Abstract
Estrogens are cholesterol-derived hormones, with four endogenous estrogens being presented in the scientific literature, namely, estradiol, estrone, estriol, and estetrol. In this study, we aim to obtain a complete thermoanalytical profile for the three most important endogenous estrogens: estradiol, estriol, and estrone. To achieve this, the TG/DTG were registered in non-isothermal conditions at five different heating rates (β = 2, 4, 6, 8, and 10 °C min−1). To describe the mechanisms of the degradation processes, a complex kinetic analysis was performed by applying a preliminary method (ASTM E698), two isoconversional methods (Flynn–Wall–Ozawa and Friedman), and the non-parametric kinetic method. The results indicate that estradiol undergoes a single-step degradation process, while estriol and estrone present a complex degradation process. The determination of the shelf life of pharmaceutical products represents a critical factor in ensuring their safety and efficacy. This parameter can be estimated from the activation energy derived from non-isothermal experiments through the application of the Arrhenius equation and appropriate kinetic models.
1. Introduction
Estrogens are sex hormones, derived from cholesterol, which, in addition to their role in the development and function of female reproductive function, present a protective effect against neurodegenerative diseases, playing a key role in bone metabolism and cardiovascular function [1,2,3,4,5]. In the literature, there are four endogenous estrogens presented: estrone, estradiol, estriol, and estetrol (their chemical structures are illustrated in Figure 1A–D) [6]. The structural differences between the four hormones are reflected by the number of hydroxyl groups. Estradiol presents the most potent hormonal activity, being secreted predominantly during the premenopausal period by the ovaries. Estrone is the second most potent hormone and is mainly produced during menopause by the adipose tissue from dehydroepiandrosterone. The third hormone, estriol, is synthesised during pregnancy by the placenta. Estetrol is the latest endogenous estrogen, discovered in 1965, and it is produced solely by the fetal liver during the pregnancy period [6,7,8,9]. The four endogenous estrogens are currently used in contraceptive therapies for premenopausal women and hormonal replacement therapies [3,8,10].
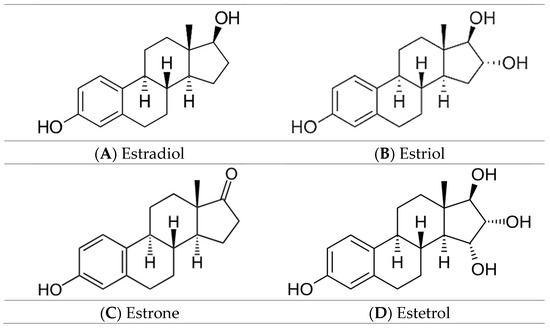
Figure 1.
The chemical structures of the four endogenous estrogens.
Despite the structural similarities that the four endogenous estrogens present, they show differences in their affinity for the three estrogen receptors (α, β, and GPR30) and pharmacokinetic properties [9,11,12,13,14]. In accordance with the intensity of the pharmacological effects, estradiol has the highest affinity for the estrogen receptors, followed by estrone, estriol, and estetrol. Concerning estetrol, it was observed that it can activate the nuclear estrogen receptor α, but when bound to the membrane estrogen receptor α, it showed antagonistic effects. As for binding to the human sex hormone-binding globulin, estetrol is the only endogenous estrogen without an affinity [15]. Regarding the bioavailability of the three most frequent endogenous estrogens (estradiol, estrone, and estriol), they present a low bioavailability of around 10%, due to an intense liver first-pass effect after oral administration [6,12], while estetrol presents the highest bioavailability among the four estrogens, namely ~90% [15,16]. The secondary metabolization processes of estetrol cannot produce any of the other three endogenous estrogens, while estradiol, estrone, and estriol can be converted into each other [6,12,15,17].
Taking into account that the doses used in the hormonal therapies are low (for endogenous hormones are around 1 mg of active pharmaceutical ingredient), it is crucial to maintain the structural integrity of the compound and to understand the possible degradation processes that may occur during non-isothermal heating conditions (in the preformulation stage and in storage conditions) to achieve an optimum biopharmaceutical profile for the intended therapeutic effect. The first step in achieving this goal is to know the complete thermoanalytical profile and the nature of the processes that occur under thermal stress for the selected molecules, to ensure an optimal biopharmaceutical profile in the preformulation stage. Accordingly, in this study, we aim to obtain a fully characterized thermoanalytical profile for three endogenous estrogens: estradiol, estrone, and estriol. To accomplish this, we have used as investigational tools the thermoanalytical methods (TG/DTG), followed by the employment of the kinetic analysis of the first degradation process observable on the DTG curve of the active pharmaceutical ingredient. Initially, a preliminary kinetic method was performed (ASTM E698), followed by two isoconversional methods: Flynn–Wall–Ozawa (FWO) and Friedman (FR). To obtain a more in-depth evaluation of the processes underlying the degradation, the modified non-parametric kinetic method (NPK) was employed.
Since the complex thermal stability profile (by heterogeneous kinetics) investigations were not previously reported for these active substances, the present study was designed to determine the kinetic parameters associated with the initial decomposition process. These parameters play a crucial role in pharmaceutical research, as they serve as the basis for calculating the shelf life and for defining appropriate processing and storage conditions of pharmaceutical formulations containing these estrogens. The findings of this article can be applied by both researchers and the pharmaceutical industry in the phase of preformulation studies and development of new dosage forms.
Non-isothermal kinetics involve the study of chemical reaction rates under changing temperature conditions and represent a powerful analytical approach in the pharmaceutical field. Non-isothermal kinetic methods mimic the storage conditions more accurately, especially when characterizing the drug stability and production methods of the pharmaceutical formulation. One of the most crucial applications of non-isothermal kinetics is to analyze the thermal stability of active pharmaceutical ingredients and drug products. Pharmaceuticals are often subjected to temperature fluctuations during production (such as drying, granulation, and synthesis), transport, and storage. Poorly controlled thermal conditions can lead to degradation of the active molecule, loss of efficacy, or the formation of toxic degradation products. Understanding how a compound behaves under these conditions is essential for ensuring that it remains effective and safe. Using thermal analysis techniques such as thermogravimetric analysis and differential scanning calorimetry, the degradation patterns can be observed, the activation energy can be calculated, and the model reaction mechanisms can be predicted. These insights help estimate a product’s shelf life under both standard and accelerated conditions.
The ASTM E698 is a preliminary kinetic method where the activation energy is determined without taking into consideration the reaction progress, and it is suitable for single-step reactions [18,19,20,21]. The activation energy is determined with the help of the following equation:
The FWO integral isoconversional kinetic method follows the changes in the values of the activation energy determined from the slope of each thermogravimetric curve (ln β vs. T−1). This method is suitable for complex decomposition processes and uses Doyle approximation [18,22,23,24]. The equation for the activation energy is as follows:
The FR differential isoconversional method is the most accurate kinetic method, presenting the advantages of the calculation of the activation energy without using any approximation, and the fact that it can be employed on isothermal and non-isothermal processes [25,26,27]. This method uses the following equation:
The NPK method, originally presented by Serra, Sempere, and Nomen [28,29,30,31] and subsequently modified by Vlase, Vlase, and Doca [32], is a kinetic method that allows the determination of the kinetic triplet without using a model for k(T) or for f(α). This method is based on the fact that the reaction rate can be expressed as the result of two independent functions; the first one is dependent only on the temperature, and the second one is dependent only on the conversion:
Another advantage of this kinetic approach is that it offers details about the type of processes that occur during the decomposition of the active pharmaceutical ingredient, chemical reactions, or physical transformations, with the help of the reaction orders. The contribution of each process to the global decomposition process is rendered by the value of the variance. This kinetic method follows the model of Šesták and Berggren [33]:
The Šesták-Berggren equation [27] represents only a generalization of the proposed and existing conversion functions presented in the scientific literature. The importance of this kinetic method stems from the fact that it does not use any approximation. The parameters m and n characterize the nature of the processes that occur under non-isothermal conditions. A value different from zero for m indicates the presence of at least one physical phenomenon, such as diffusion, recrystallization, or other phase transformations, while a value different from zero for n suggests a chemical transformation.
The novelty of the present study lies in establishing, for the first time, a complete heterogeneous kinetic profile for three widely used endogenous estrogens, which is essential for defining accurate stability-related parameters for preformulation and pharmaceutical development.
2. Materials and Methods
2.1. Samples
The three active pharmaceutical ingredients were used without any further purification and acquired as follows: micronized estradiol hemihydrate with lot 2103014-02 was obtained from EURO OTC & Audor Pharma(Bönen, Germany), estriol with lot 2111005-01 was obtained from EURO OTC & Audor Pharma, and estrone with lot 19220670 was obtained from Galeno (Comeana, Italy).
2.2. ATR-FTIR Investigations
To record the spectra of the three estrogens (estradiol hemihydrate, estriol, and estrone), an IRSpirit Fourier Transform Infrared Spectrophotometer (Shimadzu, Kyoto, Japan) was used. The measurements were performed in the spectral range of 4000 and 400 cm−1, and the spectrum was built after 32 co-added scans at a resolution of 2 cm−1.
2.3. Thermal Investigations
To obtain the thermoanalytical curves, a Setline TGA (SETARAM, Caluire, France) instrument was used with open alumina crucibles in a dynamic air atmosphere (100 mL min−1). The thermograms were registered in non-isothermal conditions at five different heating rates, namely β = 2, 4, 6, 8, and 10 °C min−1, from ambient temperature up to 800 °C, each sample weighing around 7 mg. To obtain the DSC curves, a NETZSCH DSC 204 F1 Phoenix instrument(Selb, Germany) was used. The samples were placed in sealed aluminum crucibles, and the heating process took place in an inert nitrogen medium with a flow rate of 20 mL min−1, from ambient temperature up to 300 °C, with a heating rate of β = 10 °C min−1.
2.4. Kinetic Analysis
The kinetic analysis was performed for the first decomposition process observed on the DTG curve for estriol and estrone, while for estradiol hemihydrate, the analysis was performed on the second degradation process noticed on the DTG curve (process associated with the degradation of the anhydrous estradiol). One preliminary kinetic method was used (ASTM E698), followed by two isoconversional methods: Friedman (FR) and Flynn–Wall–Ozawa (FWO). The kinetic software used was AKTS Thermokinetics software, Version 4.46 (AKTS AG TechnoArk, Siders, Switzerland). For a more in-depth evaluation of the processes that occurred during the thermal stress, the modified non-parametric method (NPK) was employed. The working protocol has already been extensively explained in the previous scientific work published by our research group [34,35,36]. The abbreviations used and the work protocol are those recommended and accepted by the ICTAC committee [37,38].
3. Results and Discussion
3.1. ATR-FTIR Investigations
In Figure 2, the spectra obtained for the three endogenous estrogens are represented, while in Table S1, the wavenumbers for each peak are presented.
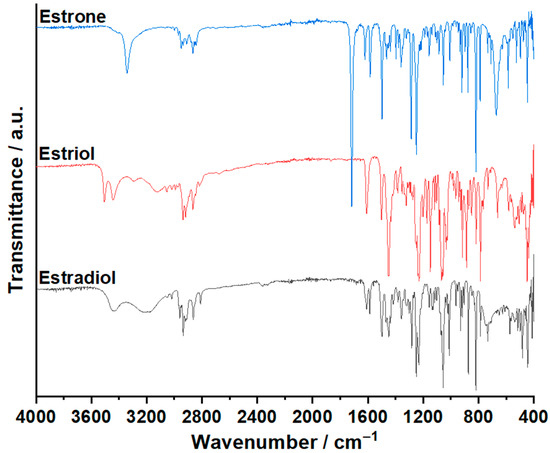
Figure 2.
The spectra for the three endogenous estrogens.
The estradiol hemihydrate FTIR spectrum highlights a broad absorption band in the spectral range of 3500–3050 cm−1, belonging to the hydroxyl group. The first peak at 3435 cm−1 is associated with the two hydroxyl radicals from the estradiol molecule, while the second at 3198 cm−1 is associated with the hydroxyl from the water of crystallization. The absorption peaks for the C–O moiety are clearly separated, at 1231 cm−1 for the phenolic hydroxyl group and at 1156 cm−1 for the alcoholic hydroxyl group. The C–H stretching vibrations are revealed at 2960, 2936, 2911, and 2863 cm−1. The bending vibrations from the same moiety are observable at 819 and 786 cm−1. The intense absorption peaks from 1609, 1586, 1469, 1448, 1437, and 1250 cm−1 belong to the aromatic ring.
The FTIR spectrum of estriol presents two intense peaks at 3505 and 3443 cm−1 characteristic of the stretching vibrations of the hydroxyl radicals; the first one can be associated with the alcoholic hydroxyl group and the second one with the phenolic hydroxyl group. The in-plane bending vibrations of the phenolic hydroxyl radical can be noticed at 1384 cm−1. The spectrum reveals one intense absorption peak at 1230 cm−1 belonging to the C–O moiety of the phenolic hydroxyl radical and another intense absorption band with three peaks at 1067, 1062, and 1056 cm−1 associated with the C–O moiety of the alcoholic hydroxyl radical. The C–H stretching vibrations are highlighted in the spectral region of 2950–2850 cm−1, with peaks at 2937, 2918, and 2866 cm−1. The C–H bending vibrations can be noticed at 818 and 786 cm−1. The vibrations from the aromatic ring can be observed at 1609, 1501, 1452, 1448, 1254, and 1248 cm−1 as intense absorption peaks.
The spectrum of estrone revealed an intense absorption peak at 3341 cm−1 caused by the stretching vibrations of the phenolic hydroxyl radical. The in-plane bending vibrations from the same group are noticeable at 1360 cm−1. The vibrations of the C–O moiety are highlighted at 1249 cm−1. The ketone group is identified as an intense absorption peak with a maximum at 1718 cm−1. The peaks associated with the aromatic ring can be identified at 1621, 1584, 1498, and 1286 cm−1. The C–H stretching vibrations are noticed in the spectral region between 2950 and 2840 cm−1, while the bending vibrations are observed at 818 and 788 cm−1.
3.2. Thermal Investigations
In Figure 3A–C, the thermoanalytical curves TG and DTG are represented, and the data obtained for the selected endogenous estrogens at a heating rate of β = 10 °C min−1 are shown in Table 1.
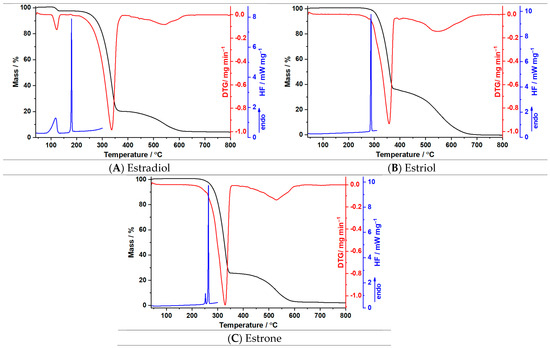
Figure 3.
The obtained thermoanalytical curves for the three endogenous estrogens recorded at a heating rate of 10 °C min−1.

Table 1.
The obtained data from the thermoanalytical curves at a heating rate of β = 10 °C min−1.
The thermal analysis for estradiol hemihydrate revealed three decomposition processes. The first one in the temperature range of 103–136 °C, accompanied by a mass loss of 3.0% and a maximum of 122 °C on the DTG curve, is characteristic of the loss of water. The same process is noticeable on the DSC curve as an endothermic peak with a maximum at 118 °C. After the dehydration, another endothermic peak can be observed at 180 °C, which can be associated with the melting of estradiol, a value in agreement with the data presented in the literature [39,40]. The degradation process of anhydrous estradiol starts at 191 °C and finishes at 382 °C with Δm = 77.6%, associated with a DTG maximum at 336 °C. The third decomposition process noticeable on the DTG curve begins at 403 °C and ends at 642 °C, with a maximum at 540 °C and a loss of mass equal to 15.3%.
Regarding estriol, the thermal decomposition takes place in two individual steps noticeable on the TG/DTG curves. The first degradation process commences at 251 °C up to 386 °C with a mass loss of more than half (Δm ≈ 65%) and a DTG maximal value of 358 °C. In the same temperature range, an intense endothermic peak can be observed on the DSC curve at 287 °C, characterizing the melting point of the active pharmaceutical ingredient, with a value in agreement with the scientific literature [41,42]. For the second process, which occurs in the temperature range of 402–712 °C, the mass loss is ~ 35% and a maximum on the DTG curve is noticed at 547 °C.
The decomposition of estrone begins at 212 °C and consists of two distinct degradation processes. The first one is observable on the DTG curve at a maximal value of 332 °C and is characterized by a mass loss of 75.3%. The second process takes place between 384 and 617 °C, with the DTG maximum being at 549 °C and the loss of mass close to one quarter. The DSC curve of estrone revealed two sharp endothermic peaks at 252 °C and 264 °C. The appearance of two phenomena is caused by the different polymorphs of estrone, presented in scientific literature as follows: Form I melts at 259 °C, Form II melts at 256 °C, and Form III melts at 254 °C [43]; our thermogram highlighted Form I and Form III. The values obtained for the melting points concur with data presented in the literature [44,45].
3.3. Kinetic Analysis
To facilitate a clear understanding of the analytical strategy, it is important to note from the outset that all three estrogens were examined using the same structured workflow. For each compound, the primary decomposition event identifiable in the DTG curve was first selected to ensure that the kinetic evaluation targeted the most relevant thermal transition. Subsequently, four complementary kinetic methods recommended by the ICTAC Committee were applied in a consistent sequence. This stepwise approach—beginning with the preliminary ASTM E698 method and followed by the Friedman, Flynn–Wall–Ozawa, and NPK analyses—enabled the construction of coherent, comparable, and mechanistically meaningful kinetic profiles for each estrogen. By maintaining an identical protocol across all active substances, the resulting data allow direct side-by-side interpretation, highlighting both similarities and intrinsic differences in their degradation behavior.
The kinetic analysis was performed on the first decomposition process observed in the DTG curve for each of the selected anhydrous estrogens; the temperature ranges selected for each heating rate are presented in Table 2.

Table 2.
The selected temperature ranges for each estrogen at the five heating rates.
The protocol selected to determine the kinetic parameters of the decomposition process for the three active substances includes four kinetic methods. These methods were chosen in accordance with ICTAC recommendations [38] and considering the different evaluation of the kinetic parameters. Thus, the ASTM E698 method estimates the activation energy under the assumption that the decomposition reaction follows a single mechanism; the Friedman method is sensitive, as it is a differential isoconversional method that analyzes the process ‘point by point’; the Flynn–Wall–Ozawa method is an integral method and provides only global activation energy values without mechanistic insight; and the NPK method requires extensive datasets and an mathematical algorithm, which may limit its applicability. The results of the kinetic analysis for the anhydrous estradiol are presented in Figure 4A–F and Figure 5, while for the other two compounds are presented in Figure 6A–F and Figure 7 (estriol), respectively Figure 8A–F and Figure 9 (estrone). By employing the preliminary method, ASTM E698, the values of the activation energy were obtained for all the active substances (Figure 4A, Figure 6A, and Figure 8A).
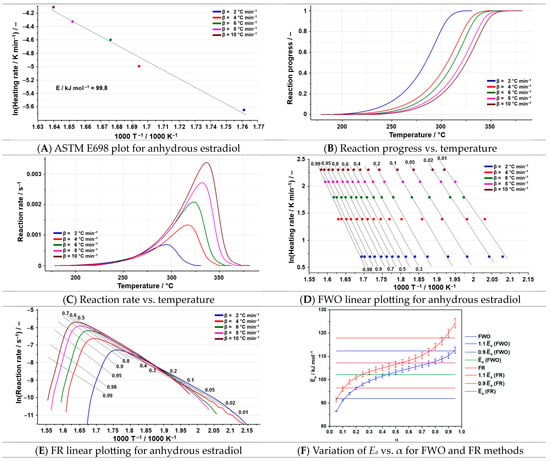
Figure 4.
The results of the kinetic analysis for the anhydrous estradiol.
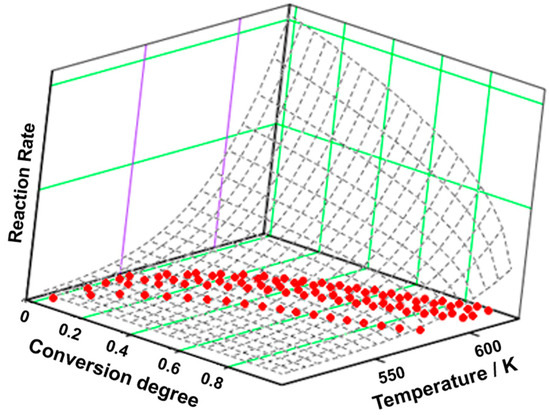
Figure 5.
The 3D transformation surface for estradiol from the NPK method.
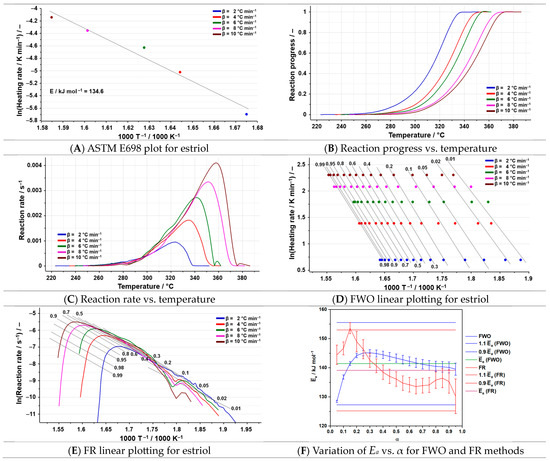
Figure 6.
The results of the kinetic analysis for estriol.
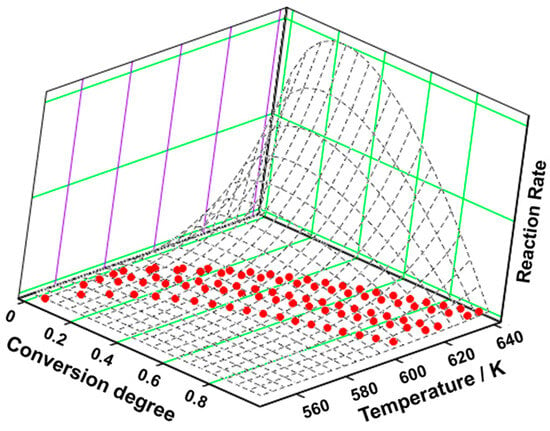
Figure 7.
The 3D transformation surface for estriol from the NPK method.
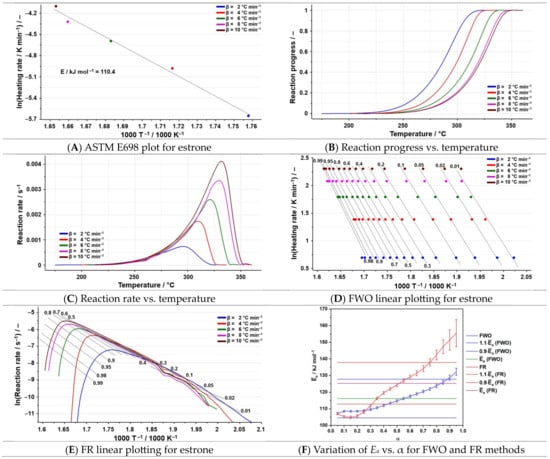
Figure 8.
The results of the kinetic analysis for estrone.
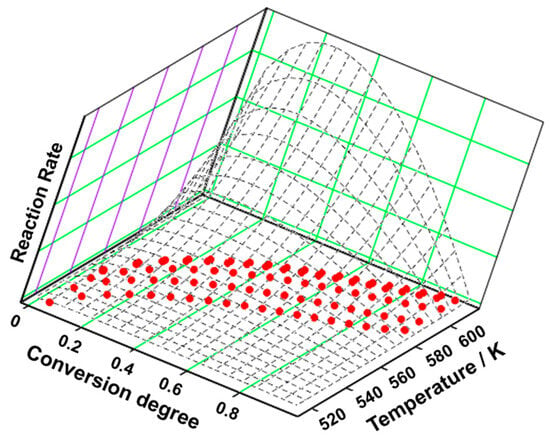
Figure 9.
The 3D transformation surface for estrone from the NPK method.
By using the work protocol recommended by the ICTAC Committee, the following applied kinetic methods are the isoconversional methods, namely FWO and FR.
The values of the activation energy obtained with the help of two isoconversional methods for each estrogen are represented in Table 3.

Table 3.
The values of the activation energy obtained with FWO and FR kinetic methods.
In the case of estradiol, the investigated degradation process is in the temperature range of 179–331 °C at a heating rate of 2 °C min−1, a degradation process characteristic of the anhydrous estradiol. With an increase in the heating rate, the selected degradation process shifts to a higher temperature due to thermal inertia. In Figure 4A–F, the results of the kinetic analysis for the anhydrous estradiol are represented. For estradiol, the obtained value of the activation energy using ASTM E698 is 99.8 kJ mol−1 (Figure 4A). The results of the two isoconversional methods are consistent with the preliminary method (Table 3), the average values of the activation energies obtained being 102.1 kJ mol−1 (FWO) and 107.2 kJ mol−1 (FR).
From the variation in Ea vs. α, it can be observed that most of the values of the activation energy are within the range of ±10%, starting with a value of approximately 90 kJ mol−1, which increases up to 120 kJ mol−1 with the growth of the conversion degree. For the FWO kinetic method, the values outside the considered range are observed at α equal to 0.05, 0.10, and 0.95. For the FR kinetic method, the values outside the interval are observed at α equal to 0.05, 0.90, and 0.95. Those results correlated with the ones of the preliminary method indicate that the decomposition of estradiol is the result of a single-step process.
In Table 4, the results of the NPK analysis for estradiol are presented, and in Figure 5, the 3D graphic of the experimental points is highlighted together with the interpolation of the reaction rate as a continuous surface.

Table 4.
The results of the NPK analysis for estradiol compared to the isoconversional methods.
The NPK kinetic method shows that the degradation of estradiol is the result of two simultaneous processes. The first one represents the greatest contribution to the global process (λ ≈ 75%) and is the result of a chemical reaction (n = 2/5). The second process, described by physical transformations (m = 1), contributes only 22.5% to the degradation of the active pharmaceutical ingredient. The activation energy is the result of the following equation: .
For the second endogenous estrogen selected, namely estriol, the highest value of the activation energy is noticed. The analyzed process is represented by the first one observed on the DTG curve. In Figure 5, the results of the kinetic analysis performed on estriol are represented.
By employing the ASTM E698 kinetic method on estriol (134.6 kJ mol−1), a value for Ea closest to that of the isoconversional methods was obtained (Figure 6A and Table 3).
By analyzing the results of the FWO method, a rapid increase in the value of the activation energy is observed up to α = 0.25, followed by a slight decrease until the end of the conversion. Those aspects suggest that a change in the degradation mechanism is possible. All the values of the activation energy can be found within the range of ± 10% for the FWO kinetic method. For the FR method, a sharp rise in the activation energy is noted up to α = 0.15, followed by a pronounced reduction in the values up to α = 0.65. After α = 0.75, another increase in the values of Ea is noted, with a rapid decrease after 0.90. These fluctuations demonstrate a change in the degradation mechanism. The differences between the fluctuations of the values of the activation energy between the two isoconversional methods may be due to the different processing of data integral for FWO and differential for FR. Outside the range of ± 10%, for the FR kinetic method, the value of the Ea corresponding to the conversion degree equal to 0.15 can be found. The inconsistency of the fluctuations indicates that estriol undergoes a complex degradation process, which consists of several simultaneous processes.
To understand the nature of the ongoing processes during the decomposition, the NPK method was employed, and in Figure 7 and Table 5, the results are presented.

Table 5.
The results of the NPK analysis for estriol compared to the isoconversional methods.
The NPK method revealed that the degradation of estriol consists of two simultaneous processes (Table 5), both consisting of chemical reactions (n = 1/3) and physical transformations (m = 1), but with different values for the activation energy; the first one presents Ea = 134.0 kJ mol−1 while the second has Ea = 189.0 kJ mol−1. Considering that the total variance value is equal to 99.5%, an accurate account of the process is provided.
The kinetic analysis performed on estrone revealed the following results, presented in Figure 8A–F.
After applying the ASTM E698 kinetic method on estrone (Figure 8A), the Ea obtained is 110.4 kJ mol−1, a value slightly smaller than the ones obtained for the two isoconversional methods (Table 3).
Regarding the variation in the activation energy, it can be noticed that for a conversion greater than 0.25 (Figure 8F), the values increase rapidly for both isoconversional methods, suggesting that a change in the degradation mechanism may occur. For the FWO kinetic method, only two values of the activation energy are noticed outside the range of ±10%, while for the FR method, the only values within this range are for α between 0.35 and 0.75. Given the results of the differential processing of the data and the fact that it is the most accurate kinetic method, it can be concluded that estrone undergoes a complex degradation process. In order to describe the decomposition process, the NPK kinetic method was also employed, and the results are highlighted in Figure 9 and Table 6.

Table 6.
The results of the NPK analysis for estrone compared to the isoconversional methods.
The degradation process of estrone is the result of two simultaneous processes, the first one presenting a contribution of 81.7% to the global process, while the second contributes only 17.9%. Both processes consist of chemical reactions (n = 1 for both processes) and physical degradations (m = 1/3 for the first one and m = 1/2 for the second one).
According to the results of all the kinetic methods applied in this study, a higher activation energy was observed for estriol, followed by estrone and then estradiol. A possible explanation for this fact is represented by the thermal stability of the three selected endogenous estrogens, which vary in the same way: the highest thermal degradation being observed for estriol (the thermal decomposition begins at 251 °C at a heating rate of 10 °C min−1), followed by estrone, which starts its degradation at 212 °C at a heating rate of 10 °C min−1, and afterwards by anhydrous estradiol (the decomposition begins at 191 °C at a heating rate of 10 °C min−1). In addition, the activation energy values vary in accordance with increasing melting point values of the three estrogens (287 °C for estriol, ≈255 °C for estrone, and 180 °C for estradiol).
Considering the results of kinetic analysis of the three estrogens, the following recommendations are proposed: due to observed dehydration and degradation temperatures for estradiol hemihydrate, storage conditions should be under tighter control (e.g., low humidity, controlled temperature) when compared to estrone and estriol. The two-step degradation patterns observed for estriol and estrone suggest the possibility of obtaining the intermediate degradation products, which can influence formulation stability and the biopharmaceutical profile of the subjected molecules.
The determination of shelf life (t90) for pharmaceutical products represents a criterion for ensuring quality, safety, and therapeutic efficacy, as it reflects the period during which the drug maintains its chemical stability and pharmacological potency within acceptable limits [46,47,48].
Estimation of shelf life based on kinetic parameters obtained from non-isothermal experiments involves determining the activation energy of degradation processes and applying the Arrhenius equation to extrapolate the rate constant (k) under storage conditions (25 °C):
Considering that the degradation kinetics follow a first-order model and using the rate constant (k) at the storage temperature (25 °C), this approach provides a solid scientific foundation for establishing the declared expiration period and defining optimal storage conditions, thereby constituting an indispensable tool in drug development and regulatory approval. The calculation of the shelf life is based on the following equation, which relates the rate constant of degradation to the time required for the drug to reach the defined stability limit under specified storage conditions, and the results are presented in Table 7:

Table 7.
The values of shelf life for the three analyzed compounds.
4. Conclusions
A study regarding the thermoanalytical profile of three endogenous estrogens, namely estradiol, estrone, and estriol, was performed. This paper represents the first systematic kinetic evaluation of these endogenous estrogens under non-isothermal conditions. The data provides valuable information about their thermal stability, which is essential for future pharmaceutical formulations. This study represents a first step in offering reliable kinetic parameters and thermal profiles for the selected compounds, which can support further technological applications.
All three compounds present high thermal stability, which varies in the following order: estriol, estrone, and estradiol. The thermal degradation of estradiol hemihydrate begins at 103 °C (β = 10 °C min−1), with the loss of water, followed by two degradation processes belonging to the decomposition of anhydrous estradiol (the first one starting at 191 °C and the second one at 403 °C, at β equal to 10 °C min−1). In the case of estriol, the decomposition begins at 251 °C (β = 10 °C min−1) and consists of two degradation processes. The mass loss of estrone starts at 212 °C (β = 10 °C min−1) and involves two degradation processes.
A detailed kinetic study was also carried out on the first degradation process of each anhydrous active pharmaceutical ingredient by using one preliminary kinetic method (ASTM E698), two isoconversional methods (FWO and FR), and, for a more in-depth evaluation of the degradation processes, the NPK kinetic method was also employed. Non-isothermal kinetics are an important tool in pharmaceutical technology, providing information regarding the thermal behavior of API, the stability, quality, and safety throughout the product lifecycle. By integrating non-isothermal kinetic methods into research and quality control processes, the pharmaceutical field can continue to deliver reliable, effective, and safe medications. The results stated that the degradation of estradiol consists of a single-step reaction, while for estriol and estrone, complex degradation processes were observed. The results showed that the values of the activation energies of the three molecules varied in the same way as their thermal stability and their melting points.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/analytica6040052/s1. Table S1. The wavenumbers for each peak observed on the spectra.
Author Contributions
Conceptualization, A.R., L.S. and F.P.; data curation, C.B., R.A.B. and I.L.; formal analysis, A.R., G.V. and I.L.; investigation, C.B., L.S., D.L.I. and F.P.; methodology, A.L., D.L.I. and T.V.; software, A.R., A.L., G.V. and T.V.; supervision, A.L. and I.L.; validation, R.A.B. and G.V.; visualization, C.B., D.L.I. and T.V.; writing—original draft, A.R., A.L. and R.A.B.; writing—review and editing, L.S., F.P. and I.L. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to acknowledge VICTOR BABES UNIVERSITY OF MEDICINE AND PHARMACY TIMISOARA for their support in covering the costs of publication for this research paper.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw data are available upon request from the corresponding author of this work.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| α | Conversion degree |
| t | Time |
| β | Linear heating rate (°C min−1) |
| A | Pre-exponential factor according to the Arrhenius kinetic model (min−1) |
| k(T) | Temperature-dependent reaction rate function |
| f(α) | Differential conversion function |
| g(α) | Integral conversion function |
| Ea | Activation energy (kJ mol−1) |
| R | Universal gas constant (J mol−1 K−1) |
| r | Reaction rate |
| T | Absolute temperature (K) |
| Δm | Mass loss over a specific temperature interval |
| FWO | Flynn–Wall–Ozawa kinetic method |
| FR | Friedman kinetic method |
References
- Cauley, J.A. Estrogen and Bone Health in Men and Women. Steroids 2015, 99, 11–15. [Google Scholar] [CrossRef]
- Simpkins, J.W.; Singh, M. More than a Decade of Estrogen Neuroprotection. Alzheimer’s Dement. 2008, 4, S131–S136. [Google Scholar] [CrossRef]
- Bustamante-Barrientos, F.A.; Méndez-Ruette, M.; Ortloff, A.; Luz-Crawford, P.; Rivera, F.J.; Figueroa, C.D.; Molina, L.; Bátiz, L.F. The Impact of Estrogen and Estrogen-Like Molecules in Neurogenesis and Neurodegeneration: Beneficial or Harmful? Front. Cell Neurosci. 2021, 15, 636176. [Google Scholar] [CrossRef]
- Scott, E.; Zhang, Q.G.; Wang, R.; Vadlamudi, R.; Brann, D. Estrogen Neuroprotection and the Critical Period Hypothesis. Front. Neuroendocrinol. 2012, 33, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Huang, Z.Y.; Xu, X.L.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen Receptor Function: Impact on the Human Endometrium. Front. Endocrinol. 2022, 13, 827724. [Google Scholar] [CrossRef] [PubMed]
- Stanczyk, F.Z. Metabolism of Endogenous and Exogenous Estrogens in Women. J. Steroid Biochem. Mol. Biol. 2024, 242, 106539. [Google Scholar] [CrossRef]
- Bennink, H.J.C. Are All Estrogens the Same? Maturitas 2004, 47, 269–275. [Google Scholar] [CrossRef]
- Coelingh Bennink, H.J.T.; Holinka, C.F.; Diczfalusy, E. Estetrol Review: Profile and Potential Clinical Applications. Climacteric 2008, 11, 47–58. [Google Scholar] [CrossRef]
- Holinka, C.F.; Diczfalusy, E.; Coelingh Bennink, H.J.T. Estetrol: A Unique Steroid in Human Pregnancy. J. Steroid Biochem. Mol. Biol. 2008, 110, 138–143. [Google Scholar] [CrossRef]
- Chaudhry, H.; Rangra, N.K.; Chawla, P.A. An Insight into the Use of Estetrol-Drospirenone as Effective Oral Contraceptive. Health Sci. Rev. 2023, 6, 100072. [Google Scholar] [CrossRef]
- Eyster, K.M. The Estrogen Receptors: An Overview from Different Perspectives. In Methods in Molecular Biology; Eyster, K.M., Ed.; Springer: New York, NY, USA, 2016; Volume 1366, pp. 1–10. ISBN 978-1-4939-3127-9. [Google Scholar]
- O’Connell, M.B. Pharmacokinetic and Pharmacologic Variation Between Different Estrogen Products. J. Clin. Pharmacol. 1995, 35, 18S–24S. [Google Scholar] [CrossRef]
- Yoh, K.; Ikeda, K.; Horie, K.; Inoue, S. Roles of Estrogen, Estrogen Receptors, and Estrogen-Related Receptors in Skeletal Muscle: Regulation of Mitochondrial Function. Int. J. Mol. Sci. 2023, 24, 1853. [Google Scholar] [CrossRef]
- Orzołek, I.; Sobieraj, J.; Domagała-Kulawik, J. Estrogens, Cancer and Immunity. Cancers 2022, 14, 2265. [Google Scholar] [CrossRef]
- Fruzzetti, F.; Fidecicchi, T.; Guevara, M.M.M.; Simoncini, T. Estetrol: A New Choice for Contraception. J. Clin. Med. 2021, 10, 5625. [Google Scholar] [CrossRef]
- Visser, M.; Coelingh Bennink, H.J.T. Clinical Applications for Estetrol. J. Steroid Biochem. Mol. Biol. 2009, 114, 85–89. [Google Scholar] [CrossRef]
- Ziegler, R.G.; Faupel-Badger, J.M.; Sue, L.Y.; Fuhrman, B.J.; Falk, R.T.; Boyd-Morin, J.; Henderson, M.K.; Hoover, R.N.; Veenstra, T.D.; Keefer, L.K.; et al. A New Approach to Measuring Estrogen Exposure and Metabolism in Epidemiologic Studies. J. Steroid Biochem. Mol. Biol. 2010, 121, 538–545. [Google Scholar] [CrossRef]
- Osman, A.I.; Fawzy, S.; Farrell, C.; Al-Muhtaseb, A.H.; Harrison, J.; Al-Mawali, S.; Rooney, D.W. Comprehensive Thermokinetic Modelling and Predictions of Cellulose Decomposition in Isothermal, Non-Isothermal, and Stepwise Heating Modes. J. Anal. Appl. Pyrolysis 2022, 161, 105427. [Google Scholar] [CrossRef]
- Fawzy, S.; Osman, A.I.; Farrell, C.; Al-Muhtaseb, A.H.; Harrison, J.; Al-Fatesh, A.S.; Fakeeha, A.H.; Doran, J.; Yang, H.; Rooney, D.W. Characterization and Kinetic Modeling for Pyrolytic Conversion of Cotton Stalks. Energy Sci. Eng. 2021, 9, 1908–1918. [Google Scholar] [CrossRef]
- Santana, N.d.S.; Monteiro, S.N.; da Silva, T.C.; Mothé, M.G. Kinetic Study of Commercial Tabletop Sweeteners Using Thermal Analysis. Biol. Life Sci. Forum 2025, 40, 35. [Google Scholar] [CrossRef]
- Sarkar, N.; Kiran, K.; Vijayan, N.; Joshi, D. Overview of Analysis on Thermal Stability and Hirshfeld Surface of Sodium Sulphamate Single Crystals. J. Mater. Sci. Mater. Electron. 2024, 35, 1674. [Google Scholar] [CrossRef]
- Khawam, A.; Flanagan, D.R. Role of Isoconversional Methods in Varying Activation Energies of Solid-State Kinetics: II. Nonisothermal Kinetic Studies. Thermochim. Acta 2005, 436, 101–112. [Google Scholar] [CrossRef]
- Baul, B.; Ledeţi, A.; Cîrcioban, D.; Ridichie, A.; Vlase, T.; Vlase, G.; Peter, F.; Ledeţi, I. Thermal Stability and Kinetics of Degradation of Moxonidine as Pure Ingredient vs. Pharmaceutical Formulation. Processes 2023, 11, 1738. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Maduka, T.O.; Suzuki, M.; Lu, S.; Wang, Q. Thermoanalytical and Kinetic Studies for the Thermal Stability of Emerging Pharmaceutical Pollutants Under Different Heating Rates. J. Xenobiotics 2024, 14, 1784–1806. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N. Interpretation and Physical Meaning of Kinetic Parameters Obtained from Isoconversional Kinetic Analysis of Polymers. Polymers 2020, 12, 1280. [Google Scholar] [CrossRef]
- Wang, B.; Yao, Z.; Reinmöller, M.; Kishore, N.; Tesfaye, F.; Luque, R. Pyrolysis Behavior, Kinetics, and Thermodynamics of Waste Pharmaceutical Blisters under CO2 Atmosphere. J. Anal. Appl. Pyrolysis 2023, 170, 105883. [Google Scholar] [CrossRef]
- Svoboda, R. Thermal Decomposition of Active Pharmaceutical Substances and Accuracy of the Related Kinetic Predictions: The Case of Nifedipine. Thermochim. Acta 2024, 738, 179790. [Google Scholar] [CrossRef]
- Serra, R.; Sempere, J.; Nomen, R. A New Method for the Kinetic Study of Thermoanalytical Data: The Non-Parametric Kinetics Method. Thermochim. Acta 1998, 316, 37–45. [Google Scholar] [CrossRef]
- Brown, M.E.; Maciejewski, M.; Vyazovkin, S.; Nomen, R.; Sempere, J.; Burnham, A.; Opfermann, J.; Strey, R.; Anderson, H.L.; Kemmler, A.; et al. Computational Aspects of Kinetic Analysis. Thermochim. Acta 2000, 355, 125–143. [Google Scholar] [CrossRef]
- Ferrer, N.; Serra, E.; Nomen, R.; Sempere, J. Thermal Decomposition of Tricyclohexylidene Triperoxide. J. Therm. Anal. Calorim. 2018, 134, 1293–1298. [Google Scholar] [CrossRef]
- Serra, R.; Nomen, R.; Sempere, J. The Non-Parametric Kinetics A New Method for the Kinetic Study of Thermoanalytical Data. J. Therm. Anal. 1998, 52, 933–943. [Google Scholar] [CrossRef]
- Vlase, T.; Vlase, G.; Doca, N.; Bolcu, C. Processing of Non-Isothermal TG Data: Comparative Kinetic Analysis with NPK Method. J. Therm. Anal. Calorim. 2005, 80, 59–64. [Google Scholar] [CrossRef]
- Šesták, J.; Berggren, G. Study of the Kinetics of the Mechanism of Solid-State Reactions at Increasing Temperatures. Thermochim. Acta 1971, 3, 1–12. [Google Scholar] [CrossRef]
- Ridichie, A.; Bengescu, C.; Ledeţi, A.; Rusu, G.; Bertici, R.; Vlase, T.; Vlase, G.; Peter, F.; Ledeţi, I.; Rădulescu, M. Thermal Stability, Preformulation, and Kinetic Degradation Studies for Gestrinone. J. Therm. Anal. Calorim. 2024, 150, 6785–6799. [Google Scholar] [CrossRef]
- Ridichie, A.; Ledeţi, A.; Peter, F.; Ledeţi, I.; Muntean, C.; Rădulescu, M. Kinetic Investigation of the Oxidative Thermal Decomposition of Levonorgestrel. Processes 2023, 11, 3210. [Google Scholar] [CrossRef]
- Ledeţi, A.; Baul, B.; Ridichie, A.; Ivan, D.; Vlase, T.; Tomoroga, C.; Dragomirescu, A.; Vlase, G.; Bertici, R.A.; Man, D.E.; et al. Thermooxidation of Four Sartans: Kinetic Analysis Based on Thermo-Gravimetric Data. Molecules 2024, 29, 5527. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Achilias, D.; Fernandez-Francos, X.; Galukhin, A.; Sbirrazzuoli, N. ICTAC Kinetics Committee Recommendations for Analysis of Thermal Polymerization Kinetics. Thermochim. Acta 2022, 714, 179243. [Google Scholar] [CrossRef]
- Koga, N.; Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Muravyev, N.V.; Pérez-Maqueda, L.A.; Saggese, C.; Sánchez-Jiménez, P.E. ICTAC Kinetics Committee Recommendations for Analysis of Thermal Decomposition Kinetics. Thermochim. Acta 2023, 719, 179384. [Google Scholar] [CrossRef]
- PubChem. Estradiol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Estradiol (accessed on 11 May 2025).
- Abdella, S.; Afinjuomo, F.; Song, Y.; Upton, R.; Garg, S. 3D Printed Bilayer Mucoadhesive Buccal Film of Estradiol: Impact of Design on Film Properties, Release Kinetics and Predicted in Vivo Performance. Int. J. Pharm. 2022, 628, 122324. [Google Scholar] [CrossRef]
- PubChem. Estriol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Estriol (accessed on 11 May 2025).
- Xu, J.; Ning, L. Study of Estriol Cocrystals. Her. Med. 2022, 41, 630–636. [Google Scholar] [CrossRef]
- Both, D. Estrone. In Analytical Profiles of Drug Substances and Excipients; Florey, K., Ed.; Analytical Profiles of Drug Substances; Academic Press: Cambridge, MA, USA, 1983; Volume 12, pp. 135–189. [Google Scholar]
- PubChem. Estrone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Estrone (accessed on 11 May 2025).
- Moodley, K.; Rarey, J.; Ramjugernath, D. Experimental Solubility for Betulin and Estrone in Various Solvents within the Temperature Range T = (293.2 to 328.2) K. J. Chem. Thermodyn. 2016, 98, 42–50. [Google Scholar] [CrossRef]
- Fisher, A.C.; Lee, S.L.; Harris, D.P.; Buhse, L.; Kozlowski, S.; Yu, L.; Kopcha, M.; Woodcock, J. Advancing Pharmaceutical Quality: An Overview of Science and Research in the U.S. FDA’s Office of Pharmaceutical Quality. Int. J. Pharm. 2016, 515, 390–402. [Google Scholar] [CrossRef]
- Yu, L.X.; Kopcha, M. The Future of Pharmaceutical Quality and the Path to Get There. Int. J. Pharm. 2017, 528, 354–359. [Google Scholar] [CrossRef]
- Capen, R.; Christopher, D.; Forenzo, P.; Ireland, C.; Liu, O.; Lyapustina, S.; O’Neill, J.; Patterson, N.; Quinlan, M.; Sandell, D.; et al. On the Shelf Life of Pharmaceutical Products. AAPS PharmSciTech 2012, 13, 911–918. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).