Thermal Stability and Degradation of Three Similar-Structured Endogenous Estrogens
Abstract
1. Introduction
2. Materials and Methods
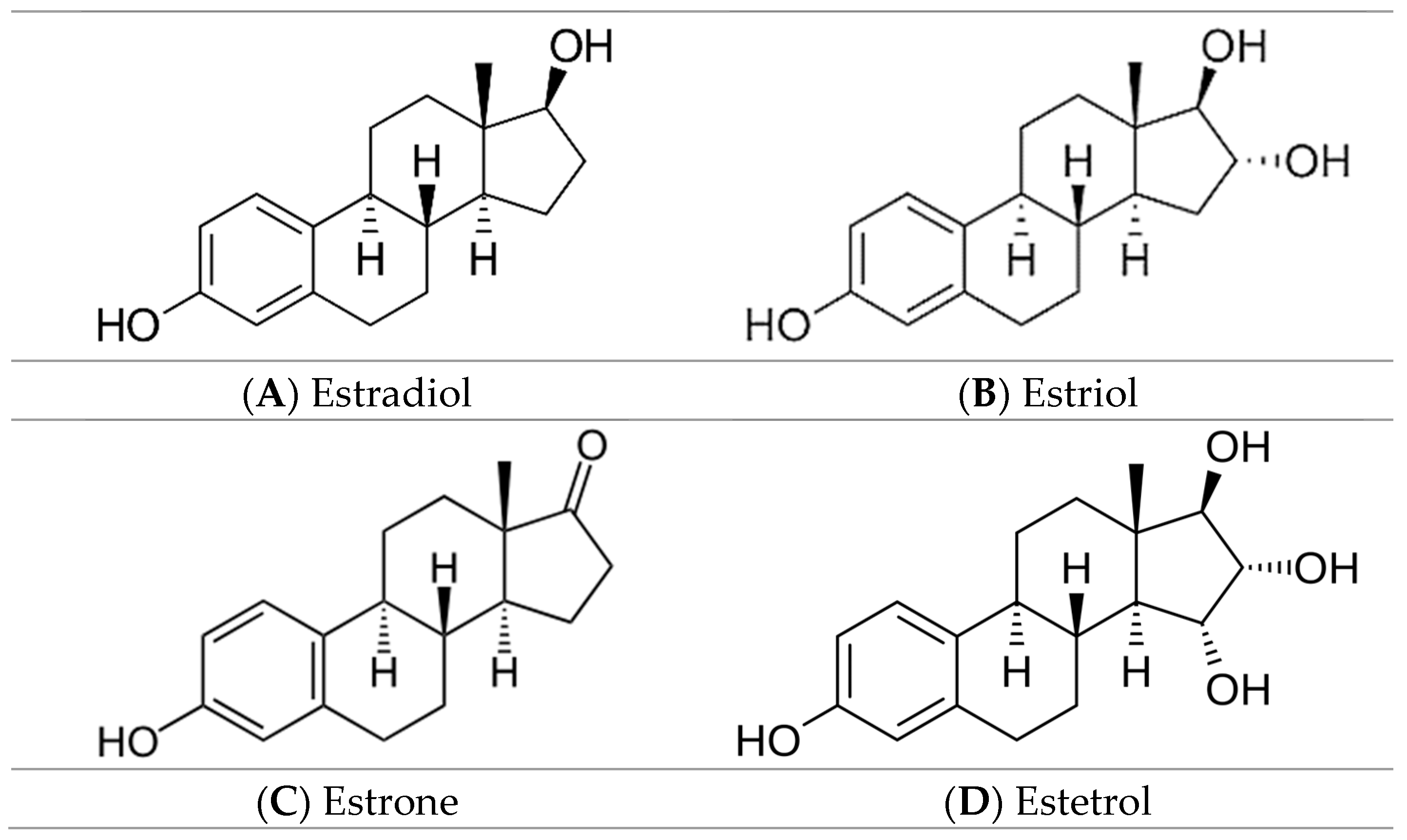
2.1. Samples
2.2. ATR-FTIR Investigations
2.3. Thermal Investigations
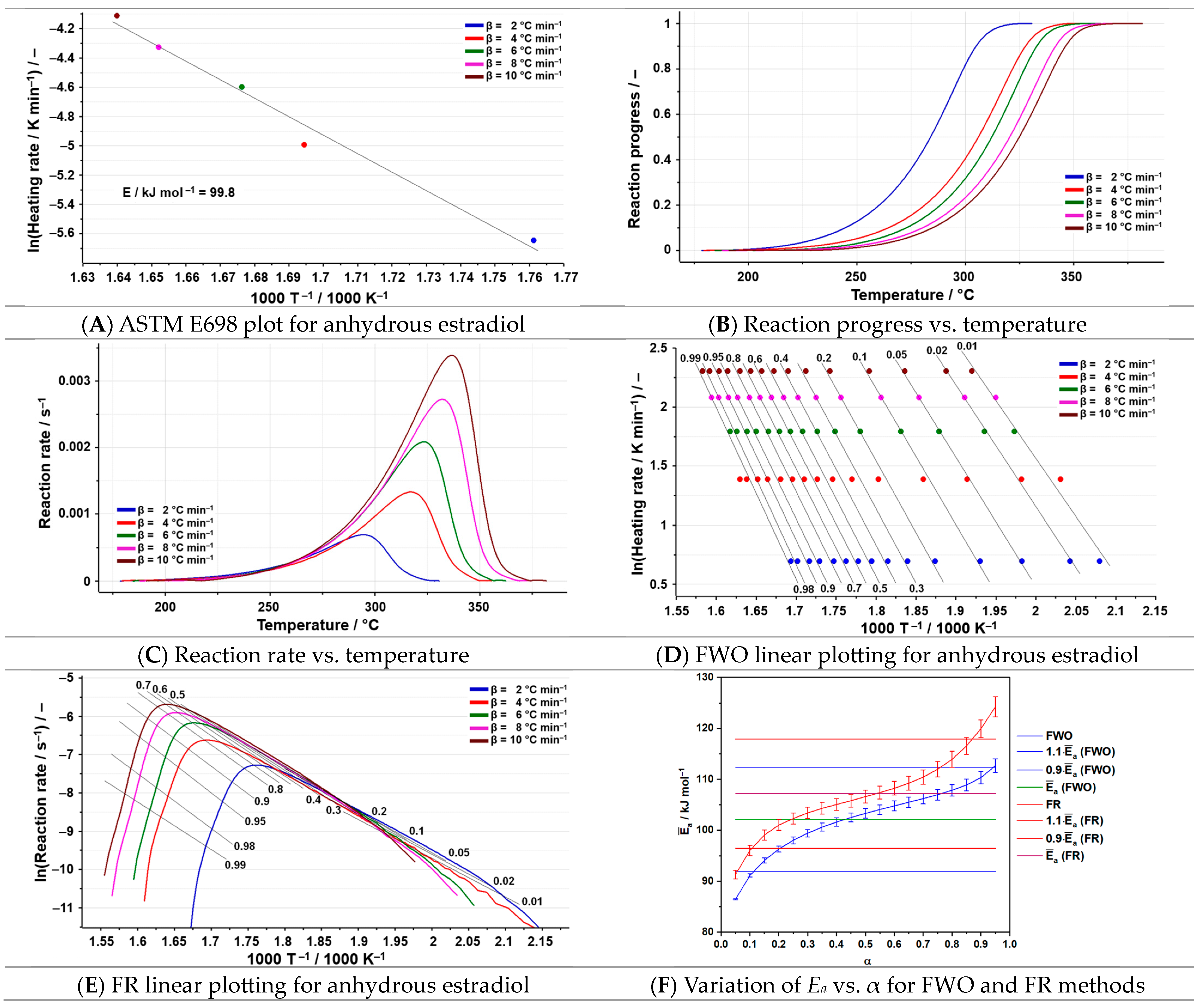
2.4. Kinetic Analysis
3. Results and Discussion
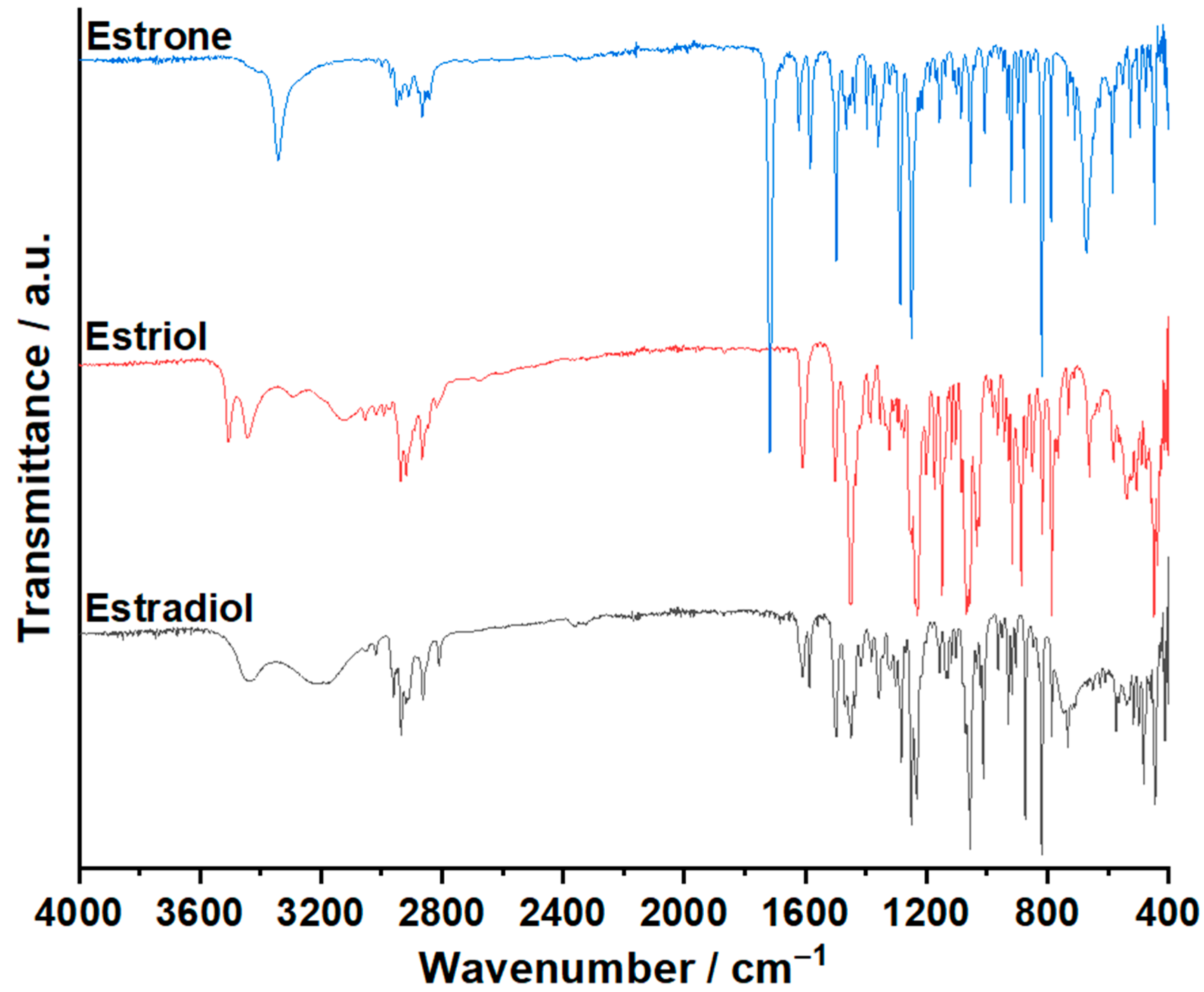
3.1. ATR-FTIR Investigations
3.2. Thermal Investigations
3.3. Kinetic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α | Conversion degree |
| t | Time |
| β | Linear heating rate (°C min−1) |
| A | Pre-exponential factor according to the Arrhenius kinetic model (min−1) |
| k(T) | Temperature-dependent reaction rate function |
| f(α) | Differential conversion function |
| g(α) | Integral conversion function |
| Ea | Activation energy (kJ mol−1) |
| R | Universal gas constant (J mol−1 K−1) |
| r | Reaction rate |
| T | Absolute temperature (K) |
| Δm | Mass loss over a specific temperature interval |
| FWO | Flynn–Wall–Ozawa kinetic method |
| FR | Friedman kinetic method |
References
- Cauley, J.A. Estrogen and Bone Health in Men and Women. Steroids 2015, 99, 11–15. [Google Scholar] [CrossRef]
- Simpkins, J.W.; Singh, M. More than a Decade of Estrogen Neuroprotection. Alzheimer’s Dement. 2008, 4, S131–S136. [Google Scholar] [CrossRef]
- Bustamante-Barrientos, F.A.; Méndez-Ruette, M.; Ortloff, A.; Luz-Crawford, P.; Rivera, F.J.; Figueroa, C.D.; Molina, L.; Bátiz, L.F. The Impact of Estrogen and Estrogen-Like Molecules in Neurogenesis and Neurodegeneration: Beneficial or Harmful? Front. Cell Neurosci. 2021, 15, 636176. [Google Scholar] [CrossRef]
- Scott, E.; Zhang, Q.G.; Wang, R.; Vadlamudi, R.; Brann, D. Estrogen Neuroprotection and the Critical Period Hypothesis. Front. Neuroendocrinol. 2012, 33, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Huang, Z.Y.; Xu, X.L.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen Receptor Function: Impact on the Human Endometrium. Front. Endocrinol. 2022, 13, 827724. [Google Scholar] [CrossRef] [PubMed]
- Stanczyk, F.Z. Metabolism of Endogenous and Exogenous Estrogens in Women. J. Steroid Biochem. Mol. Biol. 2024, 242, 106539. [Google Scholar] [CrossRef]
- Bennink, H.J.C. Are All Estrogens the Same? Maturitas 2004, 47, 269–275. [Google Scholar] [CrossRef]
- Coelingh Bennink, H.J.T.; Holinka, C.F.; Diczfalusy, E. Estetrol Review: Profile and Potential Clinical Applications. Climacteric 2008, 11, 47–58. [Google Scholar] [CrossRef]
- Holinka, C.F.; Diczfalusy, E.; Coelingh Bennink, H.J.T. Estetrol: A Unique Steroid in Human Pregnancy. J. Steroid Biochem. Mol. Biol. 2008, 110, 138–143. [Google Scholar] [CrossRef]
- Chaudhry, H.; Rangra, N.K.; Chawla, P.A. An Insight into the Use of Estetrol-Drospirenone as Effective Oral Contraceptive. Health Sci. Rev. 2023, 6, 100072. [Google Scholar] [CrossRef]
- Eyster, K.M. The Estrogen Receptors: An Overview from Different Perspectives. In Methods in Molecular Biology; Eyster, K.M., Ed.; Springer: New York, NY, USA, 2016; Volume 1366, pp. 1–10. ISBN 978-1-4939-3127-9. [Google Scholar]
- O’Connell, M.B. Pharmacokinetic and Pharmacologic Variation Between Different Estrogen Products. J. Clin. Pharmacol. 1995, 35, 18S–24S. [Google Scholar] [CrossRef]
- Yoh, K.; Ikeda, K.; Horie, K.; Inoue, S. Roles of Estrogen, Estrogen Receptors, and Estrogen-Related Receptors in Skeletal Muscle: Regulation of Mitochondrial Function. Int. J. Mol. Sci. 2023, 24, 1853. [Google Scholar] [CrossRef]
- Orzołek, I.; Sobieraj, J.; Domagała-Kulawik, J. Estrogens, Cancer and Immunity. Cancers 2022, 14, 2265. [Google Scholar] [CrossRef]
- Fruzzetti, F.; Fidecicchi, T.; Guevara, M.M.M.; Simoncini, T. Estetrol: A New Choice for Contraception. J. Clin. Med. 2021, 10, 5625. [Google Scholar] [CrossRef]
- Visser, M.; Coelingh Bennink, H.J.T. Clinical Applications for Estetrol. J. Steroid Biochem. Mol. Biol. 2009, 114, 85–89. [Google Scholar] [CrossRef]
- Ziegler, R.G.; Faupel-Badger, J.M.; Sue, L.Y.; Fuhrman, B.J.; Falk, R.T.; Boyd-Morin, J.; Henderson, M.K.; Hoover, R.N.; Veenstra, T.D.; Keefer, L.K.; et al. A New Approach to Measuring Estrogen Exposure and Metabolism in Epidemiologic Studies. J. Steroid Biochem. Mol. Biol. 2010, 121, 538–545. [Google Scholar] [CrossRef]
- Osman, A.I.; Fawzy, S.; Farrell, C.; Al-Muhtaseb, A.H.; Harrison, J.; Al-Mawali, S.; Rooney, D.W. Comprehensive Thermokinetic Modelling and Predictions of Cellulose Decomposition in Isothermal, Non-Isothermal, and Stepwise Heating Modes. J. Anal. Appl. Pyrolysis 2022, 161, 105427. [Google Scholar] [CrossRef]
- Fawzy, S.; Osman, A.I.; Farrell, C.; Al-Muhtaseb, A.H.; Harrison, J.; Al-Fatesh, A.S.; Fakeeha, A.H.; Doran, J.; Yang, H.; Rooney, D.W. Characterization and Kinetic Modeling for Pyrolytic Conversion of Cotton Stalks. Energy Sci. Eng. 2021, 9, 1908–1918. [Google Scholar] [CrossRef]
- Santana, N.d.S.; Monteiro, S.N.; da Silva, T.C.; Mothé, M.G. Kinetic Study of Commercial Tabletop Sweeteners Using Thermal Analysis. Biol. Life Sci. Forum 2025, 40, 35. [Google Scholar] [CrossRef]
- Sarkar, N.; Kiran, K.; Vijayan, N.; Joshi, D. Overview of Analysis on Thermal Stability and Hirshfeld Surface of Sodium Sulphamate Single Crystals. J. Mater. Sci. Mater. Electron. 2024, 35, 1674. [Google Scholar] [CrossRef]
- Khawam, A.; Flanagan, D.R. Role of Isoconversional Methods in Varying Activation Energies of Solid-State Kinetics: II. Nonisothermal Kinetic Studies. Thermochim. Acta 2005, 436, 101–112. [Google Scholar] [CrossRef]
- Baul, B.; Ledeţi, A.; Cîrcioban, D.; Ridichie, A.; Vlase, T.; Vlase, G.; Peter, F.; Ledeţi, I. Thermal Stability and Kinetics of Degradation of Moxonidine as Pure Ingredient vs. Pharmaceutical Formulation. Processes 2023, 11, 1738. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Maduka, T.O.; Suzuki, M.; Lu, S.; Wang, Q. Thermoanalytical and Kinetic Studies for the Thermal Stability of Emerging Pharmaceutical Pollutants Under Different Heating Rates. J. Xenobiotics 2024, 14, 1784–1806. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N. Interpretation and Physical Meaning of Kinetic Parameters Obtained from Isoconversional Kinetic Analysis of Polymers. Polymers 2020, 12, 1280. [Google Scholar] [CrossRef]
- Wang, B.; Yao, Z.; Reinmöller, M.; Kishore, N.; Tesfaye, F.; Luque, R. Pyrolysis Behavior, Kinetics, and Thermodynamics of Waste Pharmaceutical Blisters under CO2 Atmosphere. J. Anal. Appl. Pyrolysis 2023, 170, 105883. [Google Scholar] [CrossRef]
- Svoboda, R. Thermal Decomposition of Active Pharmaceutical Substances and Accuracy of the Related Kinetic Predictions: The Case of Nifedipine. Thermochim. Acta 2024, 738, 179790. [Google Scholar] [CrossRef]
- Serra, R.; Sempere, J.; Nomen, R. A New Method for the Kinetic Study of Thermoanalytical Data: The Non-Parametric Kinetics Method. Thermochim. Acta 1998, 316, 37–45. [Google Scholar] [CrossRef]
- Brown, M.E.; Maciejewski, M.; Vyazovkin, S.; Nomen, R.; Sempere, J.; Burnham, A.; Opfermann, J.; Strey, R.; Anderson, H.L.; Kemmler, A.; et al. Computational Aspects of Kinetic Analysis. Thermochim. Acta 2000, 355, 125–143. [Google Scholar] [CrossRef]
- Ferrer, N.; Serra, E.; Nomen, R.; Sempere, J. Thermal Decomposition of Tricyclohexylidene Triperoxide. J. Therm. Anal. Calorim. 2018, 134, 1293–1298. [Google Scholar] [CrossRef]
- Serra, R.; Nomen, R.; Sempere, J. The Non-Parametric Kinetics A New Method for the Kinetic Study of Thermoanalytical Data. J. Therm. Anal. 1998, 52, 933–943. [Google Scholar] [CrossRef]
- Vlase, T.; Vlase, G.; Doca, N.; Bolcu, C. Processing of Non-Isothermal TG Data: Comparative Kinetic Analysis with NPK Method. J. Therm. Anal. Calorim. 2005, 80, 59–64. [Google Scholar] [CrossRef]
- Šesták, J.; Berggren, G. Study of the Kinetics of the Mechanism of Solid-State Reactions at Increasing Temperatures. Thermochim. Acta 1971, 3, 1–12. [Google Scholar] [CrossRef]
- Ridichie, A.; Bengescu, C.; Ledeţi, A.; Rusu, G.; Bertici, R.; Vlase, T.; Vlase, G.; Peter, F.; Ledeţi, I.; Rădulescu, M. Thermal Stability, Preformulation, and Kinetic Degradation Studies for Gestrinone. J. Therm. Anal. Calorim. 2024, 150, 6785–6799. [Google Scholar] [CrossRef]
- Ridichie, A.; Ledeţi, A.; Peter, F.; Ledeţi, I.; Muntean, C.; Rădulescu, M. Kinetic Investigation of the Oxidative Thermal Decomposition of Levonorgestrel. Processes 2023, 11, 3210. [Google Scholar] [CrossRef]
- Ledeţi, A.; Baul, B.; Ridichie, A.; Ivan, D.; Vlase, T.; Tomoroga, C.; Dragomirescu, A.; Vlase, G.; Bertici, R.A.; Man, D.E.; et al. Thermooxidation of Four Sartans: Kinetic Analysis Based on Thermo-Gravimetric Data. Molecules 2024, 29, 5527. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Achilias, D.; Fernandez-Francos, X.; Galukhin, A.; Sbirrazzuoli, N. ICTAC Kinetics Committee Recommendations for Analysis of Thermal Polymerization Kinetics. Thermochim. Acta 2022, 714, 179243. [Google Scholar] [CrossRef]
- Koga, N.; Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Muravyev, N.V.; Pérez-Maqueda, L.A.; Saggese, C.; Sánchez-Jiménez, P.E. ICTAC Kinetics Committee Recommendations for Analysis of Thermal Decomposition Kinetics. Thermochim. Acta 2023, 719, 179384. [Google Scholar] [CrossRef]
- PubChem. Estradiol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Estradiol (accessed on 11 May 2025).
- Abdella, S.; Afinjuomo, F.; Song, Y.; Upton, R.; Garg, S. 3D Printed Bilayer Mucoadhesive Buccal Film of Estradiol: Impact of Design on Film Properties, Release Kinetics and Predicted in Vivo Performance. Int. J. Pharm. 2022, 628, 122324. [Google Scholar] [CrossRef]
- PubChem. Estriol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Estriol (accessed on 11 May 2025).
- Xu, J.; Ning, L. Study of Estriol Cocrystals. Her. Med. 2022, 41, 630–636. [Google Scholar] [CrossRef]
- Both, D. Estrone. In Analytical Profiles of Drug Substances and Excipients; Florey, K., Ed.; Analytical Profiles of Drug Substances; Academic Press: Cambridge, MA, USA, 1983; Volume 12, pp. 135–189. [Google Scholar]
- PubChem. Estrone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Estrone (accessed on 11 May 2025).
- Moodley, K.; Rarey, J.; Ramjugernath, D. Experimental Solubility for Betulin and Estrone in Various Solvents within the Temperature Range T = (293.2 to 328.2) K. J. Chem. Thermodyn. 2016, 98, 42–50. [Google Scholar] [CrossRef]
- Fisher, A.C.; Lee, S.L.; Harris, D.P.; Buhse, L.; Kozlowski, S.; Yu, L.; Kopcha, M.; Woodcock, J. Advancing Pharmaceutical Quality: An Overview of Science and Research in the U.S. FDA’s Office of Pharmaceutical Quality. Int. J. Pharm. 2016, 515, 390–402. [Google Scholar] [CrossRef]
- Yu, L.X.; Kopcha, M. The Future of Pharmaceutical Quality and the Path to Get There. Int. J. Pharm. 2017, 528, 354–359. [Google Scholar] [CrossRef]
- Capen, R.; Christopher, D.; Forenzo, P.; Ireland, C.; Liu, O.; Lyapustina, S.; O’Neill, J.; Patterson, N.; Quinlan, M.; Sandell, D.; et al. On the Shelf Life of Pharmaceutical Products. AAPS PharmSciTech 2012, 13, 911–918. [Google Scholar] [CrossRef] [PubMed]
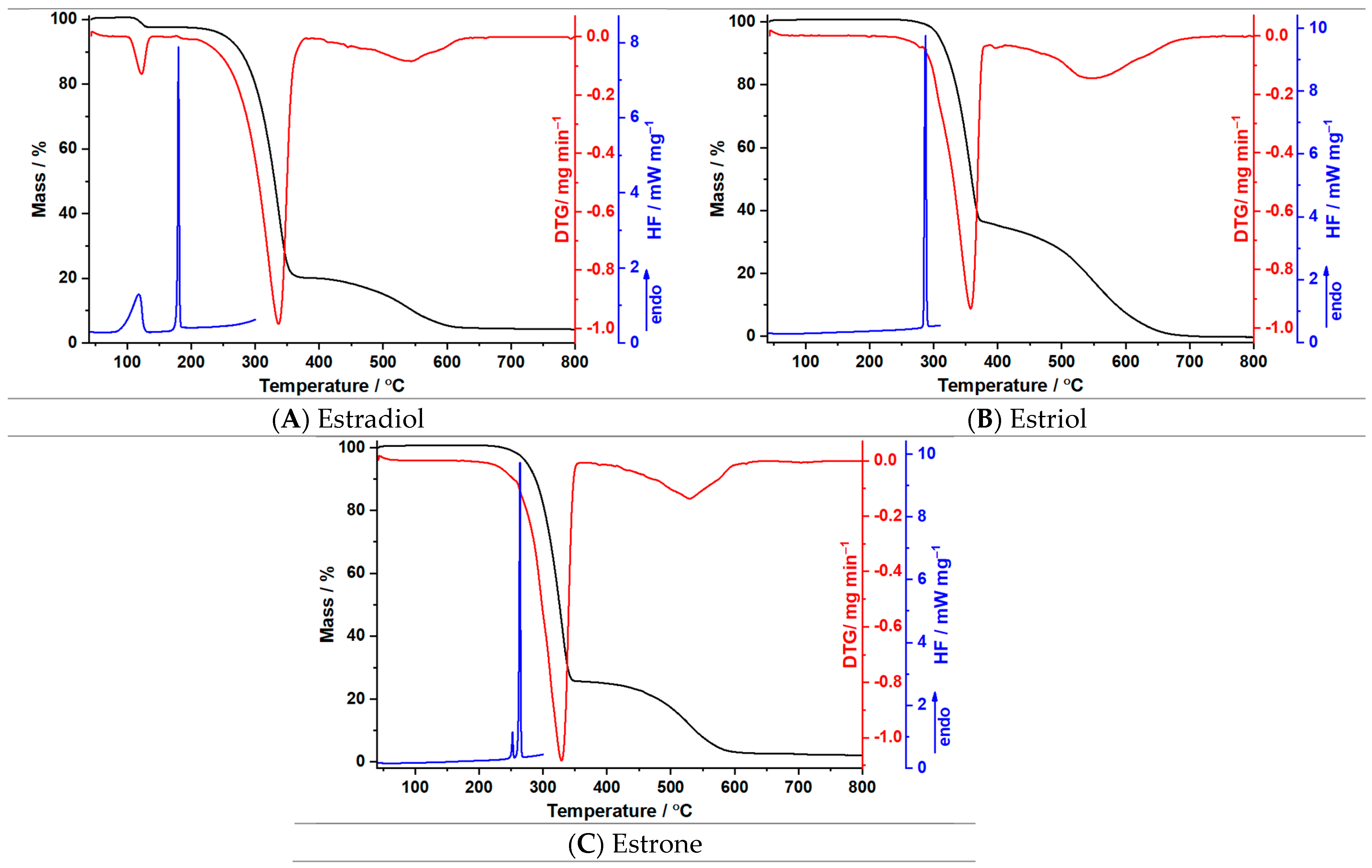
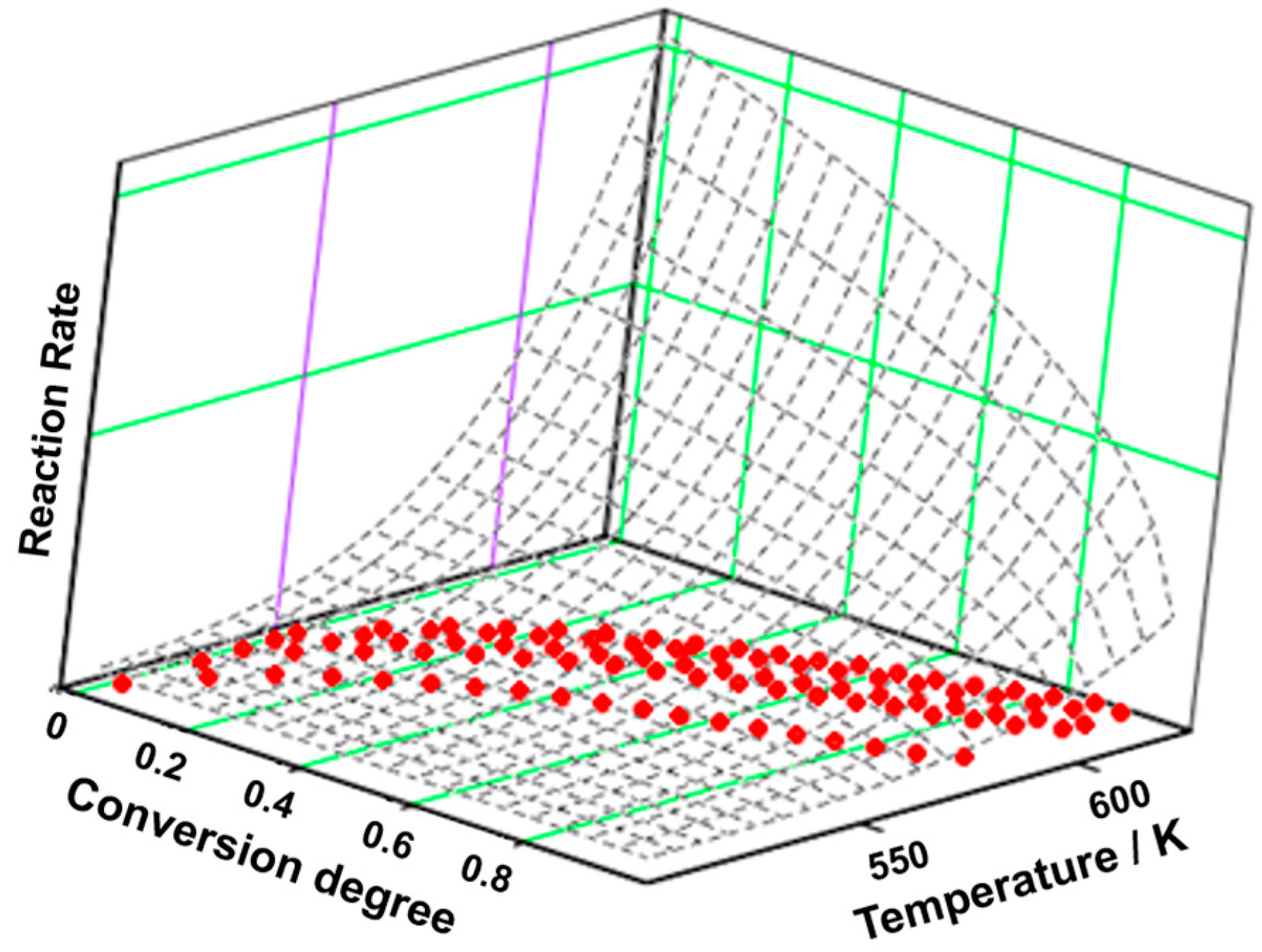
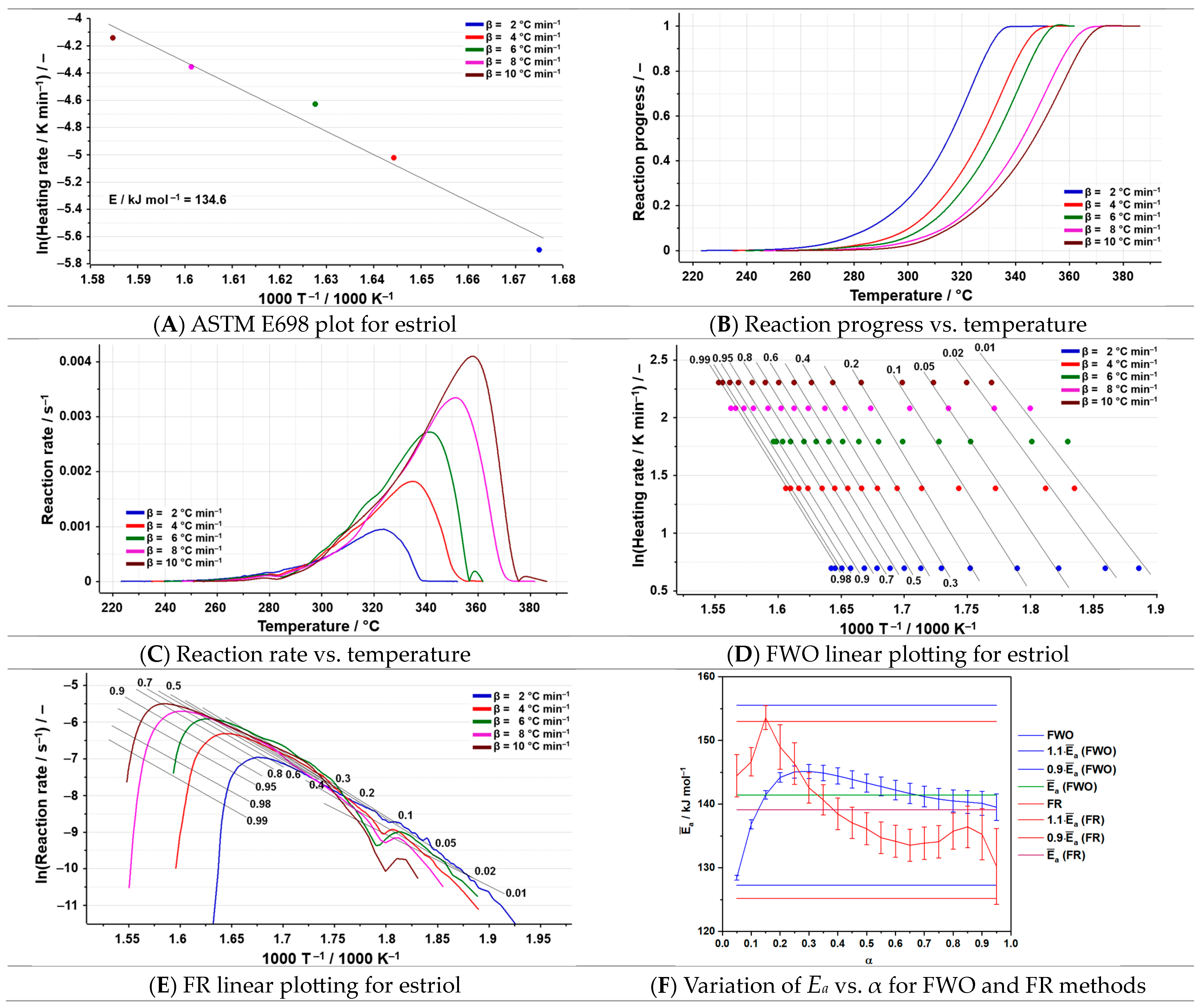

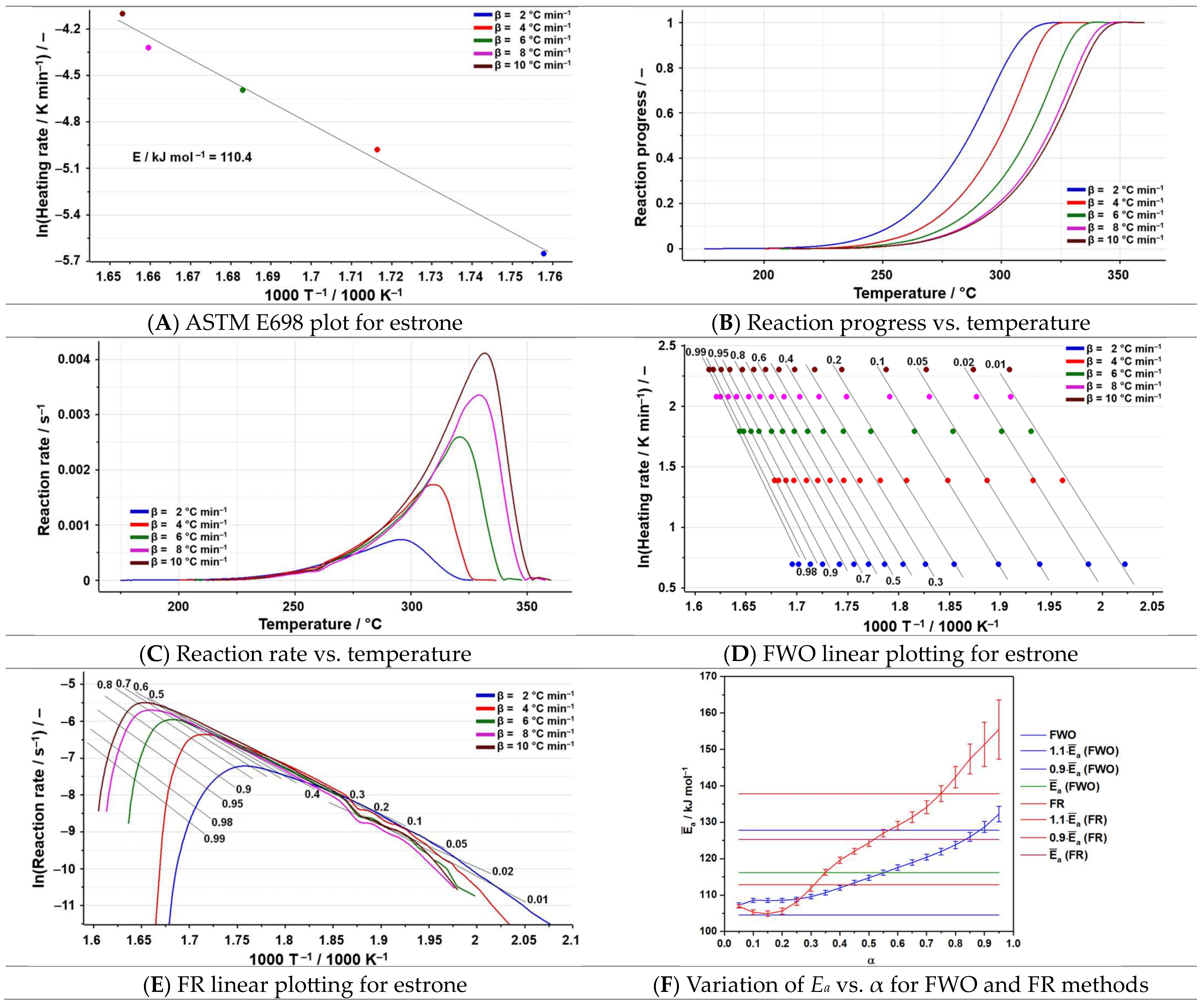
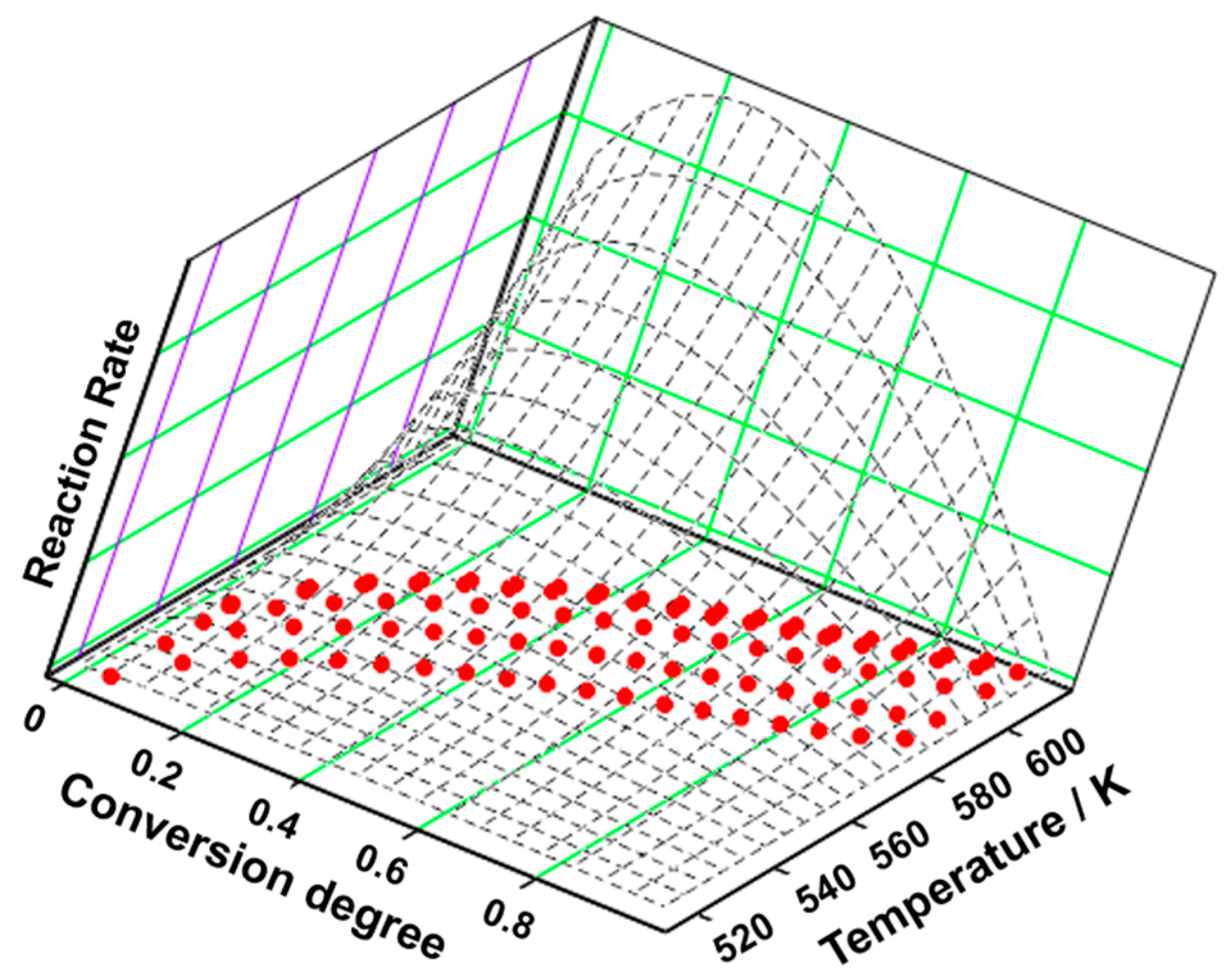
| Sample | Process | Tonset (°C) | Toffset (°C) | Tmax DTG (°C) | Δm (%) | Tmax HF (°C) | ΔH (J g−1) |
|---|---|---|---|---|---|---|---|
| Estradiol | I | 103 | 136 | 122 | 3.0 | 118 180 | 117.4 117.1 |
| II | 191 | 382 | 336 | 77.6 | |||
| III | 403 | 642 | 540 | 15.3 | |||
| Estriol | I | 251 | 386 | 358 | 65.0 | 287 | 183.2 |
| II | 402 | 712 | 547 | 35.1 | |||
| Estrone | I | 212 | 384 | 332 | 75.3 | 252 264 | 10.7 135.4 |
| II | 384 | 617 | 549 | 21.7 |
| β (°C min−1) | The Temperature Range for the Selected Process (°C) | ||
|---|---|---|---|
| ESTRADIOL | ESTRIOL | ESTRONE | |
| 2 | 179–331 | 223–352 | 175–327 |
| 4 | 179–355 | 235–362 | 200–337 |
| 6 | 185–362 | 240–362 | 207–348 |
| 8 | 188–374 | 247–382 | 211–358 |
| 10 | 191–382 | 251–386 | 212–360 |
| Ea (kJ mol−1) vs. α For | ||||||
|---|---|---|---|---|---|---|
| ESTRADIOL | ESTRIOL | ESTRONE | ||||
| α | FWO | FR | FWO | FR | FWO | FR |
| 0.05 | 86.5 ± 0.1 | 91.3 ± 0.9 | 128.4 ± 0.4 | 144.5 ± 3.3 | 107.2 ± 0.7 | 106.9 ± 0.5 |
| 0.10 | 91.1 ± 0.3 | 96.0 ± 1.0 | 136.8 ± 0.7 | 146.6 ± 2.2 | 108.6 ± 0.6 | 105.3 ± 0.6 |
| 0.15 | 94.1 ± 0.5 | 99.1 ± 1.0 | 141.5 ± 0.7 | 153.6 ± 1.9 | 108.5 ± 0.5 | 104.9 ± 0.8 |
| 0.20 | 96.3 ± 0.6 | 101.0 ± 1.0 | 144.2 ± 0.7 | 149.0 ± 3.5 | 108.5 ± 0.5 | 105.6 ± 0.9 |
| 0.25 | 98.0 ± 0.7 | 102.3 ± 1.1 | 145.1 ± 1.0 | 146.4 ± 3.3 | 108.8 ± 0.6 | 108.1 ± 0.9 |
| 0.30 | 99.4 ± 0.7 | 103.4 ± 1.1 | 145.1 ± 1.1 | 142.6 ± 2.4 | 109.5 ± 0.6 | 111.9 ± 0.9 |
| 0.35 | 100.6 ± 0.8 | 104.3 ± 1.1 | 144.9 ± 1.2 | 140.7 ± 2.4 | 110.6 ± 0.6 | 116.3 ± 0.8 |
| 0.40 | 101.6 ± 0.8 | 105.1 ± 1.2 | 144.4 ± 1.3 | 138.5 ± 2.5 | 112.0 ± 0.6 | 119.6 ± 0.8 |
| 0.45 | 102.5 ± 0.9 | 105.8 ± 1.2 | 143.9 ± 1.4 | 137.1 ± 2.5 | 113.4 ± 0.7 | 122.0 ± 0.8 |
| 0.50 | 103.3 ± 0.9 | 106.6 ± 1.2 | 143.3 ± 1.4 | 136.1 ± 2.5 | 114.7 ± 0.7 | 124.3 ± 0.9 |
| 0.55 | 104.1 ± 0.9 | 107.4 ± 1.3 | 142.8 ± 1.5 | 134.7 ± 2.5 | 116.1 ± 0.7 | 127.0 ± 1.0 |
| 0.60 | 104.8 ± 1.0 | 108.2 ± 1.3 | 142.2 ± 1.5 | 134.2 ± 2.5 | 117.5 ± 0.7 | 129.1 ± 1.2 |
| 0.65 | 105.5 ± 1.0 | 109.2 ± 1.4 | 141.7 ± 1.6 | 133.6 ± 2.5 | 118.9 ± 0.8 | 131.4 ± 1.5 |
| 0.70 | 106.2 ± 1.0 | 110.4 ± 1.4 | 141.2 ± 1.6 | 133.9 ± 2.5 | 120.4 ± 0.8 | 134.1 ± 1.8 |
| 0.75 | 107.0 ± 1.1 | 112.0 ± 1.5 | 140.8 ± 1.7 | 134.1 ± 2.6 | 122.0 ± 0.9 | 137.9 ± 2.2 |
| 0.80 | 107.8 ± 1.1 | 113.9 ± 1.6 | 140.5 ± 1.7 | 135.8 ± 2.8 | 123.8 ± 1.0 | 142.4 ± 2.9 |
| 0.85 | 108.9 ± 1.2 | 116.5 ± 1.6 | 140.3 ± 1.8 | 136.4 ± 3.3 | 126.0 ± 1.2 | 147.4 ± 4.1 |
| 0.90 | 110.3 ± 1.2 | 120.0 ± 1.7 | 140.1 ± 1.9 | 135.3 ± 4.0 | 128.7 ± 1.5 | 151.4 ± 6.1 |
| 0.95 | 112.7 ± 1.3 | 124.2 ± 2.0 | 139.5 ± 2.1 | 130.2 ± 6.0 | 132.2 ± 2.1 | 155.5 ± 8.2 |
| a (kJ mol−1) | 102.1 ± 3.9 | 107.2 ± 5.8 | 141.4 ± 6.1 | 139.1 ± 13.2 | 116.2 ± 4.0 | 125.3 ± 12.2 |
| Sample | Process | λ/% | Ea/kJ mol−1 | A/min−1 | n | m | R2 | f(α) | a (kJ mol−1) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NPK | FWO | FR | |||||||||
| Estradiol | 1 | 74.9 | 103.0 ± 1.8 | 1.2 × 109 ± 2.0 × 108 | 2/5 | 0 | 0.996 | (1 − α)2/5 | 99.4 ± 2.2 | 102.1 ± 3.9 | 107.2 ± 5.8 |
| 2 | 22.5 | 99.1 ± 0.3 | 7.3 × 108 ± 1.8 × 104 | 0 | 1 | 0.998 | α | ||||
| Sample | Process | λ/% | Ea/kJ mol−1 | A/min−1 | n | m | R2 | f(α) | a (kJ mol−1) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NPK | FWO | FR | |||||||||
| Estriol | 1 | 85.6 | 134.0 ± 1.9 | 1.9 × 1015 ± 9.5 × 106 | 1 | 1/3 | 0.988 | (1 − α) × α1/3 | 140.0 ± 1.9 | 141.4 ± 6.1 | 139.1 ± 13.2 |
| 2 | 13.9 | 189.0 ± 0.8 | 9.8 × 1014 ± 3.4 × 102 | 1 | 1/3 | 0.971 | (1 − α) × α1/3 | ||||
| Sample | Process | λ/% | Ea/kJ mol−1 | A/min−1 | n | m | R2 | f(α) | a (kJ mol−1) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NPK | FWO | FR | |||||||||
| Estrone | 1 | 81.7 | 113.0 ± 1.7 | 8.6 × 1012 ± 9.2 × 106 | 1 | 1/3 | 0.979 | (1 − α) × α1/3 | 115.0 ± 1.8 | 116.2 ± 4.0 | 125.3 ± 12.2 |
| 2 | 17.9 | 126.0 ± 0.1 | 6.3 × 1010 ± 2.0 × 102 | 1 | 1/2 | 0.984 | (1 − α) × α1/2 | ||||
| Sample | k (298.15 K) | t90 (min) | t90 (years) |
|---|---|---|---|
| Estradiol | 4.6 × 10−9 | 3.8 × 105 | 0.71 |
| Estriol | 5.6 × 10−10 | 3.1 × 106 | 5.79 |
| Estrone | 6.1 × 10−8 | 1.7 × 106 | 3.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ridichie, A.; Ledeţi, A.; Bengescu, C.; Sbârcea, L.; Bertici, R.A.; Ivan, D.L.; Vlase, G.; Vlase, T.; Peter, F.; Ledeţi, I. Thermal Stability and Degradation of Three Similar-Structured Endogenous Estrogens. Analytica 2025, 6, 52. https://doi.org/10.3390/analytica6040052
Ridichie A, Ledeţi A, Bengescu C, Sbârcea L, Bertici RA, Ivan DL, Vlase G, Vlase T, Peter F, Ledeţi I. Thermal Stability and Degradation of Three Similar-Structured Endogenous Estrogens. Analytica. 2025; 6(4):52. https://doi.org/10.3390/analytica6040052
Chicago/Turabian StyleRidichie, Amalia, Adriana Ledeţi, Cosmina Bengescu, Laura Sbârcea, Răzvan Adrian Bertici, Denisa Laura Ivan, Gabriela Vlase, Titus Vlase, Francisc Peter, and Ionuţ Ledeţi. 2025. "Thermal Stability and Degradation of Three Similar-Structured Endogenous Estrogens" Analytica 6, no. 4: 52. https://doi.org/10.3390/analytica6040052
APA StyleRidichie, A., Ledeţi, A., Bengescu, C., Sbârcea, L., Bertici, R. A., Ivan, D. L., Vlase, G., Vlase, T., Peter, F., & Ledeţi, I. (2025). Thermal Stability and Degradation of Three Similar-Structured Endogenous Estrogens. Analytica, 6(4), 52. https://doi.org/10.3390/analytica6040052














