Validated Diabetes Risk Scores and Their Associations with Lifestyle and Quality of Life in Spanish Workers
Abstract
1. Introduction
2. Methods
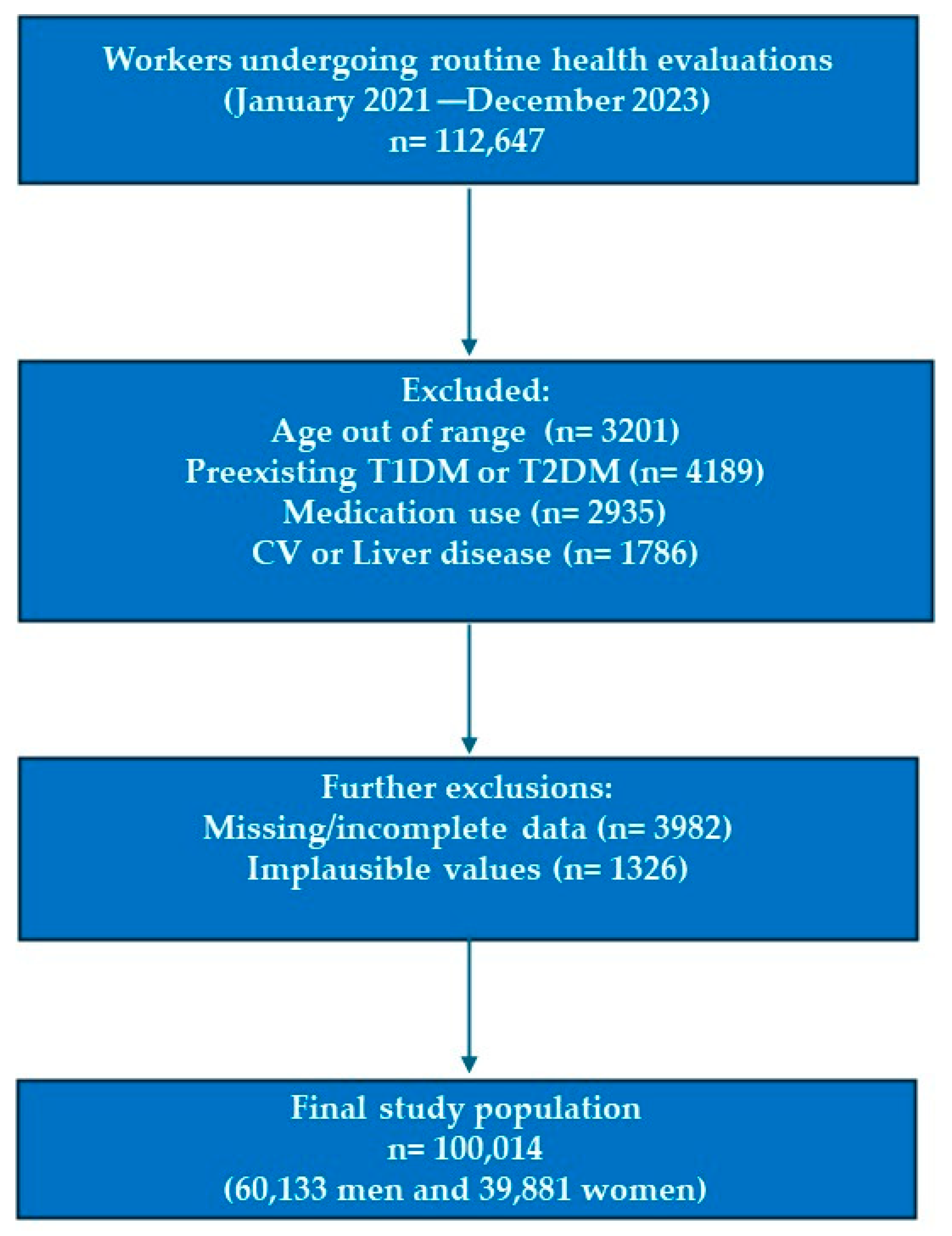
2.1. Study Design and Population
2.2. Eligibility Criteria
2.3. Anthropometric and Clinical Assessments
2.4. Diabetes Risk Assessment
- Findrisc (Finnish Diabetes Risk Score): A cumulative score (range 0–26) derived from age, BMI, waist circumference, physical activity, diet, antihypertensive medication, history of hyperglycemia, and family history of diabetes. A score ≥ 12 was considered moderate-to-high risk [38].
- QDScore: A prediction algorithm based on age, sex, ethnicity, BMI, smoking, family history, cardiovascular history, and social deprivation index. A threshold of ≥3 was defined as elevated risk [39].
- CANRISK (Canadian Diabetes Risk Questionnaire): A validated self-administered questionnaire incorporating demographic, anthropometric, lifestyle, and family history factors. Scores ≥ 21 were classified as moderate-to-high risk [40].
2.5. Lifestyle and Behavioral Variables
- Physical activity was assessed using the International Physical Activity Questionnaire—Short Form (IPAQ-SF). Participants were classified as physically active or inactive based on MET-min/week cut-offs defined by international guidelines [43].
- Smoking status was categorized as current smoker or non-smoker, based on self-report.
2.6. Sociodemographic Classification
2.7. Health-Related Quality of Life
2.8. Statistical Analysis
3. Results
4. Discussion
4.1. Comparison with Existing Literature
4.2. Strengths and Limitations
4.3. Key Contributions
4.4. Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Tinajero, M.G.; Malik, V.S. An Update on the Epidemiology of Type 2 Diabetes: A Global Perspective. Endocrinol. Metab. Clin. North Am. 2021, 50, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Galván, G.; Rosales Ronquillo, C.; Hernández Cosían, Y.; Camacho Luis, A.; López Murillo, C.P.; Rincones Monarrez, D.; Ávila, B.M.C. Identification of the polymorphic variant rs9939609 of the FTO gene in duranguense population. Acad. J. Health Sci. 2024, 39, 84–88. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, R. The Role of Obesity in Type 2 Diabetes Mellitus-An Overview. Int. J. Mol. Sci. 2024, 25, 1882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med. Sci. Sports Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Russo, S.; Kwiatkowski, M.; Govorukhina, N.; Bischoff, R.; Melgert, B.N. Meta-Inflammation and Metabolic Reprogramming of Macrophages in Diabetes and Obesity: The Importance of Metabolites. Front. Immunol. 2021, 12, 746151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yazıcı, D.; Demir, S.Ç.; Sezer, H. Insulin Resistance, Obesity, and Lipotoxicity. Adv. Exp. Med. Biol. 2024, 1460, 391–430. [Google Scholar] [CrossRef] [PubMed]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kerper, N.; Ashe, S.; Hebrok, M. Pancreatic β-Cell Development and Regeneration. Cold Spring Harb. Perspect. Biol. 2022, 14, a040741. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ikechi, I.S.; Ejike-Odeh, E.J.; Ifeanyichukwu, O.E.; Ogbu, C.; Agwu, U.U.; Obeagu, E.I. Prevalence of prediabetes among first degree relatives of type 2 diabetes individuals in Abakaliki, Ebonyi State Nigeria. Acad. J. Health Sci. 2023, 38, 85–88. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. 1), S19–S40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, M.; Hng, T.M. HbA1c: More than just a number. Aust. J. Gen. Pract. 2021, 50, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Harreiter, J.; Roden, M. Diabetes mellitus—Definition, Klassifikation, Diagnose, Screening und Prävention (Update 2023) [Diabetes mellitus: Definition, classification, diagnosis, screening and prevention (Update 2023)]. Wien. Klin. Wochenschr. 2023, 135 (Suppl. 1), 7–17. (In German) [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soriguer, F.; Valdés, S.; Tapia, M.J.; Esteva, I.; Ruiz de Adana, M.S.; Almaraz, M.C.; Morcillo, S.; Fuentes, E.G.; Rodríguez, F.; Rojo-Martinez, G. Validation of the FINDRISC (FINnish Diabetes RIsk SCore) for prediction of the risk of type 2 diabetes in a population of southern Spain. Pizarra Study. Med. Clin. 2012, 138, 371–376. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C.; Robson, J.; Sheikh, A.; Brindle, P. Predicting risk of type 2 diabetes in England and Wales: Prospective derivation and validation of QDScore. BMJ 2009, 338, b880. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, Y.; Rogers Van Katwyk, S.; Mao, Y.; Orpana, H.; Argwal, G.; de Groh, M.; Skinner, M.; Clarke, R.; Morrison, H. Assessment of dysglycemia risk in the Kitikmeot region of Nunavut: Using the CANRISK tool. Health Promot. Chronic Dis. Prev. Can. 2017, 37, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Yovera-Aldana, M.; Mezones-Holguín, E.; Agüero-Zamora, R.; Damas-Casani, L.; Uriol-Llanos, B.; Espinoza-Morales, F.; Soto-Becerra, P.; Ticse-Aguirre, R. External validation of Finnish diabetes risk score (FINDRISC) and Latin American FINDRISC for screening of undiagnosed dysglycemia: Analysis in a Peruvian hospital health care workers sample. PLoS ONE 2024, 19, e0299674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- García Samuelsson, M.; Tárraga López, P.J.; López-González, Á.A.; Busquets-Cortés, C.; Obrador de Hevia, J.; Ramírez-Manent, J.I. Evaluation of Type 2 Diabetes Risk in Individuals With or Without Metabolically Healthy Obesity. Biology 2025, 14, 608. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Młynarska, E.; Czarnik, W.; Dzieża, N.; Jędraszak, W.; Majchrowicz, G.; Prusinowski, F.; Stabrawa, M.; Rysz, J.; Franczyk, B. Type 2 Diabetes Mellitus: New Pathogenetic Mechanisms, Treatment and the Most Important Complications. Int. J. Mol. Sci. 2025, 26, 1094. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Templer, S.; Abdo, S.; Wong, T. Preventing diabetes complications. Intern. Med. J. 2024, 54, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Shukla, U.V.; Tripathy, K. Diabetic Retinopathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Hex, N.; MacDonald, R.; Pocock, J.; Uzdzinska, B.; Taylor, M.; Atkin, M.; Wild, S.H.; Beba, H.; Jones, R. Estimation of the direct health and indirect societal costs of diabetes in the UK using a cost of illness model. Diabet. Med. 2024, 41, e15326. [Google Scholar] [CrossRef] [PubMed]
- Gregg, E.W.; Pratt, A.; Owens, A.; Barron, E.; Dunbar-Rees, R.; Slade, E.T.; Hafezparast, N.; Bakhai, C.; Chappell, P.; Cornelius, V.; et al. The burden of diabetes-associated multiple long-term conditions on years of life spent and lost. Nat. Med. 2024, 30, 2830–2837. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oluchi, S.E.; Manaf, R.A.; Ismail, S.; Kadir Shahar, H.; Mahmud, A.; Udeani, T.K. Health Related Quality of Life Measurements for Diabetes: A Systematic Review. Int. J. Environ. Res. Public. Health 2021, 18, 9245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barakou, I.; Seves, B.L.; Abonie, U.S.; Finch, T.; Hackett, K.L.; Hettinga, F.J. Health-related quality of life associated with fatigue, physical activity and activity pacing in adults with chronic conditions. BMC Sports Sci. Med. Rehabil. 2025, 17, 13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Costa, D.S.J.; Mercieca-Bebber, R.; Rutherford, C.; Tait, M.A.; King, M.T. How is quality of life defined and assessed in published research? Qual. Life Res. 2021, 30, 2109–2121. [Google Scholar] [CrossRef] [PubMed]
- Niestrój-Jaworska, M.; Dębska-Janus, M.; Polechoński, J.; Tomik, R. Health Behaviors and Health-Related Quality of Life in Female Medical Staff. Int. J. Environ. Res. Public. Health 2022, 19, 3896. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Orji, C.C.; Ghosh, S.; Nwaobia, O.I.; Ibrahim, K.R.; Ibiloye, E.A.; Brown, C.M. Health Behaviors and Health-Related Quality of Life Among U.S. Adults Aged 18-64 Years. Am. J. Prev. Med. 2021, 60, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Fawkes, L.S.; Roh, T.; McDonald, T.J.; Horney, J.A.; Chiu, W.A.; Sansom, G.T. Using the 12-item short-form health survey (SF-12) to evaluate self-rated health in an environmental justice community. Arch. Public Health 2024, 82, 186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saboya, P.P.; Bodanese, L.C.; Zimmermann, P.R.; Gustavo, A.D.; Assumpção, C.M.; Londero, F. Metabolic syndrome and quality of life: A systematic review. Rev. Lat. Am. Enferm. 2016, 24, e2848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghali, H.; Elhraiech, A.; Ben Souda, H.; Karray, M.; Pavy, B.; Zedini, C. Impact of therapeutic education on quality of life in coronary patients: Interventional study. Tunis. Med. 2024, 102, 933–938. (In French) [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jankowska, A.; Golicki, D. Self-reported diabetes and quality of life: Findings from a general population survey with the Short Form-12 (SF-12) Health Survey. Arch. Med. Sci. 2021, 18, 1157–1168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gonzalez, J.S.; Krause-Steinrauf, H.; Bebu, I.; Crespo-Ramos, G.; Hoogendoorn, C.J.; Naik, A.D.; Waltje, A.; Walker, E.; Ehrmann, D.; Brown-Friday, J.; et al. Emotional distress, self-management, and glycemic control among participants enrolled in the glycemia reduction approaches in diabetes: A comparative effectiveness (GRADE) study. Diabetes Res. Clin. Pract. 2023, 196, 110229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cen, M.; Song, L.; Fu, X.; Gao, X.; Zuo, Q.; Wu, J. Associations between metabolic syndrome and anxiety, and the mediating role of inflammation: Findings from the UK Biobank. Brain Behav. Immun. 2024, 116, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ring, M. An Integrative Approach to HPA Axis Dysfunction: From Recognition to Recovery. Am. J. Med. 2025, 138, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Pesaro, A.E.; Bittencourt, M.S.; Franken, M.; Carvalho, J.A.M.; Bernardes, D.; Tuomilehto, J.; Santos, R.D. The Finnish Diabetes Risk Score (FINDRISC), incident diabetes and low-grade inflammation. Diabetes Res. Clin. Pract. 2021, 171, 108558. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C. Development and validation of QDiabetes-2018 risk prediction algorithm to estimate future risk of type 2 diabetes: Cohort study. BMJ 2017, 359, j5019. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mestre-Font, M.; Busquets-Cortés, C.; Ramírez-Manent, J.I.; Tomás-Gil, P.; Paublini, H.; López-González, A.A. Influence of sociodemographic variables and healthy habits on the values of type 2 diabetes risk scales. Acad. J. Health Sci. 2024, 39, 99–106. [Google Scholar] [CrossRef]
- Bekar, C.; Goktas, Z. Validation of the 14-item mediterranean diet adherence screener. Clin. Nutr. ESPEN 2023, 53, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.M.; Gottschall, C.B.A.; Vinholes, D.B.; Martinez-Gonzalez, M.A.; Marcadenti, A. Translation and cross-cultural adaptation of 14-item Mediterranean Diet Adherence Screener and low-fat diet adherence questionnaire. Clin. Nutr. ESPEN 2020, 39, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Mestre-Font, M.; Busquets-Cortés, C.; Ramírez-Manent, J.I.; Tomás-Gil, P.; Paublini, H.; López-González, A.A. Influence of sociodemographic variables and healthy habits on the values of overweight and obesity scales in 386,924 Spanish workers. Acad. J. Health Sci. 2024, 39, 27–35. [Google Scholar] [CrossRef]
- Aguiló Juanola, M.C.; López-González, A.A.; Tomás-Gil, P.; Paublini, H.; Tárraga-López, P.J.; Ramírez-Manent, J.I. Influence of tobacco consumption and other variables on the values of different cardiovascular risk factors in 418,343 spanish workers. Acad. J. Health Sci. 2024, 39, 89–95. [Google Scholar] [CrossRef]
- Al Omari, O.; Alkhawaldeh, A.; ALBashtawy, M.; Qaddumi, J.; Holm, M.B.; AlOmari, D. A Review of the Short Form Health Survey-Version 2. J. Nurs. Meas. 2019, 27, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Tárraga Marcos, P.J.; López-González, Á.A.; Martínez-Almoyna Rifá, E.; Paublini Oliveira, H.; Martorell Sánchez, C.; Tárraga López, P.J.; Ramírez-Manent, J.I. Risk of Insulin Resistance in 44,939 Spanish Healthcare Workers: Association with Sociodemographic Variables and Healthy Habits. Diseases 2025, 13, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mestre Font, M.; Busquets-Cortés, C.; Ramírez-Manent, J.I.; Vallejos, D.; Sastre Alzamora, T.; López-González, A.A. Influence of sociodemographic variables and healthy habits on the values of cardiometabolic risk scales in 386924 spanish workers. Acad. J. Health Sci. 2024, 39, 112–121. [Google Scholar] [CrossRef]
- Gholami, A.; Doustmohammadian, A.; Shamshirgaran, S.M.; Aminisani, N.; Azimi-Nezhad, M.; Abasi, H.; Hariri, M. Association Between Metabolic Syndrome and Health-Related Quality of Life in Older Adults: Findings from the IRanian Longitudinal Study on Ageing. Metab. Syndr. Relat. Disord. 2024, 22, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Chang, H.T.; Chiang, S.C.; Chen, H.S.; Lin, M.H.; Chen, T.J.; Hwang, S.-J. Sex differences in relationships between metabolic syndrome components and factors associated with health-related quality of life in middle-aged adults living in the community: A cross-sectional study in Taiwan. Health Qual. Life Outcomes 2018, 16, 76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Niknam, M.; Olazadeh, K.; Azami, M.; Boroumandieh, S.; Yari-Boroujeni, R.; Izadi, N.; Azizi, F.; Amiri, P. Health-related quality of life in adults with metabolic syndrome: A multi-level analysis of family and individual level variation. BMJ Open 2024, 14, e087870. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- López-González, A.A.; Ramírez Manent, J.I.; Vicente-Herrero, M.T.; García Ruiz, E.; Albaladejo Blanco, M.; López Safont, N. Prevalence of diabesity in the Spanish working population: Influence of sociodemographic variables and tobacco consumption. Sist. Sanit. Navar. 2022, 45, e0977. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kyrou, I.; Tsigos, C.; Mavrogianni, C.; Cardon, G.; Van Stappen, V.; Latomme, J.; Kivelä, J.; Wikström, K.; Tsochev, K.; Nanasi, A.; et al. Sociodemographic and lifestyle-related risk factors for identifying vulnerable groups for type 2 diabetes: A narrative review with emphasis on data from Europe. BMC Endocr. Disord. 2020, 20 (Suppl. 1), 134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amaravadi, S.K.; Maiya, G.A.K.V.; Shastry, B.A. Effectiveness of structured exercise program on insulin resistance and quality of life in type 2 diabetes mellitus-A randomized controlled trial. PLoS ONE 2024, 19, e0302831. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Tam, W.W.S.; Hounsri, K.; Kusuyama, J.; Wu, V.X. Effectiveness of Combined Aerobic and Resistance Exercise on Cognition, Metabolic Health, Physical Function, and Health-related Quality of Life in Middle-aged and Older Adults With Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2024, 105, 1585–1599. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Kerr, D.; Atiase, Y.; Samir, M.M.; Driscoll, A. Effect of a home-based physical activity program on metabolic syndrome in Ghanaian adults with type 2 diabetes: Protocol for a feasibility randomized controlled trial. Nurs. Open 2024, 11, e2180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vetrani, C.; Verde, L.; Colao, A.; Barrea, L.; Muscogiuri, G. The Mediterranean Diet: Effects on Insulin Resistance and Secretion in Individuals with Overweight or Obesity. Nutrients 2023, 15, 4524. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Celada Roldán, C.; López Diez, J.; Garrido Rider, F.; Cerezuela Abarca, M.A.; Tárraga Marcos, A.; Tárraga López, P.; et al. Impact of adherence to the Mediterranean diet on health-related quality of life in poorly controlled diabetics. Acad. J. Health Sci. 2024, 39, 103–112. [Google Scholar] [CrossRef]
- Kazukauskiene, N.; Podlipskyte, A.; Varoneckas, G.; Mickuviene, N. Health-related quality of life and insulin resistance over a 10-year follow-up. Sci Rep. 2021, 11, 24294. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hertfordshire Cohort Study. Medical Research Council, University of Southampton. Available online: https://www.mrc.soton.ac.uk/herts/ (accessed on 23 August 2025).
- Lin, Y.H.; Chang, H.T.; Tseng, Y.H.; Chen, H.S.; Chiang, S.C.; Chen, T.J.; Hwang, S.J. Changes in metabolic syndrome affect the health-related quality of life of community-dwelling adults. Sci. Rep. 2021, 11, 20267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Variable | Low Diabetes Risk | High Diabetes Risk | p-Value |
|---|---|---|---|
| Age (years) | 42.1 ± 9.5 | 47.8 ± 10.2 | <0.001 |
| Male (%) | 52.3 | 59.7 | <0.001 |
| BMI (kg/m2) | 24.6 ± 3.2 | 28.3 ± 4.1 | <0.001 |
| Mediterranean diet adherence (mean score) | 8.1 ± 2.3 | 6.7 ± 2.4 | <0.001 |
| Physical activity (high, %) | 46.8 | 32.5 | <0.001 |
| Variable | Low Diabetes Risk | High Diabetes Risk | p-Value |
|---|---|---|---|
| Good HRQoL (%) | 65.2 | 51.3 | <0.001 |
| Moderate HRQoL (%) | 22.7 | 28.4 | <0.001 |
| Poor HRQoL (%) | 12.1 | 20.3 | <0.001 |
| Predictor | OR (95% CI) | p-Value |
|---|---|---|
| Low Mediterranean diet adherence | 1.45 (1.32–1.58) | <0.001 |
| Low physical activity | 1.39 (1.27–1.52) | <0.001 |
| Poor HRQoL | 1.33 (1.22–1.47) | <0.001 |
| Male sex | 1.28 (1.15–1.42) | <0.001 |
| Older age (>45 years) | 1.52 (1.38–1.66) | <0.001 |
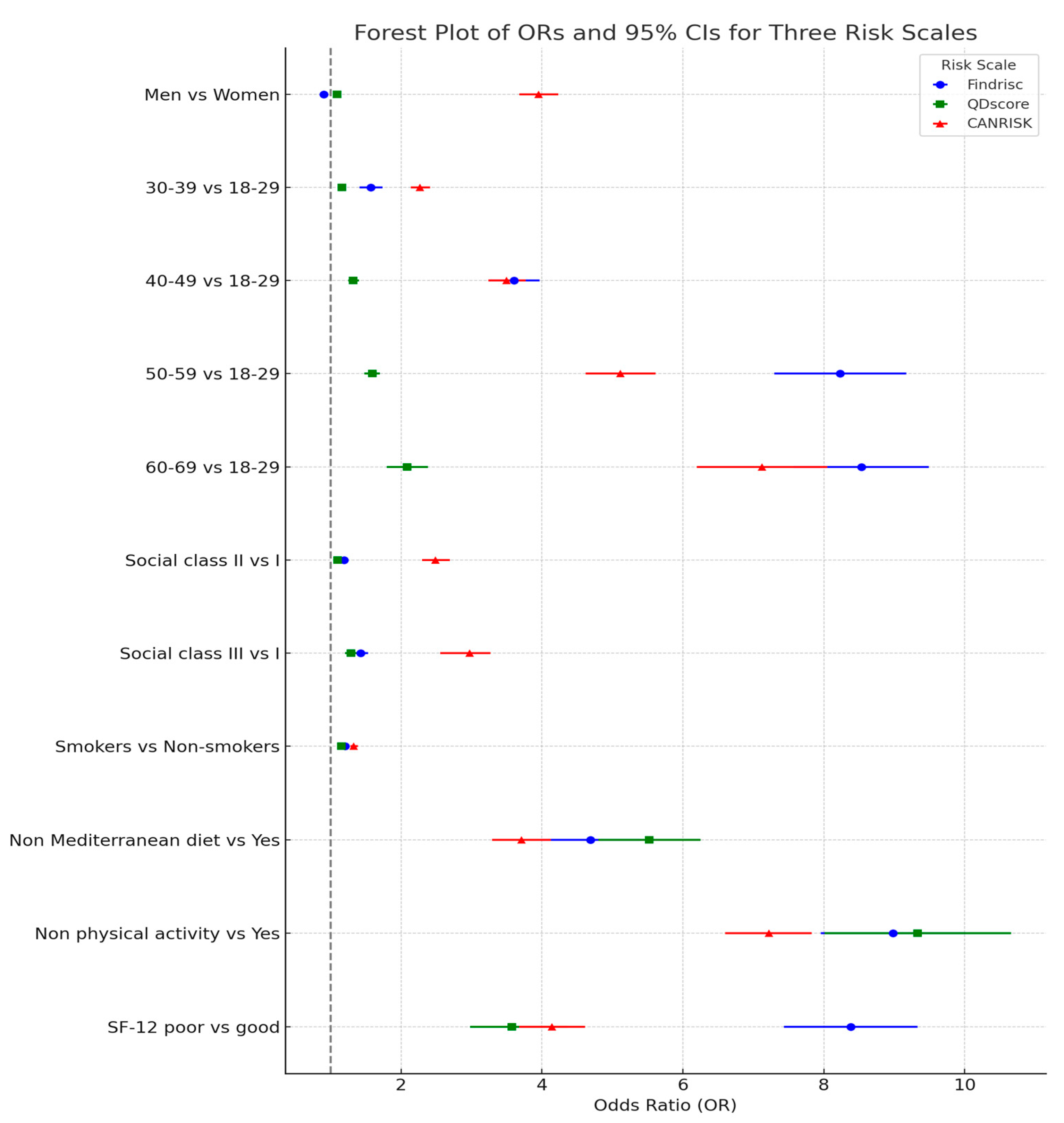
| Findrisc Moderate-High | QD-Score ≥ 3 | CANRISK Moderate-High | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Women | 1 | 1 | 1 | |||
| Men | 0.90 (0.85–0.96) | <0.001 | 1.09 (1.06–1.13) | <0.001 | 3.95 (3.68–4.23) | <0.001 |
| 18–29 years | 1 | 1 | 1 | |||
| 30–39 years | 1.57 (1.41–1.74) | <0.001 | 1.16 (1.13–1.20) | <0.001 | 2.27 (2.14–2.41) | <0.001 |
| 40–49 years | 3.60 (3.24–3.97) | <0.001 | 1.32 (1.25–1.40) | <0.001 | 3.50 (3.24–3.77) | <0.001 |
| 50–59 years | 8.23 (7.30–9.17) | <0.001 | 1.59 (1.48–1.70) | <0.001 | 5.11 (4.62–5.61) | <0.001 |
| 60–69 years | 8.53 (7.57–9.49) | <0.001 | 2.09 (1.80–2.38) | <0.001 | 7.12 (6.20–8.05) | <0.001 |
| Social class I | 1 | 1 | 1 | |||
| Social class II | 1.19 (1.15–1.24) | <0.001 | 1.10 (1.07–1.14) | <0.001 | 2.49 (2.30–2.69) | <0.001 |
| Social class III | 1.43 (1.34–1.53) | <0.001 | 1.29 (1.21–1.37) | <0.001 | 2.97 (2.56–3.27) | <0.001 |
| Non-smokers | 1 | 1 | 1 | |||
| Smokers | 1.21 (1.16–1.26) | <0.001 | 1.15 (1.10–1.21) | <0.001 | 1.33 (1.28–1.39) | <0.001 |
| Yes Mediterranean diet | 1 | 1 | 1 | |||
| Non Mediterranean diet | 4.69 (3.98–5.39) | <0.001 | 5.52 (4.80–6.25) | <0.001 | 3.71 (3.29–4.13) | <0.001 |
| Yes physical activity | 1 | 1 | 1 | |||
| Non physical activity | 8.98 (7.96–10.01) | <0.001 | 9.33 (8.01–10.66) | <0.001 | 7.22 (6.60–7.83) | <0.001 |
| SF-12 good | 1 | 1 | 1 | |||
| SF-12 poor | 8.38 (7.43–9.33) | <0.001 | 3.57 (2.98–4.37) | <0.001 | 4.14 (3.68–4.61) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansana, M.D.M.; López, P.J.T.; Miquel, J.J.G.; López-González, Á.A.; Sbert, P.R.; Busquets-Cortés, C.; Ramírez-Manent, J.I. Validated Diabetes Risk Scores and Their Associations with Lifestyle and Quality of Life in Spanish Workers. Diabetology 2025, 6, 109. https://doi.org/10.3390/diabetology6100109
Jansana MDM, López PJT, Miquel JJG, López-González ÁA, Sbert PR, Busquets-Cortés C, Ramírez-Manent JI. Validated Diabetes Risk Scores and Their Associations with Lifestyle and Quality of Life in Spanish Workers. Diabetology. 2025; 6(10):109. https://doi.org/10.3390/diabetology6100109
Chicago/Turabian StyleJansana, María Dolores Marzoa, Pedro Juan Tárraga López, Juan José Guarro Miquel, Ángel Arturo López-González, Pere Riutord Sbert, Carla Busquets-Cortés, and José Ignacio Ramírez-Manent. 2025. "Validated Diabetes Risk Scores and Their Associations with Lifestyle and Quality of Life in Spanish Workers" Diabetology 6, no. 10: 109. https://doi.org/10.3390/diabetology6100109
APA StyleJansana, M. D. M., López, P. J. T., Miquel, J. J. G., López-González, Á. A., Sbert, P. R., Busquets-Cortés, C., & Ramírez-Manent, J. I. (2025). Validated Diabetes Risk Scores and Their Associations with Lifestyle and Quality of Life in Spanish Workers. Diabetology, 6(10), 109. https://doi.org/10.3390/diabetology6100109







