Frailty in Older Patients with End-Stage Renal Disease and Undergoing Chronic Haemodialysis in Vietnam
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Outcome Variables
2.4. Predictive Variables
2.5. Covariates
2.6. Comorbidities
2.7. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Mortality Rate at the Sixth Month
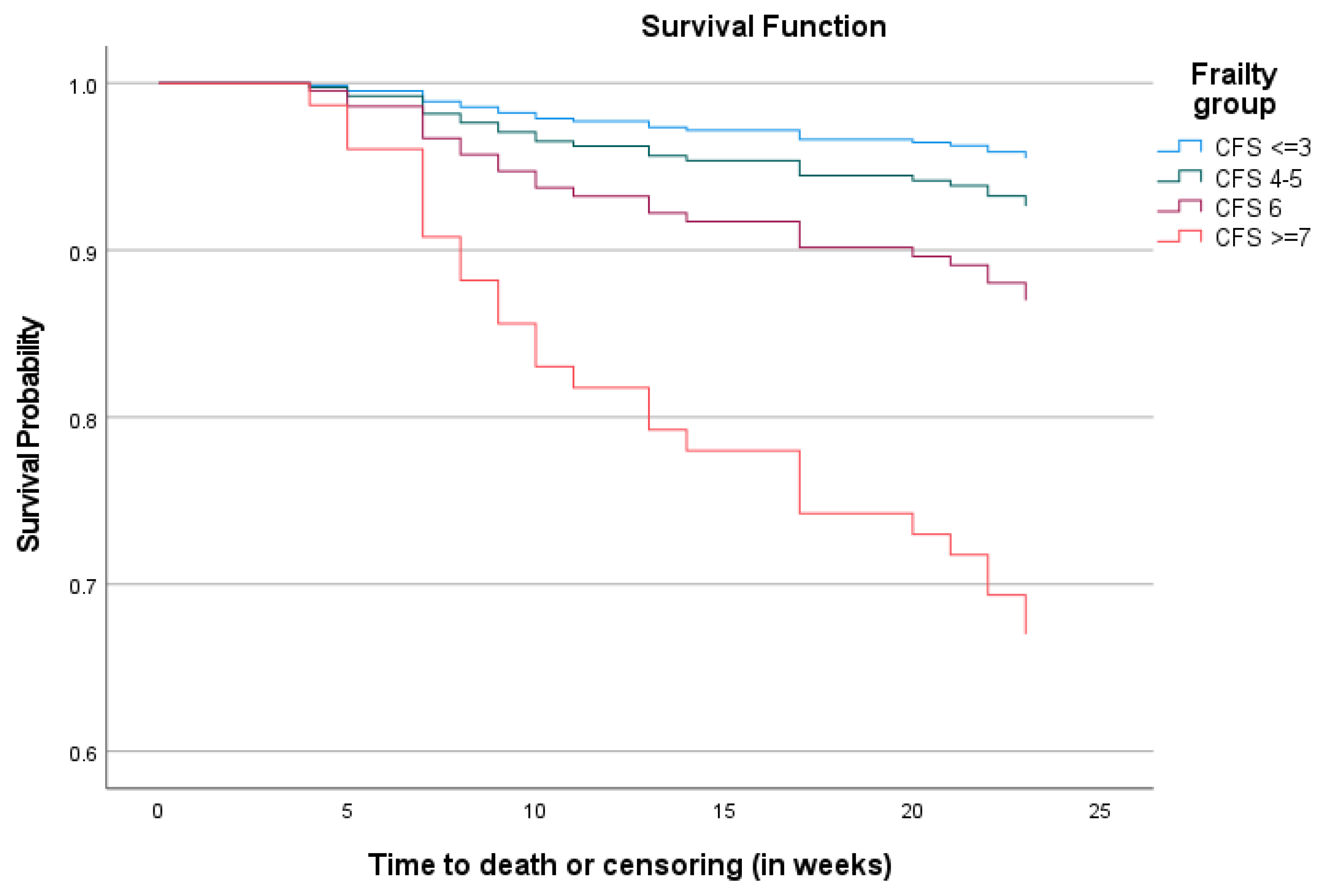
3.3. The Impact of Frailty on Mortality
4. Discussion
Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stevens, P.E.; Levin, A. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Bello, A.K.; Levin, A.; Lunney, M.; Osman, M.A.; Ye, F.; Ashuntantang, G.E.; Bellorin-Font, E.; Benghanem Gharbi, M.; Davison, S.N.; Ghnaimat, M.; et al. Status of care for end stage kidney disease in countries and regions worldwide: International cross sectional survey. BMJ 2019, 367, l5873. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Kovesdy, C.P.; Streja, E.; Rhee, C.M.; Soohoo, M.; Chen, J.L.T.; Molnar, M.Z.; Obi, Y.; Gillen, D.; Nguyen, D.V.; et al. Transition of care from pre-dialysis prelude to renal replacement therapy: The blueprints of emerging research in advanced chronic kidney disease. Nephrol. Dial. Transplant. 2017, 32 (Suppl. S2), ii91–ii98. [Google Scholar] [CrossRef] [PubMed]
- Mallappallil, M.; Friedman, E.A.; Delano, B.G.; McFarlane, S.I.; Salifu, M.O. Chronic kidney disease in the elderly: Evaluation and management. Clin. Pract. 2014, 11, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Nixon, A.C.; Bampouras, T.M.; Pendleton, N.; Woywodt, A.; Mitra, S.; Dhaygude, A. Frailty and chronic kidney disease: Current evidence and continuing uncertainties. Clin. Kidney J. 2018, 11, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, E.C.; Kennedy, C.C.; Rule, A.D.; LeBrasseur, N.K.; Kirkland, J.L.; Hickson, L.J. Frailty in CKD and Transplantation. Kidney Int. Rep. 2021, 6, 2270–2280. [Google Scholar] [CrossRef]
- Mei, F.; Gao, Q.; Chen, F.; Zhao, L.; Shang, Y.; Hu, K.; Zhang, W.; Zhao, B.; Ma, B. Frailty as a Predictor of Negative Health Outcomes in Chronic Kidney Disease: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2021, 22, 535–543.e537. [Google Scholar] [CrossRef]
- Shlipak, M.G.; Stehman-Breen, C.; Fried, L.F.; Song, X.; Siscovick, D.; Fried, L.P.; Psaty, B.M.; Newman, A.B. The presence of frailty in elderly persons with chronic renal insufficiency. Am. J. Kidney Dis. 2004, 43, 861–867. [Google Scholar] [CrossRef]
- Chowdhury, R.; Peel, N.M.; Krosch, M.; Hubbard, R.E. Frailty and chronic kidney disease: A systematic review. Arch. Gerontol. Geriatr. 2017, 68, 135–142. [Google Scholar] [CrossRef]
- Chao, C.T.; Lin, S.H. Uremic Toxins and Frailty in Patients with Chronic Kidney Disease: A Molecular Insight. Int. J. Mol. Sci. 2021, 22, 6270. [Google Scholar] [CrossRef]
- Hanna, R.M.; Ghobry, L.; Wassef, O.; Rhee, C.M.; Kalantar-Zadeh, K. A Practical Approach to Nutrition, Protein-Energy Wasting, Sarcopenia, and Cachexia in Patients with Chronic Kidney Disease. Blood Purif. 2020, 49, 202–211. [Google Scholar] [CrossRef]
- Hubbard, R.E.; Woodhouse, K.W. Frailty, inflammation and the elderly. Biogerontology 2010, 11, 635–641. [Google Scholar] [CrossRef]
- Obi, Y.; Qader, H.; Kovesdy, C.P.; Kalantar-Zadeh, K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 254–262. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Farrington, K.; Covic, A.; Aucella, F.; Clyne, N.; de Vos, L.; Findlay, A.; Fouque, D.; Grodzicki, T.; Iyasere, O.; Jager, K.J.; et al. Clinical Practice Guideline on management of older patients with chronic kidney disease stage 3b or higher (eGFR <45 mL/min/1.73 m2). Nephrol. Dial. Transplant. 2016, 31(Suppl. S2), ii1–ii66. [Google Scholar] [CrossRef] [PubMed]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Hyodo, T.; Fukagawa, M.; Hirawa, N.; Isaka, Y.; Nakamoto, H.; Van Bui, P.; Thwin, K.T.; Hy, C. Present status of renal replacement therapy in Asian countries as of 2017: Vietnam, Myanmar, and Cambodia. Renal. Replacement. Ther. 2020, 6, 65. [Google Scholar] [CrossRef]
- Ngoc, N.B.; Lin, Z.L.; Ahmed, W. Diabetes: What Challenges Lie Ahead for Vietnam? Ann. Glob. Health 2020, 86, 1. [Google Scholar] [CrossRef]
- Khue, N.T. Diabetes in Vietnam. Ann. Glob. Health 2015, 81, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.Q.; Nguyen, N.T.Y.; Nguyen, B.; Bui, Q.T.H. Quality of life assessment in patients on chronic dialysis: Comparison between haemodialysis and peritoneal dialysis at a national hospital in Vietnam. Trop. Med. Int. Health 2022, 27, 199–206. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Dent, E.; Lien, C.; Lim, W.S.; Wong, W.C.; Wong, C.H.; Ng, T.P.; Woo, J.; Dong, B.; de la Vega, S.; Hua Poi, P.J.; et al. The Asia-Pacific Clinical Practice Guidelines for the Management of Frailty. J. Am. Med. Dir. Assoc. 2017, 18, 564–575. [Google Scholar] [CrossRef]
- Fehlmann, C.A.; Nickel, C.H.; Cino, E.; Al-Najjar, Z.; Langlois, N.; Eagles, D. Frailty assessment in emergency medicine using the Clinical Frailty Scale: A scoping review. Intern. Emerg. Med. 2022, 17, 2407–2418. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Nguyen, T.X.; Nguyen, T.N.; Nguyen, T.H.T.; Pham, T.; Cumming, R.; Hilmer, S.N.; Vu, H.T.T. The impact of frailty on prolonged hospitalization and mortality in elderly inpatients in Vietnam: A comparison between the frailty phenotype and the Reported Edmonton Frail Scale. Clin. Interv. Aging 2019, 14, 381–388. [Google Scholar] [CrossRef]
- Nguyen-Thi, H.Y.; Le-Phuoc, T.N.; Tri Phat, N.; Truong Van, D.; Le-Thi, T.T.; Le, N.D.T.; Tran-Thi, H.N.; Pham Dinh, L. The Economic Burden of Chronic Kidney Disease in Vietnam. Health Serv. Insights 2021, 14, 11786329211036011. [Google Scholar] [CrossRef]
- Kennard, A.; Glasgow, N.; Rainsford, S.; Talaulikar, G. Frailty in chronic kidney disease: Challenges in nephrology practice. A review of current literature. Intern. Med. J. 2023, 53, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Nixon, A.C.; Bampouras, T.M.; Pendleton, N.; Mitra, S.; Dhaygude, A.P. Diagnostic Accuracy of Frailty Screening Methods in Advanced Chronic Kidney Disease. Nephron 2019, 141, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Matheson, K.; West, B.; Vinson, A.; West, K.; Jain, A.; Rockwood, K.; Tennankore, K. Frailty Severity and Hospitalization After Dialysis Initiation. Can. J. Kidney Health Dis. 2021, 8, 20543581211023330. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Takanashi, Y.; Harigai, T.; Sakurai, N.; Kobatake, K.; Yoshida, H.; Kobayashi, S.; Matsumoto, T.; Ueki, K. Evaluation of frailty status and prognosis in patients aged over 75 years with chronic kidney disease (CKD). Renal. Replace Ther. 2020, 6, 60. [Google Scholar] [CrossRef]
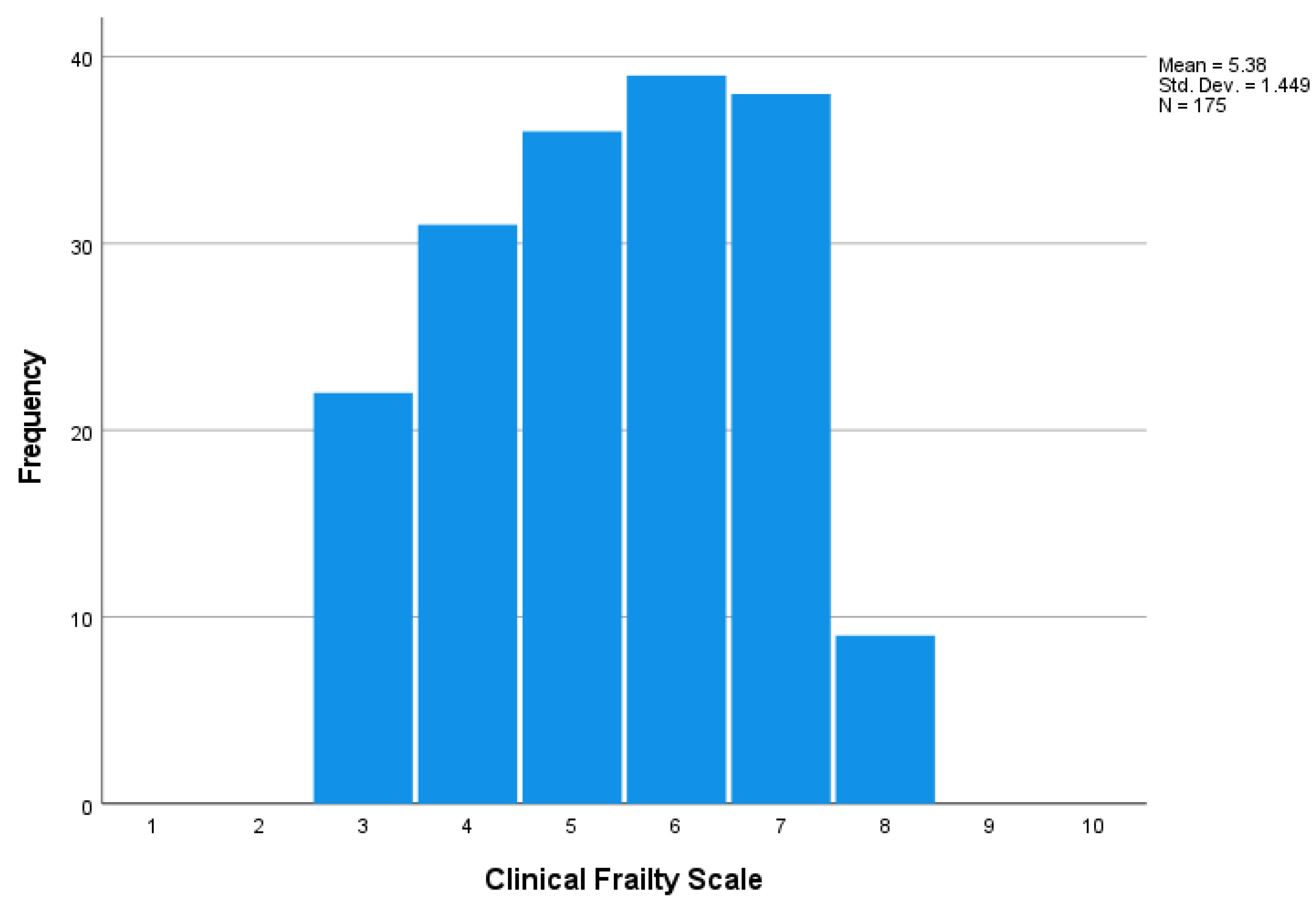
| Characteristics | All (n = 175) | CFS ≤ 3 (n = 22) | CFS 4–5 (n = 67) | CFS 6 (n = 39) | CFS ≥ 7 (n = 47) | p-Value |
|---|---|---|---|---|---|---|
| Age, years | 72.4 ± 8.5 | 67.6 ± 4.4 | 69.1 ± 7.7 | 75.8 ± 7.3 | 76.5 ± 9.0 | <0.001 |
| Female | 103 (58.9%) | 9 (40.9%) | 39 (58.2%) | 27 (69.2%) | 28 (59.6%) | 0.197 |
| Education | ||||||
| Illiterate | 6 (3.4%) | 1 (4.5%) | 2 (3.0%) | 3 (7.7%) | 0 | 0.328 |
| Primary school | 48 (27.4%) | 4 (18.2%) | 20 (29.9%) | 9 (23.1%) | 15 (31.9%) | |
| Secondary school | 43 (24.6%) | 2 (9.1%) | 16 (23.9%) | 9 (23.1%) | 16 (34.0%) | |
| High school | 41 (23.4%) | 7 (31.8%) | 15 (22.4%) | 10 (25.6%) | 9 (19.1%) | |
| Higher education | 37 (21.1%) | 8 (36.4%) | 14 (20.9%) | 8 (20.5%) | 7 (14.9%) | |
| Smoking | 50 (28.6%) | 9 (40.9%) | 19 (28.4%) | 7 (17.9%) | 15 (31.9%) | 0.255 |
| Body mass index | ||||||
| Underweight (<18.5) | 40 (22.9%) | 2 (9.1%) | 9 (13.4%) | 10 (25.6%) | 19 (40.4%) | 0.016 |
| Normal (18.5–22.9) | 84 (48%) | 14 (63.6%) | 36 (53.7%) | 16 (41.0%) | 18 (38.3%) | |
| Overweight (23.0–24.9) | 18 (10.3%) | 4 (18.2%) | 5 (7.5%) | 6 (15.4%) | 3 (6.4%) | |
| Obese (≥25.0) | 33 (18.9%) | 2 (9.1%) | 17 (25.4%) | 7 (17.9%) | 7 (14.9%) | |
| Duration of dialysis (years) | 3.6 ± 3.5 | 2.9 ± 3.5 | 4.2 ± 3.9 | 3.4 ± 2.6 | 3.4 ± 3.6 | 0.432 |
| Comorbidities | ||||||
| Anaemia | 144 (82.8%) | 16 (72.7%) | 55 (82.1%) | 32 (82.1%) | 41 (89.1%) | 0.408 |
| Diabetes | 110 (62.9%) | 12 (54.5%) | 37 (55.2%) | 23 (59.0%) | 38 (80.9%) | 0.028 |
| Heart failure | 105 (60.0%) | 6 (27.3%) | 39 (58.2%) | 26 (66.7%) | 34 (72.3%) | 0.003 |
| Dyslipidaemia | 103 (58.9%) | 14 (63.6%) | 33 (49.3%) | 26 (66.7%) | 30 (63.8%) | 0.238 |
| Ischemic heart disease | 87 (49.7%) | 8 (36.4%) | 30 (44.8%) | 18 (46.2%) | 31 (66.0%) | 0.061 |
| Stroke | 45 (25.7%) | 3 (13.6%) | 14 (20.9%) | 8 (20.5%) | 20 (42.6%) | 0.018 |
| Stomach problems | 42 (24.0%) | 7 (31.8%) | 14 (20.9%) | 8 (20.5%) | 13 (27.7%) | 0.638 |
| Chronic liver disease | 25 (14.3%) | 2 (9.1%) | 12 (17.9%) | 4 (10.3%) | 7 (14.9%) | 0.654 |
| Chronic pulmonary disease | 14 (8.0%) | 0 | 4 (6.0%) | 3 (7.7%) | 7 (14.9%) | 0.143 |
| Cancer | 7 (4.0%) | 0 | 0 | 2 (5.1%) | 5 (10.6%) | 0.020 |
| Peripheral artery disease | 7 (4.0%) | 0 | 0 | 2 (5.1%) | 5 (10.6%) | 0.020 |
| Variables | Unadjusted HRs for All-Cause Mortality (95% CI) | p-Values | Adjusted HRs for All-Cause Mortality (95% CI) | p-Values |
|---|---|---|---|---|
| Frailty severity | ||||
| CFS ≤ 3 (reference) | 1 | 1 | ||
| CFS 4–5 | 1.66 (0.19–14.23) | 1.72 (0.20–14.93) | ||
| CFS 6 | 3.03 (0.35–25.91) | 0.002 | 2.81 (0.30–26.29) | 0.029 |
| CFS ≥ 7 | 8.70 (1.15–65.90) | 6.99 (0.84–58.44) | ||
| Age (years) | 1.08 (1.03–1.13) | 0.002 | 1.03 (0.98–1.08) | 0.300 |
| Male (versus Female) | 2.35 (1.07–5.19) | 0.034 | 2.10 (0.90–4.91) | 0.087 |
| Body mass index | 0.073 | - | ||
| Underweight (reference) | 1 | |||
| Normal | 0.44 (0.19–1.01) | |||
| Overweight | 0.36 (0.08–1.64) | |||
| Obese | 1.94 (0.04–0.88) | |||
| Duration of dialysis (years) | 0.95 (0.83–1.07) | 0.375 | - | |
| Comorbidities | ||||
| Anaemia | 2.50 (0.59–10.62) | 0.213 | - | |
| Diabetes | 1.35 (0.59–3.11) | 0.478 | - | |
| Heart failure | 2.39 (0.96–5.96) | 0.061 | - | |
| Dyslipidaemia | 1.15 (0.52–2.53) | 0.733 | - | |
| Ischemic heart disease | 2.44 (1.06–5.61) | 0.036 | 1.37 (0.56–3.40) | 0.492 |
| Stroke | 1.06 (0.45–2.52) | 0.894 | - | |
| Stomach problems | 1.76 (0.79–3.96) | 0.168 | - | |
| Chronic liver disease | 0.47 (0.11–2.01) | 0.311 | - | |
| Chronic pulmonary disease | 1.56 (0.47–5.19) | 0.470 | - | |
| Cancer | 2.18 (0.51–9.21) | 0.291 | - | |
| Peripheral artery disease | 2.18 (0.51–9.21) | 0.291 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.V.; Pham, T.T.X.; Burns, M.J.; Nguyen, T.N. Frailty in Older Patients with End-Stage Renal Disease and Undergoing Chronic Haemodialysis in Vietnam. Diabetology 2023, 4, 312-322. https://doi.org/10.3390/diabetology4030027
Nguyen TV, Pham TTX, Burns MJ, Nguyen TN. Frailty in Older Patients with End-Stage Renal Disease and Undergoing Chronic Haemodialysis in Vietnam. Diabetology. 2023; 4(3):312-322. https://doi.org/10.3390/diabetology4030027
Chicago/Turabian StyleNguyen, Tan Van, Thu Thi Xuan Pham, Mason Jenner Burns, and Tu Ngoc Nguyen. 2023. "Frailty in Older Patients with End-Stage Renal Disease and Undergoing Chronic Haemodialysis in Vietnam" Diabetology 4, no. 3: 312-322. https://doi.org/10.3390/diabetology4030027
APA StyleNguyen, T. V., Pham, T. T. X., Burns, M. J., & Nguyen, T. N. (2023). Frailty in Older Patients with End-Stage Renal Disease and Undergoing Chronic Haemodialysis in Vietnam. Diabetology, 4(3), 312-322. https://doi.org/10.3390/diabetology4030027







