A Pilot Study of the Effect of Evening Almond Butter Consumption on Overnight and Fasting Interstitial Glucose
Abstract
1. Introduction
2. Materials and Methods
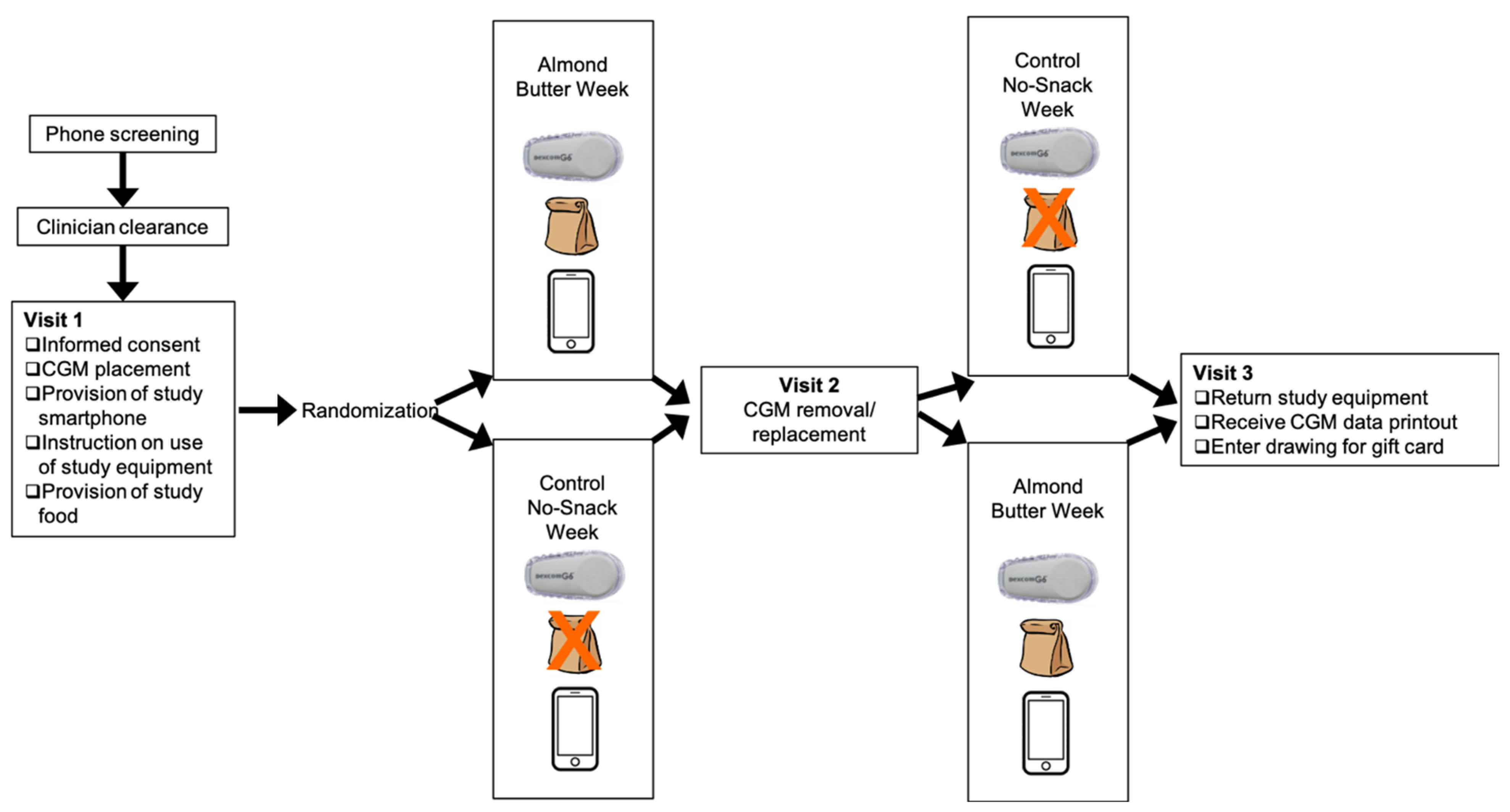
2.1. Study Design
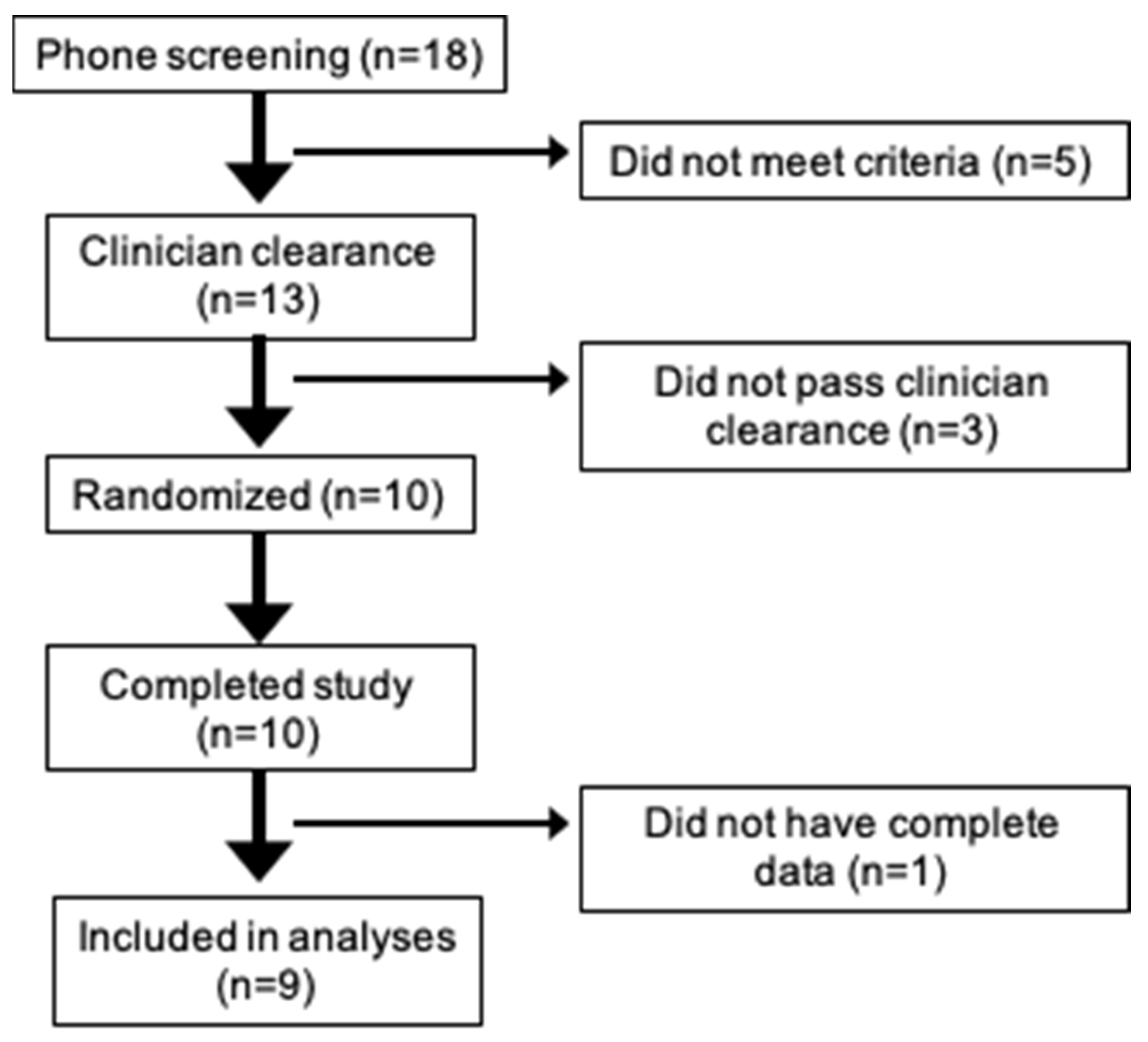
2.2. Participants
2.3. Study Food
2.4. Continuous Glucose Monitoring
2.5. Study Visits
2.6. Data Preparation
2.7. Statistical Analysis
3. Results
3.1. Primary Endpoint: Fasting Glucose (4–6 a.m.)
3.2. Secondary Endpoint: Overnight Glucose (12–3 a.m.)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. Diabetes. 2022. Available online: https://www.cdc.gov/diabetes/data/statistics-report/index.html?ACSTrackingID=DM72996&ACSTrackingLabel=New%20Report%20Shares%20Latest%20Diabetes%20Stats%20&deliveryName=DM72996 (accessed on 18 June 2022).
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.K.; Bullard, K.M.; Saaddine, J.B.; Cowie, C.C.; Imperatore, G.; Gregg, E.W. Achievement of goals in U.S. diabetes care, 1999–2010. N. Engl. J. Med. 2013, 368, 1613–1624. [Google Scholar] [CrossRef]
- American Diabetes Association. 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. S1), S40–S52. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. S1), S73–S84. [Google Scholar] [CrossRef] [PubMed]
- Best, J.D.; Drury, P.L.; Davis, T.M.; Taskinen, M.R.; Kesäniemi, Y.A.; Scott, R.; Pardy, C.; Voysey, M.; Keech, A.C.; Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study Investigators. Glycemic control over 5 years in 4900 people with type 2 diabetes: Real-world diabetes therapy in a clinical trial cohort. Diabetes Care 2012, 35, 1165–1170. [Google Scholar] [CrossRef]
- Monnier, L.; Colette, C.; Dejager, S.; Owens, D. Magnitude of the dawn phenomenon and its impact on the overall glucose exposure in type 2 diabetes: Is this of concern? Diabetes Care 2013, 36, 4057–4062. [Google Scholar] [CrossRef]
- Porcellati, F.; Lucidi, P.; Bolli, G.B.; Fanelli, C.G. Thirty years of research on the dawn phenomenon: Lessons to optimize blood glucose control in diabetes. Diabetes Care 2013, 36, 3860–3862. [Google Scholar] [CrossRef]
- Serin, Y.; Acar Tek, N. Effect of circadian rhythm on metabolic processes and the regulation of energy balance. Ann. Nutr. Metab. 2019, 74, 322–330. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Matsuda, M.; Jani, R.; Jenkinson, C.P.; Coletta, D.K.; Kaku, K.; DeFronzo, R.A. The relationship between fasting hyperglycemia and insulin secretion in subjects with normal or impaired glucose tolerance. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E401–E406. [Google Scholar] [CrossRef]
- Cavaiola, T.S.; Pettus, J.H. Management of type 2 diabetes: Selecting amongst available pharmacological agents. In Endotext; De Groot, L.J., Chrousos, G., Dungan, K., Feingold, K.R., Grossman, A., Hershman, J.M., Hofland, J., Kalra, S., Kaltsas, G., Koch, C., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Luo, C.; Zhang, Y.; Ding, Y.; Shan, Z.; Chen, S.; Yu, M.; Hu, F.B.; Liu, L. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 256–269. [Google Scholar] [CrossRef]
- Tindall, A.M.; Johnston, E.A.; Kris-Etherton, P.M.; Petersen, K.S. The effect of nuts on markers of glycemic control: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2019, 109, 297–314. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. The R Project for Statistical Computing. 2020. Available online: https://www.r-project.org/ (accessed on 8 August 2020).
- Ekstrom, C.T. MESS: Miscellaneous Esoteric Statistical Scripts. 2020. Available online: https://cran.r-project.org/web/packages/MESS/MESS.pdf (accessed on 8 August 2020).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition therapy for adults with diabetes or prediabetes: A consensus report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef] [PubMed]
- Abbie, E.; Francois, M.E.; Chang, C.R.; Barry, J.C.; Little, J.P. A low-carbohydrate protein-rich bedtime snack to control fasting and nocturnal glucose in type 2 diabetes: A randomized trial. Clin. Nutr. 2020, 39, 3601–3606. [Google Scholar] [CrossRef] [PubMed]
- Dyer-Parziale, M. The effect of extend bar containing uncooked cornstarch on night-time glycemic excursion in subjects with type 2 diabetes. Diabetes Res. Clin. Pract. 2001, 53, 137–139. [Google Scholar] [CrossRef]
- Henze, M.; Burbidge, H.; Nathan, E.; Graham, D.F. The effect of bedtime snacks on fasting blood glucose levels in gestational diabetes mellitus. Diabet. Med. 2022, 39, e14718. [Google Scholar] [CrossRef]
- Muggeo, M.; Zoppini, G.; Bonora, E.; Brun, E.; Bonadonna, R.C.; Moghetti, P.; Verlato, G. Fasting plasma glucose variability predicts 10-year survival of type 2 diabetic patients: The Verona Diabetes Study. Diabetes Care 2000, 23, 45–50. [Google Scholar] [CrossRef]
- Edelman, S.; Dailey, G.; Flood, T.; Kuritzky, L.; Renda, S. A practical approach for implementation of a basal-prandial insulin therapy regimen in patients with type 2 diabetes. Osteopath Med. Prim. Care 2007, 1, 9. [Google Scholar] [CrossRef]
- Bowen, K.J.; Sullivan, V.K.; Kris-Etherton, P.M.; Petersen, K.S. Nutrition and Cardiovascular Disease-an Update. Curr. Atheroscler. Rep. 2018, 20, 8. [Google Scholar] [CrossRef]
- Josse, A.R.; Kendall, C.W.C.; Augustin, L.S.A.; Ellis, P.R.; Jenkins, D.J.A. Almonds and postprandial glycemia—A dose-response study. Metab. Clin. Exp. 2007, 56, 400–404. [Google Scholar] [CrossRef]
- Li, S.-C.; Liu, Y.-H.; Liu, J.-F.; Chang, W.-H.; Chen, C.-M.; Chen, C.-Y.O. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metab. Clin. Exp. 2011, 60, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Mattes, R.D. Appetitive, dietary and health effects of almonds consumed with meals or as snacks: A randomized, controlled trial. Eur. J. Clin. Nutr. 2013, 67, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Benefits of nut consumption on insulin resistance and cardiovascular risk factors: Multiple potential mechanisms of actions. Nutrients 2017, 9, 1271. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Rasekhian, M.; Raciti, R.; Silva, A.S.; Annunziata, G.; Arnone, A.; Tenore, G.C.; Süntar, I.; et al. Almonds (Prunus Dulcis Mill. D. A. Webb): A Source of Nutrients and Health-Promoting Compounds. Nutrients 2020, 12, 672. [Google Scholar] [CrossRef]
- Phillips, E.; Rahman, R.; Mattfeldt-Beman, M. Relationship between diabetes knowledge, glycemic control, and associated health conditions. Diabetes Spectr. 2018, 31, 196–199. [Google Scholar] [CrossRef]
- Nielsen, S.J.; Kit, B.K.; Ogden, C.L. Nut Consumption among U.S. Adults, 2009–2010; NCHS data brief, no 176; National Center for Health Statistics: Hyattsville, MD, USA, 2014. [Google Scholar]
- Kinsey, A.W.; Ormsbee, M.J. The health impact of nighttime eating: Old and new perspectives. Nutrients. 2015, 7, 2648–2662. [Google Scholar] [CrossRef]


| Mean | Range | |
|---|---|---|
| Age | 57 | 42–73 years |
| Gender | 6 (60%) female | |
| Duration of diabetes | 5.6 years | 3–15 years |
| Oral Antihyperglycemic Medications | ||
| Yes | 7 (70%) | |
| No | 3 (30%) | |
| Home SMBG prior to study start | 6 (60%) |
| Overnight Interstitial Glucose (12–3 a.m.) | Fasting Interstitial Glucose (4–6 a.m.) | |||
|---|---|---|---|---|
| Condition | Baseline Mean (SD) | Endpoint Mean (SD) | Baseline Mean (SD) | Endpoint Mean (SD) |
| Control | 108 (32.1) mg/dL 6.0 (1.8) mmol/L | 120 (29.1) mg/dL 6.6 (1.6) mmol/L | 107 (23.1) mg/dL 6.0 (1.3) mmol/L | 128 (19.9) mg/dL 7.1 (1.1) mmol/L |
| Almond Butter | 197 (112.6) mg/dL 11.0 (6.2) mmol/L | 186 (107.2) mg/dL 10.3 (5.9) mmol/L | 193 (118.9) mg/dL 10.7 (6.6) mmol/L | 182 (98.1) mg/dL 10.1 (5.4) mmol/L |
| Metric | Overall | Fasting (4–6 a.m.) | Overnight (12–3 a.m.) | Control Week | Almond Butter Week | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range | |
| Mean glucose, mg/dL (mmol/L) | 158 (8.8) | 97–325 (5.4–18.0) | 150 (8.3) | 97–313 (5.4–17.4) | 149 (8.2) | 88–324 (4.9–18.0) | 156 (8.66) | 93–318 (5.15–17.65) | 160 (8.90) | 101–331 (5.60–18.39) |
| Coefficient of Variation (CV) mg/dL (mmol/L) | 20 (1.09) | 9–27 (0.49–1.48) | 3 (0.18) | 0–19 (0–1.07) | 3 (0.17) | 0–25 (0–1.38) | 18 (0.98) | 10–24 (0.53–1.33) | 20 (1.10) | 8–30 (0.45–1.66) |
| Standard Deviation (SD) mg/dL (mmol/L) | 28 (1.57) | 14–39 (0.80–2.17) | 5 (0.25) | 0–48 (0–2.67) | 4 (0.22) | 0–38 (0–2.13) | 26 (1.44) | 11–44 (0.62–2.44) | 28 (1.58) | 16–40 (0.89–2.23) |
| Time in Range (TIR) * | 73.2% | 0–98.71% | 77.6% | 0–100% | 77.2% | 0–100% | 72.74% | 0–98.9% | 72.49% | 0–99.94% |
| Average Number of CGM samples over study period | 3445 | 1826–3926 | - | - | - | - | 1813 | 1635–2008 | 1813 | 1485–2074 |
| Total AUC | - | - | 299 mg/dL × 120 min (16.6 mmol/L × 120 min) | 192.9–623.5 mg/dL × 120 min (10.7–34.6 mmol/L × 120 min) | 444.6 mg/dL × 180 min (24.7 mmol/L × 180 min) | 263.5–965.2 mg/dL × 180 min (14.6–53.6 mmol/L × 180 min) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnston, E.A.; Roque, N.A.; Cole, B.H.; Flanagan, M.P.; Kris-Etherton, P.M.; Petersen, K.S. A Pilot Study of the Effect of Evening Almond Butter Consumption on Overnight and Fasting Interstitial Glucose. Diabetology 2022, 3, 502-513. https://doi.org/10.3390/diabetology3040038
Johnston EA, Roque NA, Cole BH, Flanagan MP, Kris-Etherton PM, Petersen KS. A Pilot Study of the Effect of Evening Almond Butter Consumption on Overnight and Fasting Interstitial Glucose. Diabetology. 2022; 3(4):502-513. https://doi.org/10.3390/diabetology3040038
Chicago/Turabian StyleJohnston, Emily A., Nelson A. Roque, Barbara H. Cole, Michael P. Flanagan, Penny M. Kris-Etherton, and Kristina S. Petersen. 2022. "A Pilot Study of the Effect of Evening Almond Butter Consumption on Overnight and Fasting Interstitial Glucose" Diabetology 3, no. 4: 502-513. https://doi.org/10.3390/diabetology3040038
APA StyleJohnston, E. A., Roque, N. A., Cole, B. H., Flanagan, M. P., Kris-Etherton, P. M., & Petersen, K. S. (2022). A Pilot Study of the Effect of Evening Almond Butter Consumption on Overnight and Fasting Interstitial Glucose. Diabetology, 3(4), 502-513. https://doi.org/10.3390/diabetology3040038







