Abstract
Nucleosides are of significant interest to biomedical and pharmaceutical research and have been successfully separated in hydrophilic interaction liquid chromatography (HILIC). However, there have been few studies focusing on the retention mechanisms, and detailed retention mechanisms are not clearly understood. The quantitative assessment methodology based on the linear relationship between the observed retention factors and the phase ratio has been shown to be a new tool to investigate the retention mechanisms of polar compounds in HILIC. This study evaluated the retention mechanisms of 16 nucleosides on a bare silica column. The retention contributions by partitioning, adsorption, and electrostatic attractions are quantitatively determined, and the main retention mechanism can be unambiguously identified for each nucleoside. The study results indicate that the main retention mechanism can shift with the salt concentration in the mobile phase, but partitioning seems to dominate at higher salt concentrations. In addition, the partitioning coefficients are measured using the quantitative assessment methodology and have a relatively strong correlation with the log P values of the nucleosides. Considering large errors in the log P values for these very polar compounds, the partitioning coefficients measured experimentally in the HILIC system may provide a more accurate measure for polarity assessment.
1. Introduction
Nucleosides and modified nucleosides play vital roles in numerous biological and therapeutic processes, such as biomarkers for cancer, neurological and metabolic disorders [1]. Chemically modified nucleosides are critical in regulating gene expression through epigenetic mechanisms (e.g., methylation) and developing antiviral and cancer therapies [2]. For example, pseudouridine is essential in maintaining the stability of oligonucleotide therapeutics and mRNA vaccines [3]. Reversed-phase and ion-pairing reversed-phase LC methods have been developed for the analysis of nucleosides [4,5]. Due to the polar nature of nucleosides, hydrophilic interaction liquid chromatography (HILIC) has been increasingly used to retain and separate nucleosides and modified nucleosides [6,7,8,9,10,11,12]. Cytosine and uridine have been commonly used to test the retention and selectivity of polar stationary phases in HILIC [13,14]. Despite many successes of HILIC in nucleoside separation, the retention mechanisms of nucleosides and modified nucleosides have not been clearly elucidated [6].
The retention mechanisms in HILIC are complex and involve hydrophilic partitioning of polar analytes between the mobile phase and the adsorbed water layer, surface adsorption through direct polar interactions with the polar ligands (e.g., hydrogen bonding), and electrostatic interactions of ionized analytes with charged stationary phases [15]. Although these retention mechanisms may be at work simultaneously, the exact retention mechanisms or the main retention mechanism can vary depending on the chemical nature of the analytes, the type of stationary phases, and the mobile phase composition (e.g., pH, ion-pairing agents, other solvents) [16,17,18]. In addition, column temperature has a significant effect on retention and may also influence the retention mechanisms. From both theoretical and practical points of view, it is important to understand the main retention mechanism for specific analytes under specific HILIC conditions (stationary and mobile phases). However, it has been difficult to evaluate the retention mechanisms separately and assess the relative significance of each retention mechanism in HILIC. Gritti et al. proposed a model based on effective diffusion along packed beds of mesoporous particles for HILIC, which facilitates the determination of analyte concentrations in the rigid and diffuse regions of the adsorbed water layer [19]. The equilibrium constants of the analyte between the rigid and diffuse regions (Ka) and between the diffuse layer and the bulk mobile phase (Kd) can be calculated and used to evaluate the relative significance of partitioning and adsorption. However, Gritti’s model is difficult to use on a routine basis, and the relative equilibrium constants (Ka vs. Kd) do not provide the contribution of each mechanism to the overall retention in HILIC.
We have developed a quantitative assessment methodology to investigate the retention mechanisms of polar compounds in HILIC [20,21]. The quantitative assessment methodology is based on the linear relationship between the observed retention factor (kobs) of non-ionized solutes and the phase ratio (Φ) for the adsorbed water layer:
where K is the partitioning coefficient. The product of KΦ gives the retention contributed by hydrophilic partitioning (kpar), and kads represents the retention attributed to surface adsorption, which is assumed independent of the change in the adsorbed water layer (i.e., the phase ratio). The phase ratio is measured using toluene as the void marker [22]. The void volume in pure acetonitrile (VACN), and a specific mobile phase (VM) is used to calculate the phase ratio:
The phase ratio can be varied by changing the salt concentration in the mobile phase within the solubility limit. For ionized solutes, the observed retention factor deviates from the linear relationship (Equation (1)) when electrostatic interactions occur between the charged solutes and stationary phases. The contribution of electrostatic attraction of repulsion (kelec) can be calculated if the partitioning coefficient (K) and the retention contribution by adsorption (kads) are determined for the ionized solutes at sufficiently high salt concentrations when the electrostatic interactions are negligible:
Here, positive values for kelec indicate electrostatic attraction and negative values electrostatic repulsion. Detailed procedures are described elsewhere [21].
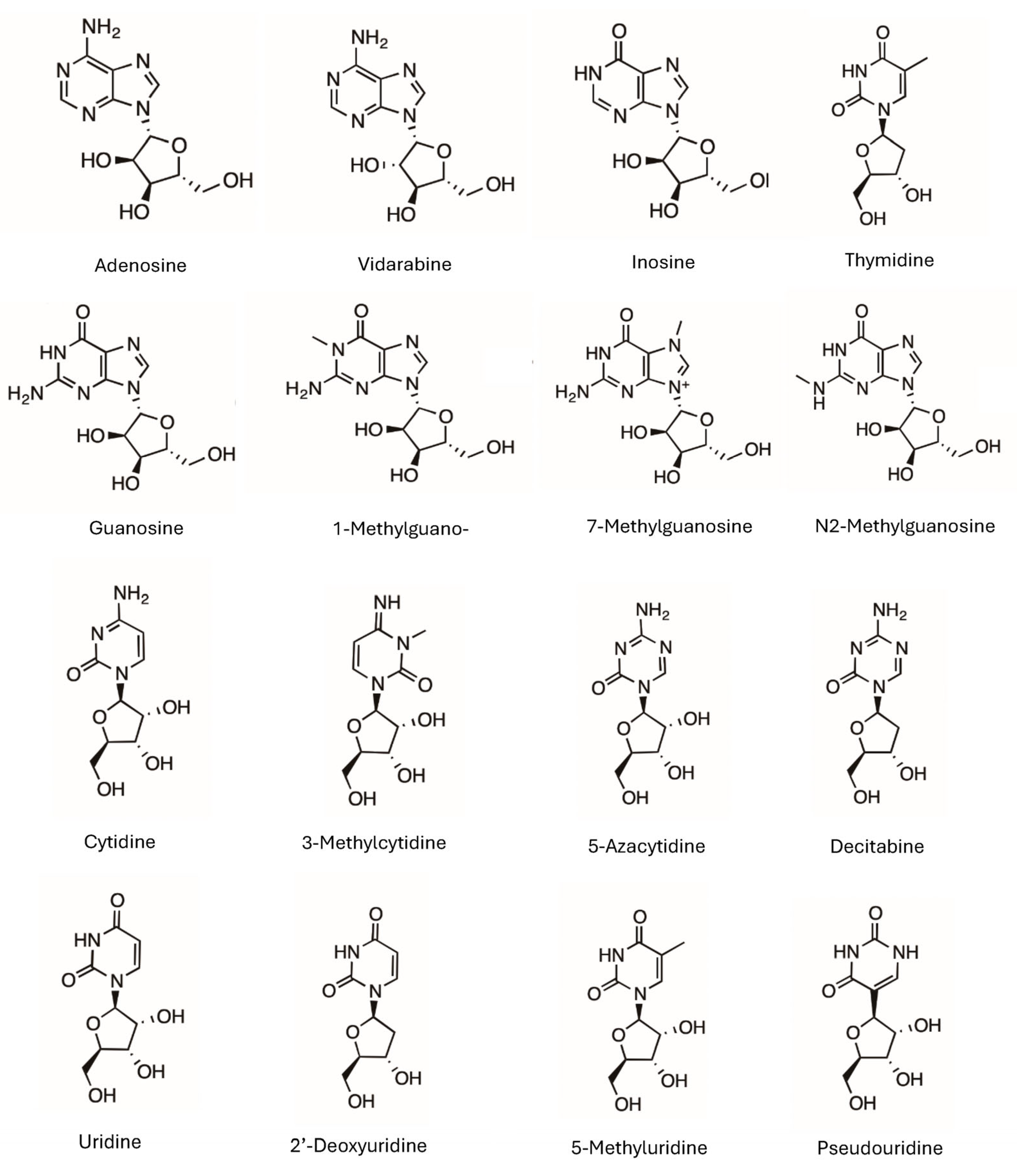
In this study, we applied the quantitative assessment methodology to evaluate the retention mechanisms of 16 native and modified nucleosides on a bare silica stationary phase in HILIC (see structures in Figure 1). The silica column was selected for this study for two reasons. First, the silica column has been used for the separation of nucleosides [7,8,12]. Second, the quantitative assessment methodology has been applied to the studies of the retention mechanisms on other types of polar stationary phases, including amide, polyacrylamide, and sulfobetaine zwitterionic phases, but not on the silica phase [22,23]. The study results provide detailed understanding of the retention mechanisms in terms of quantitative retention contributions by partitioning, adsorption, and electrostatic interactions (if applicable). In addition, experimentally determined partitioning coefficients (log P) are scarce for most modified nucleotides, and calculated log P values can vary significantly depending on the calculation methods. This makes it very challenging to compare the polarity of modified nucleosides. The partitioning coefficients (K) obtained in this study using the quantitative assessment methodology are experimentally based and can be used directly to evaluate the polarity of modified nucleosides.
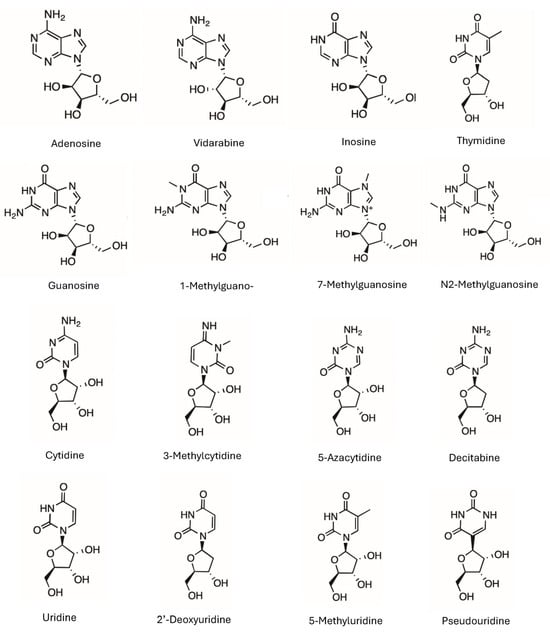
Figure 1.
The structures of the selected nucleosides. The charged form of 7-methylguanosine is shown without the counter ion, and 3-methylcytidine is presented as the amidine form without positive charge.
2. Materials and Methods
Raptor HILIC-Si column (2.7 μm particle size, 100 × 3.0 mM ID) was provided by Restek (Bellefonte, PA, USA) through a RASP grant. HPLC grade purified water and acetonitrile were purchased from Fischer Scientific (Bridgewater, NJ, USA). Ammonium acetate was supplied by TCI (Portland, OR, USA). Stock salt solution was prepared at 400 mM by dissolving appropriate amounts of ammonium acetate in purified water. The pH of the stock ammonium acetate solution was around 6.8–6.9 and was not further adjusted. Nucleoside test compounds, including adenosine, guanosine, cytidine, decitabine, inosine, thymidine, vidarabine, uridine, 5-azacytidine, 2′-deoxyuridine, and 5-methyluridine were obtained from Sigma Aldrich (St. Louis, MO, USA). Modified nucleosides 3-methylcytidine, 1-methylguanosine, 7-methylguanosine, and pseudouridine were purchased from MedChemExpress (Princeton, NJ, USA). The stock solution of each nucleoside was prepared at about 1 mg/mL by dissolving the appropriate amount of the test compound in a mixture of acetonitrile and water (50/50, v/v). The nucleoside compounds were tested in five groups. Group 1 includes guanosine, 1-methylguanosine, 7-methylguanosine, and N2-methylguanosine. Group 2 includes adenosine, vidarabine, and inosine. Group 3 includes cytidine, 3-methylcytidine, and thymidine. Group 4 includes uridine, 2′-deoxyuridine, 5-methyluridine, and pseudouridine. Group 5 includes decitabine and 5-azacytidine. Decitabine and 5-azacytidine were not stable in the sample solvent, and major degradation products were observed in the chromatograms (Supplementary Materials). The test solution for each group was prepared by mixing the stock nucleoside solutions and a small amount of acetonitrile to make sure that the test solution had about 80–85% acetonitrile. A very small amount of toluene solution in acetonitrile was also added to each test solution as the void marker.
All the experiments were performed on an Agilent 1260 HPLC system (Palo Alto, CA, USA) equipped with an online vacuum degasser, a quaternary gradient pump, an autosampler, a thermostated column compartment, and a variable wavelength UV detector. Chromatographic data was collected and processed by ChemStation for LC and LC/MS (Rev. C.01.06). The column temperature was set at 25 °C, and the flow rate was 0.5 mL/min. The injection volume was 2 μL for all the nucleosides, which were detected at 240 nm. The mobile phase was prepared by online mixing of acetonitrile, water, and ammonium acetate stock solution. Acetonitrile was kept at 85%, and ammonium acetate solution was varied from 1% to 9% to obtain the salt concentration of 4 mM to 36 mM in the mobile phase. The volume of water was adjusted accordingly to maintain the aqueous volume at 15%.
3. Results and Discussion
3.1. Purine-Related Nucleosides
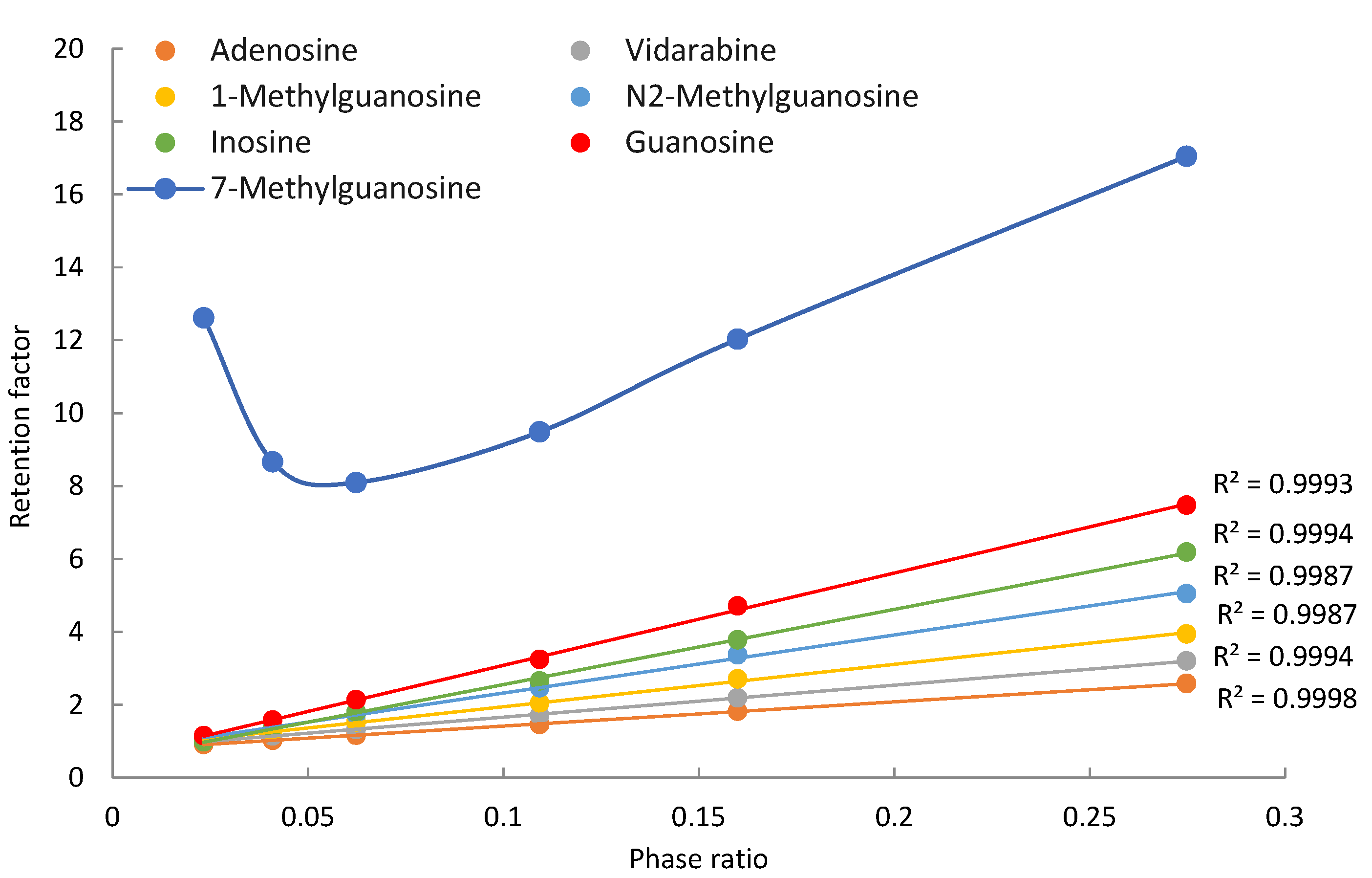
Seven purine-related nucleosides, including both unmodified and modified nucleosides, were selected for this study. In addition to adenosine and guanosine, vidarabine (an antiviral drug and a configurational isomer of adenosine) and inosine (a deamination product of adenosine) were included to increase structural diversity. Three methylated guanosines (1-methyl, 7-methyl, and N2-methylguanosine) were evaluated for the effect of methylation on retention and polarity of the nucleosides. All the purine nucleosides are neutral in the mobile phase, except for 7-methylguanosine, which carries a permanent positive charge due to methylation at the N7 position. Figure 2 shows the observed retention factors of seven purine nucleosides at various phase ratios, which were measured at the ammonium acetate concentration from 4 mM to 36 mM. Off-line experiments show that the solubility of ammonium acetate at 85% acetonitrile is about 45 mM [21]. Higher ammonium acetate concentration is not feasible due to solubility limits. Sufficient data points were obtained in the concentration range of 4–36 mM. All the nucleosides, except for 7-methylguanosine, show linear relationships between the observed retention factor and phase ratio as predicted by Equation (1) for non-ionized compounds. Methylation of guanosine at the 7-position produces a quaternary amine carrying a permanent positive charge. Electrostatic interactions between positively charged 7-methylguanosine and negatively charged surface silanol groups enhance the retention at low salt concentrations, resulting in deviation from linearity as shown in Figure 2. Positively charged 7-methylguanosine is well separated from other nucleosides due to its strong retention; however, the non-ionized nucleosides elute closely to each other, leading to insufficient resolution below 20 mM ammonium acetate. Separation of the non-ionized nucleosides is significantly improved as the salt concentration increases above 20 mM as a result of stronger retention from partitioning at higher phase ratios. This implies that salt concentration can be a useful parameter to adjust retention for desired separation.
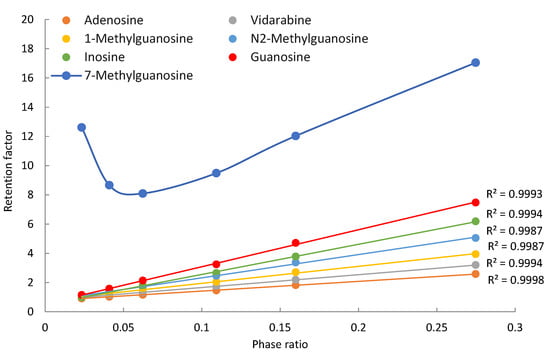
Figure 2.
The observed retention factors of the purine related nucleosides at various phase ratio. The mobile phase contains 85% (v/v) acetonitrile and 4–36 ammonium acetate.
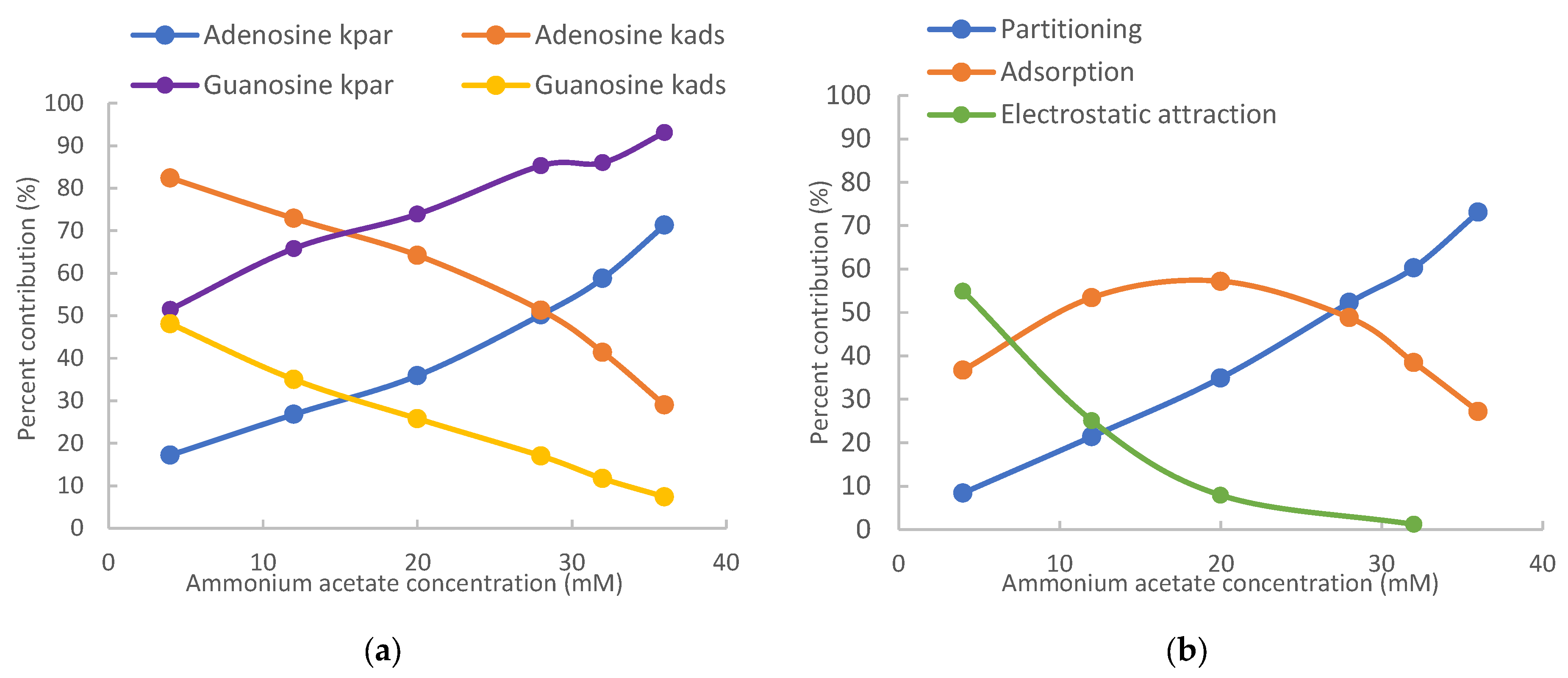
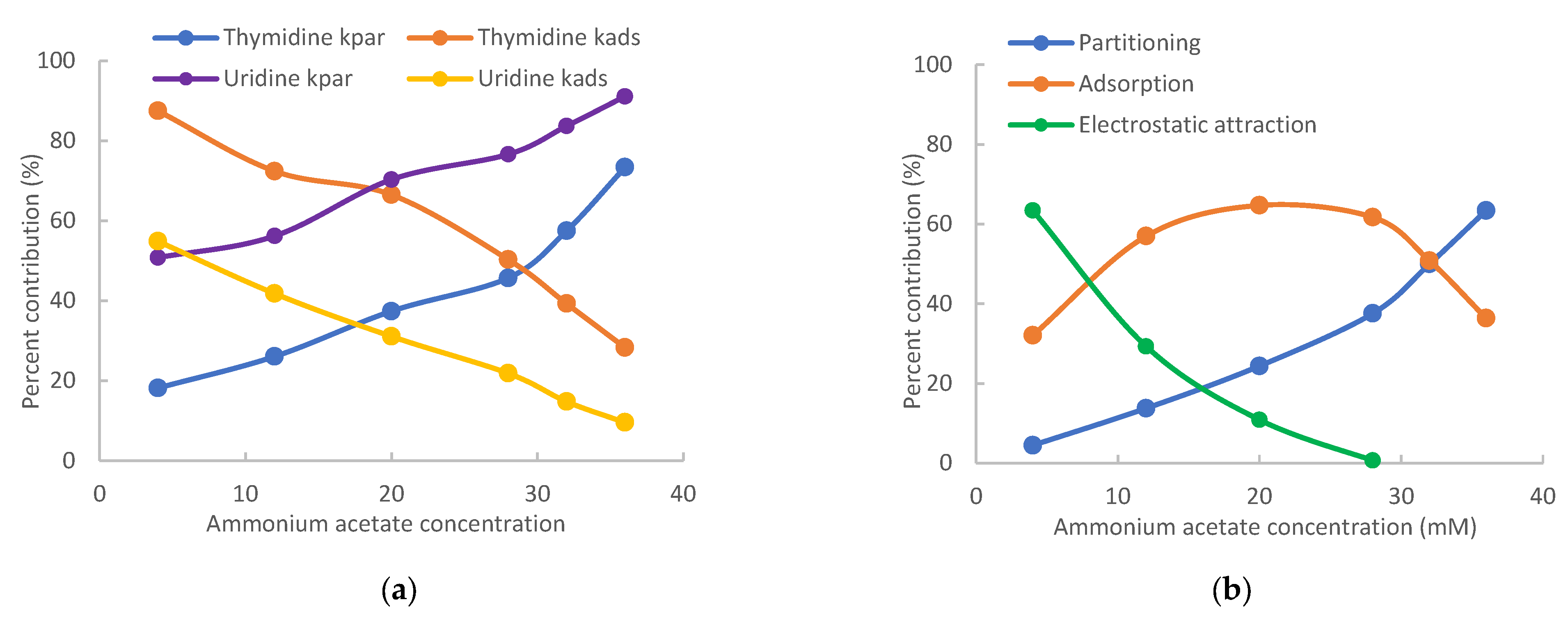
The contributions of different retention mechanisms (i.e., partitioning, adsorption, and electrostatic interactions) are determined using quantitative assessment methodology. Linearly regression of the K vs. Φ plots (Figure 2) provides the partitioning coefficients (slopes) and the retention contributed by adsorption (intercepts) for all the nucleosides except for 7-methylguanosine. The retention contribution by partitioning (kpar) is then calculated by multiplying the partitioning coefficients and phase ratio at each salt concentration. The retention contributed by partitioning and adsorption to the observed retention of the non-ionized nucleosides at the lowest (4 mM) and highest (36 mM) salt concentration is presented in Table 1. At 4 mM ammonium acetate, adsorption is the main retention mechanism for adenosine, vidarabine, 1-methyl, and N2-methylguanosine. These nucleosides have many moieties that can serve as either hydrogen bond donor or acceptor, and the surface silanol groups can be hydrogen bond acceptor if ionized or donner if protonated. Therefore, hydrogen bonding is a main contributor to surface adsorption. In addition, Dipole–dipole interactions are likely involved in surface adsorption. In comparison, partitioning and adsorption seem to contribute relatively equally to the observed retention for inosine and guanosine. At 36 mM ammonium acetate, partitioning becomes the predominant force in controlling retention for all the non-ionized nucleosides. This indicates that the retention mechanisms shift with salt concentration in the mobile phase. The trend of retention mechanism shifting is further illustrated in Figure 3. Adenosine and guanosine are selected to represent the smallest and largest contributions by partitioning at the lowest salt concentration. As shown in Figure 3a, the relatively contribution by partitioning increases with the salt concentration because the phase ratio, hence the retention contributed by partitioning (KΦ) increases with the salt concentration. In contrast, the percentage contribution by adsorption keeps decreasing since the retention contributed by adsorption is constant, but the overall retention increases with salt concentration. Although the main retention mechanism for adenosine is adsorption at 4 mM ammonium acetate, a switch occurs around 30 mM, beyond which partitioning becomes the main retention mechanism.

Table 1.
Retention contributions by partitioning and adsorption to the observed retention of the purine related nucleosides at 4 mM and 36 mM ammonium acetate.
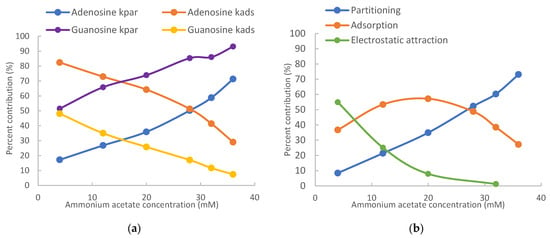
Figure 3.
Relative retention contributions of partitioning, adsorption, and electrostatic attraction at various salt concentrations for (a) adenosine and guanosine and (b) 7-methylguanosine. The mobile phase contains 85% (v/v) acetonitrile.
For positively charged 7-methylguanosine, linear regression was performed only on three data points at 28, 32, and 36 mM ammonium acetate, when the electrostatic effect is sufficiently shielded based on the K vs. Φ plot in Figure 2. The obtained partitioning coefficient and the retention contributed by adsorption (kads) were then used to calculate the contribution of the electrostatic attraction (kelec) based on Equation (3). Figure 3b clearly shows that electrostatic attraction is the main retention mechanism at 4 mM ammonium acetate; however, the electrostatic effect diminishes with increasing salt concentration and is almost completely shielded at the salt concentration above 30 mM. In comparison, the percent contribution of partitioning increases with the salt concentration due to increasing phase ratio and becomes the main retention mechanism above 30 mM. Adsorption is the main retention mechanism in the middle range of salt concentration. It is noted that the retention contributed by adsorption for 7-methylguanosine is much higher than for the other methylated guanosines (1-methyl and N2-methylguanosine). Methylation would not be sufficient to explain the enhanced adsorption for 7-methylguanosine, which might be related to the positive charge. Further investigation is needed to better understand the adsorption effect.
3.2. Pyrimidine-Related Nucleosides
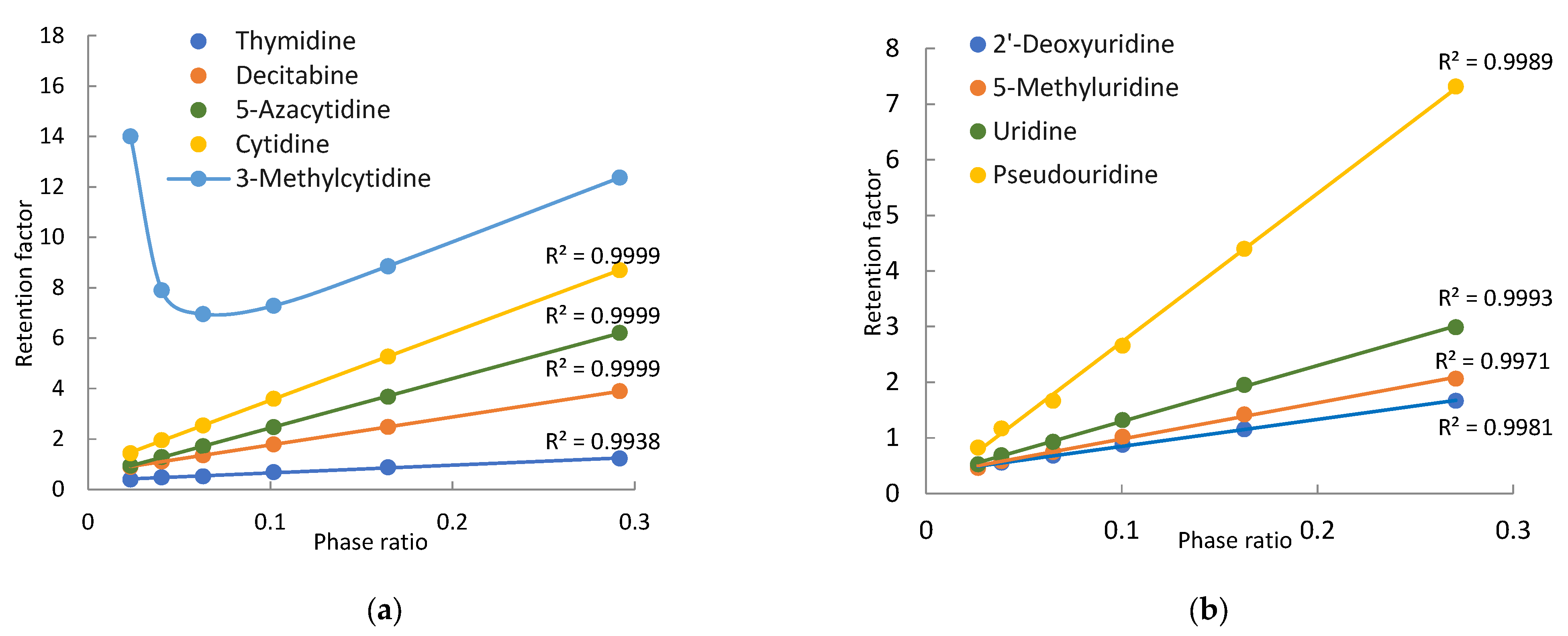
Nine pyrimidine-related nucleosides, including cytidine, thymidine, and uridine, were selected for this study. Modified pyrimidine nucleosides include two methylated nucleosides (3-methylcytidine and 5-methyluridine), two deoxy-nucleosides, 2′-deoxyuridine and decitabine (5-aza-2′-deoxycytidine), one modified cytidine (5-azacytidine), and one modified uridine (pseudouridine). Both 5-Azacytidine and decitabine are chemotherapeutic agents for hematological cancers. All the pyrimidine nucleosides are neutral in the mobile phase, except for 3-methylcytidine. Figure 1 shows the structure of 3-methylcytidine in the imine form which is a more stable tautomer. A recent quantum mechanical study indicates that 3-methylcytidine has a theoretical pKa of 9.3 (vs. the experimental pKa of 8.7) at the N4 position [24]. Therefore, 3-methylcytidine is positively charged in the mobile phase used in this study. Figure 4 shows the observed retention factors of nine pyrimidine related nucleosides at various phase ratios. Linear relationships between the observed retention factor and phase ratio are observed for all the nucleosides, except for 3-methylcytidine, indicating partitioning driving retention behaviors (Equation (1)). Similar to 7-methylguanosine, 3-methylcytidine is also a quaternary amine (Figure 1), and its retention factor deviates from linearity at low phase ratios (i.e., low salt concentrations) due to electrostatic interactions with the negatively charged silica phase. Thymidine, cytidine, and 3-methylcytidine are well separated at all the salt concentrations (phase ratios). However, decitabine and 5-azacytidine are only partially resolved due to weak retention at low salt concentrations (phase ratios), and the resolution is significantly improved at the salt concentration above 12 mM (Figure 4a). In comparison, pseudouridine is well separated from other uridines at all the salt concentrations (Figure 4b). However, uridine, 2-deoxyuridine and 5-methyluridine elute closely at low salt concentrations, and baseline separation is achieved at the salt concentration above 20 mM.
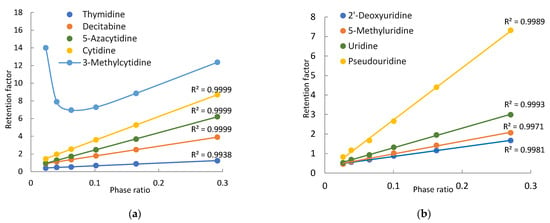
Figure 4.
Plots of the observed retention factors of the purine related nucleosides vs. the phase ratio: (a) thymidine, cytidine, decitabine, 5-azacytidine, 3-methylcytidine and (b) uridine, 2′-deoxyuridine, 5-methyluridine, pseudouridine. The mobile phase contains 85% (v/v) acetonitrile and 4–36 mM ammonium acetate.
The retention contributed by partitioning, adsorption, and electrostatic interactions are determined for the pyrimidine nucleosides using the quantitative methodology in the same way as for the purine nucleosides. Table 2 presents the retention contributed by the partitioning and adsorption mechanisms at 4 mM and 36 mM ammonium acetate. At low salt concentrations, adsorption is the main retention mechanism for cytidine, decitabine, thymidine, 2′-deoxyuridine, and 5-methyluridine. The retention of uridine and 5-azacytidine seems to be equally controlled by partitioning and adsorption. However, partitioning is the main retention mechanism for pseudouridine. In contrast, partitioning becomes the predominant mechanism for all pyrimidine nucleosides at 36 mM ammonium acetate. Particularly for pseudouridine, adsorption is negligible. The shift in the retention mechanism for pyrimidine nucleosides follows the same trend as seen for the purine nucleosides (Figure 3). As shown in Figure 5a, the relative contribution of partitioning increases while the relative contribution of adsorption decreases with the salt concentration for thymidine and uridine due to increasing phase ratio. The main retention mechanism for thymidine is adsorption below 30 mM ammonium acetate but switches to partitioning above 30 mM.

Table 2.
Retention contributions by partitioning and adsorption to the observed retention of the pyrimidine related nucleosides at 4 mM and 36 mM ammonium acetate.
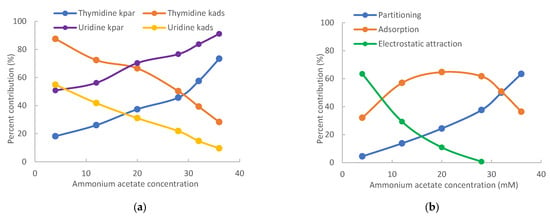
Figure 5.
Relative retention contributions of partitioning, adsorption, and electrostatic attraction at various salt concentrations for (a) thymidine and uridine and (b) 3-methylcytidine. The mobile phase contains 85% (v/v) acetonitrile.
For positively charged 3-methylcytidine, linear regression was performed only using the data points at 28, 32, and 36 mM ammonium acetate due to electrostatic attraction at lower salt concentrations. After the partitioning coefficient and the retention contributed by adsorption (kads) were obtained, the contribution of electrostatic attraction was calculated using Equation (3). As shown in Figure 5b, electrostatic attraction is the main retention mechanism at 4 mM ammonium acetate but diminishes quickly with increasing salt concentration. In comparison, the retention contributed by partitioning keeps increasing with the salt concentration due to increasing phase ratio and becomes the main retention mechanism at 36 mM ammonium acetate. Similar to 7-methylguanosine, the retention contributed by adsorption for 3-methylcytidine is much higher than that for cytidine. Adsorption is the main retention mechanism in the salt concentration range of 10–30 mM. The retention of 3-methylcytidine is equally controlled by partitioning and adsorption at 32 mM ammonium acetate.
3.3. Polarity of Modified Nucleosides
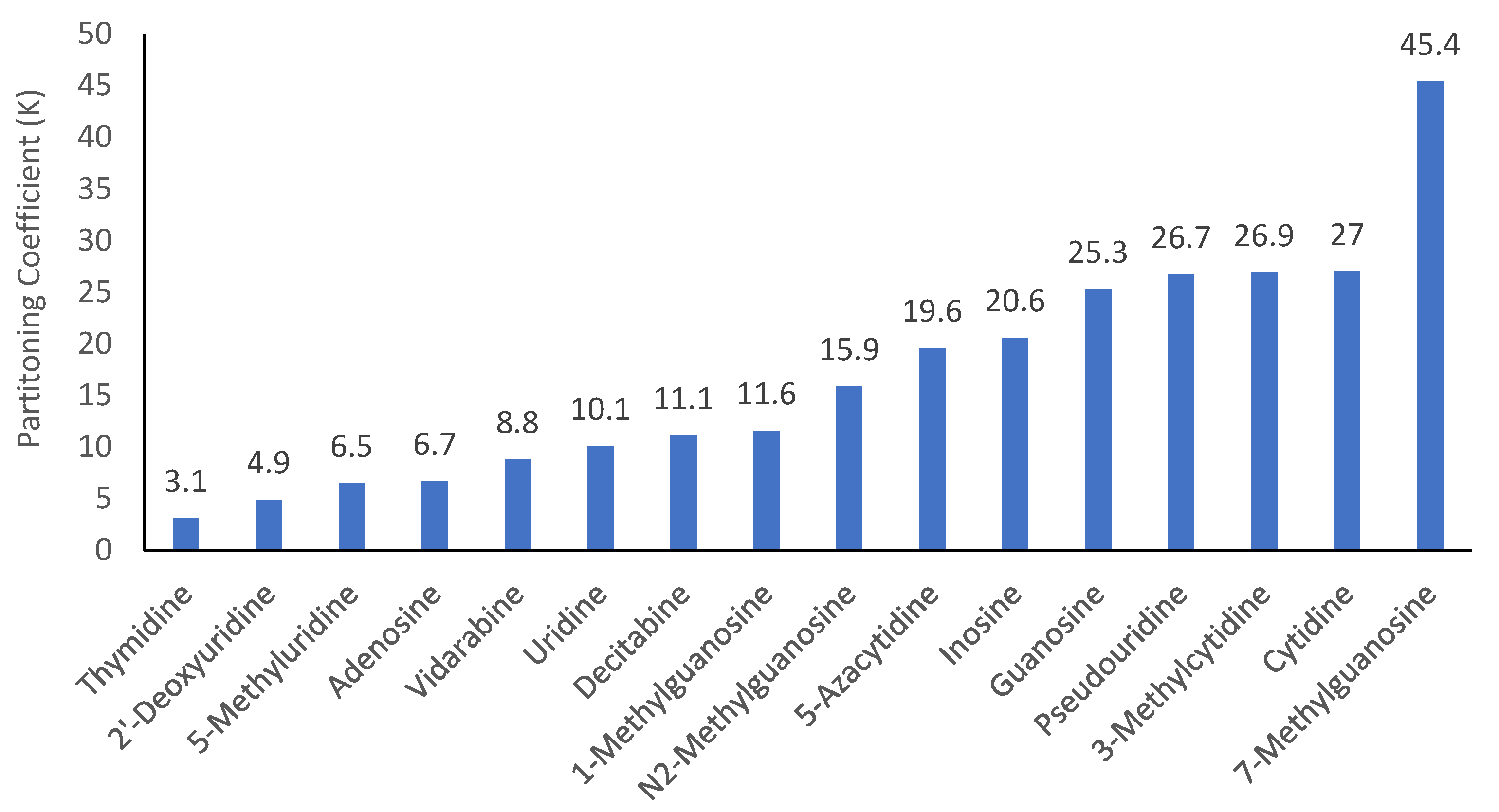
Modification of the native structures changes the polarity of nucleosides. However, it is very difficult to find experimental partitioning coefficient data (e.g., log P values) for most modified nucleosides. Calculated log P values can vary significantly depending on the calculation methods used. The quantitative assessment methodology provides a means to determine the partitioning coefficients (K) in the HILIC system, which can be used to evaluate the effect of structural modification on the polarity of nucleosides. Figure 6 shows the partitioning coefficients of all the nucleosides measured in the mobile phase containing 85% acetonitrile. Among all the nucleosides, thymidine (2′-deoxy-5-methyluridine) is less polar than 2′-deoxyuridine and 5-methyluridine, both of which are also less polar than uridine. This indicates that adding a methyl group and removing a hydroxyl group at the 2′-position of ribose reduces polarity. The effect of removing the hydroxyl group at the 2′-position on polarity is also reflected by the difference in partitioning coefficients of decitabine (2′-deoxy-5-azacytidine) and 5-azacytidine. Methylation on the purine or pyrimidine ring reduces polarity as demonstrated by the partitioning coefficients of 5-methyluridine, 1-methylguanosine, and N2-methylguanosine. However, the formation of quaternary amines by methylation does not follow this trend. In fact, 3-methylcytidine and cytidine have essentially the same partitioning coefficients, and 7-methylguanosine has a much higher partitioning coefficient than guanosine. Although adenosine and vidarabine differ only in the configuration of the hydroxyl group at the 2′-position, their partitioning coefficients are significantly different, which enables their separation at salt concentration above 20 mM. It is interesting to note that 5-azacytidine is less polar than cytidine, and pseudouridine is much more polar than uridine.
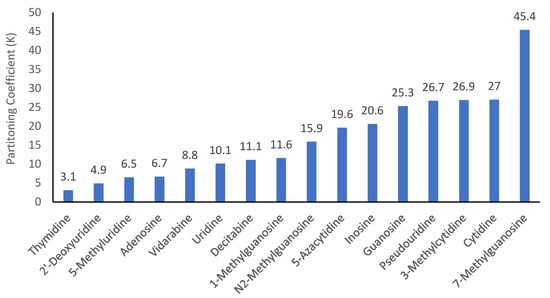
Figure 6.
Partitioning coefficients (K) of the selected nucleosides in the mobile phase containing 85% (v/v) acetonitrile.
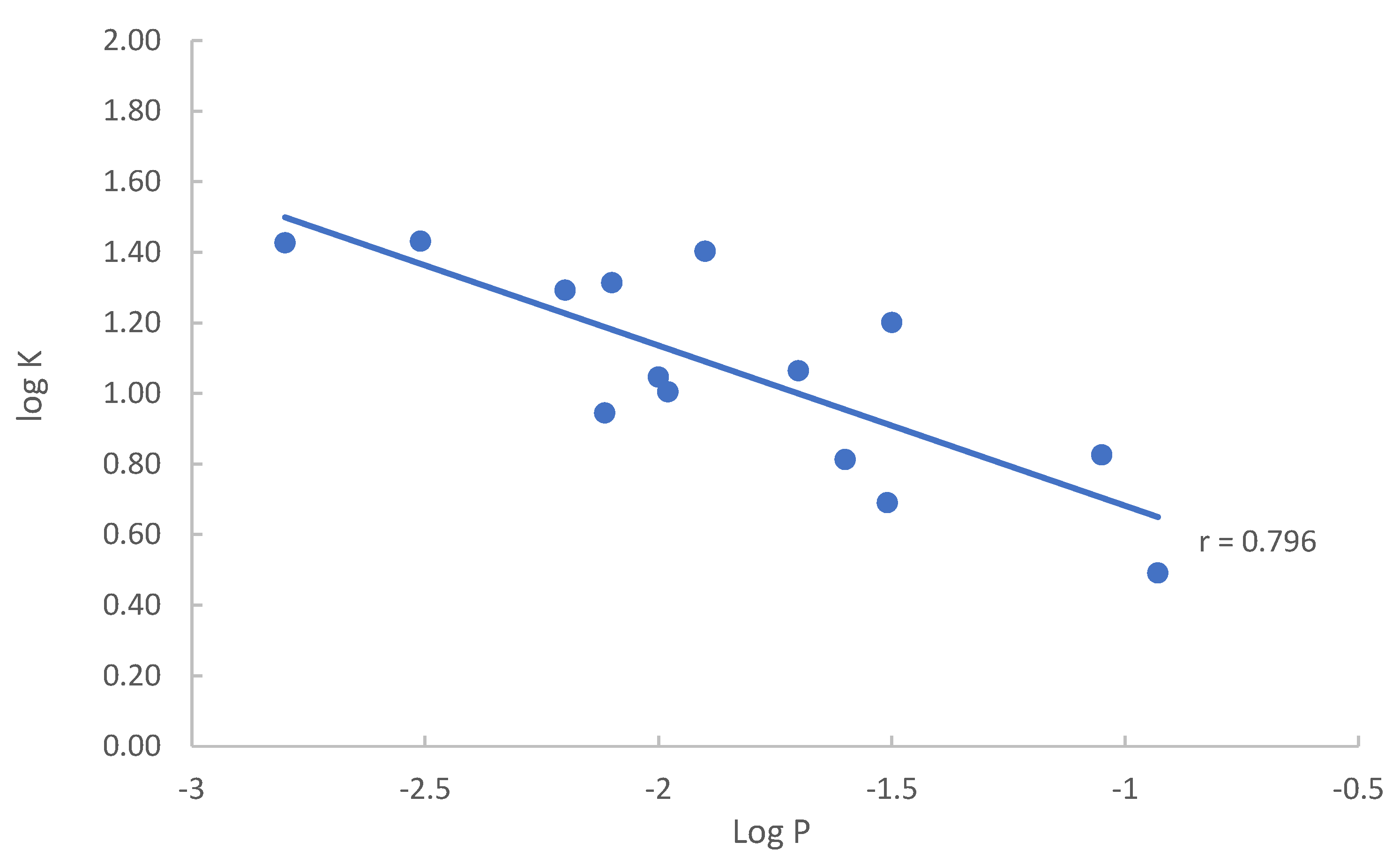
The partitioning coefficients (K) of the modified nucleosides obtained in this study are compared to the conventional log P values found in either the NIH PubChem or DrugBank database. When both the experimental and calculated log P values are available, the experimental values are selected for comparison. The log P values are plotted against the logarithmic partitioning coefficient (log K) as shown in Figure 7. The listed log P values for the quaternary amine nucleosides either has a very wide range (−1.4–−6.2) for 7-methylguanosine or appears to be too high (−1.7) for 3-methylcytidine. Thus, 3-methylcytidine and 7-methylguanosine are excluded in the plot in Figure 7. The partitioning coefficient (K) of nucleosides measured in the HILIC system reflects the solute distribution between the aqueous–organic mobile phase and the adsorbed water layer. The conventional Log P values represent the distribution of solutes between the octanol and aqueous phases. Therefore, there should be certain level of correlation between the partitioning coefficients (K) measured in HILIC and the log P values. Linear regression reveals a relatively strong correlation between log P and log K values (Pearson’s r value close to 0.80). Negative correlation between log P and log K is due to the convention used for the log P values, which are the concentration ratio between octanol and water. The traditional way of measuring the octanol–water partitioning coefficient (log P) is known to be difficult for very polar compounds with drastically different solubility in octanol and water, leading to large errors [25], and the calculated log P values are highly dependent on the calculation algorithm. The errors in the log P values may explain the relatively large residuals in linear regression as shown by the spread of data points in Figure 7. For example, adenosine and vidarabine are configurational isomers and have been shown to have similar polarity in previous HILIC studies [23]. The partitioning coefficients (K) obtained in this study are not drastically different (6.7 vs. 8.8). However, the log P values of adenosine and vidarabine are different by more than 1 (−1.05 vs. −2.11), indicating very different polarity. The elution order in HILIC and the measured partitioning coefficients (15.9 vs. 11.6) suggest that N2-methylguanosine is more polar than 1-methylguanosine. The calculated log P values of N2-methylguanosine (−1.5) and 1-methylguanosine (−1.7) indicate the opposite. These results imply that the partitioning coefficient (K) measured in the HILIC system may better reflect the polarity of nucleosides.
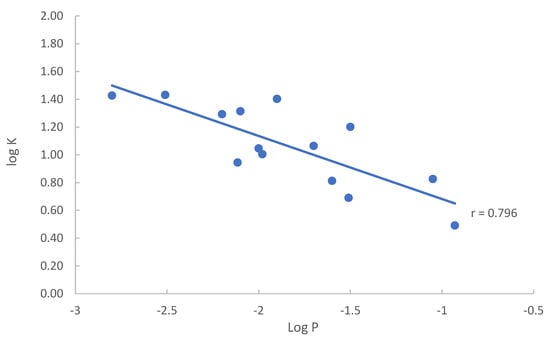
Figure 7.
Plot of log K vs. log P of the selected nucleosides in the mobile phase containing 85% (v/v) acetonitrile.
4. Conclusions
The quantitative assessment methodology has been applied to evaluate the retention mechanisms of 16 native and modified nucleosides on a bare silica column in this study. The retention mechanisms for each nucleoside are clearly delineated by retention contributed by partitioning, adsorption, and electrostatic attraction (if applicable). The main retention mechanism can shift with the ammonium acetate concentration in the mobile phase, and partitioning becomes the main mechanism at higher salt concentration due to increasing phase ratio. Identifying the main retention mechanism will not only enhance the basic understanding of HILIC separation of nucleosides but also provide practical guidance in terms of selecting the most effective chromatographic parameter to improve the separation during method development.
The partitioning coefficients (K) have been experimentally determined for all the nucleosides. Methylation generally reduces the polarity of the nucleosides, except when methylation produces positively charged quaternary amines. The removal of the hydroxyl group at the 2′-position in the ribose ring also decreases the polarity of the nucleosides. Converting uridine to pseudouridine also drastically increases polarity. Although this study was conducted only on the bare silica phase, the partitioning coefficients of the nucleosides should be the same on other types of polar stationary phases since partitioning occurs between the bulk mobile phase and the adsorbed water layer. The stationary phase only serves as the support for the adsorbed water layer in the partitioning process. However, surface adsorption may be different on other types of stationary phases. In addition, there seems to be a strong correlation between conventional log P values and the partitioning coefficients measured in the HILIC system. Due to the errors and uncertainty in the reported log P values for very polar compounds, the partitioning coefficients can be a viable alternate measure for evaluating the polarity of polar compounds, especially when the reported log P values are not available.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/analytica6040039/s1, Figure S1: chromatogram for (1) toluene, (2) 1-methylguanosine, (3) N2-methylguanosine, (4) guanosine, (5) 7-methylguanosine. Figure S2: chromatogram for (1) toluene, (4) decitabine, and (5) 5-azacytidine. Peaks 2 and 4 are main degradation products of decitabine and 5-azacytidine, respectively. Figure S3: chromatogram for (1) toluene, (2) adenosine, (3) vidarabine, and (4) inosine. Figure S4: chromatogram for (1) toluene, (2) thymidine, (3) cytidine, and (4) 3-methylcytidine. Figure S5: chromatograms for (1) toluene, (2) 2’-deoxyuridine, (3) uridine, (4) 5-methyluridine, and (5) pseudouridine.
Author Contributions
Conceptualization, Y.G.; methodology, Y.G.; formal analysis, D.K. and D.M.; investigation, D.M., Z.G. and V.A.; data curation, D.K., D.M., Z.G. and V.A.; writing—original draft preparation, Y.G.; writing—review and editing, D.K. and D.M.; supervision, Y.G.; funding acquisition, Y.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the US National Science Foundation, grant ID 2402756.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
Restek is gratefully acknowledged for providing the column used in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HILIC | Hydrophilic Interaction Liquid Chromatography |
References
- Zhang, G. Nucleosides/nucleotides biomarkers. In Targeted Biomarker Quantitation, 1st ed.; Weng, N., Jian, W., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 389–406. [Google Scholar]
- Berdis, A. Nucleobase-modified nucleosides and nucleotides: Application in biochemistry, synthetic biology, and drug discovery. Front. Chem. 2022, 10, 1051525. [Google Scholar] [CrossRef] [PubMed]
- Kariko, K.; Muramatsu, H.; Welsh, F.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.Y.; Han, H.; Wang, B.; Li, N.; Dong, T.T.X.; Zhang, T.; Tsim, K.W.K. Fast Simultaneous determination of 13 nucleosides and nucleobases in Cordyceps sinensis. Molecules 2015, 20, 21816–21825. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Sanz, A.; Scott-Ward, T.S.; Gill, H.S.; Jacoby, J.C.; Birch, R.E.; Malone-Lee, J.; Taylor, K.M.G.; Peppiatt-Wildman, C.M.; Wildman, S.S.P. Simultaneous quantitation of 12 different nucleotides and nucleosides released from renal eithelium and in human urine samples using ion-pair reversed-phase HPLC. Purinergic Signal. 2012, 8, 741–751. [Google Scholar] [CrossRef]
- Guo, Y. Separation of nucleobases, nucleosides, nucleotides and oligonucleotides by hydrophilic interaction liquid chromatography (HILIC): A state-of-the-art review. J. Chromatogr. A 2024, 1738, 465467. [Google Scholar] [CrossRef]
- Tyeca, E.; Guillarme, D.; Desmet, G. The use of individual retention modeling for gradient optimization in hydrophilic interaction chromatography: Separation of nucleobases and nucleosides. J. Chromatogr. A 2014, 1368, 125–131. [Google Scholar] [CrossRef]
- Mateos-Vivas, M.; Rodriguez-Gonzalo, E.; Carcia-Gomez, D.; Carabias-Martinez, R. Hydrophilic interaction chromatography coupled to tandem mass spectrometry in the presence of hydrophilic ion-paring reagents for the separation of nucleosides and nucleotide mono-, di- and triphosphates. J. Chromatogr. A 2015, 1414, 129–137. [Google Scholar] [CrossRef]
- Oritz-Villanueva, E.; Navarro-Reig, M.; Jaumot, J.; Tauler, R. Chemometric evaluation of hydrophilic interaction liquid chromatography stationary phases: Resolving complex mixtures of metabolites. Anal. Methods 2017, 5, 774–785. [Google Scholar] [CrossRef]
- Fang, Z.; Hu, Y.; Hong, X.; Zhang, X.; Pan, T.; Pan, C.; Zheng, S.; Guo, C. Simultaneous determination of methylated nucleosides by HILIC-MS/MS revealed their alternations in urine from breast cancer patients. Metabolites 2022, 12, 973. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Y.; Hong, X.; Wang, M.; Fang, Z.; Cao, X.; Jiang, K.; Guo, C. Determination of adenosine and its modifications in urine and plasma from breast cancer patients by hydrophilic interaction liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2022, 1209, 123428. [Google Scholar] [CrossRef]
- Cao, X.; Wang, M.; Huang, Y.; Zhang, M.; Zheng, F.; Zhang, G.; Su, J.; Yuan, Y.; Guo, C. Determination of demethylated nucleosides in serum from colorectal cancer patients by hydrophilic interaction liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2024, 1232, 123973. [Google Scholar] [CrossRef]
- Guo, Y.; Gaiki, S. Retention behavior of small polar compounds on polar stationary phases in hydrophilic interaction chromatography. J. Chromatogr. A 2015, 1074, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Kawachi, Y.; Ikegami, T.; Takubo, H.; Ikegami, Y.; Miyamoto, M.; Tanaka, N. Chromatographic characterization of hydrophilic interaction liquid chromatography stationary phases: Hydrophilicity, charge effects, structural selectivity, and separation efficiency. J. Chromatogr. A 2011, 1218, 5903–5919. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y. Recent progress in the fundamental understanding of hydrophilic interaction chromatography. Analyst 2015, 140, 6452–6466. [Google Scholar] [CrossRef] [PubMed]
- Jandera, P.; Janas, P. Recent advances in stationary phases and understanding of retention in hydrophilic interaction chromatography. A review. Anal. Chim. Acta 2017, 967, 12–32. [Google Scholar] [CrossRef]
- Jandera, P.; Hajek, T.; Sromov, Z. Mobile phase effects in reversed-phase and hydrophilic interaction liquid chromatography revisited. J. Chromatogr. A 2018, 1543, 48–57. [Google Scholar] [CrossRef]
- Khrisanfova, A.; Smagina, M.; Maksimov, G.; Tsizin, G.; Shpigun, O.; Chernobrovkina, A. Evaluating independent effect of mobile phase components on retention mechanism of ionizable analytes in hydrophilic interaction liquid chromatography. J. Chromatogr. A 2025, 1758, 466201. [Google Scholar] [CrossRef]
- Gritt, F.; Holtzel, A.; Tallarek, U.; Guiochon, G. The relative importance of the adsorption and partitioning mechanisms in hydrophilic interaction liquid chromatography. J. Chromatogr. A 2015, 1376, 112–125. [Google Scholar] [CrossRef]
- Guo, Y.; Fattal, B. Relative significance of hydrophilic partitioning and surface adsorption to the retention of polar compounds in hydrophilic interaction chromatography. Anal. Chim. Acta 2021, 1184, 339025. [Google Scholar] [CrossRef]
- Guo, Y.; Baran, D.; Ryan, L. Quantitative assessment of retention mechanisms of ionized compounds in hydrophilic interaction chromatography. Anal. Chem. 2025, 97, 4057–4065. [Google Scholar] [CrossRef]
- Guo, Y.; Bhalodia, N.; Fattal, B.; Serris, I. Evaluating the adsorbed water layer on the polar stationary phases in hydrophilic interaction chromatography. Separations 2019, 6, 6020019. [Google Scholar] [CrossRef]
- Guo, Y.; Baran, D.; Ryan, L. Insights into the selectivity of polar stationary phases based on quantitative retention mechanism assessment in hydrophilic interaction chromatography. J. Chromatogr. A 2024, 1726, 464973. [Google Scholar] [CrossRef]
- Jones, E.L.; Mlotkowski, A.J.; Hebert, S.P.; Schlegel, H.B.; Chow, C.S. Calculations of pKa values for a series of naturally occurring modified nucleobases. J. Phys. Chem. A 2022, 126, 1518–1529. [Google Scholar] [CrossRef]
- Soares, J.X.; Santos, A.; Fernandes, C.; Pinto, M.M.M. Liquid chromatography on the different methods for the determination of lipophilicity: An essential analytical tool in medicinal chemistry. Chemosensors 2022, 10, 340. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).