis, the possibility remains that concomitant therapies or unmeasured confounders contributed to this effect. The existence of this duality aligns with findings from recent experimental studies indicating that mild elevations of ROS levels may promote organ resilience rather than cause tissue damage. The interpretation of oxidative stress biomarkers, such as urinary 8-OHdG concentrations, in studies involving multiple metabolic agents must be approached cautiously because this study was limited by its relatively small sample size and the absence of a comparator arm without SGLT2 inhibitor use, which restricts the ability to draw definitive causal inferences. Moreover, we did not stratify patients by baseline oxidative stress levels or diabetes duration, which may influence redox responses, further limiting the generalizability of our findings.
Finally, any implication that SGLT2 inhibitors act through EV-mediated crosstalk should be considered hypothesis-generating. Mechanistic human studies are required before any clinical translation of this concept.
8. Conclusions
Our research highlights that SGLT2 inhibitors may contribute to maintaining redox balance, not only by suppressing oxidative stress but also by potentially engaging hormetic adaptations that upregulate endogenous antioxidant capacity. Although our clinical data suggest this effect, they do not definitively establish that SGLT2 inhibitors directly induce mitohormesis. Further mechanistic investigations are required to confirm this hypothesis and optimize redox-adaptive therapies for patients with T2DM. Recognizing oxidative stress as a modifiable signal—rather than merely a harmful byproduct—may drive a paradigm shift in diabetes care, targeting the root causes of multi-organ dysfunction through controlled redox adaptation. Future research should aim to clarify how these pathways interact with senescence biology and inter-organ crosstalk, ultimately enabling more precise, resilience-oriented diabetes care.
9. Clinical Implications and Future Directions
Figure 3 illustrates the proposed mechanism by which SGLT2 inhibitors induce mild mitochondrial oxidative stress and promote adaptive antioxidant responses, leading to reduced inflammation, decreased cellular senescence, and the protection of multiple organs in patients with T2DM. These observations caution against indiscriminate antioxidant supplementation.
While broad-spectrum antioxidant supplementation has largely failed to demonstrate clinical benefits, selective antioxidants targeting mitochondria, such as MitoQ, are under investigation. Preclinical and early clinical studies suggest that MitoQ may improve vascular function and protect against age-related oxidative damage, although its clinical utility in T2DM remains to be defined [
69,
70].
Controlled modulation of redox balance by encouraging mild ROS generation within a safe “hormetic window” may provide a novel strategy for preventing or delaying organ damage in T2DM [
9,
16]. Our findings regarding the effects of SGLT2 inhibitors align with geroscience principles that emphasize resilience building by targeting chronic inflammation, cellular senescence, and redox imbalance [
71,
72]. For clinicians, this perspective suggests that SGLT2 inhibitors might provide benefits extending beyond glycemic control by partially engaging adaptive redox pathways that preserve organ function. Notably, ketone body biology may represent an additional therapeutic axis in this context [
73,
74].
Future research should define the optimal thresholds of ROS modulation that maximize protective signaling without shifting into harmful oxidative overload. Additionally, studies should clarify how individual patient factors, such as baseline oxidative stress levels or senescent cell burden, modify responses to SGLT2 inhibitors. From a translational standpoint, EVs should currently be treated as a mechanistic model rather than a therapeutic target. The parallel evaluation of EVs and soluble mediators (adipokines, myokines, hepatokines, cardiokines, and ketone bodies) will be essential to disentangle their relative contributions to systemic redox adaptation in T2DM. Combining these agents with other hormetic interventions, such as structured exercise programs or mild caloric restriction, may further enhance resilience along the Redox–Inflammation–Organ Stress Axis.
These insights also underscore the potential utility of integrating redox biomarkers, such as urinary 8-OHdG, d-ROMs, and BAP, into routine clinical monitoring [
75,
76]. This approach might help identify individuals who demonstrate a favorable hormetic response profile and guide personalized intervention strategies. Ultimately, designing future trials that stratify participants by oxidative stress, inflammatory burden, and senescence markers will be critical for translating this concept into the clinical practice of precision metabolic medicine.
Author Contributions
Conceptualization, T.O.; writing—original draft preparation, T.O., K.-i.A. and T.T.; writing—review and editing, T.O. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We would like to thank the professional English language editors who assisted with editing this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 8-OHdG | 8-Hydroxy-2′-deoxyguanosine |
| AMPK | AMP-activated protein kinase |
| BAP | Biological antioxidant potential |
| CAVI | Cardio–ankle vascular index |
| d-ROMs | Derivatives of reactive oxygen metabolites |
| EV | Extracellular vesicle |
| FIB-4 | Fibrosis-4 index |
| GLP-1RA | Glucagon-like peptide-1 receptor agonist |
| IL | Interleukin |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MnSOD | Manganese superoxide dismutase |
| mTORC1 | Mechanistic target of rapamycin complex 1 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NOD-like receptor family pyrin domain-containing 3 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| PGC-1α | Peroxisome proliferator-activated receptor-gamma coactivator 1-alpha |
| ROS | Reactive oxygen species |
| SASP | Senescence-associated secretory phenotype |
| SGLT2 | Sodium–glucose cotransporter 2 |
| SIRT | Sirtuin |
| T2DM | Type 2 diabetes mellitus |
| TNF-α | Tumor necrosis factor-alpha |
| TNFR | Tumor necrosis factor receptor |
| UCP1 | Uncoupling protein 1 |
References
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Wang, K.; Dong, Y.; Liu, J.; Qian, L.; Wang, T.; Gao, X.; Wang, K.; Zhou, L. Effects of REDOX in regulating and treatment of metabolic and inflammatory cardiovascular diseases. Oxidative Med. Cell. Longev. 2020, 2020, 5860356. [Google Scholar] [CrossRef]
- Irazabal, M.V.; Torres, V.E. Reactive oxygen species and redox signaling in chronic kidney disease. Cells 2020, 9, 1342. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Lee, R.; Lee, E.; Choi, T.G.; Lee, A.S.; Yoon, Y.I.; Park, G.C.; Namgoong, J.M.; Lee, S.G.; et al. Therapeutic strategies for liver diseases based on redox control systems. Biomed. Pharmacother. 2022, 156, 113764. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Otoda, T.; Aihara, K.I.; Takayama, T. Lysosomal stress in cardiovascular diseases: Therapeutic potential of cardiovascular drugs and future directions. Biomedicines 2025, 13, 1053. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, Y.; Saredy, J.; Wang, X.; Drummer Iv, C.; Shao, Y.; Saaoud, F.; Xu, K.; Liu, M.; Yang, W.Y.; et al. ROS systems are a new integrated network for sensing homeostasis and alarming stresses in organelle metabolic processes. Redox Biol. 2020, 37, 101696. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Finkel, T. Mitohormesis. Cell Metab. 2014, 19, 757–766. [Google Scholar] [CrossRef]
- Ristow, M.; Schmeisser, K. Mitohormesis: Promoting health and lifespan by increased levels of reactive oxygen species (ROS). Dose Response 2014, 12, 288–341. [Google Scholar] [CrossRef]
- Radak, Z.; Chung, H.Y.; Goto, S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic. Biol. Med. 2008, 44, 153–159. [Google Scholar] [CrossRef]
- Miller, V.J.; Villamena, F.A.; Volek, J.S. Nutritional ketosis and mitohormesis: Potential implications for mitochondrial function and human health. J. Nutr. Metab. 2018, 2018, 5157645. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Abad-Jiménez, Z.; de Marañón, A.M.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: The battle continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012, 2012, CD007176. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. JAMA 2007, 297, 842–857. [Google Scholar] [CrossRef] [PubMed]
- Rossnerova, A.; Izzotti, A.; Pulliero, A.; Bast, A.; Rattan, S.I.S.; Rossner, P. The molecular mechanisms of adaptive response related to environmental stress. Int. J. Mol. Sci. 2020, 21, 7053. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormesis: A revolution in toxicology, risk assessment and medicine. EMBO Rep. 2004, 5, S37–S40. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1–Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Nogueiras, R.; Habegger, K.M.; Chaudhary, N.; Finan, B.; Banks, A.S.; Dietrich, M.O.; Horvath, T.L.; Sinclair, D.A.; Pfluger, P.T.; Tschöp, M.H. Sirtuin 1 and sirtuin 3: Physiological modulators of metabolism. Physiol. Rev. 2012, 92, 1479–1514. [Google Scholar] [CrossRef] [PubMed]
- López-Lluch, G.; Navas, P. Calorie restriction as an intervention in ageing. J. Physiol. 2016, 594, 2043–2060. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis). Exp. Gerontol. 2010, 45, 410–418. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 2014, 25, 42–52. [Google Scholar] [CrossRef]
- Tomita, I.; Kume, S.; Sugahara, S.; Osawa, N.; Yamahara, K.; Yasuda-Yamahara, M.; Takeda, N.; Chin-Kanasaki, M.; Kaneko, T.; Mayoux, E.; et al. SGLT2 inhibition mediates protection from diabetic kidney disease by promoting ketone body-induced mTORC1 inhibition. Cell Metab. 2020, 32, 404–419.e6. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span—From yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Auwerx, J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol. Metab. 2009, 20, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Mercken, E.M.; Crosby, S.D.; Lamming, D.W.; JeBailey, L.; Krzysik-Walker, S.; Villareal, D.T.; Capri, M.; Franceschi, C.; Zhang, Y.; Becker, K.; et al. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell 2013, 12, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Il’yasova, D.; Fontana, L.; Bhapkar, M.; Pieper, C.F.; Spasojevic, I.; Redman, L.M.; Das, S.K.; Huffman, K.M.; Kraus, W.E. CALERIE Study Investigators Effects of 2 Years of Caloric Restriction on Oxidative Status Assessed by Urinary F2-Isoprostanes: The CALERIE 2 Randomized Clinical Trial. Aging Cell 2018, 17, e12719. [Google Scholar]
- Heilbronn, L.K.; de Jonge, L.; Frisard, M.I.; DeLany, J.P.; Larson-Meyer, D.E.; Rood, J.; Nguyen, T.; Martin, C.K.; Volaufova, J.; Most, M.M.; et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: A randomized controlled trial. JAMA 2006, 295, 1539–1548. [Google Scholar] [CrossRef]
- Sparks, L.M.; Redman, L.M.; Conley, K.E.; Harper, M.E.; Yi, F.; Hodges, A.; Eroshkin, A.; Costford, S.R.; Gabriel, M.E.; Shook, C.; et al. Effects of 12 months of caloric restriction on muscle mitochondrial function in healthy individuals. J. Clin. Endocrinol. Metab. 2017, 102, 111–121. [Google Scholar] [CrossRef]
- Civitarese, A.E.; Carling, S.; Heilbronn, L.K.; Hulver, M.H.; Ukropcova, B.; Deutsch, W.A.; Smith, S.R.; Ravussin, E.; CALERIE Pennington Team. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007, 4, e76. [Google Scholar] [CrossRef]
- Ryan, C.P.; Corcoran, D.L.; Banskota, N.; Eckstein Indik, C.; Floratos, A.; Friedman, R.; Kobor, M.S.; Kraus, V.B.; Kraus, W.E.; MacIsaac, J.L.; et al. The CALERIE Genomic Data Resource. Nat. Aging 2025, 5, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Zarse, K.; Schmeisser, S.; Groth, M.; Priebe, S.; Beuster, G.; Kuhlow, D.; Guthke, R.; Platzer, M.; Kahn, C.R.; Ristow, M. Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab. 2012, 15, 451–465. [Google Scholar] [CrossRef]
- Kume, S.; Uzu, T.; Horiike, K.; Chin-Kanasaki, M.; Isshiki, K.; Araki, S.; Sugimoto, T.; Haneda, M.; Kashiwagi, A.; Koya, D. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J. Clin. Investig. 2010, 120, 1043–1055. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef]
- Oo, H.K.; Galicia-Medina, C.M.; Nishiuchi, T.; Tanida, R.; Goto, H.; Nakano, Y.; Takeshita, Y.; Saito, Y.; Takayama, H.; Takamura, T. Cysteine redoxome landscape in mouse brown adipose tissue under acute cold exposure. iScience 2025, 28, 112051. [Google Scholar] [CrossRef]
- He, F.; Li, J.; Liu, Z.; Chuang, C.C.; Yang, W.; Zuo, L. Redox mechanism of reactive oxygen species in exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Liu, J.; Finkel, T. Mitohormesis. Cell Metab. 2023, 35, 1872–1886. [Google Scholar] [CrossRef]
- Little, J.P.; Safdar, A.; Wilkin, G.P.; Tarnopolsky, M.A.; Gibala, M.J. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: Potential mechanisms. J. Physiol. 2010, 588, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Talbert, E.E.; Adhihetty, P.J. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J. Physiol. 2011, 589, 2129–2138. [Google Scholar] [CrossRef]
- Groennebaek, T.; Vissing, K. Impact of resistance training on skeletal muscle mitochondrial biogenesis, content, and function. Front. Physiol. 2017, 8, 713. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Reidy, P.T.; Bhattarai, N.; Sidossis, L.S.; Rasmussen, B.B. Resistance exercise training alters mitochondrial function in human skeletal muscle. Med. Sci. Sports Exerc. 2015, 47, 1922–1931. [Google Scholar] [CrossRef]
- Ramos, J.S.; Dalleck, L.C.; Tjonna, A.E.; Beetham, K.S.; Coombes, J.S. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: A systematic review and meta-analysis. Sports Med. 2015, 45, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Scribbans, T.D.; Edgett, B.A.; Vorobej, K.; Mitchell, A.S.; Joanisse, S.D.; Matusiak, J.B.; Parise, G.; Quadrilatero, J.; Gurd, B.J. Fibre-specific responses to endurance and low volume high intensity interval training: Striking similarities in acute and chronic adaptation. PLoS ONE 2014, 9, e98119. [Google Scholar] [CrossRef]
- Radak, Z.; Zhao, Z.; Koltai, E.; Ohno, H.; Atalay, M. Oxygen consumption and usage during physical exercise: The balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid. Redox Signal. 2013, 18, 1208–1246. [Google Scholar] [CrossRef]
- Misu, H.; Takayama, H.; Saito, Y.; Mita, Y.; Kikuchi, A.; Ishii, K.A.; Chikamoto, K.; Kanamori, T.; Tajima, N.; Lan, F.; et al. Deficiency of the hepatokine selenoprotein P increases responsiveness to exercise in mice through upregulation of reactive oxygen species and AMP-activated protein kinase in muscle. Nat. Med. 2017, 23, 508–516. [Google Scholar] [CrossRef]
- Oo, S.M.; Oo, H.K.; Takayama, H.; Ishii, K.A.; Takeshita, Y.; Goto, H.; Nakano, Y.; Kohno, S.; Takahashi, C.; Nakamura, H.; et al. Selenoprotein P-mediated reductive stress impairs cold-induced thermogenesis in brown fat. Cell Rep. 2022, 38, 110566. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Passos, J.F.; von Zglinicki, T.; Kirkwood, T.B. Mitochondria and ageing: Winning and losing in the numbers game. Bioessays 2007, 29, 908–917. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid. Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [PubMed]
- Basisty, N.; Kale, A.; Jeon, O.H.; Kuehnemann, C.; Payne, T.; Rao, C.; Holtz, A.; Shah, S.; Sharma, V.; Ferrucci, L.; et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020, 18, e3000599. [Google Scholar] [CrossRef]
- Minamino, T.; Orimo, M.; Shimizu, I.; Kunieda, T.; Yokoyama, M.; Ito, T.; Nojima, A.; Nabetani, A.; Oike, Y.; Matsubara, H.; et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 2009, 15, 1082–1087. [Google Scholar] [CrossRef]
- Tchkonia, T.; Morbeck, D.E.; Von Zglinicki, T.; Van Deursen, J.; Lustgarten, J.; Scrable, H.; Khosla, S.; Jensen, M.D.; Kirkland, J.L. Fat tissue, aging, and cellular senescence. Aging Cell 2010, 9, 667–684. [Google Scholar] [CrossRef]
- Katsuumi, G.; Shimizu, I.; Suda, M.; Yoshida, Y.; Furihata, T.; Joki, Y.; Hsiao, C.L.; Jiaqi, L.; Fujiki, S.; Abe, M.; et al. SGLT2 inhibition eliminates senescent cells and alleviates pathological aging. Nat. Aging 2024, 4, 926–938. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T.; Zhu, Y.; Niedernhofer, L.J.; Robbins, P.D. The clinical potential of senolytic drugs. J. Am. Geriatr. Soc. 2017, 65, 2297–2301. [Google Scholar] [CrossRef]
- Xu, M.; Palmer, A.K.; Ding, H.; Weivoda, M.M.; Pirtskhalava, T.; White, T.A.; Sepe, A.; Johnson, K.O.; Stout, M.B.; Giorgadze, N.; et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. elife 2015, 4, e12997. [Google Scholar] [CrossRef]
- Crewe, C.; Funcke, J.B.; Li, S.; Joffin, N.; Gliniak, C.M.; Ghaben, A.L.; An, Y.A.; Sadek, H.A.; Gordillo, R.; Akgul, Y.; et al. Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes. Cell. Metab. 2021, 33, 1853–1868.e11. [Google Scholar] [CrossRef]
- Nishitani, S.; Fukuhara, A.; Tomita, I.; Kume, S.; Shin, J.; Okuno, Y.; Otsuki, M.; Maegawa, H.; Shimomura, I. Ketone body 3-hydroxybutyrate enhances adipocyte function. Sci. Rep. 2022, 12, 10080. [Google Scholar] [CrossRef] [PubMed]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Kinney, B.; Yoo, H.S.; Lee, B.; Schaack, J.; Shao, J. Adiponectin increases skeletal muscle mitochondrial biogenesis by suppressing mitogen-activated protein kinase phosphatase-1. Diabetes 2012, 61, 1463–1470. [Google Scholar] [CrossRef][Green Version]
- Chau, M.D.; Gao, J.; Yang, Q.; Wu, Z.; Gromada, J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK–SIRT1–PGC-1α pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 12553–12558. [Google Scholar] [CrossRef]
- Ost, M.; Coleman, V.; Voigt, A.; van Schothorst, E.M.; Keipert, S.; van der Stelt, I.; Ringel, S.; Graja, A.; Ambrosi, T.; Kipp, A.P.; et al. Muscle mitochondrial stress adaptation operates independently of endogenous FGF21 action. Mol. Metab. 2016, 5, 79–90. [Google Scholar] [CrossRef]
- Fisher, F.M.; Maratos-Flier, E. Understanding the physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Lonn, E.; Bosch, J.; Yusuf, S.; Sheridan, P.; Pogue, J.; Arnold, J.M.; Ross, C.; Arnold, A.; Sleight, P.; Probstfield, J.; et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: A randomized controlled trial. JAMA 2005, 293, 1338–1347. [Google Scholar] [PubMed]
- Otoda, T.; Sekine, A.; Uemoto, R.; Tsuji, S.; Hara, T.; Tamaki, M.; Yuasa, T.; Tamaki, T.; Matsuhisa, M.; Aihara, K.I. Albuminuria and serum tumor necrosis factor receptor levels in patients with type 2 diabetes on SGLT2 inhibitors: A prospective study. Diabetes Ther. 2024, 15, 127–143. [Google Scholar] [CrossRef]
- Otoda, T.; Sekine, A.; Uemoto, R.; Hori, T.; Hara, T.; Tamaki, M.; Nakamura, S.; Yuasa, T.; Takayama, T.; Matsuhisa, M.; et al. Comparative effects of Imeglimin and empagliflozin on the redox-inflammation-organ stress axis in type 2 diabetes: A randomized controlled trial. Discov. Endocrinol. Metab. 2025, 1, 4. [Google Scholar] [CrossRef]
- Rossman, M.J.; Santos-Parker, J.R.; Steward, C.A.C.; Bispham, N.Z.; Cuevas, L.M.; Rosenberg, H.L.; Woodward, K.A.; Chonchol, M.; Gioscia-Ryan, R.A.; Murphy, M.P.; et al. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension 2018, 71, 1056–1063. [Google Scholar] [CrossRef]
- Graham, D.; Huynh, N.N.; Hamilton, C.A.; Beattie, E.; Smith, R.A.; Cochemé, H.M.; Murphy, M.P.; Dominiczak, A.F. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 2009, 54, 322–328. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking aging to chronic disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, H.; Yamahara, K.; Yasuda-Yamahara, M.; Kume, S. Emerging pathophysiological roles of ketone bodies. Physiology 2024, 39, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Tomita, I.; Tsuruta, H.; Yasuda-Yamahara, M.; Yamahara, K.; Kuwagata, S.; Tanaka-Sasaki, Y.; Chin-Kanasaki, M.; Fujita, Y.; Nishi, E.; Katagiri, H.; et al. Ketone bodies: A double-edged sword for mammalian life span. Aging Cell 2023, 22, e13833. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Il’yasova, D.; Scarbrough, P.; Spasojevic, I. Urinary biomarkers of oxidative status. Clin. Chim. Acta 2012, 413, 1446–1453. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).