Analytical Method Development and Validation: Calcium Butyrate
Abstract
1. Introduction
2. Experimental
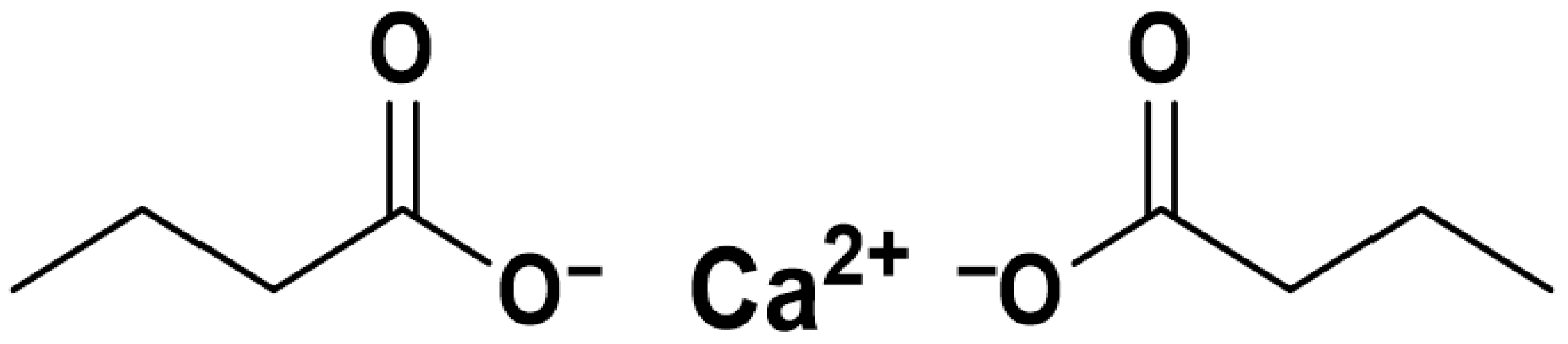
2.1. Materials
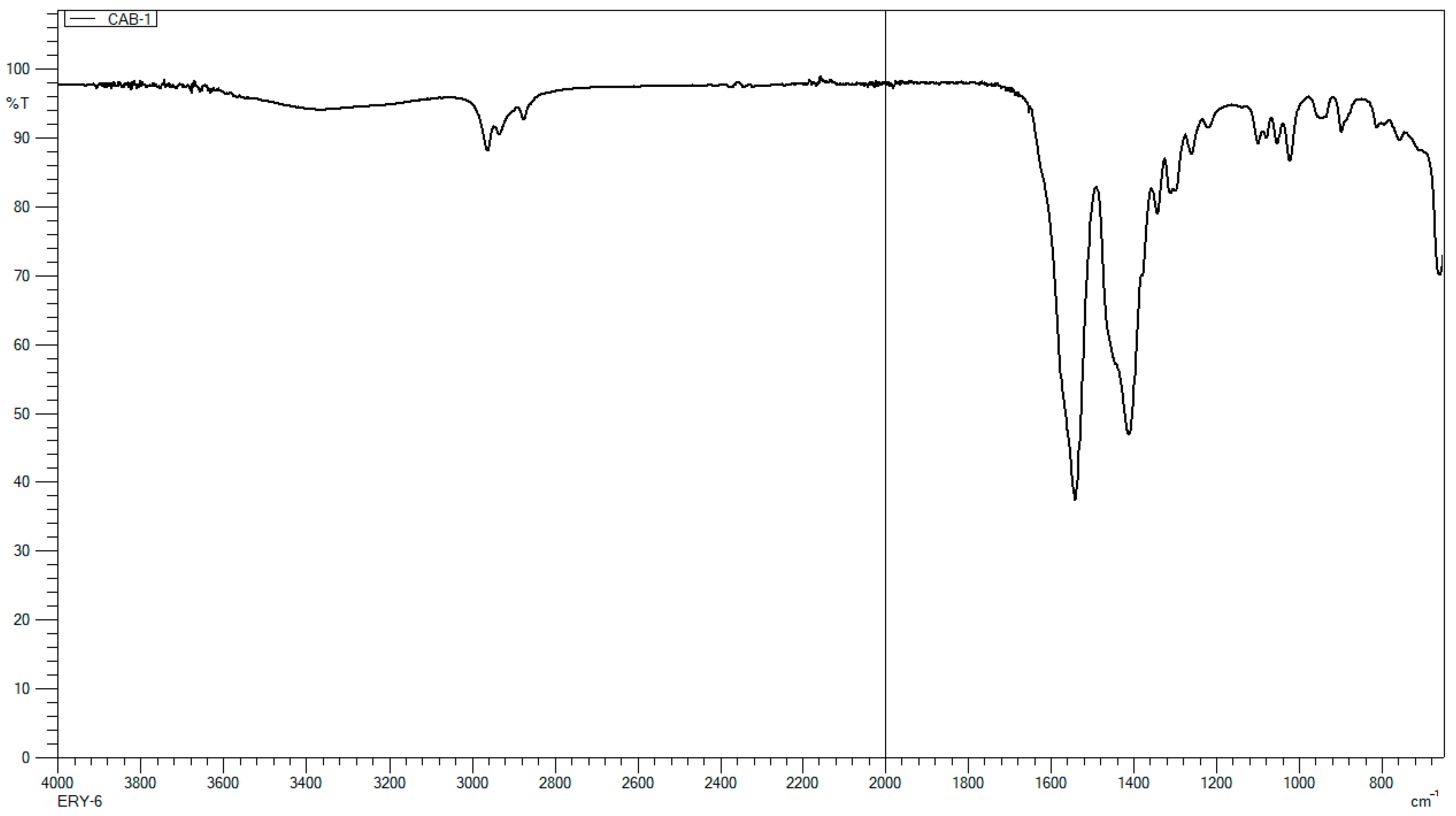
2.2. FTIR Analysis
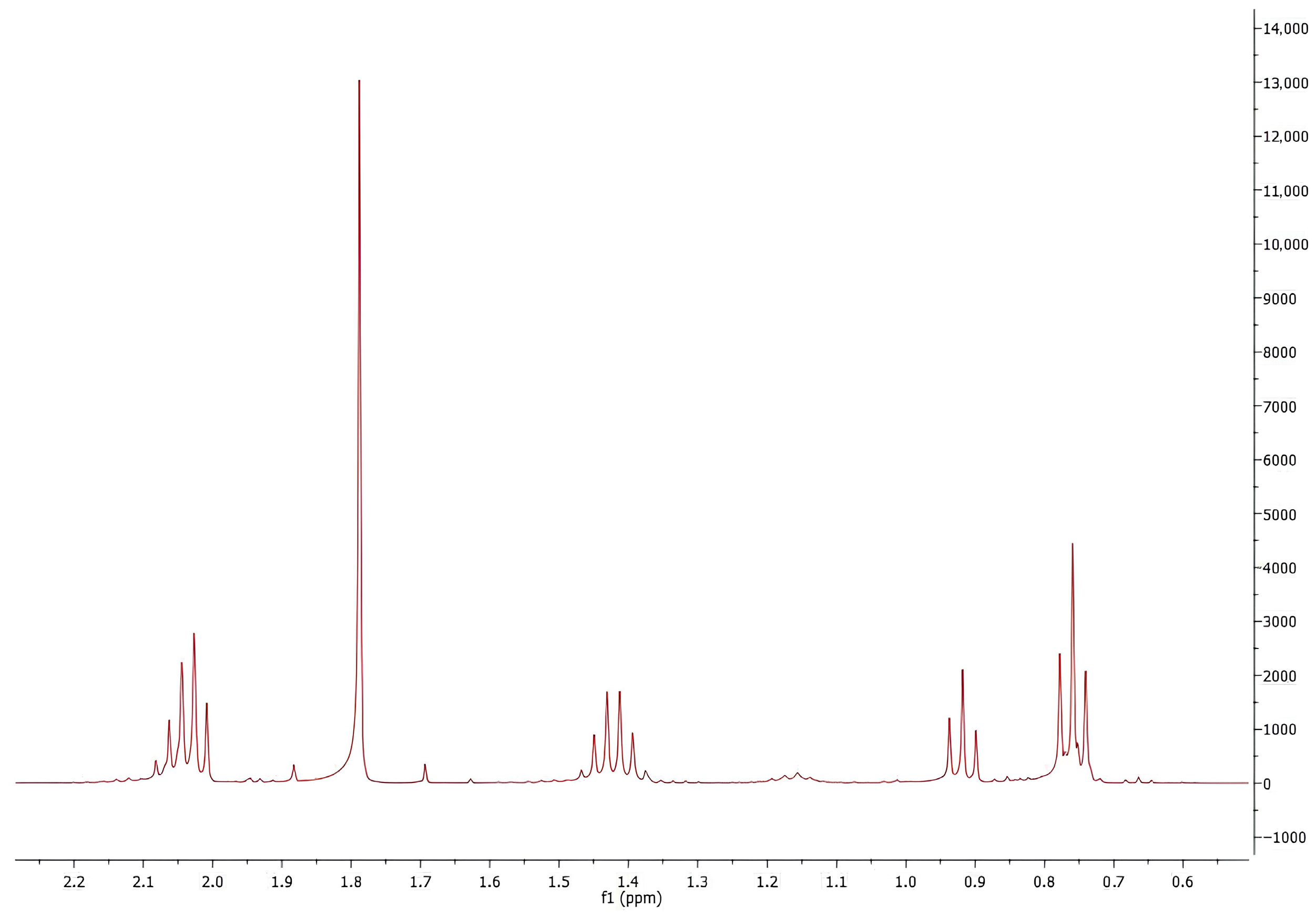
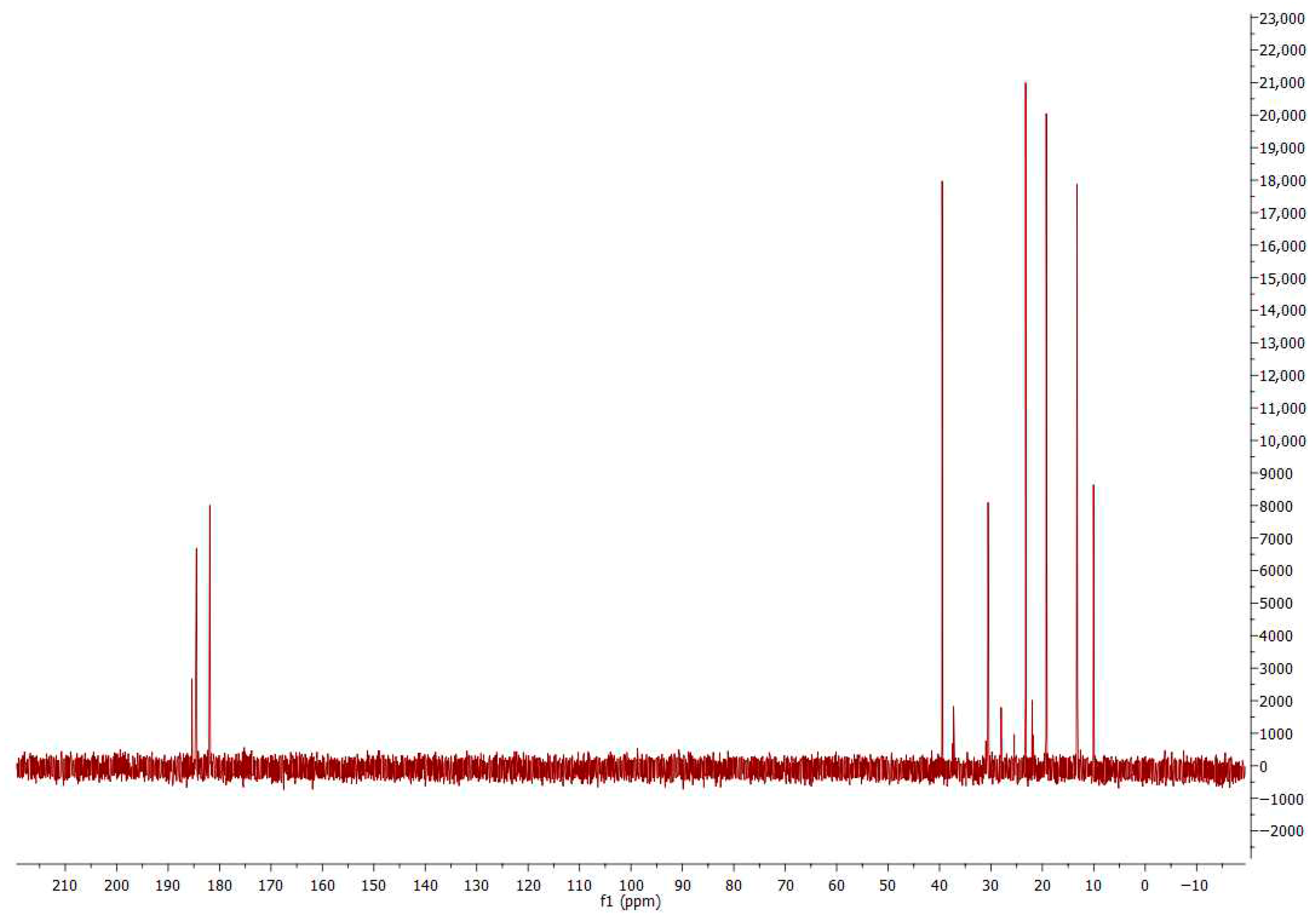
2.3. NMR Analysis
2.4. Instrumentation
2.5. Chromatographic Conditions
2.6. Preparation of Stock Solution and Standard Sample Solutions
2.7. HPLC Method Validation
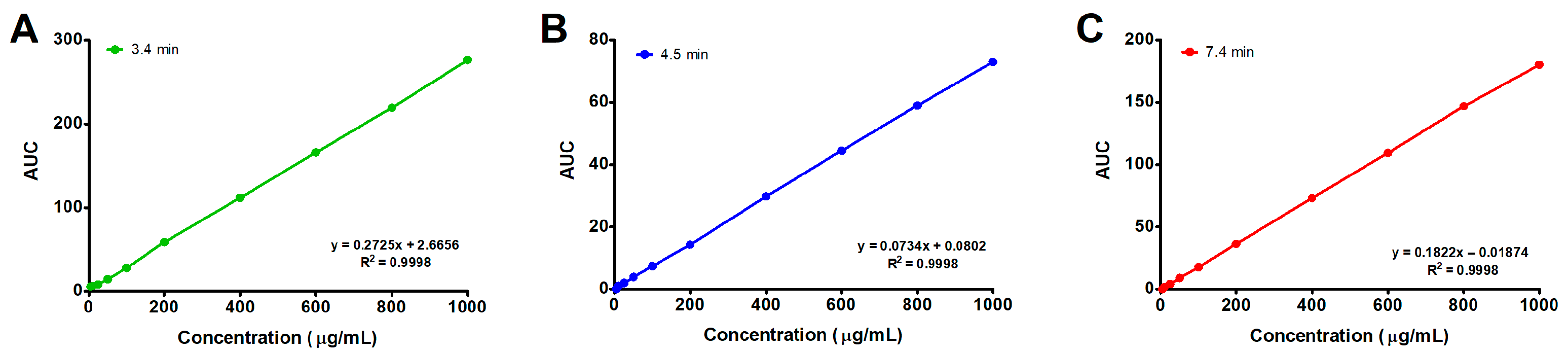
2.7.1. Linearity
2.7.2. Specificity
2.7.3. Precision
2.7.4. Accuracy
2.7.5. Robustness
2.7.6. Determination of Detection Limit and Quantification Limit
3. Results and Discussion
3.1. FTIR Analysis
3.2. NMR Analysis
3.3. HPLC Analysis and Validation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nadar, A.; Jain, V.; D’Sa, M. Stability-indicating RP-HPLC method development and validation for simultaneous quantification of ciprofloxacin, curcumin, and piperine in a novel topical dosage form. Microchem. J. 2025, 216, 114554. [Google Scholar] [CrossRef]
- Mayuri, D.; Ravindranath, S. Analytical Method Development and Validation: A review. J. Drug Deliv. Ther. 2019, 9, 563–570. [Google Scholar] [CrossRef]
- Grebe, S.K.G.; Singh, R.J. LC-MS/MS in the Clinical Laboratory—Where to from Here? Clin. Biochem. Rev. 2011, 32, 5–31. [Google Scholar]
- Gupta, M.K.; Ghuge, A.; Parab, M.; Al-Refaei, Y.; Khandare, A.; Dand, N.; Waghmare, N. A comparative review on High-Performance Liquid Chromatography (HPLC), Ultra Performance Liquid Chromatography (UPLC) & High-Performance Thin Layer Chromatography (HPTLC) with current updates. Curr. Issues Pharm. Med. Sci. 2022, 35, 224–228. [Google Scholar] [CrossRef]
- Song, M.; Jiao, H.; Zhao, J.; Wang, X.; Li, H.; Wang, P.; Ma, B.; Sun, S.; Lin, H. Dietary Supplementation of Calcium Propionate and Calcium Butyrate Improves Eggshell Quality of Laying Hens in the Late Phase of Production. J. Poult. Sci. 2022, 59, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Celasco, G.; Moro, L.; Aiello, C.; Mangano, K.; Milasi, A.; Quattrocchi, C.; DI Marco, R. Calcium Butyrate: Anti-Inflammatory Effect on Experimental Colitis in Rats and Antitumor Properties. Biomedical Reports. Available online: https://www.spandidos-publications.com/br/2/4/559 (accessed on 23 July 2025).
- Cristofori, F.; Calabrese, F.M.; Iacobellis, I.; Santamaria, M.; Celano, G.; Ferrocino, I.; Di Sabato, E.; Pergola, R.; Dargenio, V.N.; Paulucci, L.; et al. Calcium butyrate efficacy in pediatric irritable bowel syndrome: Randomized placebo-controlled multiomics-based clinical trial. J. Pediatr. Gastroenterol. Nutr. 2025, 81, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the Bacterial Butyrate Synthesis Pathways by Analyzing (Meta)genomic Data. mBio 2014, 5, e00889-14. [Google Scholar] [CrossRef]
- Klucz, E.; Dupont, B.; Mieszkowski, D.; Sikora, A.; Sikora, J.; Rogalla-Ładniak, U. Prebiotic Composition Comprising Calcium Butyrate and Magnesium Butyrate and Use Thereof. WO2019229721A1. 2018. Available online: https://patents.google.com/patent/WO2019229721A1/en (accessed on 2 October 2025).
- Engelking, L.E.; Ambrose, D.J.; Oba, M. Effects of dietary butyrate supplementation and oral nonsteroidal anti-inflammatory drug administration on serum inflammatory markers and productivity of dairy cows during the calving transition. J. Dairy Sci. 2022, 105, 4144–4155. [Google Scholar] [CrossRef] [PubMed]
- Banasiewicz, T.; Domagalska, D.; Borycka-Kiciak, K.; Rydzewska, G. Determination of butyric acid dosage based on clinical and experimental studies—A literature review. Gastroenterol. Rev. 2020, 15, 119–125. [Google Scholar] [CrossRef]
- Dobrowolska-Iwanek, J.; Lauterbach, R.; Huras, H.; Paśko, P.; Prochownik, E.; Woźniakiewicz, M.; Chrząszcz, S.; Zagrodzki, P. HPLC-DAD method for the quantitative determination of short-chain fatty acids in meconium samples. Microchem. J. 2020, 155, 104671. [Google Scholar] [CrossRef]
- Calvigioni, M.; Bertolini, A.; Codini, S.; Mazzantini, D.; Panattoni, A.; Massimino, M.; Celandroni, F.; Zucchi, R.; Saba, A.; Ghelardi, E. HPLC-MS-MS quantification of short-chain fatty acids actively secreted by probiotic strains. Front. Microbiol. 2023, 14, 1124144. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kerry, M.S. A simple liquid chromatography method running in dual modes for quantification of short and medium chain fatty acids. J. Chromatogr. A 2024, 1714, 464566. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Corona, L.R.; Parra-Saavedra, K.J.; Mora-Alonzo, R.S.; Macías-Rodríguez, M.E.; Martínez-Preciado, A.H.; Guevara-Martínez, S.J.; Zamudio-Ojeda, A.; Macias-Lamas, A.M. HPLC-DAD Development and Validation Method for Short-Chain Fatty Acids Quantification from Chicken Feces by Solid-Phase Extraction. Separations 2023, 10, 308. [Google Scholar] [CrossRef]
- De Baere, S.; Eeckhaut, V.; Steppe, M.; De Maesschalck, C.; De Backer, P.; Van Immerseel, F.; Croubels, S. Development of a HPLC–UV method for the quantitative determination of four short-chain fatty acids and lactic acid produced by intestinal bacteria during in vitro fermentation. J. Pharm. Biomed. Anal. 2013, 80, 107–115. [Google Scholar] [CrossRef] [PubMed]
- ALOthman, Z.A.; ALanazi, A.G.; Ali, I. A comparative and simultaneous analysis of indoxyl sulfate and sodium butyrate in human plasma by SPE and HPLC methods for kidney patients. J. Chromatogr. B 2020, 1159, 122356. [Google Scholar] [CrossRef]
- Reddy, B.M.; Ashok Reddy, B.R.; Kumar, A.A.; Kodipyaka, R.; Vooturi, R. A Simple Rp-Hplc Method Development and Verification for the Quantitative Estimation of Sodium Butyrate in Tablets. Asian J. Pharm. Clin. Res. 2024, 17, 45–48. [Google Scholar] [CrossRef]
- Hirun, N.; Dokmaisrijan, S.; Tantishaiyakul, V. Experimental FTIR and theoretical studies of gallic acid–acetonitrile clusters. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 86, 93–100. [Google Scholar] [CrossRef]
- Li, Z.; Yu, R.; An, W.; Chen, Q.; Zhao, W.; Xu, E. Method for Determining Butyric Acid and Sodium Butyrate by HPLC (High Performance Liquid Chromatography). CN201310755483.1A, 31 December 2013. [Google Scholar]
- Step. Committee for Medicinal Products for Human Use ICH Q2(R2) Guideline on Validation of Analytical Procedures. 2023. Available online: www.ema.europa.eu/contact (accessed on 24 July 2025).
- Koca, N.; Rodriguez-Saona, L.; Harper, W.; Alvarez, V.B. Application of Fourier Transform Infrared Spectroscopy for Monitoring Short-Chain Free Fatty Acids in Swiss Cheese. J. Dairy Sci. 2007, 90, 3596–3603. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Hanganu, A.; Chira, N.-A. When detection of dairy food fraud fails: An alternative approach through proton nuclear magnetic resonance spectroscopy. J. Dairy Sci. 2021, 104, 8454–8466. [Google Scholar] [CrossRef]
- Gunawan, R.; Nandiyanto, A.B.D. How to Read and Interpret 1H-NMR and 13C-NMR Spectrums. Indones. J. Sci. Technol. 2021, 6, 267–298. [Google Scholar] [CrossRef]
- Sacchi, R.; Paduano, A.; Caporaso, N.; Picariello, G.; Romano, R.; Addeo, F. Assessment of milk fat content in fat blends by 13 C NMR spectroscopy analysis of butyrate. Food Control 2018, 91, 231–236. [Google Scholar] [CrossRef]
- Low, G.K.C.; Duffield, A.M.; Haddad, P.R. Peak-splitting in reversed-phase, ion-pair high-performance liquid chromatography of sympathomimetic drugs and its probable mechanism. Chromatographia 1982, 15, 289–296. [Google Scholar] [CrossRef]
- Jiang, J.; Li, L.; Wang, M.; Xia, J.; Wang, W.; Xie, X. Theoretical Explanation of the Peak Splitting of Tobacco-Specific N-Nitrosamines in HPLC. Bull. Korean Chem. Soc. 2012, 33, 1722–1728. [Google Scholar] [CrossRef]
- Sarisaltik Yaşin, D.; Arslantürk Bingül, A.; Karaküçük, A.; Teksïn, Z.Ş. Development and Validation of an HPLC Method Using an Experimental Design for Analysis of Amlodipine Besylate and Enalapril Maleate in a Fixed-dose Combination. Turk. J. Pharm. Sci. 2021, 18, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, R.; Khursheed, R.; Awasthi, A.; Khurana, N.; Singh, S.K.; Khurana, S.; Sharma, N.; Gunjal, P.; Kaur, J.; et al. Development and validation of RP-HPLC method for estimation of fisetin in rat plasma. S. Afr. J. Bot. 2021, 140, 284–289. [Google Scholar] [CrossRef]
- Povetko, M.I.; Mylnikov, P.Y.; Tranova, Y.; Shchulkin, A.V.; Polupanov, A.S.; Yakusheva, E.N. Development and Validation of a Quantitative Determination Method for Sulfasalazine in Rabbit Blood Plasma and Cell Culture Medium by HPLC-MS/MS. Pharm. Chem. J. 2025, 59, 341–345. [Google Scholar] [CrossRef]
- Schmidt, A.H.; Stanic, M.; Molnár, I. In silico robustness testing of a compendial HPLC purity method by using of a multidimensional design space build by chromatography modeling—Case study pramipexole. J. Pharm. Biomed. Anal. 2014, 91, 97–107. [Google Scholar] [CrossRef]

| Test Solution | 1st Peak Recovery (%) | 2nd Peak Recovery (%) | 3rd Peak Recovery (%) |
|---|---|---|---|
| 1 | 100.151 | 105.013 | 104.984 |
| 2 | 99.781 | 107.684 | 105.265 |
| 3 | 100.707 | 107.684 | 105.828 |
| 4 | 100.707 | 105.681 | 104.984 |
| 5 | 100.522 | 105.013 | 105.547 |
| 6 | 100.893 | 103.678 | 105.828 |
| 7 | 101.078 | 104.346 | 105.547 |
| 8 | 100.893 | 104.346 | 106.109 |
| 9 | 100.707 | 104.346 | 105.265 |
| 10 | 100.893 | 104.346 | 104.984 |
| Average | 100.633 | 105.214 | 105.434 |
| SD | 0.393 | 1.409 | 0.402 |
| RSD | 0.390 | 1.339 | 0.381 |
| Test Solution | 1st Peak Recovery (%) | 2nd Peak Recovery (%) | 3rd Peak Recovery (%) | |||
|---|---|---|---|---|---|---|
| 1st Day | 2nd Day | 1st Day | 2nd Day | 1st Day | 2nd Day | |
| 1 | 100.430 | 103.733 | 102.996 | 100.952 | 100.679 | 100.953 |
| 2 | 103.366 | 99.329 | 102.996 | 100.271 | 100.405 | 99.856 |
| 3 | 101.348 | 103.182 | 103.677 | 102.315 | 100.130 | 99.856 |
| 4 | 100.063 | 102.265 | 102.996 | 102.315 | 100.405 | 100.404 |
| 5 | 102.081 | 101.898 | 103.677 | 100.952 | 100.130 | 100.679 |
| 6 | 102.081 | 102.448 | 102.996 | 103.677 | 100.130 | 100.130 |
| Average | 101.562 | 102.143 | 103.223 | 101.747 | 100.313 | 100.313 |
| SD | 1.108 | 1.396 | 0.321 | 1.250 | 0.224 | 0.448 |
| RSD | 1.091 | 1.367 | 0.311 | 1.228 | 0.223 | 0.447 |
| Test Solution | 1st Peak Recovery (%) | 2nd Peak Recovery (%) | 3rd Peak Recovery (%) | |||
|---|---|---|---|---|---|---|
| 1st Analyst | 2nd Analyst | 1st Analyst | 2nd Analyst | 1st Analyst | 2nd Analyst | |
| 1 | 100.121 | 100.893 | 101.676 | 103.678 | 97.954 | 104.422 |
| 2 | 99.595 | 101.078 | 100.340 | 101.008 | 102.172 | 101.047 |
| 3 | 100.522 | 100.522 | 99.005 | 103.011 | 98.798 | 103.859 |
| 4 | 99.039 | 99.966 | 99.673 | 102.343 | 99.641 | 103.578 |
| 5 | 99.410 | 100.707 | 101.676 | 101.008 | 100.204 | 103.016 |
| 6 | 100.707 | 100.522 | 100.340 | 100.340 | 100.766 | 103.016 |
| Average | 100.121 | 100.615 | 100.452 | 101.898 | 99.922 | 103.156 |
| SD | 0.919 | 0.384 | 1.069 | 1.313 | 1.488 | 1.163 |
| RSD | 0.917 | 0.382 | 1.065 | 1.288 | 1.489 | 1.127 |
| Concentration (µg/mL) | Test Solution | 1st Peak Recovery (%) | 2nd Peak Recovery (%) | 3rd Peak Recovery (%) |
|---|---|---|---|---|
| 200 | 1 | 102.448 | 101.008 | 100.405 |
| 2 | 102.265 | 102.343 | 100.679 | |
| 3 | 102.005 | 101.008 | 100.953 | |
| 4 | 101.263 | 102.343 | 100.130 | |
| 5 | 101.819 | 103.011 | 100.953 | |
| Mean | 101.960 | 101.943 | 100.624 | |
| SD | 0.410 | 0.801 | 0.320 | |
| RSD | 0.402 | 0.786 | 0.318 | |
| 400 | 1 | 101.225 | 101.238 | 103.814 |
| 2 | 101.503 | 105.577 | 102.970 | |
| 3 | 101.873 | 101.906 | 103.814 | |
| 4 | 101.132 | 100.571 | 103.111 | |
| 5 | 101.688 | 101.572 | 105.501 | |
| Mean | 101.484 | 102.173 | 103.842 | |
| SD | 0.277 | 1.759 | 0.900 | |
| RSD | 0.273 | 1.721 | 0.867 | |
| 1000 | 1 | 101.906 | 100.308 | 102.830 |
| 2 | 101.498 | 100.575 | 103.674 | |
| 3 | 101.239 | 100.442 | 102.774 | |
| 4 | 101.350 | 100.308 | 102.099 | |
| 5 | 101.757 | 101.243 | 102.380 | |
| Mean | 101.550 | 100.575 | 102.752 | |
| SD | 0.249 | 0.348 | 0.533 | |
| RSD | 0.245 | 0.346 | 0.519 |
| Time | 0 min | 30 min | 60 min | 120 min | 24 h | 48 h |
|---|---|---|---|---|---|---|
| 1st peak Recovery (%) | 99.039 | 101.263 | 100.707 | 100.893 | 99.329 | 102.265 |
| 100.151 | 100.522 | 100.707 | 100.707 | 101.449 | 101.078 | |
| 101.008 | 100.522 | 100.522 | 100.893 | 101.228 | 101.502 | |
| Mean | 100.066 | 100.769 | 100.645 | 100.831 | 100.669 | 101.615 |
| SD | 0.806 | 0.349 | 0.087 | 0.088 | 0.952 | 0.491 |
| RSD | 0.806 | 0.347 | 0.087 | 0.087 | 0.945 | 0.483 |
| 2nd peak Recovery (%) | 105.681 | 105.681 | 107.684 | 104.346 | 103.677 | 105.721 |
| 105.013 | 108.351 | 105.681 | 104.346 | 105.681 | 105.681 | |
| 104.346 | 108.351 | 105.013 | 104.346 | 104.346 | 104.703 | |
| Mean | 105.013 | 107.461 | 106.126 | 104.346 | 104.568 | 105.368 |
| SD | 0.545 | 1.259 | 1.135 | 0 | 0.833 | 0.471 |
| RSD | 0.519 | 1.171 | 1.069 | 0 | 0.797 | 0.447 |
| 3rd peak Recovery (%) | 104.422 | 105.828 | 105.828 | 106.109 | 104.141 | 104.422 |
| 104.984 | 105.265 | 104.984 | 105.265 | 105.013 | 104.346 | |
| 105.828 | 105.828 | 105.547 | 104.984 | 105.828 | 105.828 | |
| Mean | 105.078 | 105.640 | 105.453 | 105.453 | 104.994 | 104.865 |
| SD | 0.578 | 0.265 | 0.351 | 0.478 | 0.689 | 0.681 |
| RSD | 0.550 | 0.251 | 0.333 | 0.453 | 0.656 | 0.650 |
| 1st Peak | 2nd Peak | 3rd Peak | |
|---|---|---|---|
| Limit of detection (µg/mL) | 1.211 | 0.606 | 1.816 |
| Limit of quantification (µg/mL) | 3.670 | 1.835 | 3.676 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yağcılar, A.P.; Çağlar, E.Ş.; Üstündağ Okur, N. Analytical Method Development and Validation: Calcium Butyrate. Analytica 2025, 6, 42. https://doi.org/10.3390/analytica6040042
Yağcılar AP, Çağlar EŞ, Üstündağ Okur N. Analytical Method Development and Validation: Calcium Butyrate. Analytica. 2025; 6(4):42. https://doi.org/10.3390/analytica6040042
Chicago/Turabian StyleYağcılar, Ayşe Pınar, Emre Şefik Çağlar, and Neslihan Üstündağ Okur. 2025. "Analytical Method Development and Validation: Calcium Butyrate" Analytica 6, no. 4: 42. https://doi.org/10.3390/analytica6040042
APA StyleYağcılar, A. P., Çağlar, E. Ş., & Üstündağ Okur, N. (2025). Analytical Method Development and Validation: Calcium Butyrate. Analytica, 6(4), 42. https://doi.org/10.3390/analytica6040042







