Biological Evidence of Improved Wound Healing Using Autologous Micrografts in a Diabetic Animal Model
Abstract
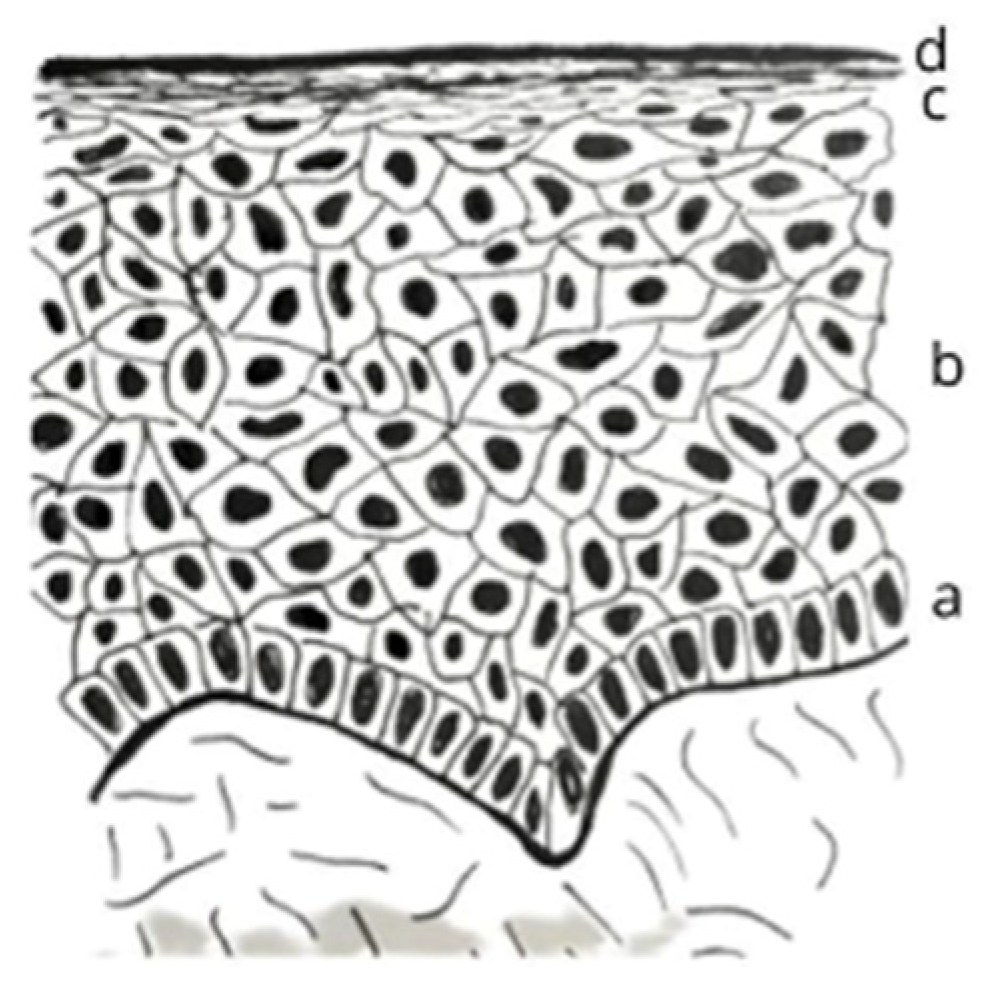
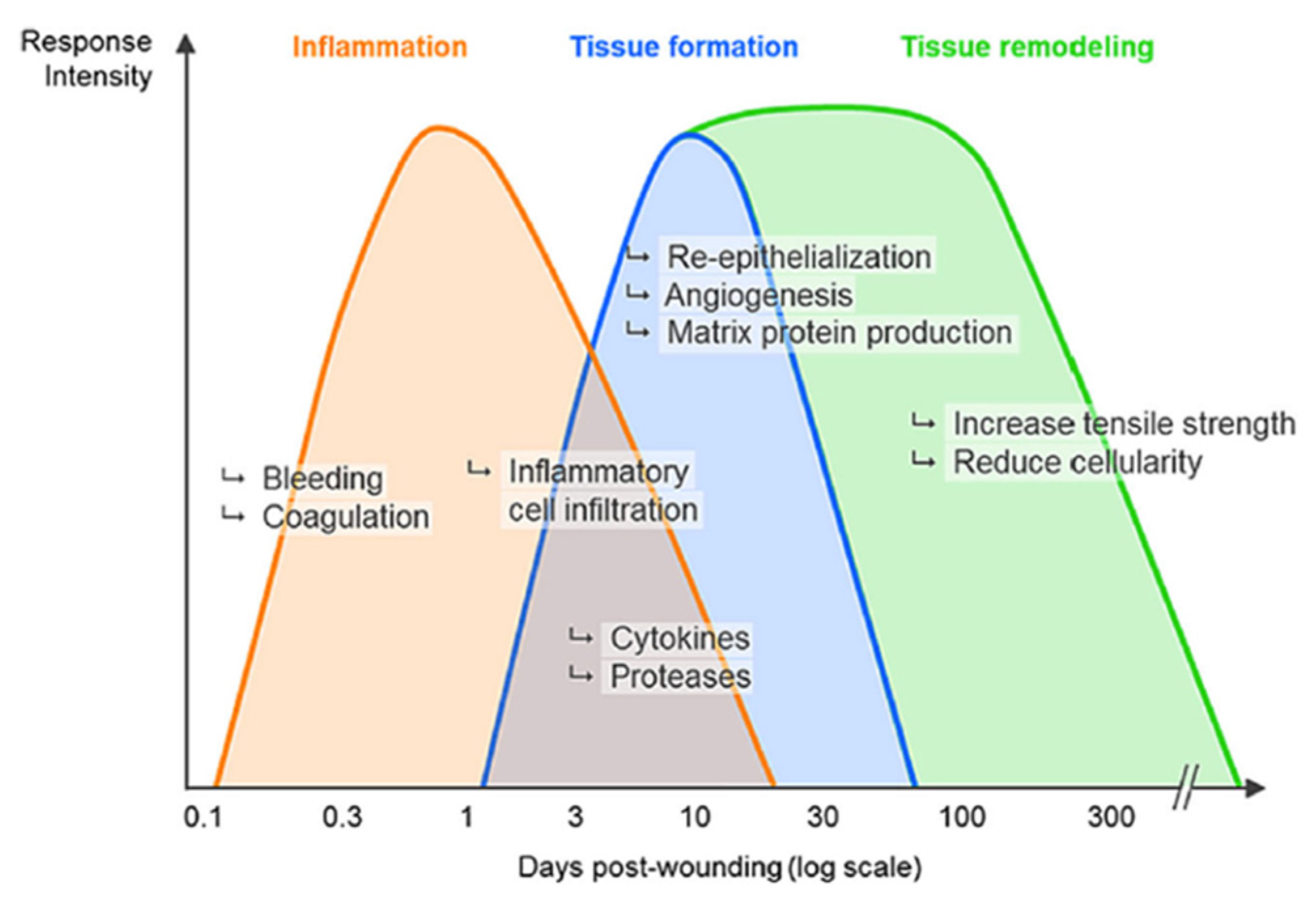
1. Introduction
2. Results
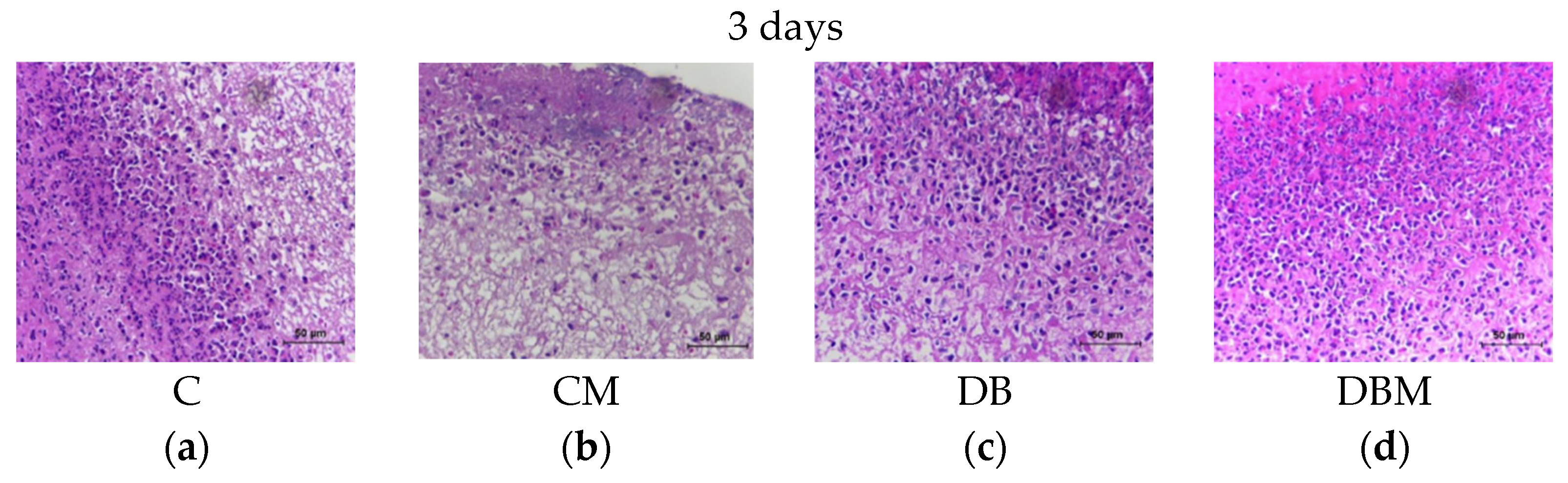
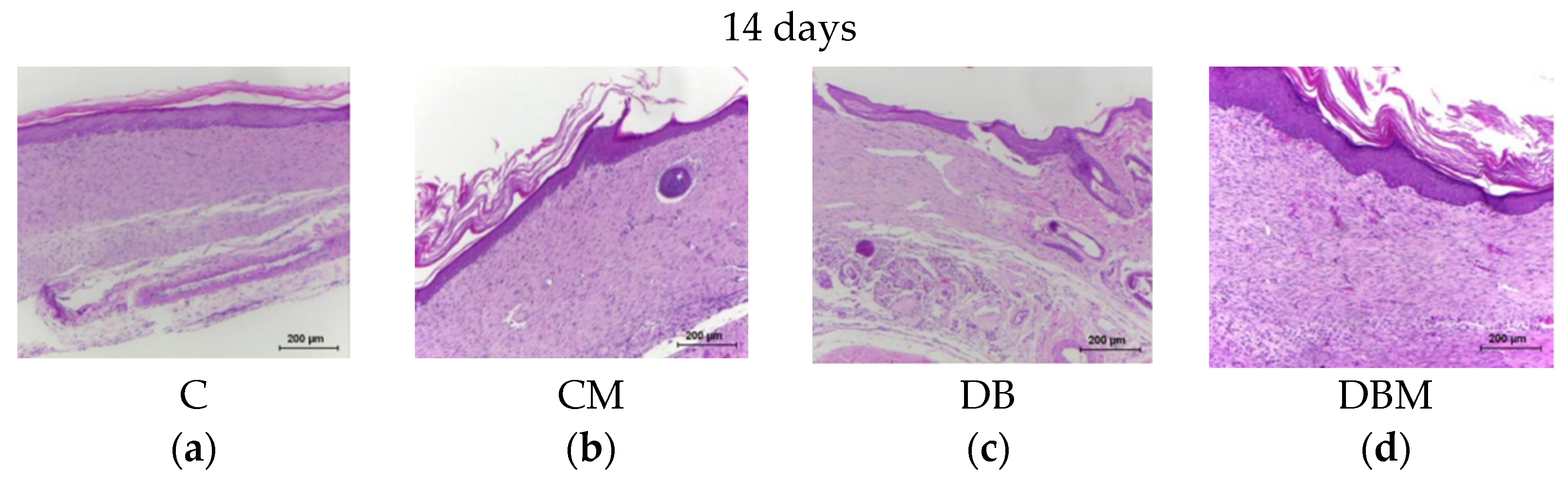
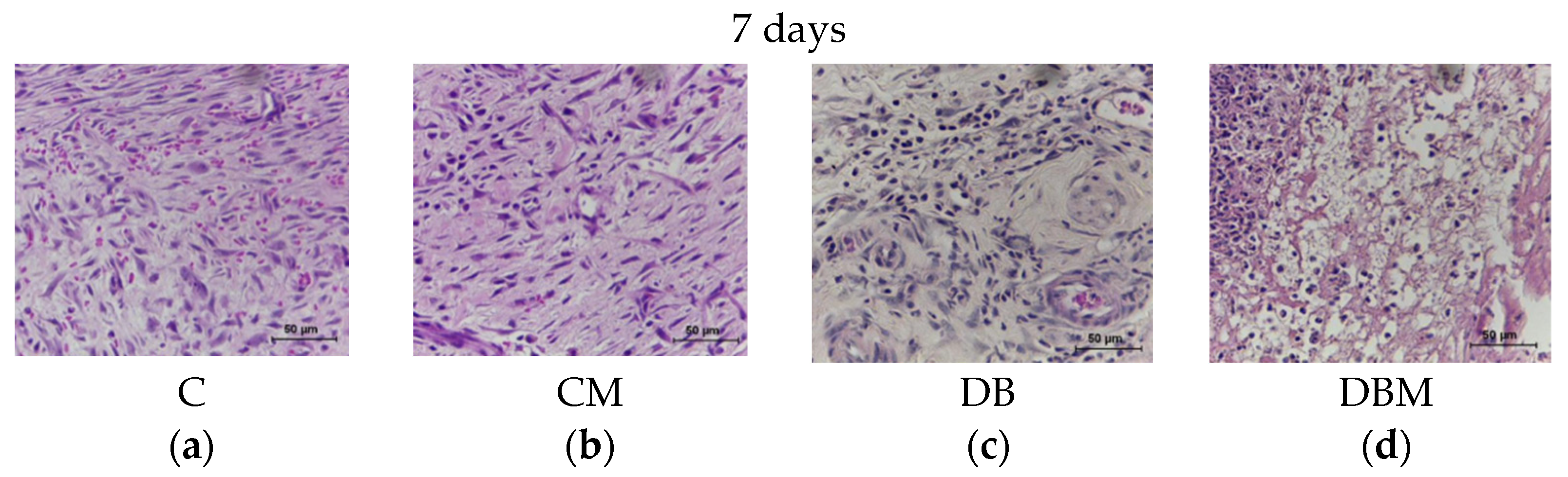
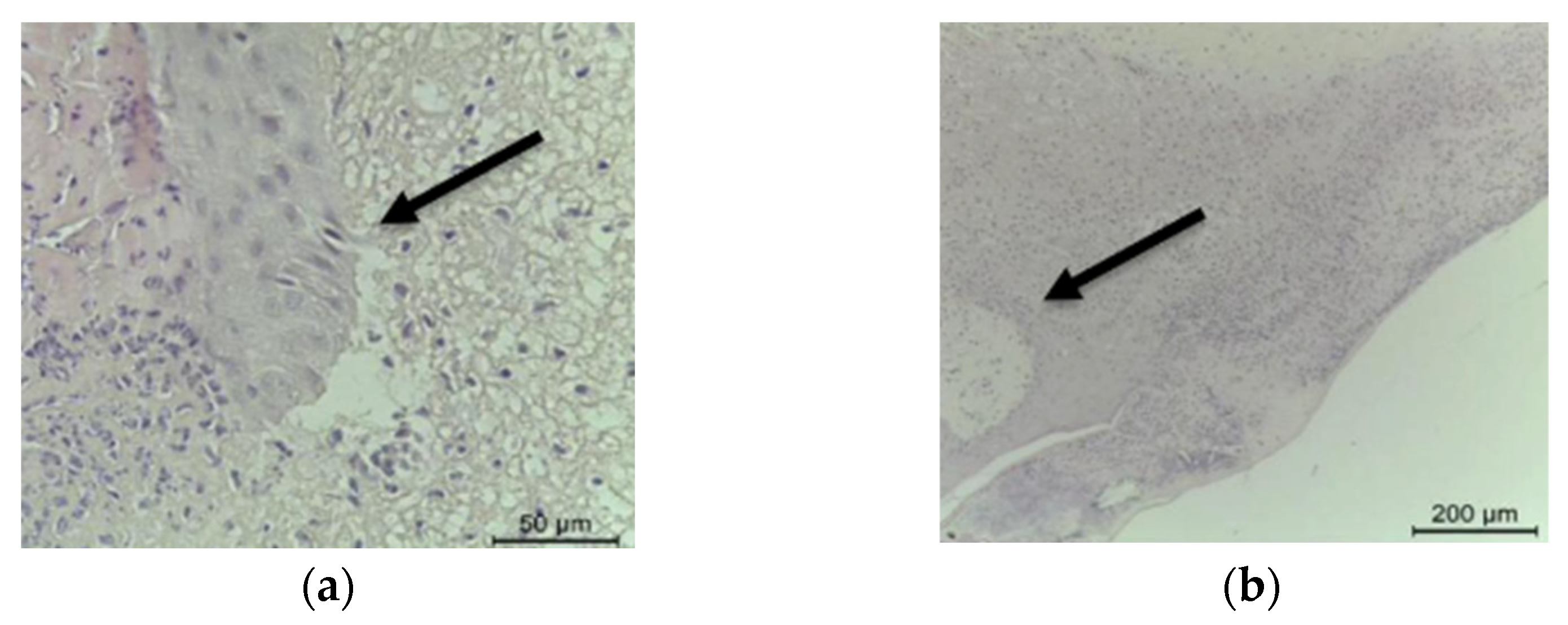
2.1. Histopathological Analysis
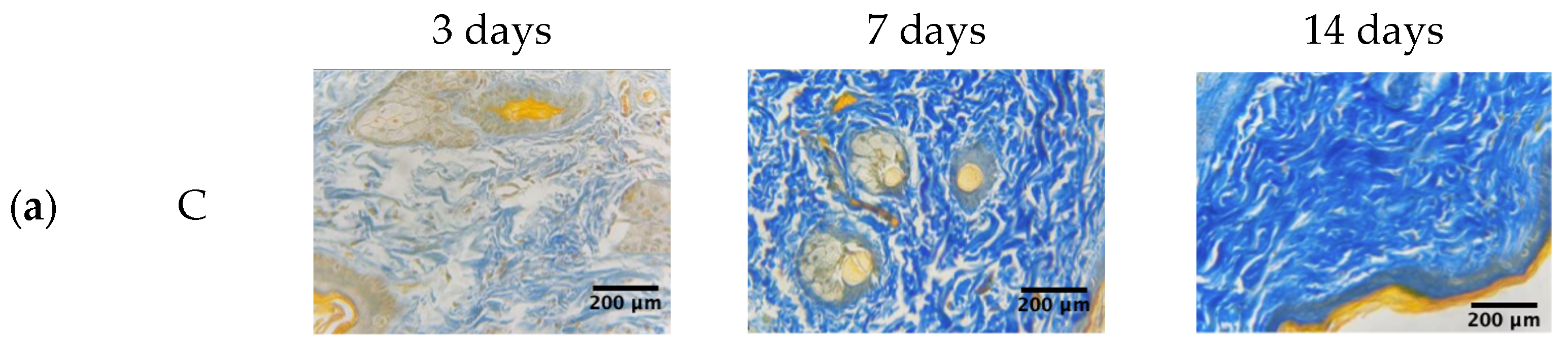
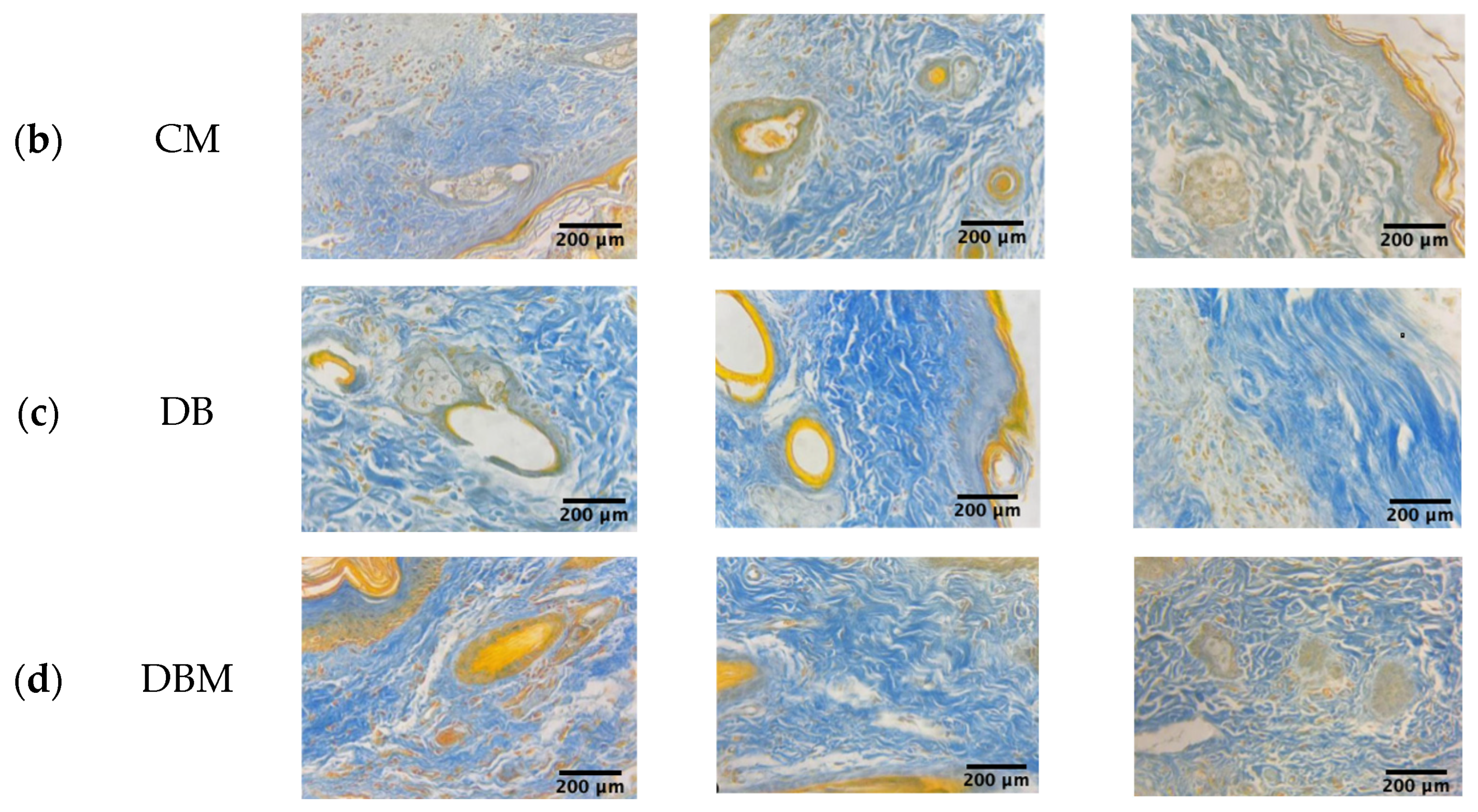
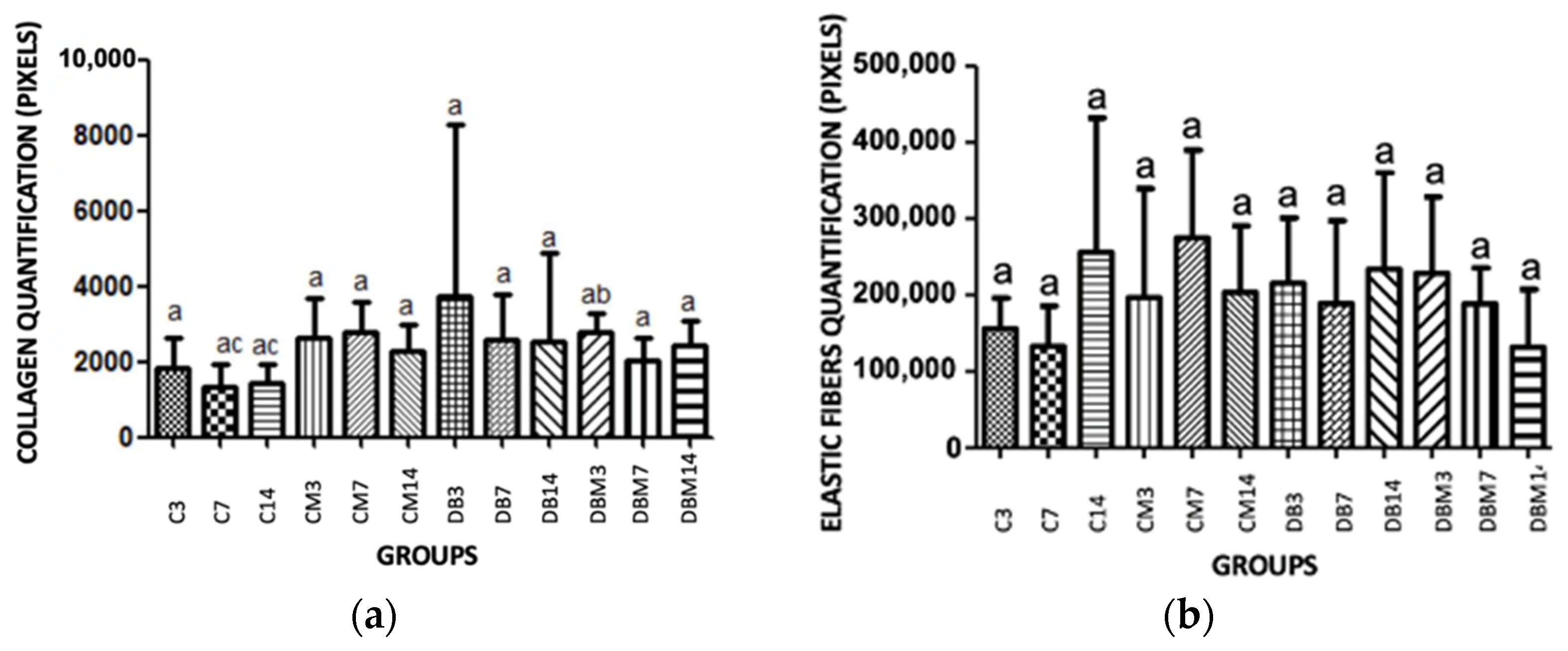
2.2. Collagen and Elastic Fibers Quantification
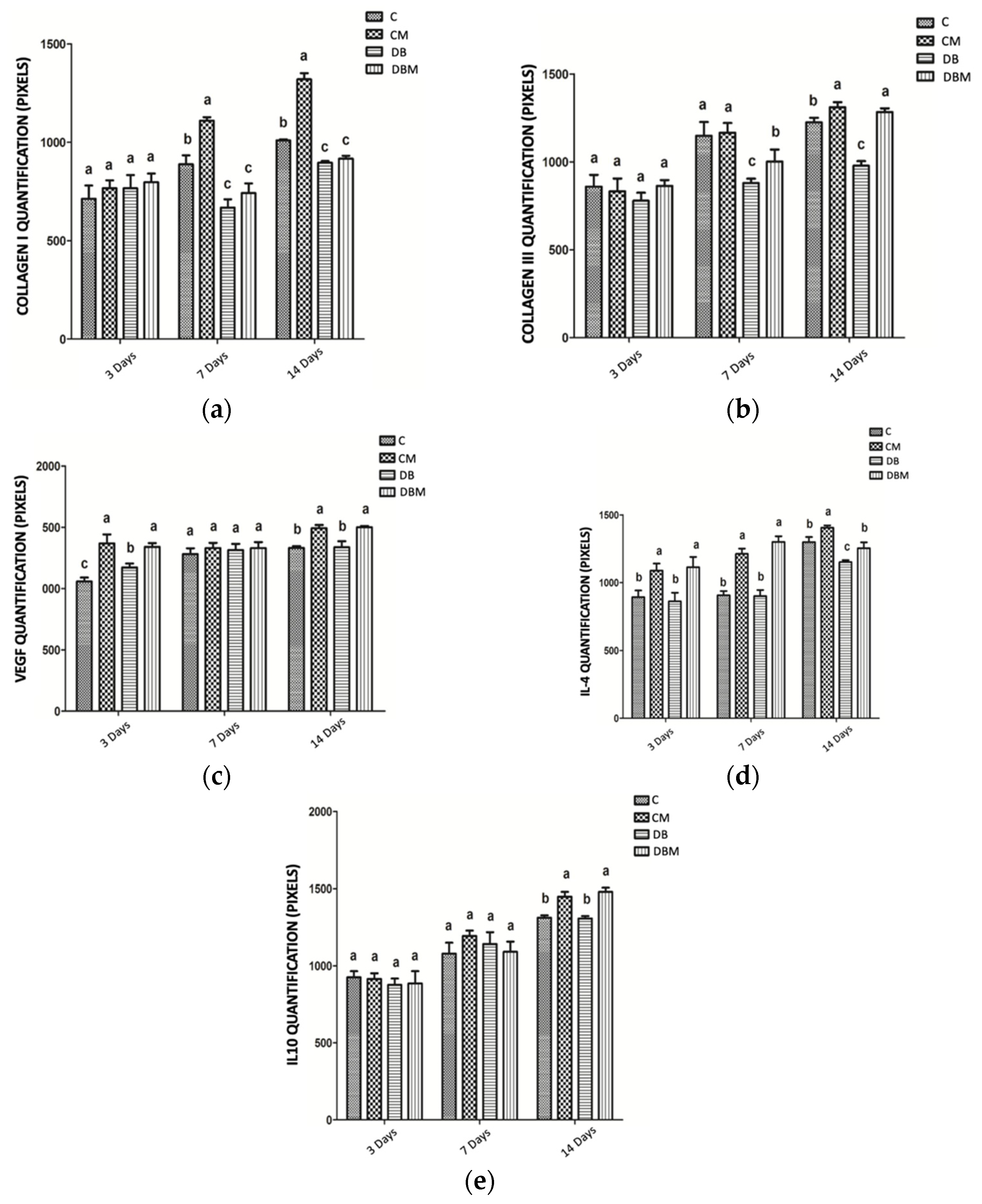
2.3. Immunohistochemical Analysis for COL1, COL3, VEGF-A, IL4, and IL10
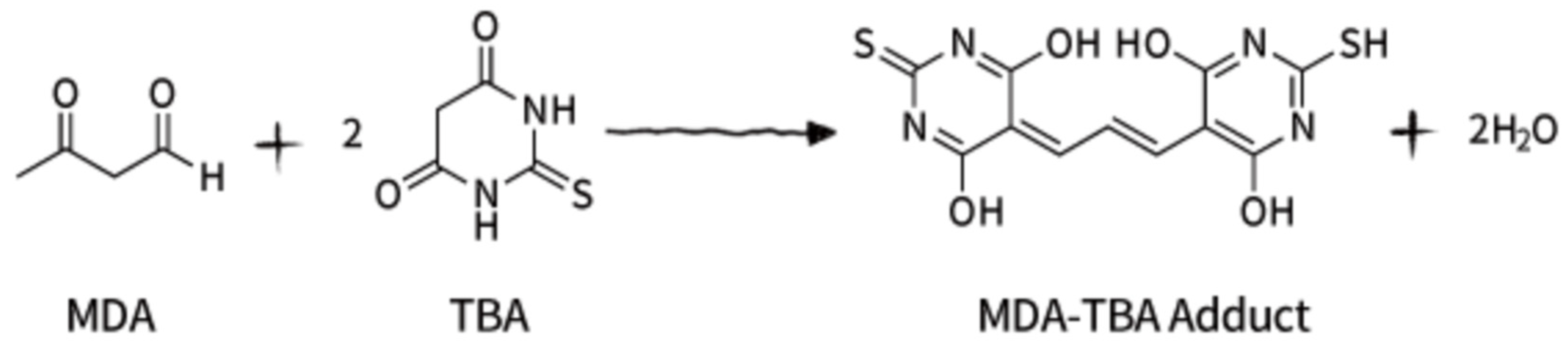
2.4. Evaluation of the Oxidative Stress—Measurement of Gluthatione (GSH)
2.5. Evaluation of Tumor Necrosis Factor-α (TNF-α)
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Diabetes Induction
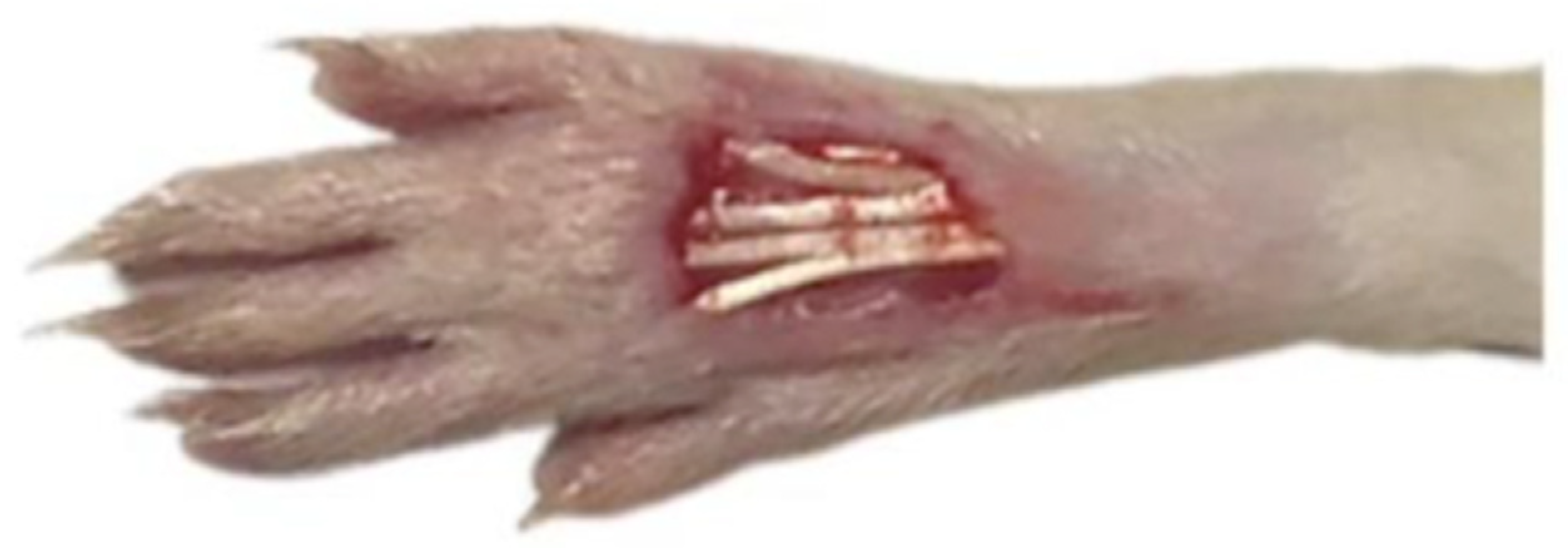
4.3. Wound Preparation
4.4. Autologous Micrografts Rigenera® Technology
4.5. Histopathological Analysis
4.6. Collagen and Elastic Fibers Quantification
4.7. Immunohistochemical Analysis for COL1, COL3, VEGF-A, IL4, and IL10
4.8. Evaluation of the Oxidative Stress—Measurement of Gluthatione (GSH)
4.9. Evaluation of Tumor Necrosis Factor-α (TNF-α)
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rittié, L. Cellular mechanisms of skin repair in humans and other mammals. J. Cell Commun. Signal. 2016, 10, 103–120. [Google Scholar] [CrossRef]
- Blair, M.J.; Jones, J.D.; Woessner, A.E.; Quinn, K.P. Skin Structure–Function Relationships and the Wound Healing Response to Intrinsic Aging. Adv. Wound Care 2020, 9, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Sun, L.; Liu, Z.; Li, M.; Cao, Y.; Han, L.; Wang, J.; Wu, X.; Sang, S. Rete ridges: Morphogenesis, function, regulation, and reconstruction. Acta Biomater. 2023, 155, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Goleva, E.; Berdyshev, E.; Leung, D.Y.M. Epithelial barrier repair and prevention of allergy. J. Clin. Investig. 2019, 129, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Pontiggia, L.; Ahuja, A.K.; Yosef, H.K.; Rütsche, D.; Reichmann, E.; Moehrlen, U.; Biedermann, T. Human Basal and Suprabasal Keratinocytes Are Both Able to Generate and Maintain Dermo–Epidermal Skin Substitutes in Long-Term In Vivo Experiments. Cells 2022, 11, 2156. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef]
- Gantwerker, E.A.; Hom, D.B. Skin: Histology and Physiology of Wound Healing. Clin. Plast. Surg. 2012, 39, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2021, 21, 8790. [Google Scholar] [CrossRef] [PubMed]
- Yardman-Frank, J.M.; Fisher, D.E. Skin pigmentation and its control: From ultraviolet radiation to stem cells. Exp. Dermatol. 2020, 30, 560–571. [Google Scholar] [CrossRef]
- Rippa, A.L.; Kalabusheva, E.P.; Vorotelyak, E.A. Regeneration of Dermis: Scarring and Cells Involved. Cells 2019, 8, 607. [Google Scholar] [CrossRef]
- Woodley, D.T. Distinct Fibroblasts in the Papillary and Reticular Dermis. Dermatol. Clin. 2017, 35, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Piccinin, M.A.; Miao, J.H.; Schwartz, J. Histology, Meissner Corpuscle; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Losquadro, W.D. Anatomy of the Skin and the Pathogenesis of Nonmelanoma Skin Cancer. Facial Plast. Surg. Clin. N. Am. 2017, 25, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Takeo, M.; Lee, W.; Ito, M. Wound Healing and Skin Regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Yang, T.; Chen, H.; Fu, D.; Hu, Y.; Wang, J.; Yuan, Q.; Yu, H.; Xu, W.; Xie, X. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2019, 20, 247–260. [Google Scholar] [CrossRef]
- Wong, T.C.; Piehler, K.; Meier, C.G.; Testa, S.M.; Klock, A.M.; Aneizi, A.A.; Shakesprere, J.; Kellman, P.; Shroff, S.G.; Schwartzman, D.S.; et al. Association Between Extracellular Matrix Expansion Quantified by Cardiovascular Magnetic Resonance and Short-Term Mortality. Circulation 2012, 126, 1206–1216. [Google Scholar] [CrossRef]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta 2014, 1840, 2506–2519. [Google Scholar] [CrossRef]
- Assis-Ribas, T.; Forni, M.F.; Winnischofer, S.M.B.; Sogayar, M.; Trombetta-Lima, M. Extracellular matrix dynamics during mesenchymal stem cells differentiation. Dev. Biol. 2018, 437, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-C.; Fuchs, E. A family business: Stem cell progeny join the niche to regulate homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 103–114. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Li, L.; Fuchs, E. Emerging interactions between skin stem cells and their niches. Nat. Med. 2014, 20, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.; Blau, H.M. Tissue Stem Cells: Architects of Their Niches. Cell Stem Cell 2020, 27, 532–556. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Abbas, J.; Costagliola, M. Barreira cutânea para reconstrução mamária com prótese. Rev. Bras. Cir. Plástica 2012, 27, 630–635. [Google Scholar] [CrossRef][Green Version]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Reinke, J.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Cooper, M.E. Mechanisms of Diabetic Complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Boniakowski, A.E.; Kimball, A.S.; Jacobs, B.N.; Kunkel, S.L.; Gallagher, K.A. Macrophage-Mediated Inflammation in Normal and Diabetic Wound Healing. J. Immunol. 2017, 199, 17–24. [Google Scholar] [CrossRef]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [PubMed]
- Bandyk, D.F. The diabetic foot: Pathophysiology, evaluation, and treatment. Semin. Vasc. Surg. 2018, 31, 43–48. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Wan, C.-D.; Cheng, R.; Wang, H.-B.; Liu, T. Immunomodulatory effects of mesenchymal stem cells derived from adipose tissues in a rat orthotopic liver transplantation model. Hepatobiliary Pancreat. Dis. Int. 2008, 7, 29–33. [Google Scholar]
- Mafi, P. Adult Mesenchymal Stem Cells and Cell Surface Characterization—A Systematic Review of the Literature. Open Orthop. J. 2011, 5, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Waszak, P.; Alphonse, R.S.; Vadivel, A.; Ionescu, L.; Eaton, F.; Thébaud, B.; Abreu, S.C.; Weiss, D.J.; Rocco, P.R.M.; Rüdiger, M.; et al. Preconditioning Enhances the Paracrine Effect of Mesenchymal Stem Cells in Preventing Oxygen-Induced Neonatal Lung Injury in Rats. Stem Cells Dev. 2012, 21, 2789–2797. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Ferrin, I.; Beloqui, I.; Zabaleta, L.; Salcedo, J.M.; Trigueros, C.; Martin, A.G. Isolation, Culture, and Expansion of Mesenchymal Stem Cells. In Stem Cell Banking; Humana Press: New York, NY, USA, 2017; pp. 177–190. [Google Scholar] [CrossRef]
- Wang, C.; Börger, V.; Sardari, M.; Murke, F.; Skuljec, J.; Pul, R.; Hagemann, N.; Dzyubenko, E.; Dittrich, R.; Gregorius, J.; et al. Mesenchymal Stromal Cell–Derived Small Extracellular Vesicles Induce Ischemic Neuroprotection by Modulating Leukocytes and Specifically Neutrophils. Stroke 2020, 51, 1825–1834. [Google Scholar] [CrossRef]
- Baena, R.R.Y.; D’Aquino, R.; Graziano, A.; Trovato, L.; Aloise, A.C.; Ceccarelli, G.; Cusella, G.; Pelegrine, A.A.; Lupi, S.M. Autologous Periosteum-Derived Micrografts and PLGA/HA Enhance the Bone Formation in Sinus Lift Augmentation. Front. Cell Dev. Biol. 2017, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; Presta, R.; Lupi, S.M.; Giarratana, N.; Bloise, N.; Benedetti, L.; De Angelis, M.G.C.; Baena, R.R.Y. Evaluation of Poly(Lactic-co-glycolic) Acid Alone or in Combination with Hydroxyapatite on Human-Periosteal Cells Bone Differentiation and in Sinus Lift Treatment. Molecules 2017, 22, 2109. [Google Scholar] [CrossRef] [PubMed]
- Marcarelli, M.; Zappia, M.; Rissolio, L.; Baroni, C.; Astarita, C.; Trovato, L.; Graziano, A. Cartilage Micrografts as a Novel Non-Invasive and Non-Arthroscopic Autograft Procedure for Knee Chondropathy: Three-Year Follow-Up Study. J. Clin. Med. 2021, 10, 322. [Google Scholar] [CrossRef]
- Marcarelli, M.; Fiammengo, M.; Trovato, L.; Lancione, V.; Novarese, E.; Indelli, P.F.; Risitano, S. Autologous Grafts in the Treatment of Avascular Osteonecrosis of the Femoral Head. 1885. Available online: https://www.actabiomedica.it (accessed on 1 June 2023).
- Hawwam, S.A.; Ismail, M.; Elhawary, E.E. The role of autologous micrografts injection from the scalp tissue in the treatment of COVID-19 associated telogen effluvium: Clinical and trichoscopic evaluation. Dermatol. Ther. 2022, 35, e15545. [Google Scholar] [CrossRef]
- Niimi, Y.; Baba, K.; Tsuchida, M.; Takeda, A. A Histological Evaluation of Artificial Dermal Scaffold Used in Micrograft Treatment: A Case Study of Micrograft and NPWT Performed on a Postoperative Ulcer Formation after Tumor Resection. Medicina 2022, 58, 73. [Google Scholar] [CrossRef]
- Andreone, A.; De Hollander, D. Case Report A Case Report on the Effect of Micrografting in the Healing of Chronic and Complex Burn Wounds. Int. J. Burn. Trauma 2020, 10, 15–20. [Google Scholar]
- Aliberti, F.; Paolin, E.; Benedetti, L.; Cusella, G.; Ceccarelli, G. 3D bioprinting and Rigenera® micrografting technology: A possible countermeasure for wound healing in spaceflight. Front. Bioeng. Biotechnol. 2022, 10, 937709. [Google Scholar] [CrossRef]
- Nummi, A.; Mulari, S.; Stewart, J.A.; Kivistö, S.; Teittinen, K.; Nieminen, T.; Lampinen, M.; Pätilä, T.; Sintonen, H.; Juvonen, T.; et al. Epicardial Transplantation of Autologous Cardiac Micrografts During Coronary Artery Bypass Surgery. Front. Cardiovasc. Med. 2021, 8, 726889. [Google Scholar] [CrossRef] [PubMed]
- Trovato, L.; Monti, M.; del Fante, C.; Cervio, M.; Lampinen, M.; Ambrosio, L.; Redi, C.A.; Perotti, C.; Kankuri, E.; Ambrosio, G.; et al. A New Medical Device Rigeneracons Allows to Obtain Viable Micro-Grafts From Mechanical Disaggregation of Human Tissues. J. Cell. Physiol. 2015, 230, 2299–2303. [Google Scholar] [CrossRef]
- Blakytny, R.; Jude, E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet. Med. 2006, 23, 594–608. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.R.; Navarro, J.; Coburn, J.C.; Mahadik, B.; Molnar, J.; Holmes, J.H.; Nam, A.J.; Fisher, J.P. Current and Future Perspectives on Skin Tissue Engineering: Key Features of Biomedical Research, Translational Assessment, and Clinical Application. Adv. Healthc. Mater. 2019, 8, 1801471. [Google Scholar] [CrossRef] [PubMed]
- Andreone, A.; Hollander, D.D. A Retrospective Study on the Use of Dermis Micrografts in Platelet-Rich Fibrin for the Resurfacing of Massive and Chronic Full-Thickness Burns. Stem Cells Int. 2019, 2019, 8636079. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Tu, C.; Lu, H.; Zhou, T.; Zhang, W.; Deng, L.; Cao, W.; Yang, Z.; Wang, Z.; Wu, X.; Ding, J.; et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials 2022, 286, 121597. [Google Scholar] [CrossRef]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and Functions of the IL-10 Family of Cytokines in Inflammation and Disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef]
- Franco, R.; Panayiotidis, M.I.; Cidlowski, J.A. Glutathione Depletion Is Necessary for Apoptosis in Lymphoid Cells Independent of Reactive Oxygen Species Formation. J. Biol. Chem. 2007, 282, 30452–30465. [Google Scholar] [CrossRef] [PubMed]
- Dall’Ago, P.; Silva, V.O.K.; De Angelis, K.L.D.; Irigoyen, M.C.; Fazan, R., Jr.; Salgado, H.C. Reflex control of arterial pressure and heart rate in short-term streptozotocin diabetic rats. Braz. J. Med. Biol. Res. 2002, 35, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Oberholzer, M.; Östreicher, M.; Christen, H.; Brühlmann, M. Methods in quantitative image analysis. Histochem. Cell Biol. 1996, 105, 333–355. [Google Scholar] [CrossRef] [PubMed]
- Bussolati, G.; Radulescu, R.T. Blocking Endogenous Peroxidases in Immunohistochemistry. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 484. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, H.M.; El-Baz, A.A.; Mahmoud, O.M.; Khalil, S.; Atta, R.; Imbaby, S. Protective effects of evening primrose oil on behavioral activities, nigral microglia and histopathological changes in a rat model of rotenone-induced parkinsonism. J. Chem. Neuroanat. 2023, 127, 102206. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandão Palma, M.; Paolin, E.; Ferreira de Melo, I.M.; De Assis Leite Souza, F.; Coelho Teixeira, Á.A.; Duarte Vieira, L.; Naro, F.; Graziano, A.; Soares, A.F. Biological Evidence of Improved Wound Healing Using Autologous Micrografts in a Diabetic Animal Model. Diabetology 2023, 4, 294-311. https://doi.org/10.3390/diabetology4030026
Brandão Palma M, Paolin E, Ferreira de Melo IM, De Assis Leite Souza F, Coelho Teixeira ÁA, Duarte Vieira L, Naro F, Graziano A, Soares AF. Biological Evidence of Improved Wound Healing Using Autologous Micrografts in a Diabetic Animal Model. Diabetology. 2023; 4(3):294-311. https://doi.org/10.3390/diabetology4030026
Chicago/Turabian StyleBrandão Palma, Mariza, Elisa Paolin, Ismaela Maria Ferreira de Melo, Francisco De Assis Leite Souza, Álvaro Aguiar Coelho Teixeira, Leucio Duarte Vieira, Fabio Naro, Antonio Graziano, and Anísio Francisco Soares. 2023. "Biological Evidence of Improved Wound Healing Using Autologous Micrografts in a Diabetic Animal Model" Diabetology 4, no. 3: 294-311. https://doi.org/10.3390/diabetology4030026
APA StyleBrandão Palma, M., Paolin, E., Ferreira de Melo, I. M., De Assis Leite Souza, F., Coelho Teixeira, Á. A., Duarte Vieira, L., Naro, F., Graziano, A., & Soares, A. F. (2023). Biological Evidence of Improved Wound Healing Using Autologous Micrografts in a Diabetic Animal Model. Diabetology, 4(3), 294-311. https://doi.org/10.3390/diabetology4030026






