Association between Altered Thyroid Function and Prediabetes in Diet-Induced Prediabetic Male Sprague Dawley Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Study Desgin and Insduction of Prediabetes
2.3. Blood Collection
2.4. Biochemical Analysis
2.5. Statistical Analysis
3. Results
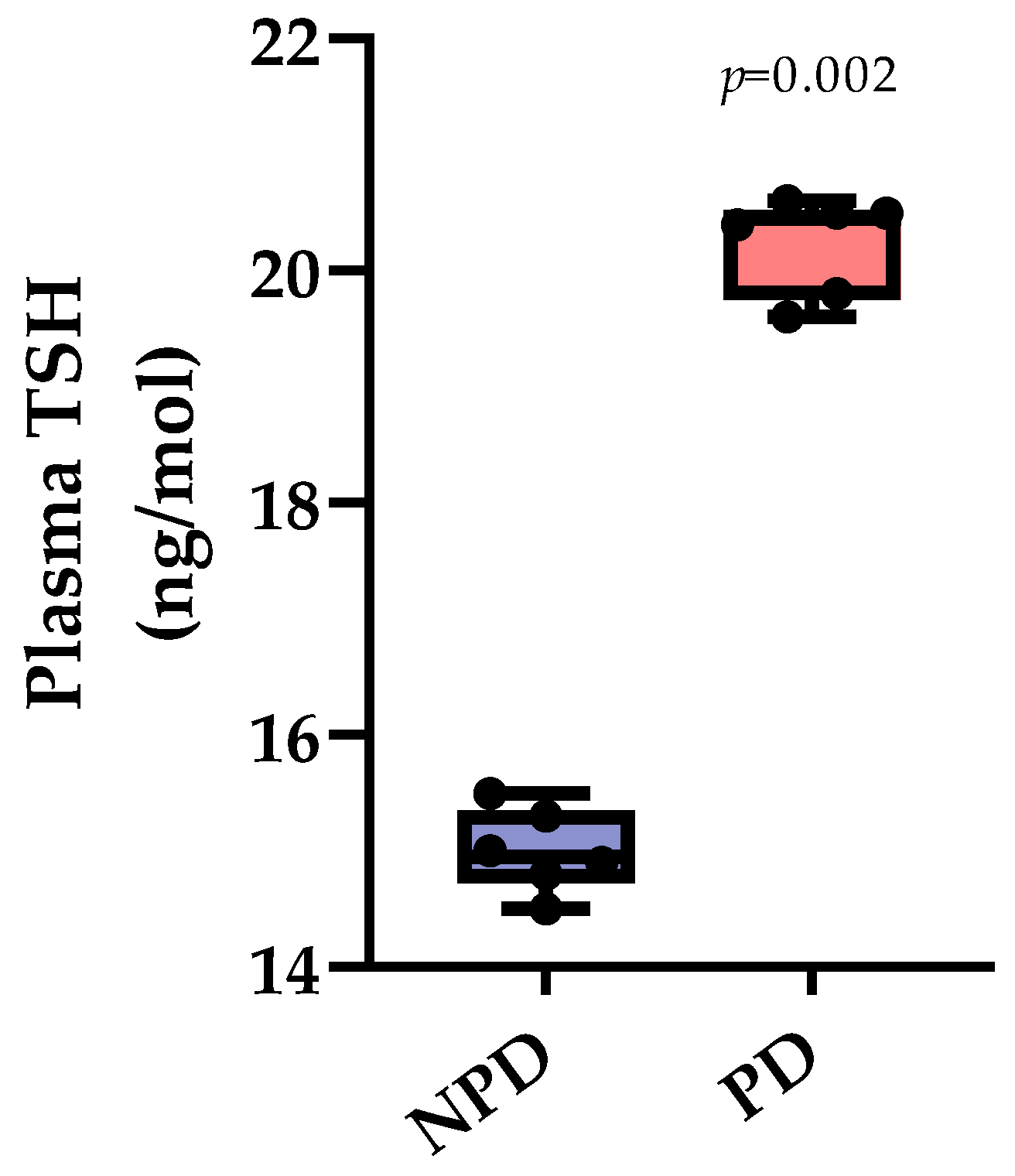
3.1. Plasma Thyroid Stimulating Hormone (TSH)
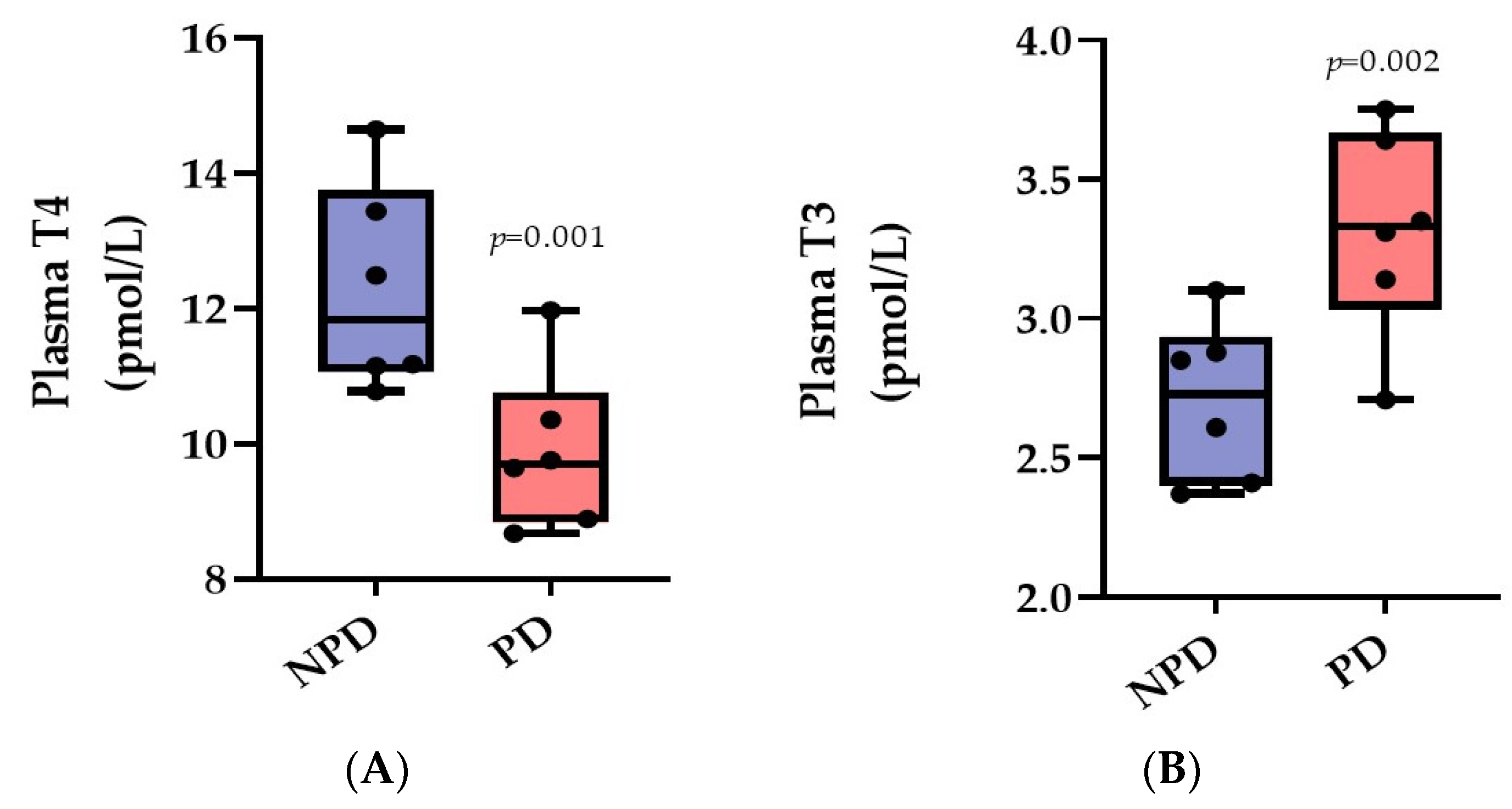
3.2. Plasma Thyroxine (T4) and Triiodothyronine (T3)
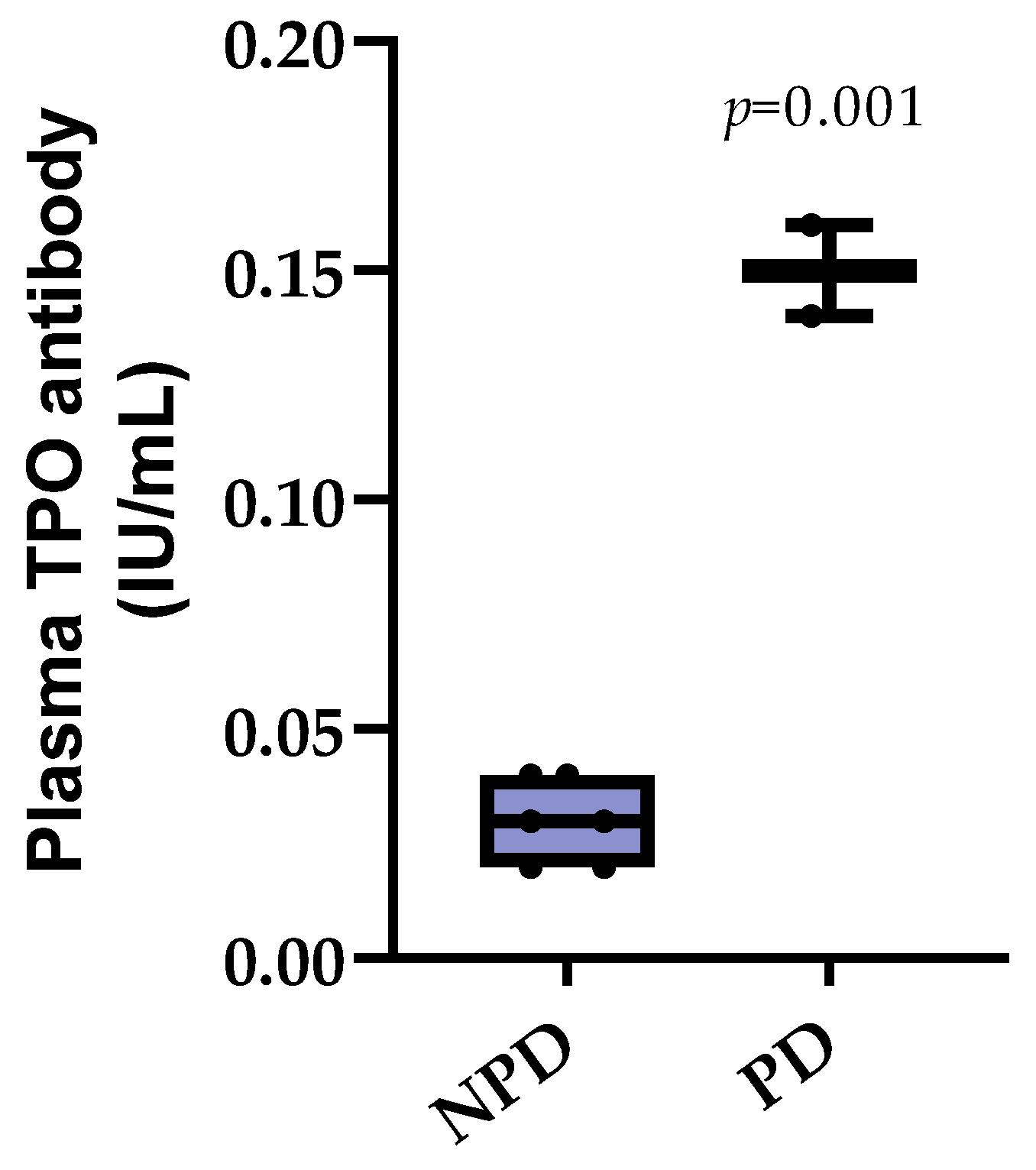
3.3. Plasma Thyroid Peroxidase (TPO) Antibody
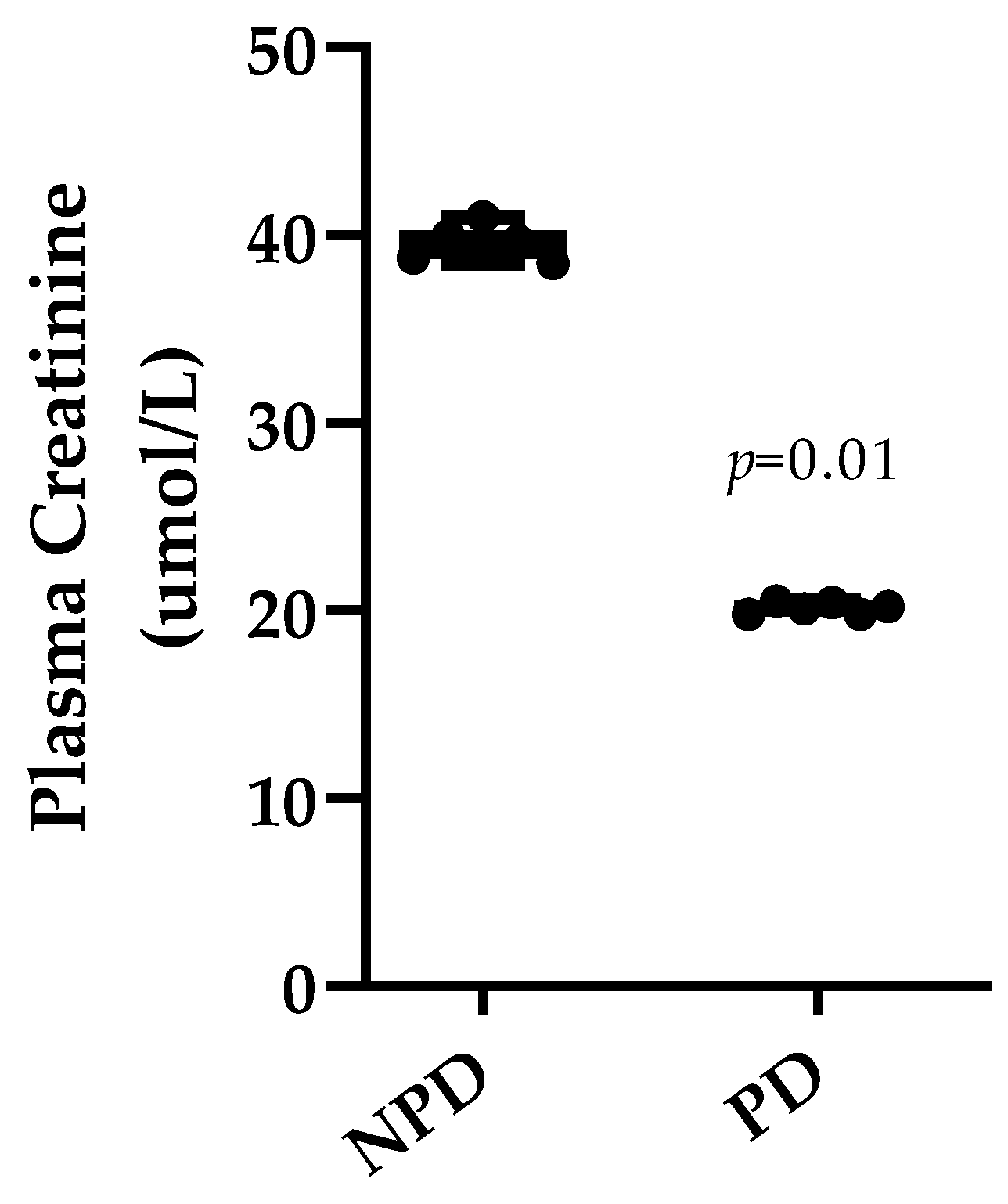
3.4. Plasma Creatinine
3.5. Plasma Insulin and Glycated Hemoglobin
3.6. HbA1c and Thyroid Hormones (T3 and T4) Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Punthakee, Z.; Goldenberg, R.; Katz, P. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can. J. Diabetes 2018, 42, S10–S15. [Google Scholar] [CrossRef]
- Shah, A.; Kanaya, A.M. Diabetes and associated complications in the South Asian population. Curr. Cardiol. Rep. 2014, 16, 476. [Google Scholar] [CrossRef]
- Dorcely, B.; Katz, K.; Jagannathan, R.; Chiang, S.S.; Oluwadare, B.; Goldberg, I.J.; Bergman, M. Novel biomarkers for prediabetes, diabetes, and associated complications. Diabetes Metab. Syndr. Obes. Targets Ther. 2017, 10, 345–361. [Google Scholar] [CrossRef]
- Tuso, P. Prediabetes and lifestyle modification: Time to prevent a preventable disease. Perm. J. 2014, 18, 88. [Google Scholar] [CrossRef]
- Bansal, N. Prediabetes diagnosis and treatment: A review. World J. Diabetes 2015, 6, 296. [Google Scholar] [CrossRef]
- Yip, W.C.; Sequeira, I.R.; Plank, L.D.; Poppitt, S.D. Prevalence of pre-diabetes across ethnicities: A review of impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) for classification of dysglycaemia. Nutrients 2017, 9, 1273. [Google Scholar] [CrossRef]
- Akinnuga, A.M.; Siboto, A.; Khumalo, B.; Sibiya, N.H.; Ngubane, P.; Khathi, A. Evaluation of the effects of bredemolic acid on selected markers of glucose homeostasis in diet-induced prediabetic rats. Arch. Physiol. Biochem. 2022, 128, 306–312. [Google Scholar] [CrossRef]
- Mkhize, B.C.; Mosili, P.; Ngubane, P.S.; Sibiya, N.H.; Khathi, A. Diet-induced prediabetes: Effects on the activity of the renin–angiotensin–aldosterone system in selected organs. J. Diabetes Investig. 2022, 13, 768–780. [Google Scholar] [CrossRef]
- Mosili, P.; Mkhize, B.C.; Ngubane, P.; Sibiya, N.; Khathi, A. The dysregulation of the hypothalamic–pituitary–adrenal axis in diet-induced prediabetic male Sprague Dawley rats. Nutr. Metab. 2020, 17, 104. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef]
- Makandar, A.; Sonagra, A.D.; Shafi, N. Study of thyroid function in type 2 diabetic and non-diabetic population. Int. J. Med. Sci. Public Health 2015, 4, 769–772. [Google Scholar] [CrossRef]
- Ahmed, R. Thyroid-insulin dysfunction during development. Int. J. Res. Stud. Zool. 2017, 3, 73–75. [Google Scholar]
- Zhao, W.; Li, X.; Liu, X.; Lu, L.; Gao, Z. Thyroid function in patients with type 2 diabetes mellitus and diabetic nephropathy: A single center study. J. Thyroid Res. 2018, 2018, 9507028. [Google Scholar] [CrossRef]
- Jali, M.; Kambar, S.; Jali, S.M.; Pawar, N.; Nalawade, P. Prevalence of thyroid dysfunction among type 2 diabetes mellitus patients. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S105–S108. [Google Scholar] [CrossRef]
- Subekti, I.; Pramono, L.A.; Dewiasty, E.; Harbuwono, D.S. Thyroid dysfunction in type 2 diabetes mellitus patients. Acta Med. Indones. 2018, 49, 314. [Google Scholar]
- Nishi, M. Diabetes mellitus and thyroid diseases. Diabetol. Int. 2018, 9, 108–112. [Google Scholar] [CrossRef]
- Luvuno, M.; Mbongwa, H.; Khathi, A. Development of a novel prediabetes animal model using a high fat high carbohydrate diet: Implications for type 2 diabetes. PLoS ONE 2017, 13, 8–14. [Google Scholar]
- Mzimela, N.C.; Ngubane, P.S.; Khathi, A. The changes in immune cell concentration during the progression of pre-diabetes to type 2 diabetes in a high-fat high-carbohydrate diet-induced pre-diabetic rat model. Autoimmunity 2019, 52, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Diabetes, Endocrinology & Metabolism. Diabetes Trial Unit. HOMA Calculator. Available online: http://www.dtu.ox.ac.uk/ (accessed on 9 July 2016).
- Gamede, M.; Mabuza, L.; Ngubane, P.; Khathi, A. The effects of plant-derived oleanolic acid on selected parameters of glucose homeostasis in a diet-induced pre-diabetic rat model. Molecules 2018, 23, 794. [Google Scholar] [CrossRef]
- Carvalho, D.P.; Dupuy, C. Thyroid hormone biosynthesis and release. Mol. Cell. Endocrinol. 2017, 458, 6–15. [Google Scholar] [CrossRef] [PubMed]
- De Vito, P.; Candelotti, E.; Ahmed, R.G.; Luly, P.; Davis, P.J.; Incerpi, S.; Pedersen, J.Z. Role of thyroid hormones in insulin resistance and diabetes. Immunol. Endocr. Metab. Agents Med. Chem. (Former. Curr. Med. Chem. Immunol. Endocr. Metab. Agents) 2015, 15, 86–93. [Google Scholar] [CrossRef]
- Rousset, B.; Dupuy, C.; Miot, F.; Dumont, J. Thyroid Hormone Synthesis and Secretion, Endotext. Mary Anne Liebert Publishers Inc. 2015. Available online: https://www.thyroidmanager.org/chapter/chapter-2-thyroid-hormone-synthesis-and-secretion (accessed on 10 March 2023).
- Liu, C.-C.; Dong, Z.-Q.; Zhou, Y.-L.; Liao, Z.-B.; RH, A.; Ming, X.; Guo, J.; Sun, X.-L. Role of HPA and HPT Axis in Anxiety Disorder Complicated with Diabetes Mellitus. Sichuan Da Xue Xue Bao. Yi Xue Ban = J. Sichuan Univ. Med. Sci. Ed. 2017, 48, 895–899. [Google Scholar]
- Fliers, E.; Bianco, A.C.; Langouche, L.; Boelen, A. Thyroid function in critically ill patients. Lancet Diabetes Endocrinol. 2015, 3, 816–825. [Google Scholar] [CrossRef]
- Annamalai, A. Medical Management of Psychotropic Side Effects; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Ramos-Leví, A.M.; Marazuela, M. Pathogenesis of thyroid autoimmune disease: The role of cellular mechanisms. Endocrinol. Nutr. 2016, 63, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.N.; Levy, D.L. Thyroid and adrenal disorders. In Rosen’s Emergency Medicine: Concepts and Clinical Practice; Mosby: St. Louis, MO, USA, 2014; pp. 1676–1693. [Google Scholar]
- Hannoush, Z.C.; Weiss, R.E. Defects of thyroid hormone synthesis and action. Endocrinol. Metab. Clin. 2017, 46, 375–388. [Google Scholar] [CrossRef]
- Vissenberg, R.; Manders, V.; Mastenbroek, S.; Fliers, E.; Afink, G.; Ris-Stalpers, C.; Goddijn, M.; Bisschop, P. Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction. Hum. Reprod. Update 2015, 21, 378–387. [Google Scholar] [CrossRef]
- Summers, R.; Macnab, R. Thyroid, parathyroid hormones and calcium homeostasis. Anaesth. Intensive Care Med. 2017, 18, 522–526. [Google Scholar] [CrossRef]
- Abdalla, S.M.; Bianco, A.C. Defending plasma T3 is a biological priority. Clin. Endocrinol. 2014, 81, 633–641. [Google Scholar] [CrossRef]
- Loh, T.P.; Tee, J.C.S.; Tee, N.W.S.; Cheng, W.L.; Thevarajah, M.; Sabir, N.; Chew, Y.Y.; Sethi, S.K.; Khoo, C.M. Association between thyroid function tests and anti-thyroid peroxidase (TPO) antibodies in pregnancy. Endocrine 2016, 53, 865–867. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. Thyroid autoimmunity: Role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front. Immunol. 2017, 8, 521. [Google Scholar] [CrossRef]
- Kumru, P.; Erdogdu, E.; Arisoy, R.; Demirci, O.; Ozkoral, A.; Ardic, C.; Ertekin, A.A.; Erdogan, S.; Ozdemir, N.N. Effect of thyroid dysfunction and autoimmunity on pregnancy outcomes in low risk population. Arch. Gynecol. Obstet. 2015, 291, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.C.; Privalsky, M.L. Thyroid hormones, t3 and t4, in the brain. Front. Endocrinol. 2014, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Dunn, D.; Turner, C. Hypothyroidism in women. Nurs. Women’s Health 2016, 20, 93–98. [Google Scholar] [CrossRef]
- Bernardes, S.S.; Guarnier, F.A.; Marinello, P.C.; Armani, A.; Simão, A.N.C.; Cecchini, R.; Cecchini, A.L. Reactive oxygen species play a role in muscle wasting during thyrotoxicosis. Cell Tissue Res. 2014, 357, 803–814. [Google Scholar] [CrossRef]
- Khathi, A.; Luvuno, M.; Mabandla, M. Diet-induced prediabetes: Effects on oxidative stress and inflammatory biomarkers as agents for vascular complications in renal function. PONTE Int. J. Sci. Res. 2019, 75. [Google Scholar] [CrossRef]
- Cicatiello, A.G.; Di Girolamo, D.; Dentice, M. Metabolic effects of the intracellular regulation of thyroid hormone: Old players, new concepts. Front. Endocrinol. 2018, 9, 474. [Google Scholar] [CrossRef]
- Duntas, L.H.; Orgiazzi, J.; Brabant, G. The interface between thyroid and diabetes mellitus. Clin. Endocrinol. 2011, 75, 1–9. [Google Scholar] [CrossRef]
- Park, E.; Jerden, D.C.; Bahouth, S.W. Regulation of phosphoenolpyruvate carboxykinase gene transcription by thyroid hormone involves two distinct binding sites in the promoter. Biochem. J. 1995, 309, 913–919. [Google Scholar] [CrossRef]
- Park, E.A.; Song, S.; Vinson, C.; Roesler, W.J. Role of CCAAT enhancer-binding protein β in the thyroid hormone and cAMP induction of phosphoenolpyruvate carboxykinase gene transcription. J. Biol. Chem. 1999, 274, 211–217. [Google Scholar] [CrossRef]
- Klieverik, L.P.; Sauerwein, H.P.; Ackermans, M.T.; Boelen, A.; Kalsbeek, A.; Fliers, E. Effects of thyrotoxicosis and selective hepatic autonomic denervation on hepatic glucose metabolism in rats. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E513–E520. [Google Scholar] [CrossRef]
- Brenta, G. Why can insulin resistance be a natural consequence of thyroid dysfunction? J. Thyroid Res. 2011, 2011, 152850. [Google Scholar] [CrossRef] [PubMed]
- Gierach, M.; Gierach, J.; Junik, R. Insulin resistance and thyroid disorders. Endokrynol. Pol. 2014, 65, 70–76. [Google Scholar] [CrossRef]
- Samy, D.M.; Ismail, C.A.; Nassra, R.A. Circulating irisin concentrations in rat models of thyroid dysfunction—Effect of exercise. Metabolism 2015, 64, 804–813. [Google Scholar] [CrossRef]
- Jayanthi, R.; Srinivasan, A.R.; Hanifah, M.; Maran, A.L. Associations among Insulin resistance, triacylglycerol/high density lipoprotein (TAG/HDL ratio) and thyroid hormone levels—A study on type 2 diabetes mellitus in obese and overweight subjects. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S121–S126. [Google Scholar] [CrossRef]
- Ağbaht, K.; Erdogan, M.F.; Emral, R.; Baskal, N.; Güllü, S. Circulating glucagon to ghrelin ratio as a determinant of insulin resistance in hyperthyroidism. Endocrine 2014, 45, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Martinez, B.; Ortiz, R.M. Thyroid hormone regulation and insulin resistance: Insights from animals naturally adapted to fasting. Physiology 2017, 32, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Gereben, B.; McAninch, E.A.; Ribeiro, M.O.; Bianco, A.C. Scope and limitations of iodothyronine deiodinases in hypothyroidism. Nat. Rev. Endocrinol. 2015, 11, 642–652. [Google Scholar] [CrossRef]

| Group | NPD | PD | p Value |
|---|---|---|---|
| Plasma Insulin (ng/mL) | 2.97 ± 0.01 | 14.25 ± 0.12 ★ | p < 0.0001 |
| Blood Glucose (mmol/L) | 4.62 ± 0.11 | 6.25 ± 0.11 ★ | p < 0.001 |
| HOMA-IR | 0.61 ± 0.01 | 3.96 ± 0.09 ★ | p < 0.0001 |
| HbA1c (%) | 3.80 ± 1.17 | 6.30 ± 0.36 ★ | p < 0.001 |
| Body Weight (g) | 368.71 ± 4.02 | 568.50 ± 8.57 ★ | p < 0.0001 |
| Groups | Correlation Analysis | Independent Variable: HbA1c vs. Dependent Variable: T3 | Independent Variable: HbA1c vs. Dependent Variable: T4 |
|---|---|---|---|
| NPD | R | −0.0680 | 0.5016 |
| p value | 0.8981 NS | 0.3107 NS | |
| N | 6 | 6 | |
| PD | R | 0.8122 | −0.8238 |
| p value | 0.0496 * | 0.0438 * | |
| N | 6 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pillay, M.; Mosili, P.; Akinnuga, A.; Sibiya, N.; Ngubane, P.; Khathi, A. Association between Altered Thyroid Function and Prediabetes in Diet-Induced Prediabetic Male Sprague Dawley Rats. Diabetology 2023, 4, 406-417. https://doi.org/10.3390/diabetology4030034
Pillay M, Mosili P, Akinnuga A, Sibiya N, Ngubane P, Khathi A. Association between Altered Thyroid Function and Prediabetes in Diet-Induced Prediabetic Male Sprague Dawley Rats. Diabetology. 2023; 4(3):406-417. https://doi.org/10.3390/diabetology4030034
Chicago/Turabian StylePillay, Malishca, Palesa Mosili, Akinjide Akinnuga, Ntethelelo Sibiya, Phikelelani Ngubane, and Andile Khathi. 2023. "Association between Altered Thyroid Function and Prediabetes in Diet-Induced Prediabetic Male Sprague Dawley Rats" Diabetology 4, no. 3: 406-417. https://doi.org/10.3390/diabetology4030034
APA StylePillay, M., Mosili, P., Akinnuga, A., Sibiya, N., Ngubane, P., & Khathi, A. (2023). Association between Altered Thyroid Function and Prediabetes in Diet-Induced Prediabetic Male Sprague Dawley Rats. Diabetology, 4(3), 406-417. https://doi.org/10.3390/diabetology4030034







