Development of Noninvasive Method for the Automated Analysis of Nine Steroid Hormones in Human Saliva by Online Coupling of In-Tube Solid-Phase Microextraction with Liquid Chromatography–Tandem Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standard Solutions
2.2. LC–MS/MS Analysis
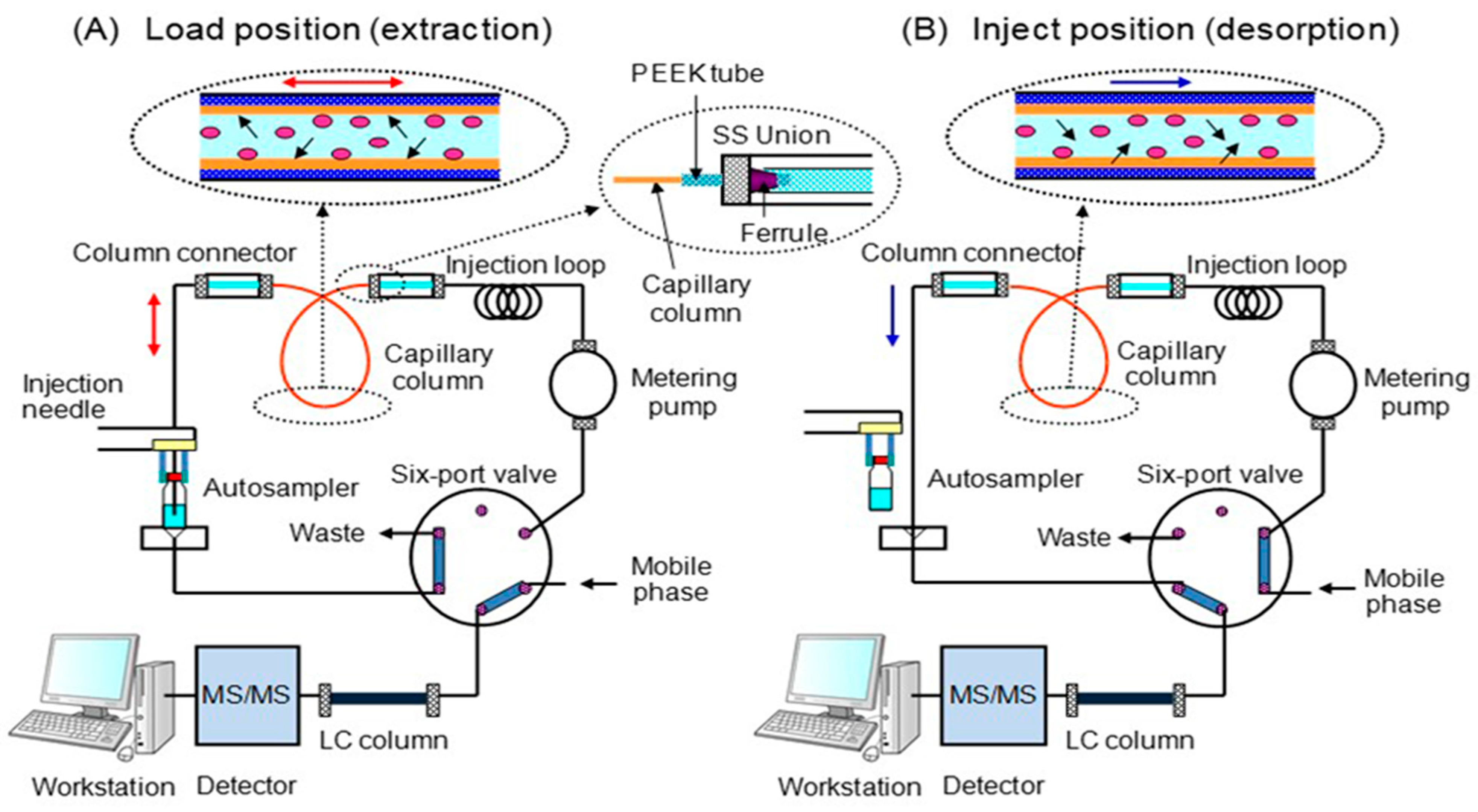
2.3. In-Tube SPME
2.4. Method Validation Study
2.5. Sampling and Preparation of Saliva Samples
3. Results
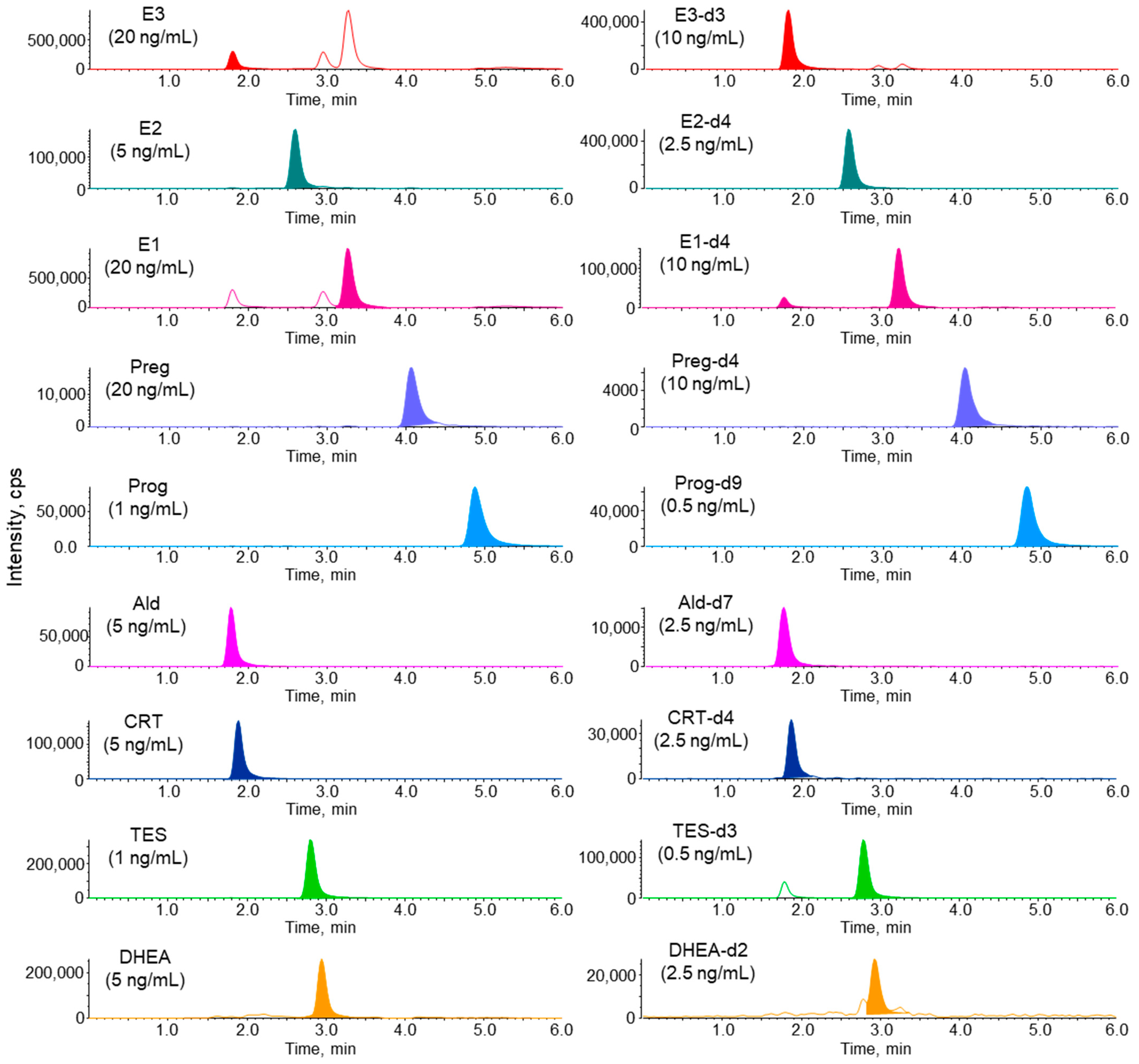
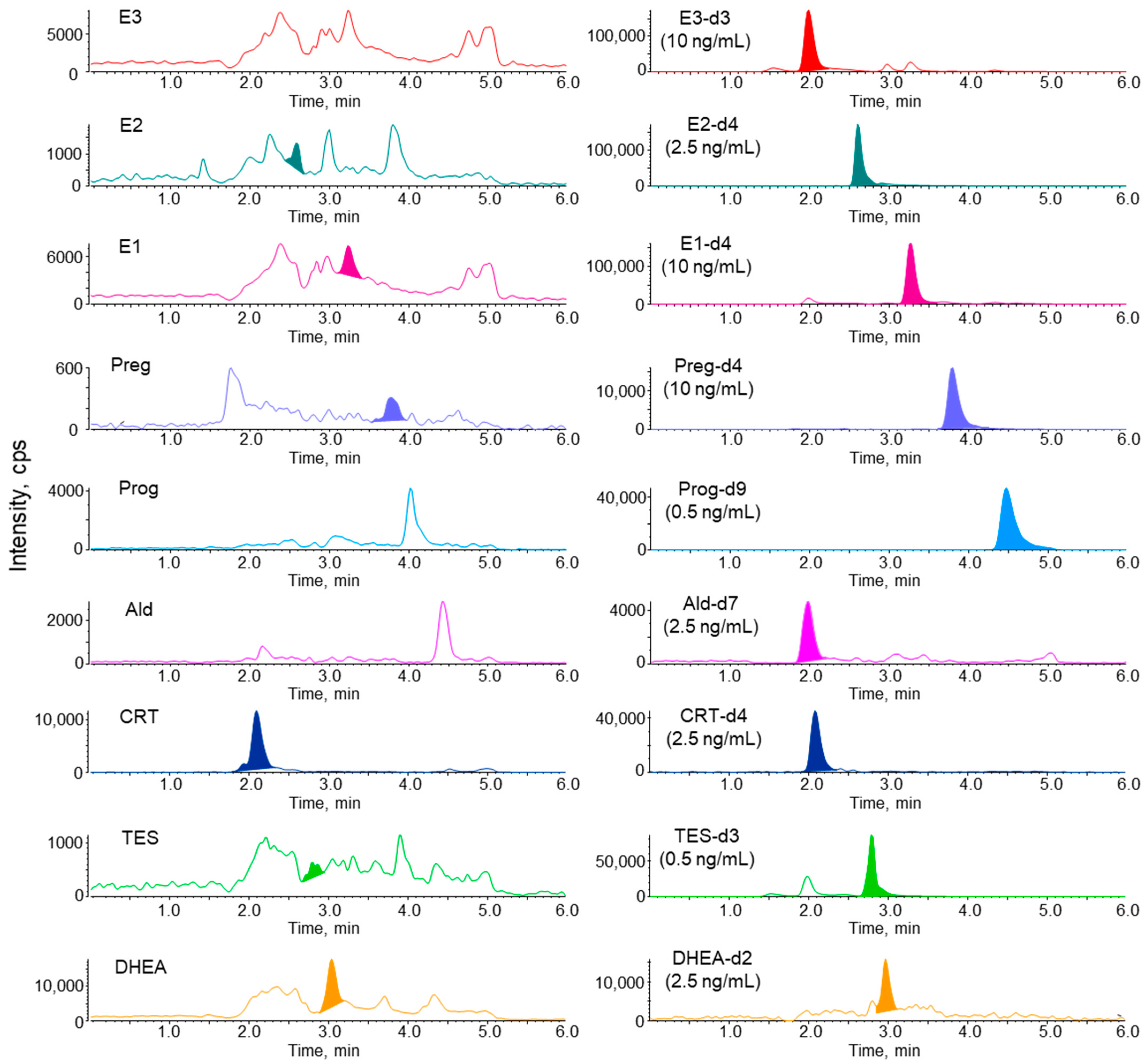
3.1. LC–MS/MS Analysis of Steroid Hormones and Their Stable Isotope-Labeled Compounds
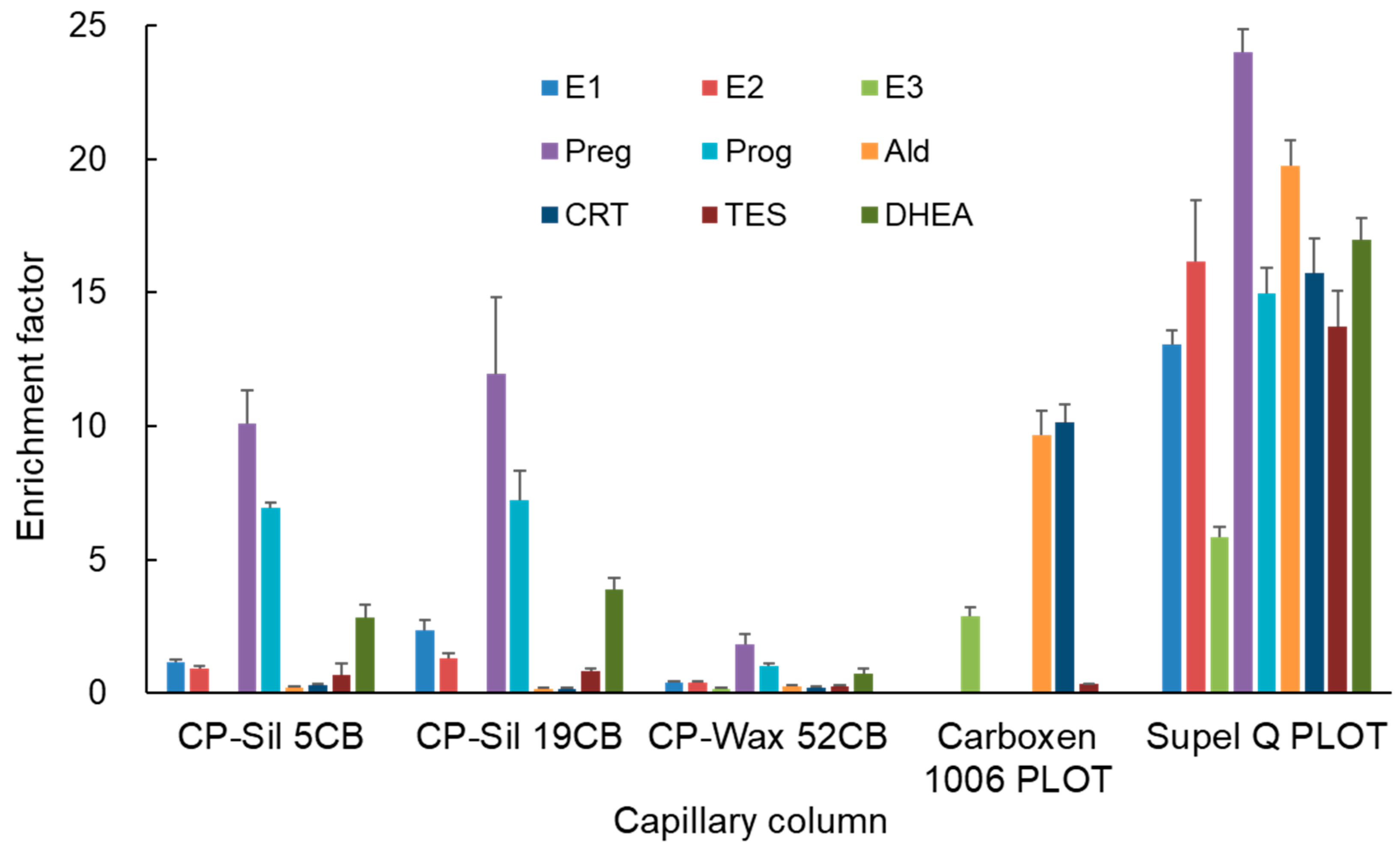
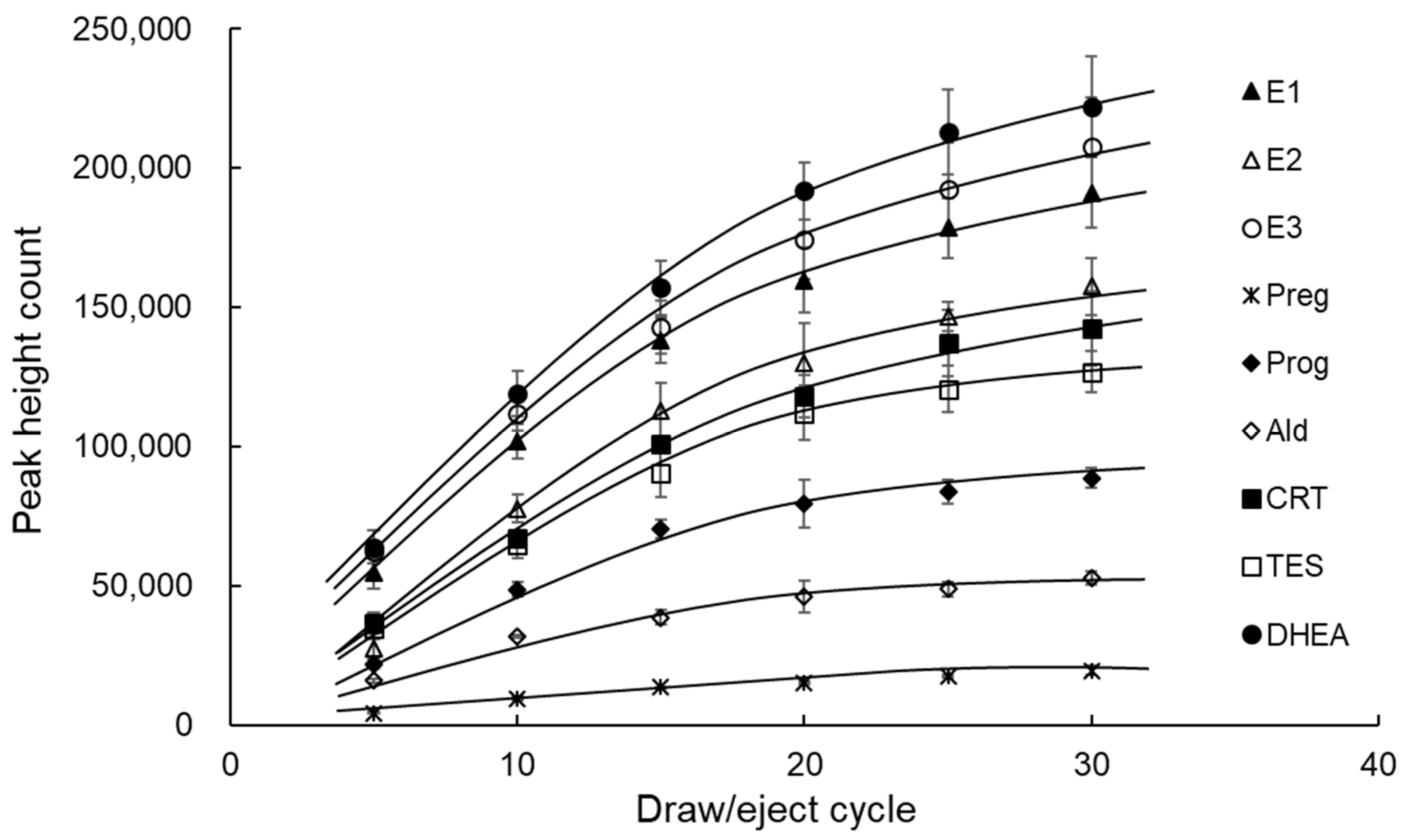
3.2. Optimization of IT-SPME and Desorption of Steroid Hormones
3.3. Linearity, Detection Limits, and Precisions of Steroid Hormones
3.4. Application to the Analysis of Saliva Samples
3.5. Comparison with Previously Reported LC–MS/MS Methods for Salivary Steroid Hormones
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schiffer, L.; Barnard, L.; Baranowski, E.S.; Gilligan, L.C.; Taylor, A.E.; Arlt, W.; Shackleton, C.H.L.; Storbecka, K.-H. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: A comprehensive review. J. Steroid Biochem. Mol. Biol. 2019, 194, 105439. [Google Scholar] [CrossRef]
- Olesti, E.; Boccard, J.; Visconti, G.; González-Ruiz, V.; Rudaz, S. From a single steroid to the steroidome: Trends and analytical challenges. J. Steroid Biochem. Mol. Biol. 2021, 206, 105797. [Google Scholar] [CrossRef] [PubMed]
- Temerdashev, A.; Dmitrieva, E.; Podolskiy, I. Analytics for steroid hormone profiling in body fluids. Microchem. J. 2021, 168, 106395. [Google Scholar] [CrossRef]
- Karashima, S.; Osaka, I. Rapidity and precision of steroid hormone measurement. J. Clin. Med. 2022, 11, 956. [Google Scholar] [CrossRef] [PubMed]
- Wudy, S.A.; Schuler, G.; Sánchez-Guijo, A.; Hartman, M.F. The art of measuring steroids: Principles and practice of current hormonal steroid analysis. J. Steroid Biochem. Mol. Biol. 2018, 179, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Mazgelytė, E.; Chomentauskas, G.; Dereškevičiūtė, E.; Rekienė, V.; Jakaitienė, A.; Petrėnas, T.; Songailienė, J.; Utkus, A.; Aušrelė, K.Z.; Karčiauskaitė, D. Association of salivary steroid hormones and their ratios with time-domain heart rate variability indices in healthy individuals. J. Med. Biochem. 2021, 40, 173–180. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wang, Y.; Liu, Z.; Xia, J.; Yin, H.; Qiu, Z.; Wang, H.; Xu, W.; Xu, Z.; Xie, J. Analysis of salivary steroid hormones in boys with autism spectrum disorder. BMC Psychiatry 2023, 23, 105. [Google Scholar] [CrossRef]
- Ouanes, S.; Popp, J. High cortisol and the risk of dementia and Alzheimer’s disease: A review of the literature. Front. Aging Neurosci. 2019, 11, 43. [Google Scholar] [CrossRef]
- Boss, L.; Kang, D.H.; Marcus, M.; Bergstrom, N. Endogenous sex hormones and cognitive function in older adults: A systematic review. West J. Nurs. Res. 2014, 36, 388–426. [Google Scholar] [CrossRef]
- Soares, N.M.; Pereira, G.M.; Altmann, V.; de Almeida, R.M.M.; Rieder, C.R.M. Cortisol levels, motor, cognitive and behavioral symptoms in Parkinson’s disease: A systematic review. J. Neural Transm. 2019, 126, 219–232. [Google Scholar] [CrossRef]
- Hackett, R.A.; Steptoe, A.; Kumari, M. Association of diurnal patterns in salivary cortisol with type 2 diabetes in the Whitehall II study. J. Clin. Endocrinol. Metab. 2014, 99, 4625–4631. [Google Scholar] [CrossRef]
- Giagulli, V.A.; Guastamacchia, E.; De Pergola, G.; Iacoviello, M.; Triggiani, V. Testosterone deficiency in male: A risk factor for heart failure, endocrine. Metab. Immune Disord. Targets 2013, 13, 92–99. [Google Scholar] [CrossRef]
- Qian, X.; Zhan, Q.; Lv, L.; Zhang, H.; Hong, Z.; Li, Y.; Xu, H.; Chai, Y.; Zhao, L.; Zhang, G. Steroid hormone profiles plus alpha-fetoprotein for diagnosing primary liver cancer by liquid chromatography tandem mass spectrometry. Clin. Chim. Acta 2016, 457, 92–98. [Google Scholar] [CrossRef]
- Fanelli, F.; Di Dalmazi, D. Serum steroid profiling by mass spectrometry in adrenocortical tumors: Diagnostic implications. Curr Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Castro, M.; Valderrábano, P.; Escobar-Morreale, H.F.; Hanzu, F.A.; Casals, G. Urine steroid profile as a new promising tool for the evaluation of adrenal tumors. Literature review. Endocrine 2021, 72, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Rege, J.; Turcu, A.F.; Else, T.; Auchus, R.J.; Rainey, W.E. Steroid biomarkers in human adrenal disease. J. Steroid Biochem. Mol. Biol. 2019, 190, 273–280. [Google Scholar] [CrossRef]
- Keevil, B.G.; Adaway, J. Assessment of free testosterone concentration. J. Steroid Biochem. Mol. Biol. 2019, 190, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Lood, Y.; Aardal, E.; Ahlner, J.; Ärlemalm, A.; Carlsson, B.; Ekman, B.; Wahlberg, J.; Josefsson, M. Determination of testosterone in serum and saliva by liquid chromatography-tandem mass spectrometry: An accurate and sensitive method applied on clinical and forensic samples. J. Pharm. Biomed. Anal. 2021, 195, 113823. [Google Scholar] [CrossRef]
- Stern, J.; Arslan, R.C.; Penke, L. Stability and validity of steroid hormones in hair and saliva across two ovulatory cycles. Compr. Psychoneuroendocrinol. 2022, 9, 100114. [Google Scholar] [CrossRef] [PubMed]
- Münzker, J.; Lindheim, L.; Adaway, J.; Trummer, C.; Obermayer-Pietsch, B. High salivary testosterone to androstenedione (T/A4) ratio and adverse metabolic phenotypes in women with PCOS. Clin. Endocrinol. 2016, 86, 567–575. [Google Scholar] [CrossRef]
- Sathyapalan, T.; Al-Qaissi, A.; Kilpatrick, E.S.; Dargham, S.R.; Adaway, J.; Keevil, B.; Atkin, S.L. Salivary testosterone measurement in women with and without polycystic ovary syndrome. Sci. Rep. 2017, 7, 3589–3597. [Google Scholar] [CrossRef] [PubMed]
- Giorgia, A.; Filippo, C.; Carlo, A.; Mariela, M.; Mario, P. Salivary cortisol and cortisone by LC–MS/MS: Validation, reference intervals and diagnostic accuracy in Cushing’s syndrome. Clin. Chim. Acta 2015, 451, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Kannankeril, J.; Carroll, T.; Findling, J.W.; Javorsky, B.; Gunsolus, I.L.; Phillips, J.; Raff, H. Prospective Evaluation of Late-Night Salivary Cortisol and Cortisone by EIA and LC-MS/MS in Suspected Cushing Syndrome. J. Endocr. Soc. 2020, 4, bvaa107. [Google Scholar] [CrossRef]
- Bäcklund, N.; Brattsand, G.; Lundstedt, S.; Aardal, E.; Bartuseviciene, I.; Berinder, K.; Höybye, C.; Burman, P.; Engström, B.E.; Isaksson, A.; et al. Salivary cortisol and cortisone in diagnosis of Cushing’s syndrome—A comparison of six different analytical methods. Comparative Study. Clin. Chem. Lab. Med. 2023, 61, 1780–1791. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, Y.; Higashi, T.; Shimada, K.; Odani, A.; Mizokami, A.; Konaka, H.; Koh, E.; Namiki, M. Simultaneous determination of salivary testosterone and dehydroepiandrosterone using LC-MS/MS: Method development and evaluation of applicability for diagnosis and medication for late-onset hypogonadism. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.M.; Keevil, B.G. Endogenous glucocorticoid analysis by liquid chromatography–tandem mass spectrometry in routine clinical laboratories. J. Steroid Biochem. Mol. Biol. 2016, 162, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiu, X.; Wang, D.; Li, Y.; Zong, Y.; Liu, Y.; Zhang, Y.; Yang, P.; Zuo, Y.; Yang, H.; et al. Quantification of 10 steroid hormones in human saliva from Chinese adult volunteers. J. Int. Med. Res. 2018, 46, 1414–1427. [Google Scholar] [CrossRef]
- Titman, A.; Price, V.; Hawcutt, D.; Chesters, C.; Ali, M.; Cacace, G.; Lancaster, G.A.; Peak, M.; Blair, J.C. Salivary cortisol, cortisone and serum cortisol concentrations are related to age and body mass index in healthy children and young people. Clin. Endocrinol. 2020, 93, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Mezzullo, M.; Pelusi, C.; Fazzini, A.; Repaci, A.; Di Dalmazi, G.; Gambineri, A.; Pagotto, U.; Fanelli, F. Female and male serum reference intervals for challenging sex and precursor steroids by liquid chromatography-tandem mass spectrometry. J. Steroid Biochem. Mol. Biol. 2020, 197, 105538. [Google Scholar] [CrossRef]
- Gregory, S.; Denham, S.G.; Lee, P.; Simpson, J.P.; Homer, N.Z.M. Using LC-MS/MS to determine salivary steroid reference intervals in a European older adult population. Metabolites 2023, 13, 265. [Google Scholar] [CrossRef]
- Keevil, B.G. LC–MS/MS analysis of steroids in the clinical laboratory. Clin. Biochem. 2016, 49, 989–997. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Peitzsch, M.; Kaden, D.; Langton, K.; Pamporaki, C.; Masjkur, J.; Tsatsaronis, G.; Mangelis, A.; Williams, T.A.; Reincke, M.; et al. Reference intervals for plasma concentrations of adrenal steroids measured by LC-MS/MS: Impact of gender, age, oral contraceptives, body mass index and blood pressure status. Clin. Chim. Acta 2017, 470, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Márta, Z.; Bobály, B.; Fekete, J.; Magda, B.; Imre, T.; Mészáros, K.V.; Bálint, M.; Szabó, P.T. Simultaneous determination of thirteen different steroid hormones using micro UHPLC-MS/MS with on-line SPE system. J. Pharm. Biomed. Anal. 2018, 150, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Rauh, M. Steroid measurement with LC–MS/MS. Application examples in pediatrics. J. Steroid Biochem. Mol. Biol. 2010, 121, 520–527. [Google Scholar] [CrossRef]
- Dorn, L.D.; Lucke, J.F.; Loucks, T.L.; Berga, S.L. Salivary cortisol reflects serum cortisol: Analysis of circadian profiles. Ann. Clin. Biochem. 2007, 44, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Cadore, E.; Lhullier, F.; Brentano, M.; Silva, E.; Ambrosini, M.; Spinelli, R.; Silva, R.; Luiz Kruel, L. Correlations between serum and salivary hormonal concentrations in response to resistance exercise. J. Sports Sci. 2008, 26, 1067–1072. [Google Scholar] [CrossRef]
- Manolopoulou, J.; Mulatero, P.; Maser-Gluth, C.; Rossignol, P.; Spyroglou, A.; Vakrilova, Y.; Petersenn, S.; Zwermann, O.; Plouin, P.F.; Reincke, M.; et al. Saliva as a medium for aldosterone measurement in repeated sampling studies. Steroids 2009, 74, 853–858. [Google Scholar] [CrossRef]
- Perogamvros, I.; Keevil, B.G.; Ray, D.W.; Bidlingmaier, M. Salivary cortisone is a potential biomarker for serum free cortisol. J. Clin. Endocrinol. Metab. 2010, 95, 4951–4958. [Google Scholar] [CrossRef] [PubMed]
- Magda, B.; Dobi, Z.; Mészáros, K.; Szabó, É.; Márta, Z.; Imre, T.; Szabó, P.T. Charged derivatization and on-line solid phase extraction to measure extremely low cortisol and cortisone levels in human saliva with liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2017, 140, 223–231. [Google Scholar] [CrossRef]
- Keevil, B.G.; Macdonald, P.; Macdowall, W.; Lee, D.M.; Wu, F.C.W. Salivary testosterone measurement by liquid chromatography tandem mass spectrometry in adult males and females. Ann. Clin. Biochem. 2014, 51 Pt 3, 368–378. [Google Scholar] [CrossRef]
- Nadarajah, N.; Skadberg, Ø.; Adaway, J.; Brede, C. Multiplexed analysis of steroid hormones in saliva by LC-MS/MS with 2-hydrazinopyridine derivatization. Clin. Mass Spectrom. 2017, 4–5, 1–10. [Google Scholar] [CrossRef]
- Higashi, T. Salivary hormone measurement using LC/MS/MS: Specific and patient-friendly tool for assessment of endocrine function. Biol. Pharm. Bull. 2012, 35, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Auchus, R.J. Steroid assays and endocrinology: Best practices for basic scientists. Endocrinology 2014, 155, 2049–2051. [Google Scholar] [CrossRef] [PubMed]
- Manaf, N.A.; Saad, B.; Mohamed, M.H.; Wilson, L.D.; Latiff, A.A. Cyclodextrin based polymer sorbents for micro-solid phase extraction followed by liquid chromatography tandem mass spectrometry in determination of endogenous steroids. J. Chromatogr. A 2018, 1543, 23–33. [Google Scholar] [CrossRef]
- Gomez, C.; Fabregat, A.; Pozo, Ó.J.; Marcos, J.; Segura, J.; Ventura, R. Analytical strategies based on mass spectrometric techniques for the study of steroid metabolism. TrAC Trends Anal. Chem. 2014, 53, 106–116. [Google Scholar] [CrossRef]
- Olesti, E.; Garcia, A.; Rahban, R.; Rossier, M.F.; Boccard, J.; Nef, S.; González-Ruiz, V.; Serge Rudaz, S. Steroid profile analysis by LC-HRMS in human seminal fluid. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1136, 121929. [Google Scholar] [CrossRef] [PubMed]
- Sobhi, H.R.; Henry, H.; Bruce, S.J.; Esrafili, A.; Rochat, B. Simple measurement of testosterone in male saliva samples using dispersive liquid–liquid microextraction followed by liquid chromatography–tandem mass spectrometry detection. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 1278–1286. [Google Scholar] [CrossRef]
- Zhao, X.-E.; Yan, P.; Wang, R.; Zhu, S.; You, J.; Bai, Y.; Liu, H. Sensitive determination of cholesterol and its metabolic steroid hormones by UHPLC-MS/MS via derivatization coupled with dual ultrasonic-assisted dispersive liquid-liquid microextraction. Rapid Commun. Mass Spectrom. 2016, 30 (Suppl. 1), 147–154. [Google Scholar] [CrossRef]
- Grau, J.; Benedé, J.L.; Chisvert, A.; Salvador, A. Modified magnetic-based solvent-assisted dispersive solid-phase extraction: Application to the determination of cortisol and cortisone in human saliva. J. Chromatogr. A 2021, 1652, 462361. [Google Scholar] [CrossRef]
- Zhan, Y.; Musteata, F.M.; Basset, F.A.; Pawliszyn, J. Determination of free and deconjugated testosterone and epitestosterone in urine using SPME and LC-MS/MS. Bioanalysis 2011, 3, 23–30. [Google Scholar] [CrossRef]
- Kataoka, H.; Ehara, K.; Yasuhara, R.; Saito, K. Simultaneous determination of testosterone, cortisol, and dehydroepiandrosterone in saliva by stable isotope dilution on-line in-tube solid-phase microextraction coupled with liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Rajska, A.; Raczak-Gutknecht, J.; Struck-Lewicka, W.; Buszewska-Forajta, M.; Wityk, P.; Verding, P.; Kowalewska, A.; Siluk, D.; Rachoń, D.; Markuszewski, M.J. Determination of urinary androgens in women with polycystic ovary syndrome using LC-QqQ/MS and the application of thin film solid-phase microextraction (TF-SPME). J. Chromatogr. A 2024, 1718, 464735. [Google Scholar] [CrossRef] [PubMed]
- Broccardo, C.J.; Schauer, W.M.; Kohrt, K.L.; Schwartz, R.S.; Murphy, J.P.; Prenni, J.E. Multiplexed analysis of steroid hormones in human serum using novel microflow tile technology and LC-MS/MS. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2013, 934, 16–21. [Google Scholar] [CrossRef]
- El-Farhan, N.; Rees, D.A.; Evans, C. Measuring cortisol in serum, urine and saliva—Are our assays good enough? Ann. Clin. Biochem. 2017, 54, 308–322. [Google Scholar] [CrossRef]
- Xu, B.; Jia, P.; Cai, J.; Gu, L. Simultaneously quantitative analysis of seven steroid hormones in human saliva. Rapid Commun. Mass Spectrom. 2021, 36, e9242. [Google Scholar]
- Kataoka, H. In-tube solid-phase microextraction: Current trends and future perspectives. J. Chromatogr. A 2021, 1636, 461787. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Matsuura, E.; Mitani, K. Determination of cortisol in human saliva by automated in-tube solid-phase microextraction coupled with liquid chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2007, 44, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Inoue, R.; Yagi, K.; Saito, K. Determination of nicotine, cotinine, and related alkaloids in human urine and saliva by automated in-tube solid-phase microextraction coupled with liquid chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2009, 49, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, R.; Ehara, K.; Saito, K.; Kataoka, H. Automated analysis of salivary stress-related steroid hormones by online in-tube solid-phase microextraction coupled with liquid chromatography−tandem mass spectrometry. Anal. Methods 2012, 4, 3625–3630. [Google Scholar] [CrossRef]
- Moriyama, E.; Kataoka, H. Automated analysis of oxytocin by on-line in-tube solid-phase microextraction coupled with liquid chromatography-tandem mass spectrometry. Chromatography 2015, 2, 382–391. [Google Scholar] [CrossRef]
- Ishizaki, A.; Uemura, A.; Kataoka, H. A sensitive method to determine melatonin in saliva by automated online in-tube solid-phase microextraction coupled with stable isotope-dilution liquid chromatography-tandem mass spectrometry. Anal. Methods 2017, 9, 3134–3140. [Google Scholar] [CrossRef]
- Kataoka, H.; Nakayama, D. Online in-tube solid-phase microextraction coupled with liquid chromatography–tandem mass spectrometry for automated analysis of four sulfated steroid metabolites in saliva samples. Molecules 2022, 27, 3225. [Google Scholar] [CrossRef]
- Kataoka, H.; Ohshima, H.; Ohkawa, T. Simultaneous analysis of multiple steroidal biomarkers in saliva for objective stress assessment by on-line coupling of automated in-tube solid-phase microextraction and polarity-switching LC-MS/MS. Talanta Open 2023, 7, 100177. [Google Scholar] [CrossRef]
- Gomez-Gomez, A.; Miranda, J.; Feixas, G.; Betegon, A.A.; Crispi, F.; Gratacós, E.; Pozo, O.J. Determination of the steroid profile in alternative matrices by liquid chromatography tandem mass spectrometry. J. Steroid Biochem. Mol. Biol. 2020, 197, 105520. [Google Scholar] [CrossRef] [PubMed]
- ICH Harmonised Guideline, ICH Q2(R2) Validation of Analytical Procedures, in International Conference for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. 2022. Available online: https://database.ich.org/sites/default/files/ICH_Q2-R2_Document_Step2_Guideline_2022_0324.pdf (accessed on 1 March 2024).
- Gröschl, M. Current status of salivary hormone analysis. Clin. Chem. 2008, 54, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Gröschl, M.; Rauh, M. Influence of commercial collection devices for saliva on the reliability of salivary steroids analysis. Steroids 2006, 71, 1097–1100. [Google Scholar] [CrossRef]

| Compound | Mass Transition (m/z) | DP 1 (V) | EP 2 (V) | CE 3 (V) | CXP 4 (V) |
|---|---|---|---|---|---|
| E1 | 271.2 → 253.5 | 70 | 10 | 15 | 10 |
| E2 | 255.3 → 159.4 | 70 | 10 | 25 | 10 |
| E3 | 271.2 → 253.5 | 70 | 10 | 15 | 10 |
| Preg | 317.5 → 159.5 | 70 | 5 | 30 | 15 |
| Prog | 315.5 → 97.2 | 75 | 5 | 30 | 10 |
| Ald | 361.4 → 315.5 | 80 | 5 | 25 | 10 |
| CRT | 363.0 → 120.9 | 70 | 10 | 30 | 10 |
| TES | 289.0 → 109.0 | 70 | 10 | 35 | 10 |
| DHEA | 289.4 → 271.4 | 40 | 10 | 13 | 10 |
| E1-d4 | 275.3 → 257.5 | 70 | 10 | 15 | 10 |
| E2-d4 | 259.4 → 161.4 | 70 | 10 | 25 | 10 |
| E3-d3 | 274.3 → 256.4 | 70 | 10 | 15 | 10 |
| Preg-d4 | 321.2 → 159.6 | 70 | 5 | 30 | 10 |
| Prog-d9 | 324.5 → 100.3 | 75 | 5 | 30 | 10 |
| Ald-d4 | 368.4 → 322.4 | 80 | 5 | 30 | 10 |
| CRT-d4 | 367.4 → 121.4 | 70 | 10 | 30 | 10 |
| TES-d3 | 292.0 → 109.4 | 70 | 10 | 35 | 10 |
| DHEA-d2 | 291.4 → 273.5 | 20 | 10 | 30 | 15 |
| Compound | Linearity | LOD 2 (pg/mL) | LOQ 3 (pg/mL) | ||
|---|---|---|---|---|---|
| Range (ng/mL) | CC 1 | Direct Injection | IT-SPME | IT-SPME | |
| E1 | 0.2-40 | 0.9990 | 270 | 8.9 | 295 |
| E2 | 0.05-10 | 0.9992 | 63 | 2.2 | 73 |
| E3 | 0.2-40 | 0.9993 | 560 | 21 | 680 |
| Preg | 0.2-40 | 0.9992 | 289 | 9.2 | 303 |
| Prog | 0.01-2 | 0.9990 | 60 | 2.3 | 77 |
| Ald | 0.05-10 | 0.9998 | 119 | 7.0 | 233 |
| CRT | 0.05-10 | 0.9998 | 83 | 4.3 | 142 |
| TES | 0.01-2 | 0.9993 | 21 | 0.7 | 24 |
| DHEA | 0.05-10 | 0.9997 | 320 | 8.1 | 268 |
| Compound | Concentration (ng/mL) | Precision (RSD 1 %), (n = 6) | |
|---|---|---|---|
| Intra-Day | Inter-Day | ||
| E1 | 1.0 | 8.1 | 11 |
| 4.0 | 3.4 | 6.8 | |
| 20 | 5.9 | 11 | |
| E2 | 0.25 | 3.5 | 5.6 |
| 1.0 | 4.4 | 7.9 | |
| 5.0 | 0.4 | 9.2 | |
| E3 | 1.0 | 5.5 | 11 |
| 4.0 | 4.3 | 8.2 | |
| 20 | 4.0 | 13 | |
| Preg | 1.0 | 6.1 | 7.4 |
| 4.0 | 7.5 | 11 | |
| 20 | 5.7 | 10 | |
| Prog | 0.05 | 1.9 | 4.0 |
| 0.2 | 2.1 | 3.1 | |
| 1.0 | 4.8 | 4.5 | |
| Ald | 0.25 | 4.0 | 8.1 |
| 1.0 | 2.2 | 4.4 | |
| 5.0 | 2.6 | 4.4 | |
| CRT | 0.25 | 0.8 | 6.3 |
| 1.0 | 1.8 | 3.5 | |
| 5.0 | 2.8 | 7.1 | |
| TES | 0.05 | 6.5 | 9.7 |
| 0.2 | 1.6 | 15 | |
| 1.0 | 4.2 | 9.9 | |
| DHEA | 0.25 | 3.0 | 4.5 |
| 1.0 | 1.5 | 10 | |
| 5.0 | 2.2 | 3.9 | |
| Compound | Spiked (ng/mL Saliva) | Recovery ± SD (%), (n = 3) |
|---|---|---|
| E1 | 10 | 97 ± 7 |
| 40 | 100 ± 8 | |
| 200 | 89 ± 11 | |
| E2 | 2.5 | 105 ± 12 |
| 10 | 96 ± 3 | |
| 50 | 94 ± 7 | |
| E3 | 10 | 106 ± 7 |
| 40 | 114 ± 1 | |
| 200 | 101 ± 10 | |
| Preg | 10 | 92 ± 12 |
| 40 | 100 ± 4 | |
| 200 | 97 ± 8 | |
| Prog | 0.5 | 82 ± 2 |
| 2 | 85 ± 3 | |
| 10 | 82 ± 4 | |
| Ald | 2.5 | 103 ± 2 |
| 10 | 104 ± 7 | |
| 50 | 102 ± 5 | |
| CRT | 2.5 | 88 ± 5 |
| 10 | 93 ± 5 | |
| 50 | 91 ± 2 | |
| TES | 0.5 | 97 ± 13 |
| 2 | 93 ± 15 | |
| 10 | 102 ± 11 | |
| DHEA | 2.5 | 89 ± 6 |
| 10 | 87 ± 8 | |
| 50 | 101 ± 14 |
| Subject | Content 1 (ng/mL Saliva) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Sex 2 | Age | E1 | E2 | E3 | Preg | Prog | Ald | CRT | TES | DHEA |
| 1 | M | 25 | <LOQ 3 | <LOQ | <LOQ | <LOQ | 0.48 | <LOQ | 0.19 | 0.27 | <LOQ |
| 2 | M | 26 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.25 | 2.42 | 0.09 | 0.43 |
| 3 | M | 27 | <LOQ | <LOQ | <LOQ | <LOQ | 0.10 | <LOQ | 2.98 | 0.09 | <LOQ |
| 4 | M | 36 | <LOQ | <LOQ | <LOQ | 0.85 | 0.58 | <LOQ | 0.31 | 0.14 | 0.83 |
| 5 | M | 65 | <LOQ | <LOQ | <LOQ | 1.1 | 2.5 | <LOQ | <LOQ | 0.06 | <LOQ |
| 6 | F | 23 | <LOQ | 0.22 | 0.75 | 0.77 | <LOQ | <LOQ | 4.15 | 0.10 | 0.53 |
| 7 | F | 24 | <LOQ | 0.26 | 0.68 | 2.3 | 3.6 | <LOQ | 0.30 | 0.07 | 0.82 |
| 8 | F | 25 | 1.1 | 0.22 | 0.79 | 1.1 | 0.11 | 0.86 | 4.83 | 0.12 | 1.06 |
| 9 | F | 34 | <LOQ | 0.25 | 0.68 | <LOQ | 0.66 | <LOQ | 0.29 | <LOQ | 0.68 |
| 10 | F | 63 | <LOQ | <LOQ | 1.1 | 1.1 | 2.1 | <LOQ | 0.31 | 0.05 | 0.99 |
| Compound | Sampling and Sample Preparation | Salivary Content | Sensitivity | Ref. |
|---|---|---|---|---|
| TES | Passive drool using Salimetrics®, OASIS MAXμElution Plate | 2–59 pg/mL in healthy adults | LOD: 2 pg/mL; LOQ: 6 pg/mL | [18] |
| TES, androstanedione (AN) | Passive drool, Isolute SLE + 400 plate, XBridge C18 cartridges | TES: 13 pmol/mL, AN: 143 pmol/mL | LOQ: 5–6.25 pmol/mL | [21] |
| CRT, cortisone (CRN) | Salivette® (cotton swab), Oasis® HLB SPE cartridges (online SPE) | CRT: 3–21 nmol/mL, CRN: 10–42 nmol/mL | LOD: 0.2–0.3 nmol/mL; LOQ: 0.51–0.55 nmol/mL | [22] |
| TES, DHEA | Passive drool, acetonitrile pretreatment, Strata-X cartridge, derivatization | 46–131 pg/mL | LOQ: 10 pg/mL | [25] |
| 10 Steroid hormones 1 | Passive drool, SPE plate using a Positive Pressure-96 Processor | 0.01–21 ng/mL | LOD: 0.8–14 pg/mL; 4.8–24 pg/mL | [27] |
| 19 Steroid and metabolites 2 | Salivette® (cotton swab), Isolute SLE + 400 96-well plates for extraction | Detected but no data listed | LLOQ: 0.05–1.25 ng/mL | [30] |
| CRT, CRN | Salivette® (polyester wool swab), LLE with ethyl acetate, derivatization | Detected but no data listed | LLOD: 2–5 pg/mL; LLOQ: 5–10 pg/mL | [39] |
| TES | Direct spitting or drool, LLE with methyl tert-butyl ether | Male: 93–378 pg/mL; female: 5–46 pg/mL | LLOQ: 5 pmol/mL | [40] |
| CRT, CRN, TES, DHEA, Prog, 17α-OH-Prog | Expectoration via polypropylene straw, LLE with methyl tert-butyl ether, derivatization | Morning: <57 nM; night: <18 | LOD: 0.011–7 pg/mL; LOQ: 0.02–20 ng/mL | [41] |
| TES, DHEA, AN, Prog, 17α-OH-Prog, Preg, 17α-OH-Preg | Direct spitting, LLE with methyl tert-butyl ether, derivatization | Detected but no data listed | LOD: 0.05–1 pg/mL; LOQ: 0.15–3 pg/mL | [55] |
| CRT, TES, DHEA | Salisoft® (polypropylene–polyethylene swab), ultrafiltration, IT-SPME | 0.032–1.07 ng/mL | LOD: 0.3–8.9 pg/mL; LOQ: 0.01–0.29 ng/mL | [51,59] |
| Sulfates of E2, Preg, CRT and DHEA | Salisoft® (polypropylene–polyethylene swab), ultrafiltration, IT-SPME | <11.9 ng/mL | LOD: 0.3–3.2 pg/mL; LOQ: 0.016–0.172 ng/mL | [62] |
| CRT, TES, DHEA, DHEA-sulfate | Salisoft® (polypropylene–polyethylene swab), ultrafiltration, IT-SPME | <7.27 ng/mL | LOD: 0.4–8.5 pg/mL; LLOQ: 0.036–0.768 ng/mL | [63] |
| E1, E2, E3, Preg, Prog, Ald, CRT, TES, DHEA | Salisoft® (polypropylene–polyethylene swab), ultrafiltration, IT-SPME | <4.83 ng/mL | LOD: 0.7–20.5 pg/mL; LOQ: 24–680 pg/mL | This method |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hitomi, T.; Kataoka, H. Development of Noninvasive Method for the Automated Analysis of Nine Steroid Hormones in Human Saliva by Online Coupling of In-Tube Solid-Phase Microextraction with Liquid Chromatography–Tandem Mass Spectrometry. Analytica 2024, 5, 233-249. https://doi.org/10.3390/analytica5020015
Hitomi T, Kataoka H. Development of Noninvasive Method for the Automated Analysis of Nine Steroid Hormones in Human Saliva by Online Coupling of In-Tube Solid-Phase Microextraction with Liquid Chromatography–Tandem Mass Spectrometry. Analytica. 2024; 5(2):233-249. https://doi.org/10.3390/analytica5020015
Chicago/Turabian StyleHitomi, Takashi, and Hiroyuki Kataoka. 2024. "Development of Noninvasive Method for the Automated Analysis of Nine Steroid Hormones in Human Saliva by Online Coupling of In-Tube Solid-Phase Microextraction with Liquid Chromatography–Tandem Mass Spectrometry" Analytica 5, no. 2: 233-249. https://doi.org/10.3390/analytica5020015
APA StyleHitomi, T., & Kataoka, H. (2024). Development of Noninvasive Method for the Automated Analysis of Nine Steroid Hormones in Human Saliva by Online Coupling of In-Tube Solid-Phase Microextraction with Liquid Chromatography–Tandem Mass Spectrometry. Analytica, 5(2), 233-249. https://doi.org/10.3390/analytica5020015







