Abstract
Nuts such as pecans, almonds, peanuts, pistachios, and walnuts are nutrient-dense foods rich in unsaturated fatty acids and antioxidant compounds. Their regular consumption has been linked to significant health benefits, including reduced risks of cardiovascular disease, diabetes, and high cholesterol. With increasing global demand, ensuring the quality of nuts before they reach consumers is critical. Conventional quality assessment methods dominate the industry but are often subjective, destructive, time-intensive, environmentally burdensome, and laborious. Therefore, there is an urgent need for rapid, non-destructive, and objective alternatives capable of meeting modern quality standards. In this systematic review, we summarize traditional approaches for evaluating nut quality parameters and introduce hyperspectral imaging as a novel technique with promising applications. We examine its use in detecting nut adulteration, assessing chemical composition, identifying defects, and evaluating other quality traits. Limitations of hyperspectral imaging in industrial settings are also discussed, along with potential solutions and future directions. Given the relatively limited research area, approximately 44 relevant studies were critically reviewed. This work provides valuable insights for researchers and industry stakeholders developing innovative technologies for nut quality assessment.
1. Introduction
Nuts are exceptionally rich in nutrients, particularly unsaturated fatty acids and other phytochemical compounds (e.g., protein, dietary fiber, essential minerals, vitamin E, and plant sterols), that are associated with elevated health benefits, including heart diseases, diabetes, and cholesterol [1,2]. Generally, nuts contain a high content of fat, ranging from 46% to 76%, and provide 20 to 30 kJ/g of energy [3]. However, some nuts, such as chestnuts, possess a low-fat content [2,4]. Notably, nuts are one of the major natural plant-based foods rich in fat and vegetable oils. Moreover, the acid composition of nuts is beneficial due to their low (about 4–16%) saturated fatty acid content (SFA), with half of the fat content consisting unsaturated [5]. Notably, nuts are available in both in-shell and shelled forms. In-shell nuts are primarily intended for fresh consumption, whereas shelled nuts are eaten raw, roasted, or used as additives in various processed food products. Overall, the unique nutritional composition and versatility of nuts make them an important component of a balanced diet and a valuable raw material for the food industry.
Conventionally, manual inspection by experts, who classify nuts based on color and the incidence of defects, is a common prevailing approach in the industry [6]. However, the approach has inherent drawbacks, such as a lack of precision and the possibility of including underdeveloped nuts in the batch, which lowers the market value. Moreover, this approach can only be used to assess the exterior quality of the in-shell nuts, while the interior (kernel) quality parameter may not be assessed. Indeed, examining the quality parameters of nuts in the industry required rigorous assessment which includes quantification of moisture, peroxide value (PV), fatty acid, protein content, and acidity index (AI) [7], which cannot be evaluated manually by visual inspection. Although chemical analyses are widely utilized to assess nut quality parameters, such approaches are time-consuming, chemically intensive, environmentally unfriendly, and costly when used for evaluating nut quality on a commercial basis [8,9]. Moreover, the destructive analysis of PV and AI requires careful data collection from representative samples to evaluate the quality of nuts. Additionally, the manual sorting and inspection of shelled and in-shell nuts can be subjective, consume time, and require experts [10]. Therefore, traditional analyses are labor-intensive and invasive and may result in lower-quality nuts due to quality variance within a consignment. Therefore, there is a need to develop a quick, reliable, and non-invasive approach to assess nut quality.
Hyperspectral imaging (HSI) has emerged as a promising tool for assessing the quality of nuts in the processing industry [11]. It is a non-destructive technique for evaluating food quality by integrating spectroscopy with digital imaging [12]. It offers detailed insights into nuts’ composition and characteristics by acquiring a wide range of spectral information and subtle changes in their chemical and physical properties, that are invisible to the human eye [13]. Another key application of HSI in the nut processing industry is the detection and defect classification, such as cracks, insect damage, and mold, which could affect the overall quality and market value [14,15]. HSI integrated with machine learning techniques can distinguish between healthy and damaged nuts by analyzing their unique spectral signature which could allow producers to sort and remove defective samples more efficiently. In addition, HSI combined with machine learning could facilitate nut maturity assessment (e.g., in peanuts) without removing exocarp of the hull [16], which is achieved by estimating the changes in spectral characteristics associated with the physiological state of nuts (or pods) which are relevant to determine the harvesting time. Similarly, HSI can help optimize post-harvesting practices and ensure that premium quality nuts are sorted in the processing line. Hence, such a capability is invaluable for quality control as it allows producers to monitor product consistency and ensure compliance with regulatory requirements. Therefore, this paper provides a systematic review on hyperspectral imaging for nut assessment in the nut processing industry. We surveyed the development of hyperspectral imaging and its application in nut adulteration, chemical composition, defect evaluation, and quality traits, and discussed the challenges and future trends of technology. This review will be beneficial to research communities in exploiting HSI technology to address challenges in nut processing industries.
2. Methods
In this systematic review, the PRISMA (preferred reporting items for systematic and meta-analysis) guideline was implemented [17], which is an evidence framework that enables systematic reviewers to transparently report the purpose of the review, what was carried out by the authors, and the findings. The focused questions, the search strategies and databases, and the article selection (inclusion and exclusion) criteria utilized followed the PRISMA guideline. The searches were meticulously focused on the various degrees of the quality assessment (physical and chemical) of the nuts using hyperspectral imaging with machine learning, which reported well documented information on accuracy, correlation coefficient (R2), ratio of prediction to deviation (RPD), and root mean square (RMSE).
Strategy
In this study, the literature was sourced from four major databases: Google Scholar, ScienceDirect, IEEE Xplore, and Web of Science. To refine search results, Boolean operators (‘AND’ and ‘OR’) were employed. Initially, individual keyword searches were conducted using terms such as “hyperspectral imaging in pecans”, “hyperspectral imaging in pistachios”, “hyperspectral imaging in macadamias”, “hyperspectral imaging in walnuts”, “hyperspectral imaging in almonds”, “hyperspectral imaging in peanuts”, “hyperspectral imaging in chestnuts”, and “hyperspectral imaging in hazelnuts”. Furthermore, the study explored various applications of hyperspectral imaging in nut processing, including adulteration detection, chemical composition analysis, defect assessment, and other related applications. To expand the search scope, Boolean operators were strategically applied: similar keywords were combined using “AND”, while different keyword groups were connected with “OR”. This approach generated comprehensive search strings such as “hyperspectral imaging in pecans AND hyperspectral imaging in nut adulteration”, “hyperspectral imaging in pecans AND hyperspectral imaging in nut chemical composition”, and “hyperspectral imaging in hazelnut nuts AND defect assessment”. From this approach, 116 articles were gathered. However, some of the articles were not relevant to this study and were discarded using the PRISMA guideline (see Figure 1). After the screening, which includes inclusion and elimination, 44 articles remained which were used in this current study.
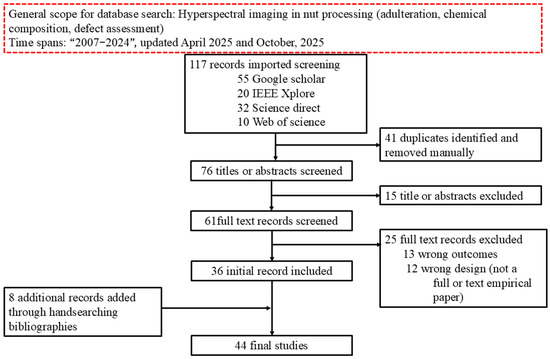
Figure 1.
The flowchart of the PRISMA guideline utilized for screening of the articles used in this review.
3. Nut Quality Parameters
Nut rancidity, color changes, mold and kernel shrinkage, and many more are the key nut defects that contribute to nut rejection by consumers, thereby lowering the market value of the produce in the global market (see Table 1) [18,19]. Notably, the defects are influenced by changes in chemical composition. Moreover, nuts possess certain attributes that make them susceptible to some degradation in their quality. For instance, nuts contain a high fat content of approximately 50–75% [20] and have a high unsaturated to saturated fatty acid ratio, which has been reported to increase the rate of oxidation [21]. However, due to high fat content, nuts are susceptible to rancidity, color changes, and off flavor when subjected to environmental stress such as nut storage at high temperature [22]. Moisture content could also affect the quality of nuts in improper storage [23]. For instance, improper storage of nuts with excess moisture could create an abode for microbial growth and fungi [24]. The fungi secretion could also result in the production of aflatoxin contamination, which is carcinogenic in nature [25]. Because of health concerns that may arise from consuming unhealthy and defective nuts, there is a need for the quality assessment of nuts.

Table 1.
Summary of quality parameters for quality assessment of nuts and the potential for HSI application.
In the nut processing industry, conventional techniques are the prevailing methods utilized to evaluate quality for premium delivery to consumers. The standard techniques involve determining the quality parameters, including the assessment of lipid oxidation level via peroxide value and thiobarbituric reactive substance assay (TBARS) [26]. They serve as quality indicators to examine the nut rancidity. The peroxide value is the indicator for the primary lipid oxidation (an early marker of oxidation deterioration). It determines the degree of fat oxidation and its tendency for rancidity [27], while TBARS serves as a secondary indicator of lipid oxidation. Oil yield in nuts’ kernels is extracted using four methods: mechanical pressing, subcritical fluid extraction, solvent extraction method, and aqueous enzymatic extraction methods. Among all, mechanical pressing is the oldest method for oil recovery and is effective with oil content yield above 20% [28], and it is beneficial owing to its low cost, lack of extreme temperature and pressure, and no solvent addition conditions [29], which contribute to preserving flavor and thermolabile compounds such as tocopherols and other phenolics. In addition, traditional approaches (manual or visual) combined with automated RGB-color inspection are commonly used for defect detection, such as sorting shells from the pulp or kernels [30,31]. Although the traditional approaches have demonstrated satisfactory outcomes in the industry, they are labor-intensive, consume time, subjective, and destructive to the sample, and they may not be able to meet the high demand of nut quality of consumers. Therefore, it is of utmost importance to develop a rapid, non-destructive, and efficient approach (such as hyperspectral imaging system) for nut quality assessment.
4. Overview of Hyperspectral Imaging
The HSI is a swift and non-destructive technique that seamlessly integrates spectroscopy with conventional digital imaging [32]. The conventional digital imaging provides spatial information of a sample, but it cannot reveal information about any component that absorbs or scatters light at wavelengths other than RGB. In contrast, spectroscopy cannot reveal spatial information about a sample but can reveal information about its chemical composition at specific wavelengths (spectral band). Therefore, integrating the two techniques enables the possibility of linking spectral data with spatial dimensions. Thus, it allows the mapping of sample chemical components. This integration results in a three-dimensional data cube (hypercube), where each slice corresponds to an image at a specific waveband. The HSI system comprises some essential components, including an illumination source that illuminates the object, a lens for accurate focus and field of view modification, a spectrograph to disperse light into distinct spectral wavelengths, a 2-D camera for capturing spatial–spectral images [33]. In addition, there is a computer with software for storing and visualization of the captured HSI images while further preprocessing, modeling, and decision making are also carried out on the computer. Hyperspectral imaging systems frequently use visible near-infrared (VNIR) (400–1000 nm), near-infrared (NIR) (900–1700 nm), and shortwave infrared (SWIR) (900–2500 nm) wavelengths for various applications, owing to sensor availability [34]. The hyperspectral imaging system captured a three-dimensional (3-D) image also known as a hypercube (x, y, λ) with spatial and spectral dimensions. The hypercube (x, y, λ) can be viewed as either a grayscale image at each wavelength or a spectrum at each pixel, conveying unique information about the object being studied. In the hypercube data, x is the horizontal spatial dimension which denotes the number of pixels across the width of the image and y is the vertical dimension that connotes the number of pixels along the height of the image while λ is the spectral dimension which represents the wavelength or bands captured by the hyperspectral sensor. The similarity of the photographs of nearby bands differs from that of distant bands, which may contain independent information. Hyperspectral imaging is useful for complete object analysis because no single wavelength image can fully convey an object’s properties.
4.1. Spectral Profile Analysis
Spectral analysis forms the foundation for hyperspectral imaging exploration. The spectral bands within the images often exhibit strong correlations, leading to intricate high-dimensional data that may be redundant, noisy, or contain irrelevant information [35]. The extraction of meaningful insights from such huge datasets necessitates meticulous analysis [36]. Multivariate data analysis techniques, such as chemometrics, play a pivotal role in correlating spectral data with target attributes to accurately visualize the sample distribution. Preprocessing steps, such as baseline corrections and the Savitzky–Golay filter to estimate derivatives, normalization, and scaling, are commonly employed to improve interpretability [37]. Among the multivariate data analyses, partial least squares regression (PLSR) is a widely utilized linear multivariate calibration technique owing to its effectiveness in handling collinear, noisy, and redundant data. However, in the case of nonlinearity, nonlinear methods such as artificial neural networks (ANNs) and support vector machine regression (SVR) are preferred [38]. Overall, proper reporting of calibration and prediction statistics, including the standard error of prediction (SEP) and coefficient of determination (R2), is essential for evaluating repeatability and accuracy in model development. Figure 2 shows a step-by-step guide on hyperspectral imaging analysis. The subsection below discusses some common machine learning methods implemented for nut quality assessment.
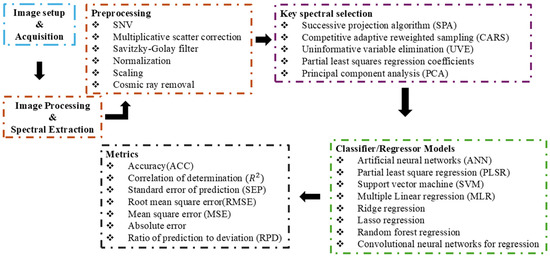
Figure 2.
Step-by-step guide on hyperspectral imaging analysis [37,38].
4.2. Artificial Intelligence in Nut Quality Assessment
As previously discussed in Section 3 and Section 4, the quality assessment of nuts (e.g., almonds, walnuts, pecans) is influenced by various factors, including moisture content, lipid oxidation, and the presence of contaminants such as fungi and aflatoxins. Conventional methods for assessing the quality attributes are often destructive to the sample or through manual inspection, which can be time-consuming and inconsistent. Recent advancements in artificial intelligence (AI), particularly through the integration of HSI and machine learning (ML) techniques, have revolutionized the field by enabling rapid, non-destructive, and accurate quality assessment of nuts. Some of the ML and deep learning methods implemented for the quality assessment of nuts are discussed as follows.
4.2.1. Machine Learning
Partial Least Squares Regression
As previously mentioned, PLSR is the most used chemometric technique for the calibration of visible-NIR [39]. PLSR has the benefit of tackling datasets with high dimensionality, multicollinearity, and small sample sizes in Vis-NIR by extracting the underlying peaks as latent variables [40]. Thereafter, the maximum covariance was extracted between the latent variables. The approach reduces noise and the extraction of essential information from the data. Thus, PLSR overcomes the problem of overfitting and other problems with high-dimensional data. Owing to its benefits, PLSR has been implemented as a statistical tool for the assessment of nut quality in the industry. For instance, Mohammadi Moghaddam et al. [41] examined the influence of hot-air roasting temperature on the textural and sensory properties of pistachio nuts and kernels. Overall, PLSR analysis of the instrumental and sensory data revealed a significant correlation between the objective and subjective properties. Zhao et al. [42] utilized PLSR to predict the contamination concentration of peanuts and walnuts in whole flour. Overall, a good standing R2 of 0.987 and RMSEP were achieved, which demonstrated the efficacy of the PLSR model in nut quality assessment. This result demonstrated the potential of building PLSR models for nut adulteration.
Artificial Neural Networks
Artificial neural networks have gained ample attention for the quality assessment of nuts owing to their capacity to handle complex, nonlinear relationships within the data. ANNs are employed to predict various quality attributes, such as size, weight, moisture content, oil content, and texture parameters, based on the input features, for example, physical characteristics and chemical composition. ANNs consist of interconnected nodes organized in layers, including the input, hidden, and output layers. Click or tap here to enter text.The network learns from the input–output patterns in the training data through a process called backpropagation, adjusting the connection weights iteratively to minimize the prediction error. ANNs can handle diverse datasets with numerous variables, capturing the intricate relationships that traditional statistical methods may overlook. Overall, the application of ANNs in nut quality assessment enhances the accuracy, efficiency, and automation of the evaluation process, contributing to better quality control, product optimization, and decision making in the nut industry. Khosa and Pasero [43], used ANNs as a classifier for quality inspection of pistachio and pine with false negative rates of 0% and 6.8% achieved for pine and pistachio, respectively. In another study, Ros and Pasero [44] implemented a functional link neural network to sort pistachio nuts to distinguish unhealthy nuts from healthy nuts. Overall, an accuracy of 99.6% was achieved. In addition, Ganganagowdar and Siddaramappa [45] utilized an ANN to grade white whole cashew kernels into four classes with an accuracy of 88.93%. The results achieved while implementing ANNs as a classifier for the quality assessment of nuts indicated the efficacy of the model in yielding an objective assessment in the nut processing industry.
Support Vector Machine
The support vector machine is another commonly used nonlinear model for the quality assessment of nuts in the industry. SVM is a robust method for assessing the quality of nuts, leveraging its adeptness in handling complex data and nonlinear relationships [46]. The data could be the size, color, texture, and defects obtained from samples of an image. Preprocessing methods can be adopted on the data to refine, clean any inconsistencies, and normalize features for uniformity. Feature selection or extraction techniques are then applied to identify the most influential attributes in determining nut quality. SVM is usually trained on segmented data (2-class) where it discerns patterns and establishes a hyperplane that optimally segregates different quality categories while maximizing the margins between them [47,48]. Parameter tuning, which is crucial for SVM optimization, involves selecting appropriate kernel functions and regularization parameters using techniques such as cross-validation. SVM has the capacity to handle complex data and is resistant to overfitting when properly regularized. Therefore, SVM stands out as a powerful tool for nut quality assessment because it provides reliability and efficiency for detecting quality changes among nut varieties. Khosa and Pasero [49] utilized ANNs and SVMs as classifiers to differentiate between healthy and unhealthy pine nuts, with accuracies of 87.6% and 86.3%, respectively. In another study, Onaran et al. [50] used an SVM as a classifier to differentiate between underdeveloped hazelnuts and developed hazelnuts with an accuracy of 97%. The results show the robustness and reliability of SVM in the quality assessment of nuts.
4.2.2. Deep Learning Models
Deep learning (DL), a branch of machine learning, employs multi-layered neural networks to learn hierarchical data representations, thereby enabling analysis and interpretation of complex data [51]. DL utilizes ANNs inspired by the structure and function of the human brain. The main advantage of DL is its self-feature extraction capability from raw data, thereby eliminating the handcrafted feature extraction approach as required in traditional machine learning. DL could identify and represent relevant patterns from data. DL models develop hierarchical representations of data. The first layers extract simple features such as the edges of an image, and the next layers combine the features to identify more complex patterns such as shapes or objects. DL model training requires large amounts of data during which the network iteratively adjusts its weights and biases through a process called backpropagation [52]. The optimization technique minimizes the error between predicted and actual outputs, quantified by a loss function. DL models typically demand substantial computational resources and large data to perform effectively, prompting the adoption of specialized hardware, such as graphic processing units (GPUs), to speed up both training and interference [53]. Some of the DL models that could be used in nut quality assessments are discussed below.
Convolutional Neural Network
A convolutional neural network (CNN) consists of multiple layers that utilize convolution operations to input data. The operation utilizes filters or kernels to detect features such as edges, textures, and patterns [54]. As the filter scans across the input image, it creates feature maps which emphasize the important parts of the data as regions of interest (e.g., edges, texture, and patterns). Following the convolution layer, a pooling (sub-sampling) layer is introduced. The primary role of pooling is to reduce the number of trainable parameters and to provide translation variance. This is achieved by selecting a window and applying a pooling function to the input elements within it, which produces a new output vector. Several pooling methods exist (e.g., average pooling and max pooling). Max pooling is the most utilized pooling method due to its strong effectiveness in minimizing feature maps. Notably, during backpropagation, the error is not propagated to the winning unit, since it does not contribute to the forward pass. After the convolution and pooling stages, the process advances to the fully connected layer. At this stage, the feature maps produced earlier are flattened and passed as input, where a dot product between the weight vector and the input vector is calculated to generate the final output. Figure 3 depicts the structural representation of a convolutional neural network.
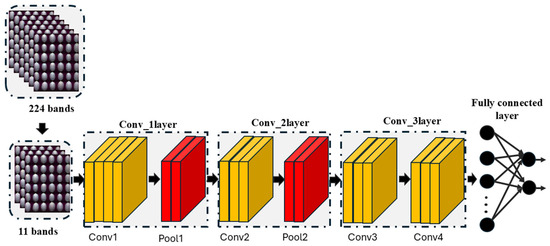
Figure 3.
Structural representation of convolutional neural network.
Recurrent Neural Network
Recurrent neural networks (RNNs) are deep learning models that can identify patterns in sequential data. It retains information from previous inputs through feedback connections [55,56]. The RNN comprises an input layer, a hidden layer, and an output layer [57]. In contrast to the feedforward neural networks, RNN has recurrent connections (memory mechanism), as depicted in Figure 4. This memory mechanism enables RNNs to capture temporal dependencies, making them effective for applications such as time-series forecasting. However, conventional RNNs face challenges such as vanishing and exploding gradients, which limit their ability to learn long-term dependencies [58]. To overcome the issues, advanced variants such as Long Short-term Memory (LSTM) networks and Gated Recurrent Units (GRUs) were introduced, offering improved performance in sequence modeling. More recently, attention-based architectures such as Transformers have emerged, further advancing sequential data processing by enabling parallelization and capturing long-term dependencies more effectively. Figure 4 shows the architecture representation of the recurrent neural network. In Olaniyi et al. [59], hybrid CNN–LSTM was integrated with hyperspectral data (VNIR and NIR) to intelligently sort pecan shelled materials (shell, kernel, and inner-wall) with satisfactory results achieved. The model was further improved by implementing a fusion system using the VNIR and NIR-HSI data built on CNN–CNN–LSTM. The results achieved demonstrated the efficacy of implementing recurrent neural networks for nut quality assessment.
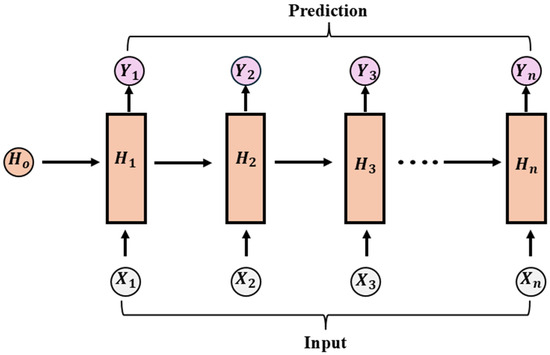
Figure 4.
Architectural representation of the basic recurrent network.
Transformer Neural Network
The transformer neural network is a form of deep learning algorithm that analyzes sequential data and images [60]. It handles data in parallel utilizing self-attention, which is different from RNN and CNN, which use recurrent and convolution layers. This allows for more efficient collection of long-range dependencies and contextual interactions. A transformer has essential components such as embedded layers, positional encoding layers, fully connected layers, multi-head self-attention layers, and layer normalization. The self-attention layer ensures that the model weighs the importance of each element in the input sequence relative to others, effectively learning contextual representations without being constrained by sequence order, while positional encoding compensates for the lack of inherent sequence order by allowing the model to identify the relative positions of the input elements. Transformers can be implemented as only encoder, decoder, or encoder–decoder configurations depending on the task. Transformers have demonstrated superior performance in a variety of tasks, from machine translation and text generation to image classification. The transformer neural network can quickly evaluate enormous amounts of data and can also handle multiple types of input. This has made transformer models a key part of modern deep learning research and applications. Figure 5 shows the structural representation of transformer neural networks.
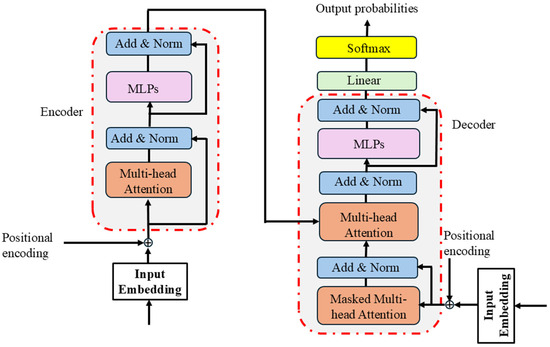
Figure 5.
Structural representation of transformer (input embedding, positional encoding, encoder, and decoder).
4.3. Application of Hyperspectral Imaging in Nut Quality Assessment
Hyperspectral imaging provides a diverse approach to nut quality assessment across multiple domains, such as nut adulteration detection, identification of nut defects (e.g., cracks, mold, broken kernels, and insect damage), nut chemical composition analysis, and assessment of quality characteristics. In the case of nut adulteration, hyperspectral imaging distinguishes between authentic nuts and adulterants by recording spectral signatures specific to each component [61]. This capacity contributes to maintaining product integrity and ensures consumer confidence. Furthermore, HSI aids in the detection of nut defects, such as cracks, broken kernels, and insect damage, by identifying small differences in spectral patterns related to the defects [62]. Producers can improve product quality while reducing waste by properly identifying defects. Furthermore, HSI offers insights into nut chemical composition by quantifying essential components such as moisture, oil, and protein content via spectral analysis [63]. This information is critical for optimizing the processing procedures and achieving quality requirements. Finally, HSI enables the assessment of other quality traits of the nuts, such as size, color, and maturity, by linking spectral data to the desired properties. Using HSI, stakeholders in the nut sector could improve quality control methods, verify product authenticity, and meet consumer expectations effectively. The next subsections discussed the applications of hyperspectral imaging in different areas of nut quality assessment in the industry.
4.3.1. Hyperspectral Imaging for Nut Adulteration Assessment
Food fraud adulteration is a long-standing problem. Food fraud, characterized by the intentional misrepresentation of food products, poses significant challenges to consumers and the food industry [64]. Food fraud is a growing concern among the authorities, the food sector, and consumers. It is primarily driven by economic gain and has the potential to harm customer health, leading to a high level of distrust in the food supply chain and a major economic impact [65,66]. One of the most common forms of fraud is adulteration, addition, or replacement of a component to produce less expensive products. So, to put an end to the long-standing fraudulence in the industry, HSI has become a necessary tool and has gained acceptance (Table 2).
Netto et al. [67] explored three infrared spectroscopies (NIR) with spectral wavelengths (900–1700 nm, 950–1650 nm, and 1350–2500 nm) to verify the authenticity of almond flour. In this study, 54 different varieties of almond flours were adulterated with low-cost flours (made from oats, cutuaba, guarana powder, nuts, peanuts, wheat protein, all in unknown proportions). Soft independent modeling of class analogies (SIMCA), data-driven SIMCA, and one-class partial least squares were used for classification, whereas PLSR was utilized to authenticate the sample. A sensitivity of 100% and specificity of 95% were achieved in the classifier model, while the PLS model achieved a coefficient of determination (R2) of 0.90 and an RMSE between 3.2% and 4.8%. The results demonstrated that NIR spectroscopy is viable for the detection of adulteration and authentication of almond flours. However, an important wavelength selection approach can be employed to improve the model’s performance. Faqeerzada et al. [61] investigated the use of shortwave infrared hyperspectral imaging (SWIR-HSI) to authenticate almond powder. By imaging two almond varieties adulterated with apricot and peanut powder at varying concentrations, this study employs a one-class classifier technique called data-driven soft independent modeling of class analogy (DD-SIMCA) for analysis. The results showed high sensitivity (100%) and specificity (89–100%) for different sets of adulterated samples using DD-SIMCA. Additionally, a partial least squares regression (PLSR) model predicted adulterant concentrations, yielding low error rates (<1%) for apricot adulteration but higher (2.5% to 4.4%) for peanut adulteration. This study demonstrated that SWIR-HSI combined with DD-SIMCA can provide effective high-throughput screening for the potential adulteration of almond powder. Similarly, Ref. [68] investigated the efficacy of the NIR-HSI system (900–1700 nm) to detect 448 almonds adulterated with the apricot kernel (see Figure 6). The spectra from the HSI images were extracted and developed using PLS-DA coupled with a preprocessing method. PLS-DA achieved an accuracy of 97% on the full spectral spectrum, while 85% accuracy was achieved based on a pixel-wise classification approach. In Mishra et al. [69] the NIR-HSI system (1000–2500 nm) was used to detect and quantify peanut traces in wheat flour. Spectral features were extracted from the HSI images and principal component analysis (PCA) was applied to reduce redundant information. In addition, a standard normal variate and Savitzky–Golay smoothing were employed to eliminate global differences in the spectra. Overall, a coefficient of determination (R2 = 0.946) was achieved. Zhao et al. [70] investigated the potential of the Vis-NIR HSI system (935.61–1720.23 nm) to detect low-level peanut contamination in two types of whole flour (spring wheat flour and winter wheat flour). The spectral features were extracted from the HSI images, and standard normal variate with Savitzky–Golay were employed as preprocessing techniques, while competitive adaptive reweighted sampling (CARS) was employed to select key wavelengths developed on PLSR. Overall, the coefficients of determination (R2) were 0.993 and 0.991, and root mean square errors were 0.251% and 0.285%, respectively, for contaminated spring wheat flour and winter wheat flour. The results demonstrated the efficacy of the NIR-HSI technique in detecting low levels of peanut powder in wheat flour. However, further research is required to determine the location of contamination. Furthermore, Vega-Castellote et al. [71] utilized two hyperspectral imaging systems (VNIR and SWIR) to investigate the cross-contamination of peanuts in chopped nut products (hazelnuts, almonds, and walnuts). Two strategies were implemented (Strategy I: spectral of individual pixel, while Strategy II: mean spectrum of each individual nut piece). PLS-DA models were developed based on the two strategies and accuracies of 98.3% and 99.8% were achieved using Strategy I, while 100% was achieved using Strategy II. While HSI systems demonstrated potential in detecting adulteration, a better spectral extraction strategy is required to obtain the effective and efficient spectral that could have a great benefit to the models for prediction. In summary, HSI integrated with chemometric or machine learning models consistently outperforms conventional NIR in adulteration detection. However, there are still challenges with scaling, spectral feature selection, and real-world application.
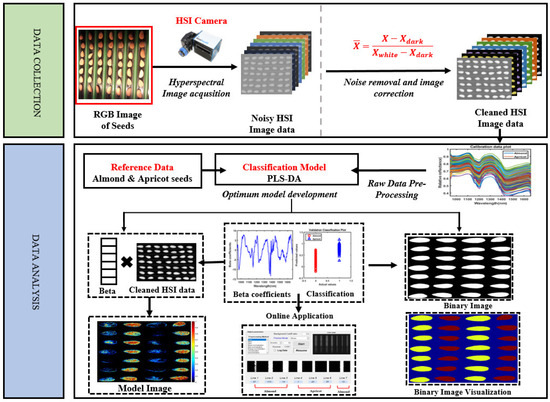
Figure 6.
An example of a schematic representation for the hyperspectral imaging application in nut adulteration [68].

Table 2.
Summary of hyperspectral imaging for assessment of nut adulteration.
Table 2.
Summary of hyperspectral imaging for assessment of nut adulteration.
| Product | Sample No. | Appl. | Spectral Range (nm) | Model | Perf. | Reference |
|---|---|---|---|---|---|---|
| Almond flour | 124 | Adulteration | 900–1700 950–1650 1350–2500 | PLSR | R2 ≥ 0.90 Acc = 100% | [67] |
| Almond | 448 | Adulteration | 900–1700 | PLS-DA | Acc = 85% | [68] |
| Almond powder and apricot powder | NA | Adulteration | 900–2494 | DD-SIMCA PLSR | Acc = 100%, R2 = 0.99 | [61] |
| Whole wheat flour | Adulteration | 950–1700 | R2 = 0.987 | [42] | ||
| Peanut + wheat flour | 11 | Adulteration | 1000–2500 | Pixel-wise | R2 = 0.946 | [69] |
| Peanut | Adulteration | 935.61–1720.23 | PLSR | R2 = 0.993 RMSE = 0.991 | [70] | |
| Pine nuts | 63 | Identification of chemical distribution and composition | 940–1625 | SIMCA | Acc = 84–100% | [72] |
| Peanut, hazelnut, almond, and walnut | 800 | Cross-contamination | 419–1007 and 842–2532 | PLS-DA | Acc = 98.3%, 99.8% 100% | [71] |
4.3.2. Hyperspectral Imaging for Assessment of Nut Chemical Composition
Nuts are nutritionally beneficial for a healthy diet; however, their high unsaturated oil content renders them susceptible to rancidity. The nutrient content of nuts is an essential aspect of their health benefits [73]. However, evaluating both the rancidity and nutrient content of nuts consumes time and is costly [74]. Therefore, HSI systems have recently gained ample attention in the nut processing industry for the rapid and non-destructive evaluation of nuts (see Table 3). For instance, Han et al. [75] explored the potential of the HSI system (388.9–1005.33 nm) to estimate the quality level of Canarium indicum based on PV. In this study, 2300 sub-images of 289 C. indium were acquired using the HSI system. The spectral features were extracted and developed on a CNN with an overall accuracy of 93.48% and R2 of 0.67. This study demonstrates the potential of combining deep learning with spectral information to estimate PV levels in nuts. However, further studies are required to determine how to improve the regression model. Panda et al. [76] used the NIR-HSI system (900–1700 nm) to determine the moisture content and rancidity (free fatty acid and peroxide value (PV)) in 589 almond kernels. Samples (354 and 235) were treated with moisture and rancidity, respectively. Extracted spectral features were developed on PLSR with R2 values of 0.957, 0.970, and 0.955 for moisture content, free fatty acid, and PV, respectively. Thereafter, a competitive adaptive reweighted sampling method (CARS) was employed to select the key wavelength and built on multiple linear regression. An overall R2 of 0.941, 0.903, and 0.886 for MC, FFA, and PV, respectively, was achieved. The full spectrum achieved better model performance than the selected features. This could be due to the potential of PLSR in terms of regression ability compared to MLR. The authors could have used the same regression model to better compare the results.
Moreover, Zhang et al. [77] evaluated kernel protein content in 30 walnuts based on two HSI systems (863–1704 nm and 382–1027 nm). In this study, an improved whale optimized algorithm (IWOA) was proposed to reduce redundancy in the data. Thereafter, eight (8) key wavelengths combined with textural features were developed on SVM, BPNN, and RF, with RF achieving the best R2 of 0.8537 with an RMSE of 11.1383 g/kg. Nogales-Bueno et al. [78] explored the efficacy of the NIR-HSI system (900–1700 nm) to evaluate total fat, MUFA, and PUFA in 200 samples of five varieties of walnuts (see Figure 7). The extracted spectral profiles were built on modified PLSR with a standard error prediction of 2.12% for PUFA and 13.08% for MUFA. The results demonstrate that the NIR-HSI system is suitable for the evaluation of total fat, MUFA, and PUFA. However, further studies are required to estimate tocopherols and triglycerides using this system. Mohammadi-Moghaddam et al. [79] utilized a visible/NIR-HSI system (400–1000 nm) to develop a calibration model for predicting the moisture content and textural characteristics (e.g., hardness, fracture force, and compressive energy) of pistachio kernels roasted at different temperatures. Several preprocessing techniques were implemented on the extracted spectral profile and then built on PLSR and ANN. Overall, ANN achieved the highest result compared with PLSR, with an R2 of 0.957. The results demonstrated the efficacy of the Vis/NIR HSI system in predicting the moisture content and textural characteristics.
In addition, Tahmasbian et al. [80] utilized the Vis/NIR-HSI system (400–1000 nm) to predict the rancidity (PV and FFA) in 390 samples comprising Canarium’s (blanched and unblanched) and macadamia. The spectra extracted from the HSI images were developed using SVC and PLSR. Overall, an accuracy of 87% and R2 of 0.60–0.76 were achieved for predicting PV and FFA. The results demonstrate that Vis/NIR-HSI has the potential to predict mixed nuts. However, there is a need to improve the model’s performance. This could be achieved by increasing the number of samples and employing deep learnin models. In another study, Bai et al. [7] investigated the potential of the HSI system (400–1000 nm) to predict the rancidity of two batches of Canarium indicum kernels based on PV. Key spectral features were selected using the β-coefficient to discard irrelevant information and develop the key spectral on PLSR for rancidity prediction. The PLSR model achieved a strong prediction (R2 = 0.72 for PV in the first batch and R2 values of 0.81, 0.80, 0.75, 0.51, 0.81, 0.71, 0.76, and 0.62 for total nitrogen, iron, potassium, magnesium, manganese, sulfur, and zinc, respectively, for the second batch). These results demonstrated the efficacy of the HSI in predicting the chemical composition of nuts. Generally, robust models with improved results are essential for rapid, accurate, and effective assessments in the nut processing industry.
In another study, Ezenarro et al. [81] investigated the potential of the NIR-HSI system to monitor the hazelnut oxidation in plastic bags under different atmospheric conditions (air, nitrogen, and vacuum). The result achieved shows time as the main oxidation driver, while atmosphere and light significantly impact the oxidation rate. It was observed that vacuum storage reduces the oxidation while light under ambient conditions elevates oxidation through photo-oxidation. Indeed, NIR-HSI demonstrated itself as a reliable tool for evaluating hazelnut quality. Zhao et al. [82] utilized two HSI (VNIR and NIR) systems in combination with low-level (LLF) and mid-level fusion (MLF) strategies to predict indicators across storage periods (6 and 18 months) and classify walnuts. Three models were developed (PLSR, PSO–SVR, and RF) after preprocessing and key wavelength selection with of 0.8706 was achieved with MLF on RF, while the LLF strategy achieved an improved of 0.9694 on PSO–SVR combined with UVE–CARS, and the RF achieved a storage period classification accuracy of 100%. Thus, the results demonstrated the efficacy of fusing hyperspectral data for the quality assessment of nuts.
In summary, the investigations have shown that HSI can be utilized to monitor rancidity, moisture, protein, lipid composition, and storage stability in nuts (see Figure 7). The conventional machine learning models such as PLSR and SVM demonstrated a strong prediction capacity, while integration of deep learning, feature selection algorithms, and data fusion strategies have helped to enhance the accuracy and robustness across nut varieties and quality indicators. However, most studies are constrained by small sample sizes, controlled laboratory conditions, and variabilities in model transferability across nuts types and storage environments. Consequently, subsequent research must focus on gathering huge, diverse data and building strong, generalizable models that can be used in real time in industry. Ultimately, HSI demonstrated strong potential to transform nut quality monitoring, ensuring both product safety and consumer confidence.
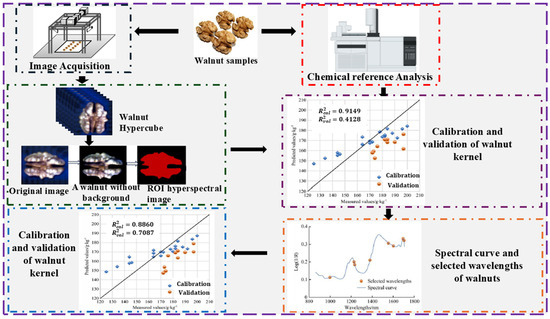
Figure 7.
Overview of nut chemical composition analysis [77,78,82].

Table 3.
Summary of hyperspectral imaging for assessment of nut chemical composition.
Table 3.
Summary of hyperspectral imaging for assessment of nut chemical composition.
| Product | Sample No. | Appl. | Spectral Range (nm) | Model | Perf. | Reference |
|---|---|---|---|---|---|---|
| Canarium | 2300 | Quality estimation | 388.9−1005.33 | CNN | Acc = 93.48% R2 = 0.67 | [75] |
| Almond | 354 and 235 | Moisture content and rancidity estimation | 900−1700 | PLSR and MLR | Full spectral: R2 = 0.957, 0.97, 0.955 CARS selected spectral: R2 = 0.941, 0.903, 0.886 | [76] |
| Walnut | 200 | Evaluation of fat, MUFA, PUFA | 900−1700 | MPLSR | SEP = 2.12% for PUFA SEP = 13.08% for MUFA | [78] |
| Walnut | 30 | Assessment of walnut kernel protein content | 863−1704 and 382−1027 | RF | R2 = 0.8537, RMSE = 11.1382 g/kg | [77] |
| Pistachio kernel | Moisture content and textural properties prediction | 400−1000 | PLSR and ANN | Best model: ANN: R2 = 0.957 | [79] | |
| Canarium and macadamia | 390 | Prediction of PV and FFA | 400−1000 | SVC, PLSR | Acc = 87%, R2 = 0.60–0.76 | [80] |
| Canarium | 107 | Prediction of PV, nitrogen, and mineral nutrients | 400−1000 | PLSR | [7] | |
| Hazelnut | 216 | Prediction of oxidation | 1000−1600 | ASCA PLSR | R2 = 0.78 | [81] |
| Walnut | 150 | Prediction of fat content, acid value, and storage time | 400−1000 and 900−1700 | RF PSO–SVR | R2 = 0.8706 and 0.9694 Acc = 100% | [82] |
4.3.3. Hyperspectral Imaging for Defect Evaluation in Nuts
In the nut processing industry, fine broken kernels are commonly detected using mesh screening. Similarly, other defects, including insect damage and malformations, often require manual picking [83]. Such approaches consume time, are subjective, and are labor-intensive. However, the HSI technique has recently gained considerable attention in the industry. The HSI technique is a rapid, non-destructive, and objective method for assessing defects in nuts (see Table 4).
Xu et al. [84] utilized VNIR (370–1042 nm) to detect mildew in 120 walnuts. The spectra from the HSI images were pre-processed using SNV and extracted using PCA. The extracted spectral data were built on SVM, and the overall accuracy was obtained. The results obtained demonstrate the efficacy of HSI spectral analysis with SVM; however, more machine learning approaches can be used to validate the results. In addition, a spectral selection approach can be employed to improve the performance of the model. In another study, Nakariyakul and Casasent [62] utilized the HSI system (700–1400 nm) to detect 454 internally damaged almond nuts (mission variety almond) from healthy ones. In this study, fast ratio feature selection was employed to discard bands with similar information. Thereafter, the selected spectra were built using an SVM with a Gaussian kernel. Overall, an accuracy of 91.2% was achieved. This study demonstrated the significance of selecting key features for model development. However, more effort is required to improve the performance of the model. To improve the model performance, Ref. [85] implemented an adaptive branch and bound (ABB) algorithm to select key spectral data from almond nuts captured with the HSI system (700–1400 nm). Thereafter, the SVM was developed using a Gaussian kernel on the selected spectra. An improved accuracy of 94.2% was achieved. This shows that the ABB algorithm is more effective than the previously used fast ratio feature selection. In addition, Chen et al. [83] explored the potential of the Vis-HSI system (400–1000 nm) to distinguish insect-damaged almonds from undamaged kernels and assess almond freshness (Figure 8). To achieve an optimal performance, key bands were selected from the extracted spectral signatures of the HSI images. One- and two-dimensional CNNs were combined to allow the concurrent extraction of spectral and spatial characteristics for insect damage identification. PCA was implemented and the first 30 bands were obtained as the vital bands for freshness assessment. Overall, the identification model achieved an accuracy of 99.05%, whereas the freshness evaluation model achieved an accuracy of 97.68%. This study introduces precision techniques for defect identification and freshness assessment in the almond industry.
Bonifazi et al. [86] explored the SWIR-HSI system (1000–2500 nm) to detect contaminants among pistachio products with six classes (edible, inedible, shells, husks, twigs, and stones). In this study, PLS-DA, PCA–DA, PCA–kNN, and Classification and Regression Tree (CART) were the multivariate classifiers employed. Overall, the best accuracy, between 92 and 99% was achieved on the PCA–kNN model, followed by PLS-DA and PCA–DA. The results demonstrate the efficacy of SWIR-HSI in the detection of contamination in pistachio nuts as a promising and profitable quality strategy for online and offline applications. In another study, Kheiralipour et al. [87] explored the potential of a long-wave NIR-HSI system (700–1700 nm) to assess the infection of 681 pistachio kernels by two isolates of Aspergillus flavus: KK11 and R5. The extracted spectral features were selected and built on LDA and QDA classifiers. Overall, QDA achieved the highest result (91.7%) compared to LDA. This result demonstrated that the HSI has the potential to detect aflatoxin contamination in pistachio kernels. However, further research is required to improve the accuracy of the models. The implementation of deep learning methods could be another approach to achieving better results.
Qiao et al. [88] explored the SWIR-HSI system (967–2499 nm) to identify moldy peanuts of three breeds. Spectral features were extracted by nonparametric weighted feature extraction (NWFE) and selected by analysis of variance (ANOVA). The selected features were used to develop a pixel-wise SVM model. The model achieved 96.32%, 94.2%, and 97.51% accuracy for breeds A, B, and C, respectively. The results showed the efficacy of the SWIR-HSI system in identifying contamination in peanuts. However, efforts could also be made to determine factors such as moisture content and oil content for more robust and reliable moldy peanut identification systems in the nut industry. Jiang et al. [89] utilized the NIR-HSI system (970–2570 nm) to identify 70 moldy peanuts. PCA was employed to select important bands from the extracted spectral data. Thereafter, they segmented the images into kernel-scale objects in the spatial dimension using a marker-controlled watershed algorithm. Overall, an accuracy of 98.73% was achieved in pixel-wise classification and identification of moldy peanuts.
Overall, the HSI has offered an efficient, objective, and beneficial way for inspecting defects in nuts. Studies on walnuts, almonds, pistachios, and peanuts have shown consistent accuracies that exceed 90% while integrating HSI with deep learning and applying feature selection approach enhanced the accuracy to around 97–99%. Meanwhile, the conventional approaches such as manual inspection remain laborious and subjective. HSI offers scalable solutions for industrial nut processing. However, broader validation, larger datasets, and more generalizable models are still needed to ensure reliable, real-time deployment across diverse nut varieties and processing conditions.
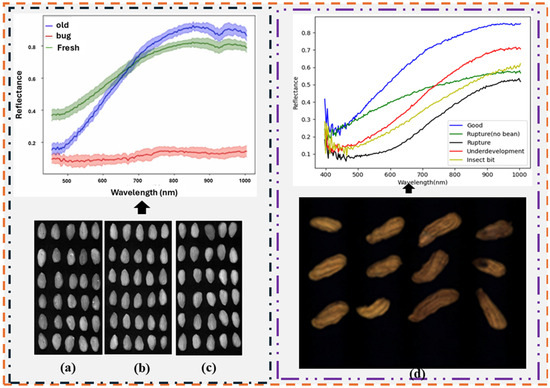
Figure 8.
An example of application of hyperspectral imaging for defect evaluation in nut spectral extraction of the almond and peanut samples: (a) insect-damaged almond in hyperspectral images, (b) fresh almonds in hyperspectral images, (c) expired almonds in hyperspectral images, and (d) peanuts in hyperspectral images [83,90].

Table 4.
Summary of hyperspectral imaging for defect assessment in nuts.
Table 4.
Summary of hyperspectral imaging for defect assessment in nuts.
| Product | Sample No. | Appl. | Spectral Range (nm) | Model | Perf. | Reference |
|---|---|---|---|---|---|---|
| Walnuts | 120 | Moldy walnuts assessment | 370−1042 | SVM | 93% | [84] |
| Almond kernels | 1245 | Assessment of defect and freshness | 400−1000 | VGGNet, MobileNet, CNN | Acc = 99.05% Acc = 97.68% Acc = 99.05% | [83] |
| Almond kernels | 454 | Assessment of internal damage | 700−1400 | SVM | Acc = 94.2% | [85] |
| Almond nuts | 454 | Assessment of internal damage | 700−1400 | SVM | Acc = 91.2% | [62] |
| Pistachio nuts | 99 | Detection of contaminated edible pistachios | 1000−2500 | PLS-DA, PCA–DA, PCA–kNN, CART | Best: PCA–kNN Acc = 0.92–0.99% | [86] |
| Pistachio kernels | 681 | Classifying heathy and infected pistachio kernels | 900−1700 | LDA and QDA | Best model: QDA Acc = 91.7% | [87] |
4.3.4. Hyperspectral Imaging for Aflatoxin in Nuts
Aflatoxin naturally occurs; it is toxic in nature and a carcinogenic compound with a fluorescence characteristic which is produced by fungi that contaminates specific foods [91]. It poses a persistent threat to global health, with nuts (especially peanuts) being the most common source. Studies have shown that consumption of aflatoxin-contaminated food can increase the risk of death from liver cancer, especially in individuals that are also infected with hepatitis B virus (HBV) [92]. It thrives in humid environments, such as inadequately controlled storage facilities, and can rapidly spread to other food products once it develops. Due to its colorless and tasteless characteristics, it is difficult to detect and because of its extreme heat resistance it can survive at high temperature which makes it difficult to be destroyed or removed by cooking or boiling. Conventional approaches, including mass spectrometry, gas chromatography, thin layer chromatography, high performance liquid chromatography, microcolumn techniques, and enzyme linked immunosorbent assay, have been widely utilized for aflatoxin analysis in nuts. While the approaches provide reliable results, however, they are complex and destructive to the sample and unsuitable for online detection.
Recently, non-destructive techniques such as the HSI system (see Table 5) are widely gaining attention for aflatoxin detection [93,94]. Zhongzhi and Limiao [95] investigated the efficacy of using an HSI system (400–700 nm) under 365 nm ultraviolet light to identify the degree of aflatoxin contamination in 250 peanut samples. In this study, five concentrations of aflatoxin solution were added to peanut kernels. The spectra were extracted, and the Fisher Discrimination method was employed to select the key bands. Thereafter, the selected features were developed using RBF-SVM for the regression and determination of the degree of contamination. Overall, the SVM achieved an accuracy of 95.5% and R2 of 0.9785. The results demonstrated the importance of spectral selection for model development. Qi et al. [96] explored the SWIR-HSI system (967–2499 nm) to identify fungal contamination in two peanut varieties. The key bands sensitive to fungal contamination were selected by successive projections and built on a joint sparse representation and SVM classifier. Classification accuracies of 99.2% and 98.8% were achieved by the JSRC model, whereas accuracies of 98.4% and 96.8% were achieved by the SVM classifier for the two varieties. Although JSRC showed better accuracy than the SVM classifier, further work needed to be considered for peanut varieties and factors, such as moisture and oil content, to develop a robust and reliable model.
Yang et al. [97] investigated the potential of the Raman HSI system (732–1024 nm) to identify and differentiate infected peanut kernels from healthy kernels. In this study, 600 peanuts from two peanut varieties were utilized, and three artificial inoculation strains of Aspergillus flavus (A. flavus 142,801, A. flavus 142,803, and A. flavus 336,156) were used to infect the two peanut kernel varieties. Spectra were extracted, preprocessed, and selected. Thereafter, the selected spectra were developed on the SVM for 2-class and 3-class classification. Overall, the 2-class model achieved the highest accuracy of 99% while the 3-class model achieved 88.9% for the A peanut variety and 92.4% for the B peanut variety. An accuracy of 74.8% was achieved for the mixed peanut variety (varieties A and B). The Raman HSI system used in this study has demonstrated great potential for identifying contamination within a peanut variety. However, more effort is needed to identify contamination in mixed and combined peanut kernel varieties.
In another study, Mishra et al. [98] explored the potential of the HSI system in the NIR range (900–1700 nm) to develop an efficient non-destructive method to predict AFB1 content in single almond kernels in a sample of 500. In the study, CARS selected hyperspectral features were built on PLSR and MLR with R2 of 0.958 and 0.948, respectively, while RMSE of 0.089 µg/g and 0.090 µg/g were achieved. Again, Mishra et al. [99] investigated the potential of a multispectral imaging system for the detection of aflatoxin in single-kernel almonds. The wavelengths used were selected from the HSI system (900–1700 nm). In this study, five concentrations of AFB1 (0.25, 0.50, 0.75, and 1 µg/kernel) were used to test the sorter. The AFB1 concentration levels were predicted by ANN, while four discriminant analyses, SVM, LR, LDA, and QDA, were employed to distinguish the contaminated kernels from the healthy ones. Overall, the ANN achieved an accuracy of 95.70% and 86.20% and the lowest error rates of 0.109 µg/g and 0.187 µg/g, respectively. The LR effectively discriminates the contaminated from non-contaminated kernels with an accuracy of 90.8% and 95% with a threshold of 0.25 µg/g. These studies demonstrated that integrating a robust feature selection approach with optimized machine learning models could enable scalable, non-destructive aflatoxin detection in the nut industry.
Recently, Kabir et al. [100] utilized a shortwave infrared hyperspectral imaging system (900–1700 nm) to acquire almond kernels contaminated with aflatoxin of different concentrations (250, 500, 750, and 1000 ppb). The almond kernels contained three sets of data in which dataset 1 comprises 122 controls and 98, 90, 89, and 90 samples at 250 ppb, 500 ppb, 750 ppb, and 1000 ppb, while dataset 2 consists of 183 controls, 43, 48, 48, and 143 samples at 250 ppb, 500 ppb, 750 ppb, and 1000 ppb. Dataset 3 comprises 260 controls, 164, 158, 151, and 155 samples at 250 ppb, 500 ppb, 750 ppb, and 1000 ppb, respectively. The almond kernels spectral extracted were built on the proposed correlation awareness evolutionary sparse hybrid spectral band selection algorithm which outperformed other conventional classifiers such as RF, Adaboost, ReliefF, and others. Williams et al. [101] employed an HSI system within the VNIR range (400–1000 nm) to categorize the AFB1 contamination of 300 pistachio nuts into three classes: <8 ppn, >160 ppn, and >300 ppn. The spectral features were constructed using k-Means and ResNet, with accuracies of 84.38% and 96.67%, respectively. In another study, Ref. [102] demonstrated the potential of the HSI system with 900–1700 nm range in detecting AFB1 contamination on 5400 almonds. The extracted spectra were built on a 3D inception-ResNet model to 2-class classification (contaminated and non-contaminated almonds). Indeed, recent studies have demonstrated the efficacy of HSI systems in both VNIR (400–1000 nm) and SWIR (900–1700 nm) ranges; combined with advanced feature selection and deep learning models, they can achieve high accuracy in classifying aflatoxin-contaminated nuts. Future research could explore integrating the deep learning approaches for multi-class detection across different nut types and contamination levels to enhance generalizability and robustness.

Table 5.
Summary of hyperspectral imaging for assessment of aflatoxin contamination in nuts.
Table 5.
Summary of hyperspectral imaging for assessment of aflatoxin contamination in nuts.
| Product | Sample No. | Appl. | Spectral Range (nm) | Model | Perf. | Reference |
|---|---|---|---|---|---|---|
| Peanut | 600 | Fungal identification | 723–1024 | SVM | Acc for 3-class: A variety: 88.9%, B variety: 92.4%, Acc for 2-class: 99% Mixture: Acc = 74.8% | [97] |
| Peanut | NA | Identification of fungi-contaminated peanuts | 967–2499 | SVM | Pixel-wise Acc = 96.32%, 94.2%, and 97.51% for A, B, and C | [88] |
| Peanut | 70 | Detection of moldy peanut | 970–2570 | Pixel-wise classifier | Acc = 98.73% | [89] |
| Peanut | 250 | Identification of degree of aflatoxin contamination | 400–720 | RBF-SVM | Acc = 95.5% R2 = 0.9785 MSE = 0.0223 | [95] |
| Almond kernel | 500 | Prediction of aflatoxin B1 | 900–1700 | PLSR and MLR | R2 = 0.958 and 0.948 RMSE = 0.089 µg/g and 0.090 µg/g | [98] |
| Almond kernel | 400 | Assessment of contaminated kernel | 900–1700 | SVM, LDA, QDA, and LR | Acc = 58.3% Acc = 81.7% Acc = 45% Acc = 95% | [99] |
| Almond kernel | 5400 | Aflatoxin B1 contamination | 900–1700 | 3D Inception-ResNet | Acc = 90.81%, F1 = 0.899 | [102] |
| Pistachio | 300 | Identification of the degree of contamination | 400–1000 | k-Means and ResNet | Acc = 84.38% and 96.67% | [101] |
4.3.5. Hyperspectral Imaging for Moisture Content Assessment in Nuts
Moisture content is a crucial quality parameter for tree nuts which directly influences the taste, oil composition, and storage properties. Low moisture content decreases economic value for growers while the high moisture content is one of the prime reasons behind initiating hydrolytic rancidity [9]. Additionally, high moisture content encourages microbial growth (such as fungi), leading to the production of enzymes that hydrolyze fats into free fatty acids. Conventional techniques such as gravimetry oven, electronic nose moisture analyzer [103], and Karl Fischer titration [104] are commonly used for the assessment of moisture content in nuts. However, the methods are labor-intensive, consume time, and are destructive to samples. Therefore, there is an urgent need for rapid, reliable, efficient, and non-destructive techniques for assessing the moisture content in nuts.
Hyperspectral imaging is recently gaining ample attention in the food industry. Its application has been adopted to assess the moisture content in tree nuts (see Table 6). For instance, Jin et al. [105] utilized HSI systems in two spectral ranges, VNIR (400–1000 nm) and SWIR (1000–2500 nm), to predict the moisture content of five varieties (Huayu, Luohanguo, Zhonghua, Dabaisha, and Xiaobaisha) of peanut kernels. A total of 150 samples (100 for calibration while 50 samples for prediction) were used in this study. The full spectral were extracted from the HSI images and built on PLSR yielding R2 of 0.908 and 0.906, and RMSE of 0.063% for both, respectively. To optimize the model, optimal wavelengths were selected using regression coefficients of the PLSR (RC-PLSR), leading to six optimal wavelengths. The optimal wavelengths were built on PLSR and achieved improved performances of R2 0.910 and 0.90 with RMSE of 0.061 and 0.06%, respectively. In another study, Ref. [79] investigated the efficacy of VNIR-HSI system (400–1000 nm) to predict the moisture content and texture characteristics of roasted pistachio kernels under different conditions (temperature, time, and air velocities). The extracted spectral were subjected to two preprocessing techniques: normalization (multiplicative scatter correction (MSC), standard normal variate transformation (SNV), smoothing) and differentiation (first derivative and second derivative). The preprocessed data were then built on PLSR and ANN. Overall, the MSC preprocessed data built on ANN achieved the best performance in predicting moisture content (R2 = 0.907, RMSEP = 0.179). The result obtained demonstrated the efficacy of the hyperspectral imaging system for the prediction of moisture content.
In another study, Ref. [106] investigated the potential of VNIR (400–1000 nm) to assess moisture concentrations of macadamia nuts (485 in-shell and 529 kernels). The HSI images were acquired in two orientations of the macadamia nuts (base-up, base-down) and data were extracted automatically and manually. The extracted data (base-up, base-down, and combined) were built on PLSR, ANN, and Gaussian process regression (GPR). Overall, PLSR built on 10 selected wavelengths achieved the best accuracy (R2 = 0.96, RMSE = 1.20%, RPD = 5.15) in in-shell while in kernel, it achieved R2 of 0.99, RMSE of 0.308%, and RPD of 11.05. Meanwhile, ANN and GPR achieved the same results (R2 of 0.99). The results obtained demonstrated the potential of hyperspectral imaging in predicting moisture content. Moreover, Jiang et al. [107] utilized the Vis-HSI system (400–1000 nm) to evaluate moisture content in 188 Chinese walnuts. The spectral extracted from the HSI images built on PLSR achieved R2 of 0.7161, RMSEP of 0.6005, RPD of 1.35, and RER of 8.73. To optimize the model, wavelength selection methods (2D correlation spectroscopy, regression coefficients, and competitive adaptive reweighted sampling (CARS)) were implemented. Among all, CARS with the nine best wavelengths was preferred and modeled on PLSR with R2 = 0.6921, RMSEP = 0.6084, RPD = 1.33, and RER = 8.62.

Table 6.
Summary of hyperspectral imaging for assessment of moisture content in nuts.
Table 6.
Summary of hyperspectral imaging for assessment of moisture content in nuts.
| Product | Sample No | Appl. | Spectral Range (nm) | Model | Perf. | Reference |
|---|---|---|---|---|---|---|
| Peanut kernels | 150 | Moisture content prediction | 400–1000 1000–2500 | PLSR RC-PLSR | R2 = 0.910 and 0.90 RMSE = 0.061% and 0.06% | [105] |
| Roasted pistachio kernels | 62 g/100 g | Moisture content prediction | 400–1000 | PLSR and ANN | R2 = 0.907, RMSEP = 0.179 | [79] |
| Macadamia nuts | 485 in-shell and 529 kernels | Assessment of moisture concentration | 400–1000 | PLSR ANN GPR | R2 = 0.96, RMSE = 1.20%, RPD = 5.15 R2 = 0.99, RMSE = 0.308% RPD = 11.05 R2 = 0.99 | [106] |
| Chinese walnuts | 188 | Moisture content assessment | 400–1000 | CARS-PLSR | R2 = 0.6921, RMSEP = 0.6084, RPD = 1.33 and RER = 8.62 | [107] |
4.3.6. Hyperspectral Imaging for Other Quality Traits in Nuts
Other quality traits of nuts include the discrimination of pulp from shell, distinguishing nut varieties, and quality grading of the nuts. Hyperspectral imaging has been explored in this area for the rapid, objective, and non-destructive assessment of nuts (see Table 7). For instance, Jiang et al. [108] investigated the potential of a hyperspectral fluorescence imaging system (425–775 nm) to discriminate pulp from the shells of 6257 black walnuts. The images acquired through the hyperspectral fluorescence system were built using a Gaussian kernel SVM, and an accuracy of 90.3% was achieved. Although good model performance was achieved, the model could be improved by selecting the optimal parameter in the kernel function, which could enable a more robust model. Jiang et al. [109] explored the potential of the HSI system (425–775 nm) to discriminate between the shells and meat of 5496 black walnuts. PCA was implemented to extract and select the key features from the HSI images. Thereafter, the selected spectral features were developed using a Gaussian mixture model (GMM)-based Bayesian classifier. Overall, an accuracy of 95.6% was achieved. The results obtained demonstrated improved accuracy compared to their previous research. However, further study is needed to consider other statistical methods that can best fit the images.
Jiang et al. [110] explored the potential of HSI system (400–1000 nm) to effectively identify and visualize 400 Chinese walnut of two varieties. PCA was applied to spectral profiles for effective identification. Thereafter, PCA with selected CARS features was developed using three models (k-nearest neighbor, PLS-DA, and SVM). Overall, the full spectra achieved an accuracy of 92%, whereas the selected features achieved 91% accuracy. The selected features yielded lower results than the full spectral, which could be due to the loss of relevant information during the spectral selection. Therefore, more robust spectral selection methods are required to obtain satisfactory results. In another study Zhong et al. [111] investigated the efficacy of the HSI system (935–1720 nm) to identify the quality of 170 chestnuts. PCA was employed to analyze the spectra extracted for the chestnut quality assessment, followed by three preprocessing techniques. Thereafter, spectra were developed using conventional machine learning and deep learning models. The best accuracy of 99.72% was achieved with FD-LSTM, while key wavelengths selected by UVE and developed on CNN (FD-UVE-CNN) achieved a satisfactory accuracy of 97.33%. However, it is expected that the key wavelength would yield a higher accuracy than the full spectral range. This study demonstrated the potential of key wavelength adoption over the full spectral implementation. Although full wavelength yielded better accuracy than the key wavelength selection, selecting the wavelength enables rapid assessment with satisfactory results. Similarly, Li et al. [112] utilized the HSI system (400–1000 nm) to identify 417 Chinese chestnuts from three geographical regions. Initially, PCA was employed to extract the mean spectra from different regions. Thereafter, the full spectral extract was developed on ID-CNN, PLS-DA, and PSO-SVM, whereas the spectral selection approach (CARS and SPA) was implemented to select the key wavelengths. Selected wavelengths were built on 1D-CNN, PLS-DA, and PSO-SVM. Overall, the full spectrum achieved the best performance with 97.12%, 97.12%, and 95.68% accuracy on 1D-CNN, PLS-DA, and PSO-SVM, respectively, whereas the selected spectral developed on 1D-CNN and PLS-DA achieved improved results of 97.12%. However, similar results were obtained for the full and selected spectra developed using the classification model. Although the two approaches achieved the same result (97.12%), the selection approach is more rapid and beneficial for industrial application than the full spectral approach.
Feng et al. [113] utilized the HSI system (400–900 nm) to discriminate between the shells and kernels of 213 Chinese hickory nuts. In this study, the spectral features of the HSI images were developed on a 2D-CNN and long short-term memory (LSTM) to differentiate Chinese hickory nuts at the pixel level, while conventional machine learning models (PCA–kNN and SVM) were implemented as identification models. Overall, 2D-CNN–LSTM achieved the highest performance of 99%, whereas SVM and PCA–kNN yielded satisfactory accuracies of 93% and 94.1%, respectively. The results emphasize the significance of extracting deep features from HSI images to obtain optimal results. However, spectral selection could be a way to improve the performance of the conventional machine learning implemented in this study. Recently, Ref. [59] fused spectral features from two HSI systems VNIR (400–1000 nm) and NIR (900–1700 nm) and built on hybrid CNN–CNN–LSTM to sort pecan shelled products (shell, inner-wall, and kernel) with an accuracy of 99.29% achieved. In another study, Moscetti et al. [114] explored the SWIR-HSI system (850–2500 nm) to sort 2400 hazelnuts based on four quality grades (extra class, class I, class II, and waste). In this study, the full spectrum extracted from the HSI images was developed using PLS-DA, while the spectral pretreatment was optimized via an iterative routine. Overall, an accuracy > 90% was achieved based on the selected model. The results demonstrated that the spectral and spatial features of HSI images are promising for the sorting of hazelnuts. However, further study is needed to improve performance as misclassification is common in low-quality grades (class II and waste) as compared to high-quality grades (class extra and class I). These studies have demonstrated that combining spectral fusion, deep feature extraction, and targeted optimization for difficult classes would be key to developing robust nut sorting solutions.
Overall, HSI has proven effective for nut variety discrimination, shell–kernel separation, and quality grading, with accuracy frequently exceeding 90–99% across walnuts, chestnuts, hickory nuts, hazelnuts, and pecans. Meanwhile, integrating HSI with deep learning models (e.g., CNN, LSTM, and hybrid networks) has proven to outperform traditional methods and spectral fusion and feature selection further enhance efficiency. Still, there are problems with incorrectly categorizing lower-quality grades and losing important information when choosing wavelengths. In the future, our focus would be on strong spectral–spatial fusion, sophisticated feature extraction, and optimization for difficult classes so that nut sorting can be performed reliably and in real time in industrial settings.

Table 7.
Summary of hyperspectral imaging for assessment of nut quality traits.
Table 7.
Summary of hyperspectral imaging for assessment of nut quality traits.
| Product | Sample No. | Appl. | Spectral Range (nm) | Model | Perf. | Reference |
|---|---|---|---|---|---|---|
| Black walnut | 6257 | Discrimination of walnut shell and pulp | 425–775 | SVM | Acc = 90.3% | [108] |
| Black walnut | 5496 | Differentiating walnut shell and meat | 425–775 | PCA–GMM-BC | Acc = 95.65% | [109] |
| Chinese walnut | 400 | Walnut variety identification | 400–1000 | PLS-DA, KNN, SVM | Full spectra: CCR = 92%, Selected spectra: CCR = 91% | [110] |
| Chestnut | 170 | Grading walnuts | 935–1720 | CNN | Full spectra: Acc = 99.72% Key spectra: Acc = 97.33% | [111] |
| Chinese hickory nut | 213 | Differentiate the shell from kernel | 400–1000 | 2D-CNN–LSTM, SVM, and PCA–kNN | Acc = 99% Acc = 93%, and Acc = 94.1% | [113] |
| Chinese chestnut | 417 | Identification of geographical origin | 400–1000 | PLS-DA 1D-CNN | Acc = 90% | [112] |
| Hazelnut kernel | 2400 | Quality grading | 850–1870 | PLS-DA | Acc = > 90% | [114] |
| Pecan | 576 for shelled and 576 for in-shell | Pecan variety identification | 400–1000 and 900–1700 | RF, DT, PLSDA, GB, SVM, and LDA | Acc > 90% | [115] |
| Pecan | 2484 | Sorting of pecan shelled products | 400–1000 And 900–1700 | DT, GB, RF, SVM, CNN–LSTM, and CNN–CNN–LSTM | Acc > 90% | [59] |
5. Challenges and Future Outlook
Hyperspectral imaging is becoming a great alternative to traditional techniques for the quality assessment of nuts in the nut processing industry. Despite several merits of this technology, it still suffers some inherent limitations. For instance, obtaining an accurate outcome from HSI depends on the accuracy of wet chemistry. Therefore, this is an indirect method. HSI requires complicated multivariate calibration [116]. Accurate outcomes in hyperspectral imaging require intricate multivariate calibrations. It is essential to select an appropriate multivariate data mining analysis for the application. Calibration transfer across instruments is required because of the differences [117]. This means that models designed for one instrument may not be appropriate for others. Therefore, calibration transfer is crucial. However, maintaining precision and accuracy during this procedure may be difficult. In addition, maintaining uniformity among instruments requires careful attention to detail and reliable techniques. Indeed, more effort and researchers are required to transition from lab-based offline calibration to industrial-scale online situations. HSI has the capacity to measure hundreds of thousands of variables simultaneously. However, not all variables contain key information for model development. The redundant (uninformative and interfering) variables can lead to model instability. Therefore, an effort is required to select the key information for model development. Variable selection can improve model stability by increasing model accuracy and speeding up prediction [118,119]. It is anticipated that a robust and reliable model for assessing nuts in the processing industry will be achievable with selected variables and advanced optical technology (e.g., HSI).
The illumination source is another major challenge in implementing hyperspectral and imaging-based quality assessment systems. Indeed, halogen lamps are commonly used as illumination sources which provide a broad and continuous spectrum [120]. However, it has several inherent limitations such as producing high heat, limited operational lifespans, and intensity instability over time [121]. Such limitations could compromise measurement consistency and increase maintenance requirements. Moreover, in industrial settings, additional factors such as dust, vibration, and variable humidity could further exacerbate the challenges by affecting both the light source and imaging sensors. Dust accumulation on optical components (e.g., lenses) or protective covers could scatter light, reduce image clarity, and distort spectral measurements [122]; therefore, demanding regular calibration and cleaning is essential to maintain data accuracy. The combined effects of environmental instability and hardware limitations underscore the need for more robust, energy-efficient, and stable illumination systems. Future advancements, such as LED- or laser-based lighting technologies, could provide better spectral stability, longer lifespan, and lower thermal output which could improve reliability and performance in harsh industrial conditions while reducing maintenance demand time [121].
Furthermore, the high cost of hyperspectral imaging systems is still a major barrier to their widespread utilization, especially among small and medium sized processors. The HSI system requires substantial initial investment in specialized cameras, illumination units, and data processing infrastructures, as well as expenses related to maintenance, calibration, and skilled personnel. Large industrial processors could often justify the costs due to their higher production volumes, automation capacity, and potential for rapid return on investment. In contrast, small processors typically operate with limited financial resources and tighter profit margins, making it difficult to implement or sustain such advanced technology. The economic disparity creates a technological division that restricts access to the benefits of hyperspectral imaging, such as non-destructive quality assessment and process optimization, to larger enterprises. Consequently, widespread industrial deployment remains limited, especially in decentralized or small-scale operations. To address this challenge, future efforts should focus on developing cost-effective, portable, and scalable hyperspectral imaging solutions, possibly using low-cost sensors, compact optical components, and AI-based data compression. The development of new concepts could advance imaging technologies which would help small processors improve product quality control and stay competitive.
5.1. Integrating Hyperspectral Imaging with Robotics
Automation is the key for industrial efficiency and effectiveness; therefore, integrating HSI with robotics for adequate automation of nut quality assessment is essential. This approach will tackle issues related to efficiency and precision [123] in nut quality assessment. For instance, this approach could enable automated sorting and defect detection in real-time processing lines. Notably, identifying nut quality parameters (moisture content, oil content, and rancidity) are essential for healthier consumption by nut lovers. Therefore, integrating robotic systems with HSI could effectively benefit the nut industry in optimizing the product quality. Several robotic solutions could be effectively applied: robotic arms could be integrated with conveyor-based HSI systems to pick and sort nuts on spectral classification results. Mobile robotic platforms could be deployed for in-line inspection and monitoring which could reduce manual labor requirements. In addition, collaborative robots (Cobots) equipped with HSI cameras can assist operators in tasks requiring flexibility and precision such as handling irregular nut shapes or detecting subtle defects invisible to RGB imaging. Machine learning models, such as support vector machines (SVM,) and convolutional neural networks (CNN,), can be embedded within the robotic control frameworks to enable adaptive decision making and high accuracy classification [83,86]. Such hybrid systems minimize human error, ensure consistent quality standards, and maintain scalability in high-throughput operations.
Additionally, the integration of robotics with HSI systems could mitigate scalability and operational issues encountered by nut processing facilities. Automated systems integrated with HSI can run all the time, which would increase throughput while ensuring good quality. Most importantly, this integration would be essential to meet the high demand for nuts in the global market while manual or traditional methods may not meet the demand. For instance, an efficient and reliable processing system could be achieved when robotic arms are directed by real-time HSI data. This could enhance the handling and sorting of nuts and assure optimal production flow by minimizing the bottleneck. Notably, sorting lines that integrate robotics with HSI data could achieve classification accuracies that surpass 90% in nut defect identification, even in difficult conditions such as overlapping spectral signatures or varied nut morphologies [114]. The recent decline in the cost of HSI and robotic components would encourage considerable potential to improve sustainability and competition globally in the nut processing sector. This would facilitate the development of more efficient, automated, and precise quality evaluation methods.
5.2. Need for Real-Time and In-Line Monitoring
The application of HSI for real-time, in-line monitoring in nut processing demonstrated the improvement in quality assurance practices. In contrast to the conventional batch sampling approach, the in-line HSI provides continuous nut assessment during processing, thereby allowing real-time defect, contamination, and quality variation identification. HSI systems acquire comprehensive spectral data from individual nuts, facilitating the swift assessment of parameters including moisture content, oil composition, and surface defects without disrupting production. This capability facilitates the prompt elimination of substandard nuts, minimizing waste and averting the distribution of defective products, thereby protecting consumer trust and brand reputation. Furthermore, the implementation of in-line HSI monitoring enhances batch consistency while ensuring compliance with stringent regulatory standards. The system provides a rapid, non-destructive, and objective quality assessment, ensuring consistency across entire batches and addressing critical industry challenges such as product variability and cross-contamination. In addition, real-time data collection enables producers to establish detailed records of product quality, which improves traceability and ensures adherence to regulatory standards. HSI effectively detects and classifies aflatoxin-contaminated nuts and differentiates moldy samples from healthy ones, which is crucial for compliance with food safety standards in international markets [96]. The growing demand for automation and digitalization in the food industry necessitates the integration of HSI with current processing lines, providing a scalable solution consistent with Industry 4.0 principles. This technological shift enhances operational efficiency and bolsters the nut industry’s capacity to satisfy consumer and regulatory requirements for consistent, high-quality products.
5.3. Digital Twin Needs in Nut Processing
Digitalization has recently emerged as a sweeping megatrend across all industries, driving a re-evaluation of how digital insights can enhance process development, design, and operation [124]. Generally, digitalization applications in industries could be used to increase throughput, efficiency, product quality, monitoring, and traceability of the physical representation in virtual environments. Digital twin (DT) was originally proposed by Grives and Vickers in 2002 to describe the digital construct of the physical entity [125]. DT can be described as a virtual representation of physical products (e.g., nuts) developed using data acquired by sensors [126]. The adoption of digital twin (DT) technology in the tree nut processing and export industry is crucial. In real-life export situations, sensors are usually set up in a particular location inside the shipping container to monitor the condition (temperature and relative humidity) of the produce via transit. However, such measurements could not provide an accurate reflection of the nut’s conditions because the sensor could be sensitive to the nut around it but be insensitive to nuts in the remote area of the container. Additionally, the nut surface temperature tends to be high due to convective heat transfer, while there could be elevated internal temperature due to conduction and metabolic heat from respiration. The inconsistency could make it difficult to accurately determine the real produce temperature. This could result in quality degradation during the supply chain.
Subsequently, employing DT could ensure accurate prediction of the internal temperature distribution throughout the nut bulk. This would enable a better quality assessment of nut evolution and remaining shelf life. Furthermore, simulating the product volume by DT would ensure the identification of the elevated or reduced temperature zone, thereby revealing the potential heat accumulation areas and guiding interventions to prevent quality and nutrient degradation. This makes DT a powerful tool for optimizing postharvest management and ensuring the delivery of high-quality nuts to the market. Recently, several efforts have been used in fruit processing industries to utilize DT applications in monitoring batches of fruits during food chains. For instance, Ref. [127] developed the digital fruit twin using mechanistic modeling to minimize the environmental impact of the cold chain and enhance logistics by providing deeper insight into how fruit quality changes during individual shipments. Pattanaik and Jenamani [128] developed digital twins for the three most exported mango varieties (Alphonso, Totapuri, and Kesar) to simulate the cooling behavior of real mango based on airflow rate and its temperature. Despite the efforts that have been made and the fact that some success has been recorded in the adoption of DT in the fruit processing industry, no effort has been recorded in the nut processing industries.
6. Conclusions
This systematic review focuses on the huge potential of hyperspectral imaging as a rapid, non-destructive, and objective method for assessing tree nut quality in the processing industry. The evaluation focuses on the HSI’s ability to provide detailed information about the composition and quality of nuts, such as the peroxide value, acidity index, and chemical composition. This technology enables the detection and classification of defects and assessment of nut maturity, all of which are required to maintain high quality and market value. However, despite its various benefits, HSI deployment has inherent limitations which must be addressed. Therefore, this review recommends that overcoming limitations may necessitate additional research into more robust multivariate calibration models, improved algorithm development, and calibration transfer methodologies across HSI systems. We have suggested approaches that could be implemented by integrating HSI systems with robotics for efficient and accurate in-line sorting and defect detection. Similarly, we suggest incorporating HSI systems (as sensors) with digital twins to accurately and efficiently monitor nut quality in the industry and during chain supply. In summary, while hyperspectral imaging has the potential to transform the nut processing industry, its widespread adoption will be dependent on technological development and the elimination of operational complexities. This will ultimately improve the industry’s capacity to maintain product quality and safety, while adhering to regulatory standards and consumer expectations.
Author Contributions
Conceptualization, C.K. and E.O.O.; methodology, C.K. and E.O.O.; validation, C.K. and F.K.; formal analysis, E.O.O.; investigation, E.O.O. and C.K.; resources, C.K.; data curation, E.O.O.; writing—original draft preparation, E.O.O.; writing—review and editing, C.K. and F.K.; supervision, C.K. and F.K.; project administration, C.K.; funding acquisition, C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Georgia Agricultural Commodity Commission for Pecans.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Abbreviations
| ANN | Artificial neural network |
| ASCA | ANOVA-simultaneous component analysis |
| CART | Classification and regression tree |
| CNN | Convolutional neural network |
| CNN–LSTM | Convolutional neural network–long short-term memory |
| Conv | Convolutional |
| 3D inception-ResNet | 3D-inception residual network |
| DD-SIMCA | Data-driven soft independent modeling class analogy for analysis |
| DT | Decision tree |
| GB | Gradient boosting |
| GMM | Gaussian mixture model |
| GPR | Gaussian process regression |
| LDA | Linear discriminant analysis |
| MLR | Multiple linear regression |
| MPLSR | Modified partial least squares regression |
| PCA–DA | Principal component analysis with discriminant analysis |
| PCA–GMM-BC | Principal component analysis–Gaussian mixture model-based Bayesian classifier |
| PCA–kNN | Principal component analysis with k-nearest neighbor |
| PLS-DA | Partial least squares discriminant analysis |
| Pool | Max-pooling |
| PSO–SVR | Particle swarm optimization with support vector regression |
| R2 | Coefficient of determination |
| ResNet | Residual network |
| RF | Random forest |
| RMSE | Root means square error |
| RPD | ratio of prediction to deviation |
| SVC | Support vector classification |
| VGGNet | Very deep convolutional networks |
References
- Chun, J.; Lee, J.; Eitenmiller, R.R. Vitamin E and oxidative stability during storage of raw and dry roasted peanuts packaged under air and vacuum. J. Food Sci. 2005, 70, C292–C297. [Google Scholar] [CrossRef]
- Gonçalves, B.; Pinto, T.; Aires, A.; Morais, M.C.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Cosme, F. Composition of Nuts and Their Potential Health Benefits—An Overview. Foods 2023, 12, 942. [Google Scholar] [CrossRef] [PubMed]
- Buthelezi, N.M.D.; Tesfay, S.Z.; Ncama, K.; Magwaza, L.S. Destructive and non-destructive techniques used for quality evaluation of nuts: A review. Sci. Hortic. 2019, 247, 138–146. [Google Scholar] [CrossRef]
- Alina, M.; Mureșan, C.; Pop, A.; Georgiana Smaranda, M.; Mureșan, A.; Puscas, A.; Postolache, A.; Stoica, F.; Crivei, I.; Veleşcu, I.; et al. An Overview of the Characteristics, Advantages, and Uses of Nuts. In Nut Consumption and Its Usefulness in the Modern World; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Ros, E. Health benefits of nut consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef]
- Chen, S.; Dai, D.; Zheng, J.; Kang, H.; Wang, D.; Zheng, X.; Gu, X.; Mo, J.; Luo, Z. Intelligent grading method for walnut kernels based on deep learning and physiological indicators. Front. Nutr. 2022, 9, 1075781. [Google Scholar] [CrossRef]
- Bai, S.H.; Tahmasbian, I.; Zhou, J.; Nevenimo, T.; Hannet, G.; Walton, D.; Randall, B.; Gama, T.; Wallace, H.M. A non-destructive determination of peroxide values, total nitrogen and mineral nutrients in an edible tree nut using hyperspectral imaging. Comput. Electron. Agric. 2018, 151, 492–500. [Google Scholar] [CrossRef]
- Alvarado, M.C.; Sanchez, P.D.C.; Polongasa, S.G.N. Emerging rapid and non-destructive techniques for quality and safety evaluation of cacao: Recent advances, challenges, and future trends. Food Prod. Process. Nutr. 2023, 5, 40. [Google Scholar] [CrossRef]
- Canneddu, G.; Júnior, L.C.C.; de Almeida Teixeira, G.H. Quality evaluation of shelled and unshelled macadamia nuts by means of near-infrared spectroscopy (NIR). J. Food Sci. 2016, 81, C1613–C1621. [Google Scholar] [CrossRef]
- Omid, M. Design of an expert system for sorting pistachio nuts through decision tree and fuzzy logic classifier. Expert Syst. Appl. 2011, 38, 4339–4347. [Google Scholar] [CrossRef]
- Puneet, M.; Diezma Iglesias, B.; Barreiro Elorza, P. NIR hyperspectral imaging for detection of nut contamination. New Food 2015, 18, 30–33. [Google Scholar]
- Marín-Méndez, J.-J.; Luri Esplandiú, P.; Alonso-Santamaría, M.; Remirez-Moreno, B.; Urtasun Del Castillo, L.; Echavarri Dublán, J.; Almiron-Roig, E.; Sáiz-Abajo, M.-J. Hyperspectral imaging as a non-destructive technique for estimating the nutritional value of food. Curr. Res. Food Sci. 2024, 9, 100799. [Google Scholar] [CrossRef]
- Farrar, M.B.; Martinez, M.; Jones, K.; Omidvar, N.; Wallace, H.M.; Chen, T.; Hosseini Bai, S. The Potential for Hyperspectral Imaging and Machine Learning to Classify Internal Quality Defects in Macadamia Nuts. Horticulturae 2024, 10, 1129. [Google Scholar] [CrossRef]
- Moscetti, R.; Monarca, D.; Cecchini, M.; Haff, R.P.; Contini, M.; Massantini, R. Detection of Mold-Damaged Chestnuts by Near-Infrared Spectroscopy. Postharvest Biol. Technol. 2014, 93, 83–90. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Fan, S.; Huang, W.; Zhao, C.; Liu, C.; Huang, D. Hyperspectral imaging combined with multivariate analysis and band math for detection of common defects on peaches (Prunus persica). Comput. Electron. Agric. 2015, 114, 14–24. [Google Scholar] [CrossRef]
- Zou, S.; Tseng, Y.-C.; Zare, A.; Rowland, D.; Tillman, B.; Yoon, S.-C. Peanut maturity classification using hyperspectral imagery. Biosyst. Eng. 2019, 188, 165–177. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. bmj 2021, 372, 71. [Google Scholar] [CrossRef]
- Ampofo, J.; Grilo, F.S.; Langstaff, S.; Wang, S.C. Oxidative Stability of Walnut Kernel and Oil: Chemical Compositions and Sensory Aroma Compounds. Foods 2022, 11, 3151. [Google Scholar] [CrossRef]
- Tournas, V.H.; Niazi, N.S.; Kohn, J.S. Fungal Presence in Selected Tree Nuts and Dried Fruits. Microbiol. Insights 2015, 8, 1–6. [Google Scholar] [CrossRef]
- Grilo, F.S.; Wang, S.C. Walnut (Juglans regia L.) Volatile Compounds Indicate Kernel and Oil Oxidation. Foods 2021, 10, 329. [Google Scholar] [CrossRef]
- Aondoakaa, I.P.; Martini, S.; Akoh, C.C. Physical and chemical properties of low saturated zero trans-fat soft margarine formulated with blends of enzymatically modified soybean oil and mango kernel fat. Food Chem. 2025, 492, 145255. [Google Scholar] [CrossRef] [PubMed]
- Aruwajoye, N.N.; Buthelezi, N.M.D.; Mditshwa, A.; Tesfay, S.Z.; Magwaza, L.S. Assessing the Impact of Roasting Temperatures on Biochemical and Sensory Quality of Macadamia Nuts (Macadamia integrifolia). Foods 2023, 12, 2116. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Peng, X.; Huang, Y.; Hu, H.; Li, C.; Chen, Y.; Yu, Q.; Wang, Y. Recent advances in the occurrence, mechanisms, influence factors and control strategies of process contaminants in nuts: A comprehensive review. Food Control 2024, 159, 110265. [Google Scholar] [CrossRef]
- Alsuhaiban, A. Effects of Storage Periods and Temperature on Mold Prevalence and Aflatoxin Contamination in Nuts. Pak. J. Nutr. 2018, 17, 219–227. [Google Scholar] [CrossRef][Green Version]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A Global Concern for Food Safety, Human Health and Their Management. Front. Microbiol. 2016, 7, 2170. [Google Scholar] [CrossRef]
- Yildiz, G.; Wehling, R.L.; Cuppett, S.L. Comparison of four analytical methods for the determination of peroxide value in oxidized soybean oils. J. Am. Oil Chem. Soc. 2003, 80, 103–107. [Google Scholar] [CrossRef]
- Ityotagher, A.P.; Terhile, C. Production and quality evaluation of margarine from blends of melon and palm kernel oils. World 2020, 4, 72–79. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, J.; Su, X.; Li, Q.; Su, C.; Li, Y.; Ma, G.; Zhang, S.; Yu, X. Extraction, chemical components, bioactive functions and adulteration identification of walnut oils: A review. Grain Oil Sci. Technol. 2024, 7, 30–41. [Google Scholar] [CrossRef]
- Ghiasi, P.; Sohrabi, O.; Rahmati, E.; Najafi, G.; Mohamed, M.; Ghasemnezhad, A. Modeling for extraction of oil from walnut and sesame using batch flow cold press oil extraction system. Food Sci. Nutr. 2022, 10, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Ataş, M.; doğan, Y. Classification of Closed and Open Shell Pistachio Nuts by Machine Vision. In Proceedings of the International Conference on Advanced Technology Sciences, Antalya, Turkey, 4–7 August 2015. [Google Scholar]
- Fan, X.; Zhou, J. Nondestructive Detection and Quality Grading System of Walnut Using X-Ray Imaging and Lightweight WKNet. Foods 2025, 14, 2346. [Google Scholar] [CrossRef]
- Olaniyi, E.O.; Kucha, C. Advances in Precision Systems Based on Machine Vision for Meat Quality Detection. Food Eng. Rev. 2025, 1–26. [Google Scholar] [CrossRef]
- Peng, Y.; Dhakal, S. Optical Methods and Techniques for Meat Quality Inspection. Trans. ASABE 2015, 58, 1371–1386. [Google Scholar] [CrossRef][Green Version]
- Stuart, M.B.; McGonigle, A.J.S.; Willmott, J.R. Hyperspectral Imaging in Environmental Monitoring: A Review of Recent Developments and Technological Advances in Compact Field Deployable Systems. Sensors 2019, 19, 3071. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Islam, M.R.; Uddin, M.P.; Ulhaq, A. A Deep Learning-Based Hyperspectral Object Classification Approach via Imbalanced Training Samples Handling. Remote Sens. 2023, 15, 3532. [Google Scholar] [CrossRef]
- Paul, S.; Kumar, D.N. Partial informational correlation-based band selection for hyperspectral image classification. J. Appl. Remote Sens. 2019, 13, 046505. [Google Scholar] [CrossRef]
- Yan, C. A review on spectral data preprocessing techniques for machine learning and quantitative analysis. iScience 2025, 28, 112759. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yan, Z.; Zhang, L. A comparative study of 11 non-linear regression models highlighting autoencoder, DBN, and SVR, enhanced by SHAP importance analysis in soybean branching prediction. Sci. Rep. 2024, 14, 5905. [Google Scholar] [CrossRef] [PubMed]
- Saeys, W.; Nguyen Do Trong, N.; Van Beers, R.; Nicolaï, B.M. Multivariate calibration of spectroscopic sensors for postharvest quality evaluation: A review. Postharvest Biol. Technol. 2019, 158, 110981. [Google Scholar] [CrossRef]
- Silalahi, D.D.; Midi, H.; Arasan, J.; Mustafa, M.S.; Caliman, J.-P. Kernel partial diagnostic robust potential to handle high-dimensional and irregular data space on near infrared spectral data. Heliyon 2020, 6, e03176. [Google Scholar] [CrossRef]
- Mohammadi Moghaddam, T.; Razavi, S.M.; Taghizadeh, M.; Sazgarnia, A. Sensory and instrumental texture assessment of roasted pistachio nut/kernel by partial least square (PLS) regression analysis: Effect of roasting conditions. J. Food Sci. Technol. 2016, 53, 370–380. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.; Ni, X.; Chu, X.; Li, Y.-F.; Sun, C. Evaluation of Near-Infrared Hyperspectral Imaging for Detection of Peanut and Walnut Powders in Whole Wheat Flour. Appl. Sci. 2018, 8, 1076. [Google Scholar] [CrossRef]
- Khosa, I.; Pasero, E. Artificial neural network classifier for quality inspection of nuts. In Proceedings of the 2014 International Conference on Robotics and Emerging Allied Technologies in Engineering (iCREATE), Islamabad, Pakistan, 22–24 April 2014; pp. 103–108. [Google Scholar]
- Ros, P.M.; Pasero, E. Defects detection in pistachio nuts using artificial neural networks. In Proceedings of the Neural Nets and Surroundings: 22nd Italian Workshop on Neural Nets, WIRN 2012, Salerno, Italy, 17–19 May 2013; Springer: Berlin/Heidelberg, Germany, 2013; pp. 147–156. [Google Scholar]
- Ganganagowdar, N.V.; Siddaramappa, H.K. Recognition and classification of White Wholes (WW) grade cashew kernel using artificial neural networks. Acta Sci. Agron. 2016, 38, 145–155. [Google Scholar] [CrossRef]
- Baldomero-Naranjo, M.; Martínez-Merino, L.I.; Rodríguez-Chía, A.M. A robust SVM-based approach with feature selection and outliers detection for classification problems. Expert Syst. Appl. 2021, 178, 115017. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Gweon, G.; Yoo, Y.J. Supervised diagnostic classification of cognitive attributes using data augmentation. PLoS ONE 2024, 19, e0296464. [Google Scholar] [CrossRef]
- Olaniyi, E.O.; Oyedotun, O.K.; Khashman, A. Heart Diseases Diagnosis Using Neural Networks Arbitration. Int. J. Intell. Syst. Appl. 2015, 7, 72–79. [Google Scholar] [CrossRef]
- Khosa, I.; Pasero, E. A Machine Vision System for Quality Inspection of Pine Nuts. Int. J. Adv. Comput. Sci. Appl. 2016, 7, 253–267. [Google Scholar] [CrossRef]
- Onaran, I.; Pearson, T.; Yardimci, Y.; Cetin, A.E. Detection of underdeveloped hazelnuts from fully developed nuts by impact acoustics. Trans. ASABE 2006, 49, 1971–1976. [Google Scholar] [CrossRef]
- Chakraborty, C.; Bhattacharya, M.; Pal, S.; Lee, S.-S. From machine learning to deep learning: Advances of the recent data-driven paradigm shift in medicine and healthcare. Curr. Res. Biotechnol. 2024, 7, 100164. [Google Scholar] [CrossRef]
- Razavi, S. Deep learning, explained: Fundamentals, explainability, and bridgeability to process-based modelling. Environ. Model. Softw. 2021, 144, 105159. [Google Scholar] [CrossRef]
- Khan, W.; Daud, A.; Khan, K.; Muhammad, S.; Haq, R. Exploring the frontiers of deep learning and natural language processing: A comprehensive overview of key challenges and emerging trends. Nat. Lang. Process. J. 2023, 4, 100026. [Google Scholar] [CrossRef]
- Alzubaidi, L.; Zhang, J.; Humaidi, A.J.; Al-Dujaili, A.; Duan, Y.; Al-Shamma, O.; Santamaría, J.; Fadhel, M.A.; Al-Amidie, M.; Farhan, L. Review of deep learning: Concepts, CNN architectures, challenges, applications, future directions. J. Big Data 2021, 8, 53. [Google Scholar] [CrossRef]
- Alhajeri, M.S.; Ren, Y.M.; Ou, F.; Abdullah, F.; Christofides, P.D. Model predictive control of nonlinear processes using transfer learning-based recurrent neural networks. Chem. Eng. Res. Des. 2024, 205, 1–12. [Google Scholar] [CrossRef]
- Tsantekidis, A.; Passalis, N.; Tefas, A. Chapter 5—Recurrent neural networks. In Deep Learning for Robot Perception and Cognition; Iosifidis, A., Tefas, A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 101–115. [Google Scholar]
- Mienye, I.D.; Swart, T.G.; Obaido, G. Recurrent Neural Networks: A Comprehensive Review of Architectures, Variants, and Applications. Information 2024, 15, 517. [Google Scholar] [CrossRef]
- Waqas, M.; Humphries, U.W. A critical review of RNN and LSTM variants in hydrological time series predictions. MethodsX 2024, 13, 102946. [Google Scholar] [CrossRef]
- Olaniyi, E.O.; Kucha, C.; Dahiya, P.; Niu, A. Intelligent sorting of pecan shelled products using hyperspectral fingerprints and deep learning. J. Food Eng. 2025, 395, 112533. [Google Scholar] [CrossRef]
- Chitty-Venkata, K.T.; Mittal, S.; Emani, M.; Vishwanath, V.; Somani, A.K. A survey of techniques for optimizing transformer inference. J. Syst. Archit. 2023, 144, 102990. [Google Scholar] [CrossRef]
- Faqeerzada, M.A.; Lohumi, S.; Kim, G.; Joshi, R.; Lee, H.; Kim, M.S.; Cho, B.-K. Hyperspectral Shortwave Infrared Image Analysis for Detection of Adulterants in Almond Powder with One-Class Classification Method. Sensors 2020, 20, 5855. [Google Scholar] [CrossRef]
- Nakariyakul, S.; Casasent, D.P. Classification of internally damaged almond nuts using hyperspectral imagery. J. Food Eng. 2011, 103, 62–67. [Google Scholar] [CrossRef]
- Dodevska, M.; Markovic, J.K.; Sofrenic, I.; Tesevic, V.; Jankovic, M.; Djordjevic, B.; Ivanovic, N.D. Similarities and differences in the nutritional composition of nuts and seeds in Serbia. Front. Nutr. 2022, 9, 1003125. [Google Scholar] [CrossRef]
- Lees, M.; Morin, J.-F. Foodintegrity Handbook: A Guide to Food Authenticity Issues and Analytical Solutions; Eurofins Analytics France: Nantes Cedex, France, 2018. [Google Scholar]
- Meerza, S.I.A.; Giannakas, K.; Yiannaka, A. Markets and welfare effects of food fraud. Aust. J. Agric. Resour. Econ. 2019, 63, 759–789. [Google Scholar] [CrossRef]
- Meerza, S.I.A.; Gustafson, C.R. Consumers’ Response to Food Fraud. J. Agric. Resour. Econ. 2020, 45, 219–231. [Google Scholar]
- Netto, J.M.; Honorato, F.A.; Celso, P.G.; Pimentel, M.F. Authenticity of almond flour using handheld near infrared instruments and one class classifiers. J. Food Compos. Anal. 2023, 115, 104981. [Google Scholar] [CrossRef]
- Faqeerzada, M.A.; Perez, M.; Lohumi, S.; Lee, H.; Kim, G.; Wakholi, C.; Joshi, R.; Cho, B.-K. Online Application of a Hyperspectral Imaging System for the Sorting of Adulterated Almonds. Appl. Sci. 2020, 10, 6569. [Google Scholar] [CrossRef]
- Mishra, P.; Herrero-Langreo, A.; Barreiro, P.; Roger, J.M.; Diezma, B.; Gorretta, N.; Lleó, L. Detection and Quantification of Peanut Traces in Wheat Flour by near Infrared Hyperspectral Imaging Spectroscopy Using Principal-Component Analysis. J. Near Infrared Spectrosc. 2015, 23, 15–22. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.; Ni, X.; Chu, X.; Li, Y.-F.; Lu, C. Utilising near-infrared hyperspectral imaging to detect low-level peanut powder contamination of whole wheat flour. Biosyst. Eng. 2019, 184, 55–68. [Google Scholar] [CrossRef]
- Vega-Castellote, M.; Sánchez, M.-T.; Kim, M.S.; Hwang, C.; Pérez-Marín, D. Investigating the detection of peanuts in chopped nut products using hyperspectral imaging systems. J. Food Eng. 2025, 388, 112378. [Google Scholar] [CrossRef]
- Faqeerzada, M.A.; Lohumi, S.; Joshi, R.; Kim, M.S.; Baek, I.; Cho, B.-K. Non-Targeted Detection of Adulterants in Almond Powder Using Spectroscopic Techniques Combined with Chemometrics. Foods 2020, 9, 876. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Reina, R.; Callejón, R.M.; Amigo, J.M. Feasibility of a rapid and non-destructive methodology for the study and discrimination of pine nuts using near-infrared hyperspectral analysis and chemometrics. Food Control 2021, 130, 108365. [Google Scholar] [CrossRef]
- Gama, T.; Wallace, H.M.; Trueman, S.J.; Hosseini-Bai, S. Quality and shelf life of tree nuts: A review. Sci. Hortic. 2018, 242, 116–126. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Z.; Khoshelham, K.; Bai, S.H. Quality estimation of nuts using deep learning classification of hyperspectral imagery. Comput. Electron. Agric. 2021, 180, 105868. [Google Scholar] [CrossRef]
- Panda, B.K.; Mishra, G.; Ramirez, W.A.; Jung, H.; Singh, C.B.; Lee, S.-H.; Lee, I. Rancidity and moisture estimation in shelled almond kernels using NIR hyperspectral imaging and chemometric analysis. J. Food Eng. 2022, 318, 110889. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Z.; Ma, W.; Zhang, M.; Yang, L. Hyperspectral detection of walnut protein contents based on improved whale optimized algorithm. Int. J. Agric. Biol. Eng. 2022, 15, 235–241. [Google Scholar] [CrossRef]
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Hernández-Hierro, J.M.; Garcia, R.; Barroso, J.M.; Heredia, F.J.; Rato, A.E. Assessment of Total Fat and Fatty Acids in Walnuts Using Near-Infrared Hyperspectral Imaging. Front. Plant Sci. 2021, 12, 729880. [Google Scholar] [CrossRef]
- Mohammadi-Moghaddam, T.; Razavi, S.M.A.; Taghizadeh, M.; Pradhan, B.; Sazgarnia, A.; Shaker-Ardekani, A. Hyperspectral imaging as an effective tool for prediction the moisture content and textural characteristics of roasted pistachio kernels. J. Food Meas. Charact. 2018, 12, 1493–1502. [Google Scholar] [CrossRef]
- Tahmasbian, I.; Wallace, H.M.; Gama, T.; Hosseini Bai, S. An automated non-destructive prediction of peroxide value and free fatty acid level in mixed nut samples. LWT 2021, 143, 110893. [Google Scholar] [CrossRef]
- Ezenarro, J.; Saouabi, I.; García-Pizarro, Á.; Schorn-García, D.; Mestres, M.; Amigo, J.M.; Busto, O.; Boqué, R. NIR-HSI for the non-destructive monitoring of in-bag hazelnut oxidation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 333, 125906. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Qiu, J.; Liu, X.; Chen, M.; Cao, W.; Chang, S.; Zhao, X. Combining VNIR and NIR hyperspectral imaging techniques with a data fusion strategy for the determination of fat content, acid value, and storage time of walnuts. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 343, 126355. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Wang, M.-Y.; Kuo, Y.-M.; Chan, Y.-C.; Chen, Y.-C. Almond defect and freshness inspection system using hyperspectral imaging and deep learning techniques. Postharvest Biol. Technol. 2024, 211, 112837. [Google Scholar] [CrossRef]
- Xu, J.; Xu, D.; Bai, X.; Yang, R.; Cao, J. Non-Destructive Detection of Moldy Walnuts Based on Hyperspectral Imaging Technology. Molecules 2022, 27, 6776. [Google Scholar] [CrossRef]
- Nakariyakul, S. Internal damage inspection of almond nuts using optimal near-infrared waveband selection technique. J. Food Eng. 2014, 126, 173–177. [Google Scholar] [CrossRef]
- Bonifazi, G.; Capobianco, G.; Gasbarrone, R.; Serranti, S. Contaminant detection in pistachio nuts by different classification methods applied to short-wave infrared hyperspectral images. Food Control 2021, 130, 108202. [Google Scholar] [CrossRef]
- Kheiralipour, K.; Ahmadi, H.; Rajabipour, A.; Rafiee, S.; Javan-Nikkhah, M.; Jayas, D.; Siliveru, K. Detection of fungal infection in pistachio kernel by long-wave near-infrared hyperspectral imaging technique. Qual. Assur. Saf. Crops Foods 2016, 8, 129–135. [Google Scholar] [CrossRef]
- Qiao, X.; Jiang, J.; Qi, X.; Guo, H.; Yuan, D. Utilization of spectral-spatial characteristics in shortwave infrared hyperspectral images to classify and identify fungi-contaminated peanuts. Food Chem. 2017, 220, 393–399. [Google Scholar] [CrossRef]
- Jiang, J.; Qiao, X.; He, R. Use of Near-Infrared hyperspectral images to identify moldy peanuts. J. Food Eng. 2016, 169, 284–290. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Wu, Y.-C.; Kuo, Y.-M.; Zhang, R.-H.; Cheng, T.-Y.; Chen, Y.-C.; Chu, P.-Y.; Kang, L.-W.; Lin, C. Automated peanut defect detection using hyperspectral imaging and deep learning: A real-time approach for smart agriculture. Smart Agric. Technol. 2025, 11, 100943. [Google Scholar] [CrossRef]
- Mahmoud, Y.A.G.; Elkaliny, N.E.; Darwish, O.A.; Ashraf, Y.; Ebrahim, R.A.; Das, S.P.; Yahya, G. Comprehensive review for aflatoxin detoxification with special attention to cold plasma treatment. Mycotoxin Res. 2025, 41, 277–300. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, F. Global burden of aflatoxin-induced hepatocellular carcinoma: A risk assessment. Environ. Health Perspect. 2010, 118, 818–824. [Google Scholar] [CrossRef]
- Zhongzhi, H.; Limiao, D. Application driven key wavelengths mining method for aflatoxin detection using hyperspectral data. Comput. Electron. Agric. 2018, 153, 248–255. [Google Scholar] [CrossRef]
- Teena, M.; Manickavasagan, A.; Mothershaw, A.; El Hadi, S.; Jayas, D.S. Potential of Machine Vision Techniques for Detecting Fecal and Microbial Contamination of Food Products: A Review. Food Bioprocess Technol. 2013, 6, 1621–1634. [Google Scholar] [CrossRef]
- Zhongzhi, H.; Limiao, D. Aflatoxin contaminated degree detection by hyperspectral data using band index. Food Chem. Toxicol. 2020, 137, 111159. [Google Scholar] [CrossRef]
- Qi, X.; Jiang, J.; Cui, X.; Yuan, D. Identification of fungi-contaminated peanuts using hyperspectral imaging technology and joint sparse representation model. J. Food Sci. Technol. 2019, 56, 3195–3204. [Google Scholar] [CrossRef]
- Yang, G.; Tian, X.; Fan, Y.; Xiang, D.; An, T.; Huang, W.; Long, Y. Identification of Peanut Kernels Infected with Multiple Aspergillus flavus Fungi Using Line-Scan Raman Hyperspectral Imaging. Food Anal. Methods 2024, 17, 155–165. [Google Scholar] [CrossRef]
- Mishra, G.; Panda, B.K.; Ramirez, W.A.; Jung, H.; Singh, C.B.; Lee, S.-H.; Lee, I. Application of SWIR hyperspectral imaging coupled with chemometrics for rapid and non-destructive prediction of Aflatoxin B1 in single kernel almonds. LWT 2022, 155, 112954. [Google Scholar] [CrossRef]
- Mishra, G.; Panda, B.K.; Ramirez, W.A.; Jung, H.; Singh, C.B.; Lee, S.-H.; Lee, I. Detection of aflatoxin contamination in single kernel almonds using multispectral imaging system. J. Food Compos. Anal. 2024, 125, 105701. [Google Scholar] [CrossRef]
- Kabir, M.A.; Lee, I.; Singh, C.B.; Mishra, G.; Panda, B.K.; Lee, S.-H. Correlation awareness evolutionary sparse hybrid spectral band selection algorithm to detect aflatoxin B1 contaminated almonds using hyperspectral images. Food Chem. 2025, 476, 143381. [Google Scholar] [CrossRef]
- Williams, L.; Shukla, P.; Sheikh-Akbari, A.; Mahroughi, S.; Mporas, I. Measuring the Level of Aflatoxin Infection in Pistachio Nuts by Applying Machine Learning Techniques to Hyperspectral Images. Sensors 2025, 25, 1548. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.A.; Lee, I.; Lee, S.-H. Deep Learning-Based Detection of Aflatoxin B1 Contamination in Almonds Using Hyperspectral Imaging: A Focus on Optimized 3D Inception–ResNet Model. Toxins 2025, 17, 156. [Google Scholar] [CrossRef] [PubMed]
- Kandala, C.V.K.; Butts, C.L.; Lamb, M.C. Moisture content determination for in-shell peanuts with a low-cost impedance analyzer and capacitor sensor. Trans. ASABE 2008, 51, 1377–1381. [Google Scholar] [CrossRef]
- Vera Zambrano, M.; Dutta, B.; Mercer, D.G.; MacLean, H.L.; Touchie, M.F. Assessment of moisture content measurement methods of dried food products in small-scale operations in developing countries: A review. Trends Food Sci. Technol. 2019, 88, 484–496. [Google Scholar] [CrossRef]
- Jin, H.; Li, L.; Cheng, J. Rapid and Non-destructive Determination of Moisture Content of Peanut Kernels Using Hyperspectral Imaging Technique. Food Anal. Methods 2015, 8, 2524–2532. [Google Scholar] [CrossRef]
- Farrar, M.B.; Omidvar, R.; Nichols, J.; Pelliccia, D.; Al-Khafaji, S.L.; Tahmasbian, I.; Hapuarachchi, N.; Bai, S.H. Hyperspectral imaging predicts macadamia nut-in-shell and kernel moisture using machine vision and learning tools. Comput. Electron. Agric. 2024, 224, 109209. [Google Scholar] [CrossRef]
- Jiang, H.; Xie, C.; Li, J.; Liu, W.; Li, T.; Zhou, H. Non-destructive assessment of moisture content of single Chinese walnut using hyperspectral imaging integrated with chemometric tools. J. Food Meas. Charact. 2025, 19, 7796–7808. [Google Scholar] [CrossRef]
- Jiang, L.; Zhu, B.; Rao, X.; Berney, G.; Tao, Y. Discrimination of black walnut shell and pulp in hyperspectral fluorescence imagery using Gaussian kernel function approach. J. Food Eng. 2007, 81, 108–117. [Google Scholar] [CrossRef]
- Jiang, L.; Zhu, B.; Jing, H.; Chen, X.; Rao, X.; Tao, Y. Gaussian Mixture Model-Based Walnut Shell and Meat Classification in Hyperspectral Fluorescence Imagery. Trans. ASABE 2007, 50, 153–160. [Google Scholar] [CrossRef]
- Jiang, H.; Ye, L.; Li, X.; Shi, M. Variety Identification of Chinese Walnuts Using Hyperspectral Imaging Combined with Chemometrics. Appl. Sci. 2021, 11, 9124. [Google Scholar] [CrossRef]
- Zhong, Q.; Zhang, H.; Tang, S.; Li, P.; Lin, C.; Zhang, L.; Zhong, N. Feasibility Study of Combining Hyperspectral Imaging with Deep Learning for Chestnut-Quality Detection. Foods 2023, 12, 2089. [Google Scholar] [CrossRef]
- Li, X.; Jiang, H.; Jiang, X.; Shi, M. Identification of Geographical Origin of Chinese Chestnuts Using Hyperspectral Imaging with 1D-CNN Algorithm. Agriculture 2021, 11, 1274. [Google Scholar] [CrossRef]
- Feng, Z.; Li, W.; Cui, D. Detection of endogenous foreign bodies in Chinese hickory nuts by hyperspectral spectral imaging at the pixel level. Int. J. Agric. Biol. Eng. 2022, 15, 204–210. [Google Scholar] [CrossRef]
- Moscetti, R.; Saeys, W.; Keresztes, J.C.; Goodarzi, M.; Cecchini, M.; Danilo, M.; Massantini, R. Hazelnut Quality Sorting Using High Dynamic Range Short-Wave Infrared Hyperspectral Imaging. Food Bioprocess Technol. 2015, 8, 1593–1604. [Google Scholar] [CrossRef]
- Olaniyi, E.; Kucha, C.; Dahiya, P.; Niu, A. Precision variety identification of shelled and in-shell pecans using hyperspectral imaging with machine learning. Infrared Phys. Technol. 2024, 142, 105570. [Google Scholar] [CrossRef]
- Stefansson, P.; Fortuna, J.; Rahmati, H.; Burud, I.; Konevskikh, T.; Martens, H. Chapter 2.12—Hyperspectral time series analysis: Hyperspectral image data streams interpreted by modeling known and unknown variations. In Data Handling in Science and Technology; Amigo, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 32, pp. 305–331. [Google Scholar]
- Workman, J.J. A Review of Calibration Transfer Practices and Instrument Differences in Spectroscopy. Appl. Spectrosc. 2018, 72, 340–365. [Google Scholar] [CrossRef]
- Huan, K.; Chen, X.; Song, X.; Dong, W. Variable selection in near-infrared spectra: Application to quantitative non-destructive determination of protein content in wheat. Infrared Phys. Technol. 2021, 119, 103937. [Google Scholar] [CrossRef]
- Guo, P.; Li, T.; Gao, H.; Chen, X.; Cui, Y.; Huang, Y. Evaluating Calibration and Spectral Variable Selection Methods for Predicting Three Soil Nutrients Using Vis-NIR Spectroscopy. Remote Sens. 2021, 13, 4000. [Google Scholar] [CrossRef]
- Zahavi, A.; Palshin, A.; Liyanage, D.; Tamre, M. Influence of Illumination Sources on Hyperspectral Imaging. In Proceedings of the 20th International Conference on Research and Education in Mechatronics (REM), Wels, Austria, 23–24 May 2019; pp. 1–5. [Google Scholar]
- Ks, P.; Basha, S.; Chattopadhyay, A.; Mahato, K.K. Recent advancements of light-emitting diodes in dairy industries. Trends Food Sci. Technol. 2025, 160, 105018. [Google Scholar] [CrossRef]
- Pettit, R.; Freese, J.M. Wavelength dependent scattering caused by dust accumulation on solar mirrors. Sol. Energy Mater. 1980, 3, 1–20. [Google Scholar] [CrossRef]
- Hassoun, A.; Dankar, I.; Bhat, Z.; Bouzembrak, Y. Unveiling the relationship between food unit operations and food industry 4.0: A short review. Heliyon 2024, 10, e39388. [Google Scholar] [CrossRef]
- Ghosh, S.; Hughes, M.; Hodgkinson, I.; Hughes, P. Digital transformation of industrial businesses: A dynamic capability approach. Technovation 2022, 113, 102414. [Google Scholar] [CrossRef]
- Grieves, M.; Vickers, J. Digital Twin: Mitigating Unpredictable, Undesirable Emergent Behavior in Complex Systems. In Transdisciplinary Perspectives on Complex Systems: New Findings and Approaches; Kahlen, F.-J., Flumerfelt, S., Alves, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 85–113. [Google Scholar]
- Kucha, C.; Olaniyi, E.O. Applications of hyperspectral imaging in meat tenderness detection: Current research and potential for digital twin technology. Food Biosci. 2024, 58, 103754. [Google Scholar] [CrossRef]
- Defraeye, T.; Tagliavini, G.; Wu, W.; Prawiranto, K.; Schudel, S.; Assefa Kerisima, M.; Verboven, P.; Bühlmann, A. Digital twins probe into food cooling and biochemical quality changes for reducing losses in refrigerated supply chains. Resour. Conserv. Recycl. 2019, 149, 778–794. [Google Scholar] [CrossRef]
- Pattanaik, S.; Jenamani, M. Numerical Analysis of Cooling Characteristics of Indian mangoes using Digital Twin. In Proceedings of the IECON 2020 The 46th Annual Conference of the IEEE Industrial Electronics Society, Singapore, 18–21 October 2020; pp. 3095–3101. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).