Abstract
Melamine (MEL) has broad applications and can be released to the aquatic environment from various sources, including industry, agriculture, traffic, and household articles. In addition, MEL derivatives ammeline (AMN), ammelide (AMD), and cyanuric acid (CYA) as well as potential precursors cyromazine (CYRO) and hexa(methoxymethyl)melamine (HMMM) are relevant related substances. However, occurrence and transformation in water resources has not yet been thoroughly investigated. Here, we developed a sensitive analytical method for quantification of these analytes by hydrophilic interaction liquid chromatography (HILIC) coupled to tandem mass spectrometry (MS/MS). Direct injection achieved limits of quantification (LOQs) of 0.1 µg/L (AMN 0.2 µg/L; CYA 1 µg/L), while LOQs could be improved to 0.01 µg/L (CYA 0.05 µg/L) by applying evaporation for analyte pre-concentration. The method was extensively validated, showing good recovery, repeatability, and linearity. The evaluation of the matrix effects revealed method applicability for various water matrices, including surface water and wastewater. During proof-of-concept measurements, HMMM in combination with MEL and its derivatives was found in multiple samples, emphasizing the importance of including precursors. In the future, the developed method with its novelty of covering both MEL derivatives and precursors can be applied for comprehensive monitoring programs elucidating MEL sources and transformation in water resources.
1. Introduction
Melamine (MEL; CAS number 108-78-1) and melamine-based resins are used for a broad variety of applications in industry (e.g., manufacture of plastics, laminates, adhesives, surface coatings, tires, and textiles). Therefore, MEL is a high-production-volume chemical. Although acute toxicity of MEL is low [1], it is suspected to be carcinogenic [2]. Melamine is under assessment as endocrine-disrupting by the European Chemicals Agency (ECHA) [2]. Recent publications, however, have stated that the substance does not meet the criteria for endocrine disruption [3,4].
The relevant derivatives of MEL are ammeline (AMN), ammelide (AMD), and cyanuric acid (CYA), which are all synthesis by-products and transformation products of MEL [5,6], formed by hydrolysis under elimination of ammonia (Figure 1). Especially the simultaneous occurrence of MEL and CYA has been shown to elevate substance toxicity substantially by formation of crystal complexes resulting in kidney stones [1,7]. In addition, MEL may be formed by degradation of precursors. MEL was shown to be the main metabolite of cyromazine (CYRO), an insecticide which can be transformed to MEL by dealkylation [8] (Figure 1). Also, hexa(methoxymethyl)melamine (HMMM), used as a cross-linking agent in coatings and vulcanizer in tires, is suspected to degrade to MEL [9,10].
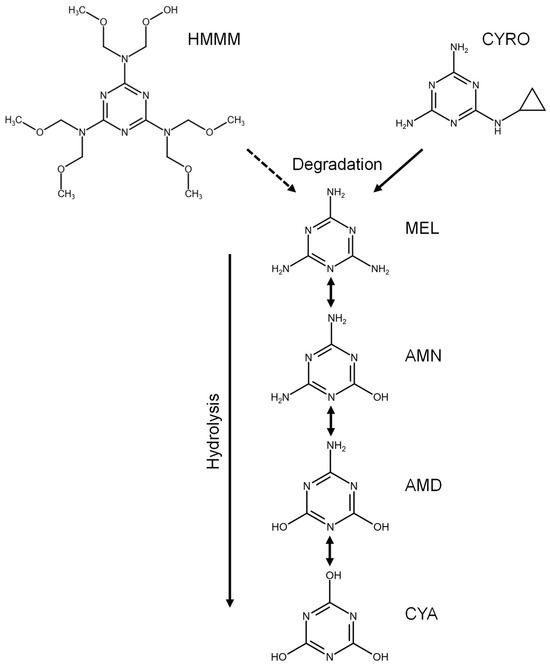
Figure 1.
Structures and possible transformations of melamine (MEL), ammeline (AMN), ammelide (AMD), cyanuric acid (CYA), hexa(methoxymethyl)melamine (HMMM), and cyromazine(CYRO).
Melamine has received substantial political and scientific attention since contamination incidents in 2007 and 2008. Milk products and gluten in pet food were found to be illegally contaminated with MEL and its derivatives, resulting in severe renal failure in both humans and animals [11,12,13]. This led to the development and publication of numerous analytical methods for detection and quantification of MEL and its derivatives in the following years. During a comprehensive literature review, 84 relevant publications describing analytical methods for MEL and at least one of its derivatives were evaluated. Different analytical methods have been applied, mostly based on gas chromatography (GC) or (high-performance) liquid chromatography ((HP)LC). Gas chromatography can be coupled with mass spectrometry (MS) [14,15] or MS/MS detection [16,17], while (HP)LC has been applied in combination with ultraviolet (UV) detection [18], diode array detection (DAD) [19,20], MS [21,22], or MS/MS [23,24].
Although a multitude of applications have been published, the majority is targeting foodstuffs and pet food (43 of 84 relevant publications), with special emphasis on milk and infant formula, e.g., [16,17,22,25,26]. However, MEL and its related substances can also be released to the aquatic environment by discharge and transformation processes which are not yet well understood. Industrial discharges as well as release from the use phase of household products are possible entry pathways [27], defining MEL as an indicator for anthropogenic activity [28]. Monitoring data for MEL occurrence in the aquatic environment is scarce [27] and only a few studies on the analysis and occurrence of MEL and derivatives in water matrices have been published so far [19,23,29,30,31]. To our knowledge, only Zhu and Kannan [29] measured all four derivatives with limits of quantification (LOQs) below 0.5 µg/L in aquatic matrices. Most published methods either resulted in higher LOQ values or did not include all derivatives. In most publications, solid-phase extraction (SPE) with both cation and anion exchange has been applied for sample preparation [29,30,32]. Moreover, analysis of MEL precursors in water matrices in addition to its transformation products has received very limited scientific attention. Cyromazine has occasionally been included in analytical methods [19,23,30,33]. Hexa(methoxymethyl)melamine has been analyzed in combination with MEL [10,34] but not, however, with its transformation products.
Thus, to gain information about distribution of MEL and its related substances in water resources as well as to elucidate transformation pathways, a sensitive, robust, and efficient quantitative analytical method covering MEL and its derivatives AMN, AMD, and CYA, as well as precursors CYRO and HMMM is needed. Here, we developed a sensitive multi-method for quantification of these six target analytes in various water matrices, including drinking water, surface water, and wastewater. Analysis was conducted by HPLC-MS/MS with chromatographic separation by hydrophilic interaction liquid chromatography (HILIC). The method was validated and showed good recovery, linearity, and repeatability. While already sensitive with direct injection, method sensitivity can be further enhanced by applying evaporation during sample preparation.
2. Materials and Methods
2.1. Reagents and Stock and Standard Solutions
Certified reference materials of analytical standards and stable isotope-labeled internal standards (ISs) were obtained from Dr. Ehrenstorfer (Augsburg, Germany; MEL, CYA, CYRO, and MEL-13C3), Cambridge Isotope Laboratories (Tewksbury, MA, USA; AMN and AMD), and Toronto Research Chemicals Inc. (Toronto, ON, Canada; HMMM, AMN-13C3, AMD-13C3, CYA-13C3, and CYRO-d4). For HMMM, no isotope-labeled IS was commercially available. Ultrapure water from a Sartorius AriumPro system (Goettingen, Germany); methanol from Honeywell (Charlotte, NC, USA); acetonitrile from Supelco (Bellefonte, PA, USA); diethylamine, ammonium acetate, and ammonium formate from Sigma-Aldrich (Saint Louis, MO, USA); acetic acid from Roth (Karlsruhe, Germany); and formic acid from VWR (Darmstadt, Germany) were used for preparation of HPLC eluents and stock and standard solutions (all except for ammonium acetate LC-MS grade; ammonium acetate > 98% purity).
The standards and ISs of MEL, CYA, CYRO, and HMMM were dissolved in methanol. Ammeline and AMD were dissolved in a mixture of ultrapure water:diethylamine (80:20, v:v) due to low solubility in methanol. From these stock solutions, standard solutions with a concentration of 1 mg/L were prepared in methanol for all standards and ISs. These standard solutions were determined to be stable for one year. Combined calibration standards of all the substances were prepared daily in acetonitrile:water (90:10, v:v). As the water matrix, the local drinking water was used, which was free of the analytes under investigation.
2.2. Instrumentation
Sample evaporation was performed with a vacuum pre-concentrator (RVC 2-33 CDplus, Martin Christ Gefriertrocknungsanlagen GmbH, Osterode, Germany). LC-MS/MS analysis was implemented on an HPLC Infinity 1290 system (Agilent Technologies, Santa Clara, CA, USA) coupled to an API5500 Triple Quad system with electrospray ionization (AB Sciex, Framingham, MA, USA). For the final method, a Luna HILIC chromatography column (150 × 2 mm, 3 µm; Phenomenex, Torrance, CA, USA) with a HILIC security guard cartridge (4 × 2 mm; Phenomenex) was applied.
2.3. Method Optimization
For sample preparation, both evaporation and SPE were assessed as possible methods for analyte pre-concentration. The SPE protocol was adapted from previous publications [24,29] and varied, using cation exchange (Waters Oasis MCX, 150 mg, 6 mL, 30 µm; Milford, MA, USA) and anion exchange cartridges (Waters Oasis MAX, 150 mg, 6 mL, 30 µm). The details of the tested SPE protocol are given in Table S1.
Aside from the chosen Luna HILIC column, another HILIC column (Sequant ZIC-HILIC, 20 × 2.1 mm, 5 µm; Merck, Darmstadt, Germany) and two mixed-mode columns (Obelisc R, 150 × 2.1 mm, 5 μm; SIELC, Wheeling, IL, USA, and Raptor Polar, 100 × 2.1 mm, 2.7 μm; Restek, Centre County, PA, USA) were tested. However, the mixed-mode columns were excluded from further method development due to insufficient separation of analytes and poor signal intensities. Chromatographic separation of the analytes was also achieved on the ZIC-HILIC column, but peak shapes and sensitivity were slightly better on the chosen Luna HILIC column. Different eluent compositions with an acetonitrile content between 60 and 95 vol% during isocratic elution and a gradient with variation between 100 and 80 vol% were tried out on the HILIC columns to achieve optimum sensitivity and analyte peak shapes. In addition, ammonium acetate, ammonium formate, acetic acid, and formic acid were evaluated as eluent modifiers to increase method sensitivity. The injection volume was varied between 5 and 40 µL to optimize peak shapes.
2.4. Determination of Limits of Detection and Quantification
After optimization, limits of detection (LODs) and LOQs were determined for both the direct injection method and the evaporation method in various matrices, i.e., drinking water, surface water, and wastewater (influent and effluent of wastewater treatment plant (WWTP)), based on signal-to-noise ratios (S/N). The LOD was defined at S/N = 3 and LOQ at S/N = 10.
2.5. Method Validation
The method was validated including both direct injection and analyte pre-concentration by evaporation. The validation protocol included the determination of recovery during analyte pre-concentration, repeatability, calibration linearity, and matrix effects of the method. Recovery of pre-concentration compared to direct injection in matrix drinking water was considered acceptable between 75 and 125% [35]. Calibration linearity was determined for each analyte in a concentration range between 0.5 and 10 × LOQ and the resulting coefficient of determination r2 was required to exceed 0.99. The matrix effects of surface water and wastewater (influent and effluent of WWTP) were examined and required to remain below a threshold of 25% compared to drinking water as the reference value. Method repeatability was determined by analyzing five replicate samples with a concentration of 5 × LOQ, whereas standard deviation had to remain below 25%.
2.6. Stability Test
Stability of analytes was assessed over 28 days in sample containers consisting of glass, polyethylene (PE), and polystyrene (PS) stored at room temperature (20 °C), refrigerated (4 °C), and frozen (−18 °C). To this end, separate analyte solutions with 100 µg/L were prepared in drinking water, and samples were taken on days 0, 1, 2, 5, 7, 14, 21, and 28 for MEL, AMN, AMD, and CYA. Additionally, samples for CYRO and HMMM were taken on days 0 and 28.
2.7. Proof of Concept
To show the applicability of the developed method and obtain the first results, 10 samples exhibiting different water matrices were analyzed. These included drinking water, groundwater, surface water, road run-off, and wastewater (detailed information on the samples is given in Table S2). Drinking water and groundwater were measured with evaporation as pre-concentration, while all the other samples were measured with direct injection.
3. Results
3.1. Sample Preparation
Both evaporation and SPE proved successful for pre-concentration of the analytes under investigation. During SPE, MEL, AMN, CYRO, and HMMM were pre-concentrated by cation exchange and AMD and CYA by anion exchange (Table S1). However, pre-concentration by SPE was by far more labor-intensive and material-consuming due to the use of two separate cartridges and several conditioning and elution steps. The LOQ values achieved with SPE after optimization (Table S1) were similar to the values after evaporation, thereby not enhancing method sensitivity compared to simple evaporation of the water. Therefore, evaporation was selected as the preferable method for analyte pre-concentration, with an initial sample volume of 10 mL. After evaporation, the dry residue was reconstituted in acetonitrile:ultrapure water (90:10, v:v) to match the eluent composition of the chromatographic method. Alternatively, samples can be measured by direct injection if maximizing sensitivity is not crucial. In this case, water samples need to be diluted with acetonitrile to achieve the matrix of acetonitrile:water (90:10, v:v; Table 1).

Table 1.
Sample preparation steps with direct injection and pre-concentration by evaporation.
3.2. Optimum Chromatographic and MS Conditions
While multiple chromatographic parameters were optimized, exemplary chromatograms are shown in Figure 2, depicting the impact of isocratic eluent variation on CYA detection. Here, isocratic elution with acetonitrile:ultrapure water (90:10, v:v) was determined as the best eluent composition, with addition of 10 mM ammonium acetate and 0.1% formic acid to the water phase. The sensitivity and peak shape improved with increasing acetonitrile content. From acetonitrile:ultrapure water 90:10 (v:v; Figure 2C) to 95:5 (v:v; Figure 2D), only minimal improvement was achieved, and the higher acetonitrile content would also increase the necessary sample dilution during direct injection to match the eluent composition and sample matrix; therefore, a ratio of 90:10 (v:v) was determined as optimum. Optimum peak shapes were achieved with an injection volume of 10 µL. The flow was set to 0.4 mL/min and the column temperature to 30 °C. The method resulted in retention times between 1.2 and 3.4 min (Figure 3) and was continued until 10 min to avoid contamination. The analytical standards and isotope-labeled ISs of the respective analytes showed simultaneous elution. The MS parameters for detection of target analytes and ISs are listed in Table 2.
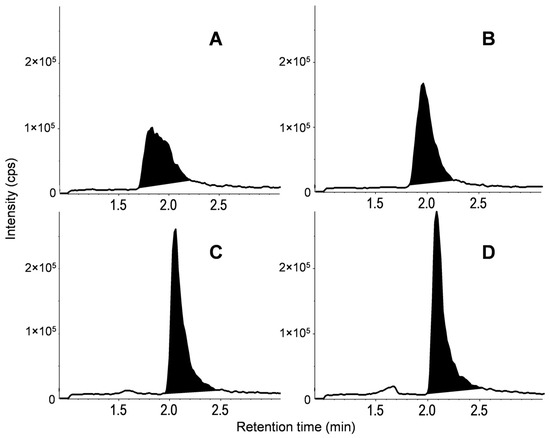
Figure 2.
Comparison of the chromatographic signal of CYA as the most challenging analyte during method development. Signals with eluent ratios acetonitrile:ultrapure water with 10 mM ammonium acetate and 0.1% formic acid of (A) 70:30 v:v, (B) 80:20 v:v, (C) 90:10 v:v, and (D) 95:5 v:v are shown, with all other parameters kept constant. (C) was chosen for all further optimization steps.
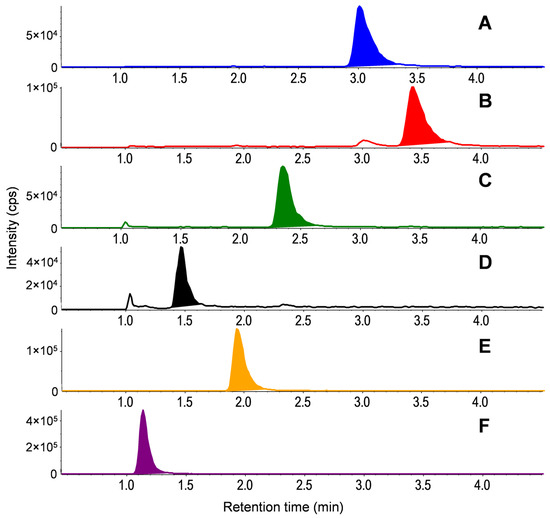
Figure 3.
Extracted ion chromatograms of quantifier ions for (A) MEL, (B) AMN, (C) AMD, (D) CYA, (E) CYRO, and (F) HMMM with the final method. Standards with concentrations of 10 µg/L in drinking water were measured with direct injection. Chromatographic separation was achieved on a Luna HILIC column (150 × 2 mm, 3 µm; Phenomenex, Torrance, CA, USA) with a HILIC security guard cartridge (4 × 2 mm; Phenomenex) and isocratic elution with acetonitrile:ultrapure water with 10 mM ammonium acetate and 0.1% formic acid (90:10, v:v). The flow was set to 0.4 mL/min and the column temperature to 30 °C. An injection volume of 40 µL was applied.

Table 2.
Retention times and MS parameters (m/z at quadrupole 1 (Q1), m/z at quadrupole 3 (Q3), declustering potential (DP), entrance potential (EP), collision energy (CE), and collision cell exit potential (CXP)) for detection of target analytes and available ISs.
3.3. Limits of Detection and Quantification
With direct injection, LOQ values of 0.1 µg/L were achieved for MEL, AMD, CYRO, and HMMM. For AMN and CYA, direct injection resulted in LOQs of 0.2 µg/L and 1.0 µg/L (2.0 µg/L for CYA in wastewater), respectively. By applying evaporation for analyte pre-concentration, LOQ values in the drinking and surface water were lowered to 0.01 µg/L for all analytes but for CYA, where the LOQ was 0.05 µg/L. In the wastewater samples, which had to be diluted in ultrapure water prior to analysis with evaporation (by factor 5 for WWTP effluents and factor 10 for WWTP influents), LOQ differences between direct injection and evaporation were not as significant (Table 3; LOD values in Table S3).

Table 3.
Limits of quantification for target analytes in different water matrices with direct injection and evaporation.
3.4. Method Validation
3.4.1. Recovery of Evaporation
Recoveries of target analytes after pre-concentration by evaporation ranged between 76.1% (HMMM) and 103.2% (MEL; Table 4). Consequently, all the analytes showed sufficient recovery during sample preparation.

Table 4.
Recoveries of target analytes after pre-concentration by evaporation (corrected by internal standards, except for HMMM).
3.4.2. Calibration Linearity
All the analytes showed linear calibrations with r2 ≥ 0.99 for both direct injection and evaporation (Table S4). Linearity was therefore ascertained in the concentration range of 0.5 to 10 × LOQ.
3.4.3. Matrix Effects
All the analytes except for HMMM remained well below the threshold of 25% for acceptable matrix effects in surface water and wastewater with both direct injection and evaporation (Table S5). For HMMM, considerably higher matrix effects were observed with evaporation for WWTP effluents (76.6%) and WWTP influents (68.7%). These matrix effects could not be corrected because no IS for HMMM was commercially available and all other applied ISs were unsuitable for HMMM matrix correction. Therefore, it is not recommended to apply the evaporation method for HMMM measurements in wastewater samples. These samples should be measured for HMMM only with direct injection. Also, the application of SPE might be suitable for analysis of HMMM in wastewater samples, leading to the removal of interfering compounds.
3.4.4. Repeatability
Standard deviations of five replicate samples with concentrations of 5 × LOQ remained within 25% of the spiked value for all target analytes (Table S6). Consequently, the method showed sufficient repeatability.
3.5. Stability Test
All the target analytes were stable in water over the test period of 28 days in all the tested sample containers (glass, PE, and PS) and under all the tested temperature conditions (room temperature, 4 °C, and −18 °C). Analytes were considered stable if the concentrations remained within a deviation of ±25% from the initial concentration (Figures S1–S3). These deviations can be attributed to measurement uncertainties. Also, no decreasing trends over time, which would indicate degradation or transformation, were observed. As further confirmation, samples were analyzed for all six target analytes even though only one standard was added. Since some of the analytes are transformation products of other analytes, occurrence and increasing concentrations of such other analytes would suggest degradation. However, no other target analytes were detected, supporting the findings of analyte stability.
3.6. Proof of Concept
Of the 10 analyzed proof-of-concept samples, all except two contained MEL, with concentrations between 0.015 µg/L (groundwater) and 6.9 µg/L (WWTP influent). The MEL concentrations were below the LOQ only in the drinking water and one groundwater sample. Ammeline was not detected in any sample, but AMD and CYA were found in road run-off samples and CYA additionally in both WWTP effluents, with concentrations up to 2.1 µg/L (AMD) and 12 µg/L (CYA). Furthermore, HMMM was detected in road run-off, wastewater, and one surface water sample, with concentrations up to 1.6 µg/L (Table 5).

Table 5.
Concentrations of target analytes in samples measured as proof of concept.
4. Discussion
In this study, a sensitive and robust method for analysis of MEL; its derivatives AMN, AMD, and CYA; and its potential precursors CYRO and HMMM was developed and validated for various water matrices. Depending on the required sensitivity and expected concentration ranges, samples can either be measured with direct injection or after evaporation as analyte pre-concentration. Only HMMM, for which no isotope-labeled IS is commercially available, should not be measured in wastewater with evaporation due to the possibility of severe matrix effects, such as signal suppression.
Even with evaporation, sample preparation is more efficient in terms of required time and material when compared to methods applying SPE. In addition, with a total run-time of 10 min per sample for separation of all analytes, the chromatographic method is fast while still achieving good sensitivity. Furthermore, the required volumes (1 mL for direct injection; 10 mL for evaporation) are low compared to methods including SPE as analyte pre-concentration, where usually between 20 and 100 mL is necessary to perform both cation and anion exchange [29,32]. However, it is still recommended to collect at least 50 mL of sample, if possible, to allow for repeated measurements including analyte pre-concentration.
Several implications can be drawn from the conducted stability tests, guiding sampling and sample transport strategies. The observed stability of all the analytes under the tested conditions renders addition of stabilizers and sample cooling unnecessary. Still, samples should not be exposed to temperatures above 25 °C and shielded from sunlight. Addition of pH modifiers should also be avoided because both acidic and basic conditions can facilitate transformation between MEL and its derivatives [24,36]. With all the tested sample container materials (glass, PE, and PS) determined as suitable, sampling in PE or PS containers is recommended to avoid glass breakage during sample transport. Samples should be analyzed within 28 days after sampling to remain within the timeframe of ascertained analyte stability.
During proof-of-concept measurements, MEL was found in all the surface water, road run-off, and wastewater samples and additionally in one groundwater sample. These results are in accordance with recent findings, showing almost ubiquitous occurrence of MEL throughout the water cycle [28]. Releases to the aquatic environment may stem from a variety of anthropogenic activities, including, among others, industrial discharges [28], agricultural applications [30], traffic due to tire wear [37,38,39], laundry (dissolution of wrinkle-proof substances in textiles [40]), concrete additives (superplasticizers [41,42]), and use of MEL-containing household items [27]. In our study, the highest MEL concentration was observed in the WWTP influent, with a concentration decrease towards the effluent. Reduction in MEL concentrations by wastewater treatment has been previously reported [29,43,44], with adsorption on activated sludge determined as a main removal mechanism while microbial degradation was negligible [44]. However, various strains of bacteria are capable of degrading MEL finally into CO2 and ammonia [6,45,46]. In contrast, other studies reported an increase in MEL concentrations in WWTPs [10,30]. These opposing results suggest the simultaneous occurrence of several degradation, formation, or release processes, which have not yet been thoroughly investigated.
Of the three MEL derivatives, CYA occurred most abundantly during the proof-of-concept measurements, which was also observed by Zhu and Kannan during measurement of various water matrices in the United States [29]. This may either be due to MEL transformation with CYA as final hydrolytic product or to a discharge by separate sources, e.g., presence of CYA as an impurity in dishwashing detergents [26,29] or as an additive in swimming pools [47]. Cyromazine was likely not detected in the measured samples because of seasonal applications in agriculture [8]. The observed occurrence of HMMM in wastewater, road run-off, and surface water has been previously reported [10,34,43]. In the proof-of-concept study, all the samples with HMMM contained MEL and partially AMD and CYA as well. While the number of samples measured as proof of concept during this study is not sufficient to establish statistical correlations between the measured compounds, these findings still suggest some connections. Consequently, in addition to contributing to available monitoring data on MEL, the application of the developed method to various water samples can also elucidate pathways and favoring conditions for transformation of precursors to MEL or its hydrolysis products, which so far remain unclear. Especially HMMM is of interest since it is frequently detected in the aquatic environment and has usually not been included in analytical methods in addition to MEL and its derivatives.
In conclusion, the developed method with efficient sample preparation and sensitive measurement of MEL, derivatives, and precursors is applicable to conduct large-scale studies and monitoring programs. The method adds novelty by covering both derivatives and several potential precursors in addition to MEL, which has not been previously reported. Thus, the method contributes to an improved understanding of MEL distribution in the aquatic environment and its transformation in water resources and therefore to securing water quality.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/analytica6030027/s1. Table S1: Details of tested SPE protocol (adapted from [29]); Table S2: Sample information on proof-of-concept samples; Table S3: Limits of detection for target analytes in different water matrices with direct injection and evaporation; Table S4: Linearity (described by correlation coefficient r2 and linear equation) of target analytes for direct injection and evaporation in the concentration range 0.5–10 × LOQ (corrected by IS, except for HMMM); Table S5: Matrix effects of analytes at a concentration of 5 × LOQ for direct injection and evaporation in matrices, surface water, WWTP effluent, and WWTP influent, compared to drinking water (corrected by IS, except for HMMM); Table S6: Standard deviation of five replicate measurements of analytes with concentrations of 5 × LOQ for direct injection and evaporation (corrected by IS, except for HMMM); Figure S1: Results of stability tests (corrected by IS) of MEL (top) and AMD (bottom) in the tested container materials glass (left), PE (middle), and PS (right) at tested temperature conditions, room temperature (red), refrigerated (black), and frozen (blue). The green area depicts a deviation of ±25% from the concentration measured at day 0; Figure S2: Results of stability tests (corrected by IS) of AMN (top) and CYA (bottom) in the tested container materials glass (left), PE (middle), and PS (right) at tested temperature conditions, room temperature (red), refrigerated (black), and frozen (blue). The green area depicts a deviation of ±25% from the concentration measured at day 0; Figure S3: Results of stability tests of CYRO (top; corrected by IS) and HMMM (bottom) in the tested container materials glass (left), PE (middle), and PS (right) at tested temperature conditions, room temperature (red), refrigerated (black), and frozen (blue). The green area depicts a deviation of ±25% from the concentration measured at day 0.
Author Contributions
Conceptualization, F.S. and M.K.; methodology, M.K. and L.N.; validation, L.N., M.K. and F.S.; formal analysis, M.K.; investigation, L.N. and M.K.; writing—original draft preparation, M.K.; writing—review and editing, F.S. and L.N.; visualization, M.K.; supervision, F.S.; project administration, F.S.; funding acquisition, F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Melamine Producers Association (EMPA) during the project “Development of a standardised analytical method for the detection and quantitation of melamine and related substances in aqueous media.” The APC was also funded by EMPA.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; or in the writing of the manuscript.
Abbreviations
The following abbreviations are used in this manuscript:
| AMD | Ammeline |
| AMN | Ammelide |
| CE | Collision energy |
| CXP | Collision cell exit potential |
| CYA | Cyanuric acid |
| CYRO | Cyromazine |
| DAD | Diode array detector |
| DP | Declustering potential |
| ECHA | European Chemicals Agency |
| GC | Gas chromatography |
| EP | Entrance potential |
| HMMM | Hexa(methoxymethyl)melamine |
| HILIC | Hydrophilic interaction liquid chromatography |
| HPLC | High-performance liquid chromatography |
| IS | Isotope-labeled internal standards |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| MEL | Melamine |
| MS | Mass spectrometry |
| MS/MS | Tandem mass spectrometry |
| PE | Polyethylene |
| PS | Polystyrene |
| REACH | Registration, evaluation, authorization, and restriction of chemicals |
| S/N | Signal-to-noise ratio |
| SPE | Solid-phase extraction |
| UV | Ultraviolet detector |
| WWTP | Wastewater treatment plant |
References
- Wu, Y.; Zhang, Y. Analytical chemistry, toxicology, epidemiology and health impact assessment of melamine in infant formula: Recent progress and developments. Food. Chem. Toxicol. 2013, 56, 325–335. [Google Scholar] [CrossRef] [PubMed]
- ECHA (European Chemicals Agency). Substance Infocard Melamine. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.003.288 (accessed on 15 April 2025).
- Charron, I.; Magueresse-Battistoni, B.L.; Habert, R.; Canivenc-Lavier, M.C.; Mhaouty-Kodja, S.; Michel-Caillet, C. Melamine regulatory assessment for endocrine disruption. Environ. Int. 2024, 194, 109188. [Google Scholar] [CrossRef] [PubMed]
- Ministère de la Transition Écologique et de la Cohésion des Territoires. Regulatory Management Option Analysis Conclusion Document. 2024. Available online: https://echa.europa.eu/documents/10162/1f5fde3d-96ae-340a-78bd-61a650ab3fb4 (accessed on 12 June 2025).
- Ono, S.; Funato, T.; Inoue, Y.; Munechika, T.; Yoshimura, T.; Morita, H.; Rengakuji, S.-I.; Shimasaki, C. Determination of melamine derivatives, melame, meleme, ammeline and ammelide by high-performance cation-exchange chromatography. J. Chromatogr. A 1998, 815, 197–204. [Google Scholar] [CrossRef]
- Shelton, D.R.; Karns, J.S.; McCarty, G.W.; Durham, D.R. Metabolism of melamine by Klebsiella terragena. Appl. Environ. Microbiol. 1997, 63, 2832–2835. [Google Scholar] [CrossRef]
- Dobson, R.L.M.; Motlagh, S.; Quijano, M.; Cambron, R.T.; Baker, T.R.; Pullen, A.M.; Regg, B.T.; Bigalow-Kern, A.S.; Vennard, T.; Fix, A.; et al. Identification and characterization of toxicity of contaminants in pet food leading to an outbreak of renal toxicity in cats and dogs. Toxicol. Sci. 2008, 106, 251–262. [Google Scholar] [CrossRef]
- Pote, D.H.; Daniel, T.C.; Edwards, D.R.; Mattice, J.D.; Wickliff, D.B. Effect of drying and rainfall intensity on cyromazine loss from surface-applied caged-layer manure. J. Environ. Qual. 1994, 23, 101–104. [Google Scholar] [CrossRef]
- Rauert, C.; Kaserzon, S.L.; Veal, C.; Yeh, R.Y.; Mueller, J.F.; Thomas, K.V. The first environmental assessment of hexa(methoxymethyl)melamine and co-occurring cyclic amines in Australian waterways. Sci. Total Environ. 2020, 743, 140834. [Google Scholar] [CrossRef]
- Alhelou, R.; Seiwert, B.; Reemtsma, T. Hexamethoxymethylmelamine—A precursor of persistent and mobile contaminants in municipal wastewater and the water cycle. Water Res. 2019, 165, 114973. [Google Scholar] [CrossRef]
- Tyan, Y.-C.; Yang, M.-H.; Jong, S.-B.; Wang, C.-K.; Shiea, J. Melamine contamination. Anal. Bioanal. Chem. 2009, 395, 729–735. [Google Scholar] [CrossRef]
- Tittlemier, S.A. Methods for the analysis of melamine and related compounds in foods: A review. Food Addit. Contam. Part A 2010, 27, 129–145. [Google Scholar] [CrossRef]
- Puschner, B.; Reimschuessel, R. Toxicosis caused by melamine and cyanuric acid in dogs and cats: Uncovering the mystery and subsequent global implications. Clin. Lab. Med. 2011, 31, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ma, X.; Li, Z.; Chen, M. Fast derivatization followed by gas chromatography–mass spectrometry for simultaneous detection of melamine, ammeline, ammelide, and cyanuric acid in fish and shrimp. Food Anal. Methods 2016, 9, 16–22. [Google Scholar] [CrossRef]
- Wong, Y.-L.; Mok, C.-S. A single analytical procedure for the simultaneous and confirmatory determination of melamine and related compounds in various food matrices by isotope dilution gas chromatography-mass spectrometry (ID-GC-MS). Anal. Methods 2013, 5, 2305–2314. [Google Scholar] [CrossRef]
- Miao, H.; Fan, S.; Zhou, P.P.; Zhang, L.; Zhao, Y.F.; Wu, Y.N. Determination of melamine and its analogues in egg by gas chromatography-tandem mass spectrometry using an isotope dilution technique. Food Addit. Contam. 2010, 27, 1497–1506. [Google Scholar] [CrossRef]
- Tzing, S.-H.; Ding, W.-H. Determination of melamine and cyanuric acid in powdered milk using injection-port derivatization and gas chromatography–tandem mass spectrometry with furan chemical ionization. J. Chromatogr. A 2010, 1217, 6267–6273. [Google Scholar] [CrossRef]
- Wang, H.; Lin, L.; Sun, Q.; Lin, Q.; Xiong, X.; Wu, K.; Yu, C.-P. Simultaneous determination of cyromazine, melamine and their biodegradation products by ion-pair high-performance liquid chromatography. Int. J. Environ. Anal. Chem. 2014, 94, 1173–1182. [Google Scholar] [CrossRef]
- Sun, H.; Qin, X.; Ge, X.; Wang, L. Effective separation and sensitive determination of cyanuric acid, melamine and cyromazine in environmental water by reversed phase high-performance liquid chromatography. Enivron. Technol. 2011, 32, 317–323. [Google Scholar] [CrossRef]
- Ehling, S.; Tefera, S.; Ho, I.P. High-performance liquid chromatographic method for the simultaneous detection of the adulteration of cereal flours with melamine and related triazine by-products ammeline, ammelide, and cyanuric acid. Food Addit. Contam. 2007, 24, 1319–1325. [Google Scholar] [CrossRef]
- Vinas, P.; Campillo, N.; Férez-Melgarejo, G.; Hernández-Córdoba, M. Determination of melamine and derivatives in foods by liquid chromatography coupled to atmospheric pressure chemical ionisation mass spectrometry and diode array detection. Anal. Lett. 2012, 45, 2508–2518. [Google Scholar] [CrossRef]
- Draher, J.; Ehling, S.; Cellar, N.; Reddy, T.; Henion, J.; Sousou, N. Determination of emerging nitrogenous economic adulterants in milk proteins by high-performance liquid chromatography/compact mass spectrometry. Rapid Commun. Mass Spectrom. 2016, 30, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Kolkman, A.; Vughs, D.; Sjerps, R.; Kooij, P.J.F.; van der Kooi, M.; Baken, K.; Louisse, J.; de Voogt, P. Assessment of highly polar chemicals in Dutch and Flemish drinking water and its sources: Presence and potential risks. Environ. Sci. Technol. Water 2021, 1, 928–937. [Google Scholar] [CrossRef]
- Zhu, H.; Kannan, K. Melamine and cyanuric acid in foodstuffs from the United States and their implications for human exposure. Environ. Int. 2019, 130, 104950. [Google Scholar] [CrossRef] [PubMed]
- García-Miguel, E.; Meza-Márquez, O.G.; Osorio-Revilla, G.; Téllez-Medina, D.I.; Jiménez-Martínez, C.; Cornejo-Mazón, M.; Hernández-Martínez, D.M.; Gallardo-Velazquez, T. Detection of cyanuric acid and melamine in infant formula powders bei mid-FTIR spectroscopy and multivariate analysis. J. Food Qual. 2018, 2018, 7926768. [Google Scholar] [CrossRef]
- Braekevelt, E.; Lau, B.P.-Y.; Feng, S.; Ménard, C.; Tittlemier, S.A. Determination of melamine, ammeline, ammelide and cyanuric acid in infant formula purchased in Canada by liquid chromatography-tandem mass spectrometry. Food Addit. Contam. 2011, 28, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Lütjens, L.H.; Pawlowski, S.; Silvani, M.; Blumenstein, U.; Richter, I. Melamine in the environment: A critical review of available information. Environ. Sci. Eur. 2023, 35, 2. [Google Scholar] [CrossRef]
- Warner, W.; Licha, T. Melamine—A PMT/vPvM substance as a generic indicator for anthropogenic activity and urbanisation? An explorative study on melamine in the water cycle and soil. Chemosphere 2025, 370, 143918. [Google Scholar] [CrossRef]
- Zhu, H.; Kannan, K. Occurrence and distribution of melamine and its derivatives in surface water, drinking water, precipitation, wastewater, and swimming pool water. Environ. Pollut. 2019, 258, 113743. [Google Scholar] [CrossRef]
- Liu, S.-S.; Cai, Q.-S.; Li, C.; Cheng, S.; Wang, Z.; Yang, Y.; Ying, G.-G.; Sweetman, A.J.; Chen, C.-E. In situ measurement of an emerging persistent, mobile and toxic (PMT) substance—Melamine and related triazines in waters by diffusive gradient in thin-films. Water Res. 2021, 206, 117752. [Google Scholar] [CrossRef]
- Neuwald, I.J.; Hübner, D.; Wiegand, H.L.; Valkov, V.; Borchers, U.; Nödler, K.; Scheurer, M.; Hale, S.E.; Arp, H.P.H.; Zahn, D. Occurence, distribution, and environmental behavior of persistent, mobile, and toxic (PMT) and very persistent and very mobile (vPvM) substances in the sources of German drinking water. Environ. Sci. Technol. 2022, 56, 10857–10867. [Google Scholar] [CrossRef]
- Schulze, S.; Zahn, D.; Montes, R.; Rodil, R.; Quintana, J.B.; Knepper, T.P.; Reemtsma, T.; Berger, U. Occurrence of emerging persistent and mobile organic contaminants in European water samples. Water Res. 2019, 153, 80–90. [Google Scholar] [CrossRef]
- He, L.; Su, Y.; Shen, X.; Zheng, Y.; Guo, H.; Zeng, Z. Solid-phase extraction of melamine from aqueous samples using water-compatible molecularly imprinted polymers. J. Sep. Sci. 2009, 32, 3310–3318. [Google Scholar] [CrossRef]
- Johannessen, C.; Helm, P.; Metcalfe, C.D. Detection of selected tire wear compounds in urban receiving waters. Environ. Pollut. 2021, 287, 117659. [Google Scholar] [CrossRef]
- GTFCH (Gesellschaft für Toxikologische und Forensische Chemie). Requirements for the Validation of Analytical Methods. 2009. Available online: https://www.gtfch.org/cms/images/stories/files/Appendix%20B%20GTFCh%2020090601.pdf (accessed on 12 June 2025).
- Gong, H.; Tang, S.; Zhang, T. Catalytic hydrolysis of waste residue from the melamine process and the kinetics of melamine hydrolysis in NaOH solution. React. Kinet. Mech. Catal. 2016, 118, 377–391. [Google Scholar] [CrossRef]
- Johannessen, C.; Parnis, J.M. Environmental modelling of hexamethoxymethylmelamine, its transformation products, and precursor compounds: An emerging family of contaminants from tire wear. Chemosphere 2021, 280, 130914. [Google Scholar] [CrossRef] [PubMed]
- Dsikowitzky, L.; Schwarzbauer, J. Hexa(methoxymethyl) melamine: An emerging contaminant in German rivers. Water Environ. Res. 2015, 87, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, C.; Helm, P.; Metcalfe, C.D. Runoff of the tire-wear compound hexamethoxymethyl-melamine into urban watersheds. Archives Environ. Contam. Toxicol. 2022, 82, 162–170. [Google Scholar] [CrossRef]
- Zhu, H.; Kannan, K. Determination of melamine and its derivatives in textiles and infant clothing purchased in the United States. Sci. Total Environ. 2020, 710, 136396. [Google Scholar] [CrossRef]
- Judzentiene, A.; Zdaniauskiene, A.; Ignatjev, I.; Druteikiene, R. Evaluation of physico-chemical characteristics of cement superplasticizer based on polymelamine sulphonate. Materials 2024, 17, 1940. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Xiong, W.; Liu, X.; Zhang, Z. Synthesis and the effects of new melamine superplasticizer on the properties of concrete. Int. Sch. Res. Notices 2013, 1, 708063. [Google Scholar] [CrossRef]
- Seitz, W.; Winzenbacher, R. A survey on trace organic chemicals in a German water protection area and the proposal of relevant indicators for anthropogenic influences. Environ. Monit. Assess. 2017, 189, 244. [Google Scholar] [CrossRef]
- An, H.; Li, X.; Yang, Q.; Wang, D.; Xie, T.; Zhao, J.; Xu, Q.; Chen, F.; Zhong, Y.; Yuan, Y.; et al. The behavior of melamine in biological wastewater treatment system. J. Hazard. Mater. 2017, 322, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Jutzi, K.; Cook, A.M.; Hütter, R. The degradative pathway of the s-triazine melamine. The steps to ring cleavage. Biochem. J. 1982, 208, 679–684. [Google Scholar] [CrossRef]
- Takagi, K.; Fujii, K.; Yamazaki, K.; Harada, N.; Iwasaki, A. Biodegradation of melamine and its hydroxy derivatives by a bacterial consortium containing a novel Nocardioides species. Appl. Microbiol. Biot. 2012, 94, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Su, Y.; Chen, J.; Li, Z.; Wang, T. Study on the health risk of cyanuric acid in swimming pool water and its prevention and control measures. Front. Public Health 2024, 11, 1294842. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).