3.1. Physicochemical Characterization of Biosorbents
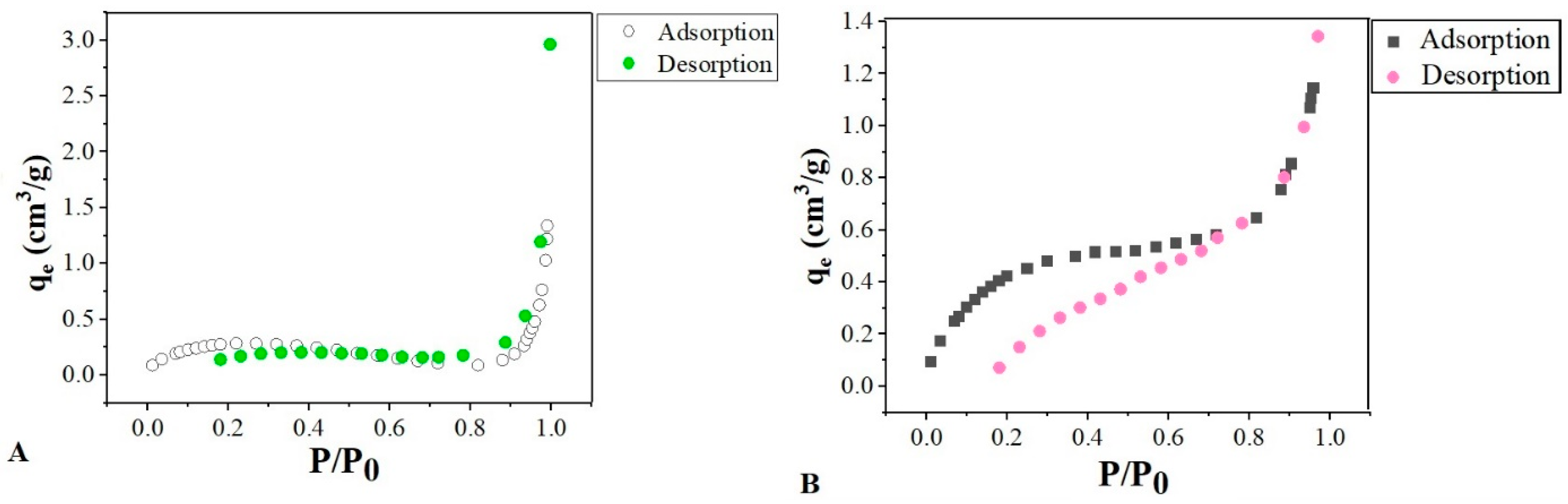
The surface area of SOH showed changes in SS, as seen in
Figure 1A,B. The isotherm obtained using the BET method shows a linear relationship between the increase in relative pressure and the adsorption of N
2. According to the IUPAC classification, Type II is the isotherm that best represents the biomass’s behavior from this study. This isotherm is specific for mesoporous and macroporous adsorbents, in which N
2 adsorption occurs in multilayers. This category of isotherm is characterized by having two inflection points. The first, concave to the P/P
0 axis, indicates that the monolayer has been filled, while the second, with inflection convex to the P/P
0 axis, designates the saturation point. The linear part between the two inflection points marks the multilayer adsorption [
27].
The SS biomass isotherm,
Figure 1A, obtained a first slightly concave inflection; this suggests that the monolayer may not be filled before adsorption in the upper layers occurs. The SOH biomass presented loops of hysteresis of the H3 type and the SS of the H4 type. According to IUPAC, these types of hysteresis are observed in aggregates of plate-like particles, which give rise to slit-shaped pores and presents swelling of a nonrigid porous structure due to the isotherm presenting a hysteresis loop at low pressures [
27].
It is possible to verify that the isotherms obtained for biomasses with relative pressure close to one obtain a more significant vertical rise, reflecting the presence of macropores. If the extension of these macropores is large, the vertical line tendency is greater [
27].
Table 2 shows that the BET method’s mean diameter revealed a predominance of mesopores for both biomasses. Comparing SOH with SS, the former showed a decrease in pore size. Consequently, there was a minor increase in the specific surface area of SOH concerning SS.
With this result, it is possible to infer that the chemical modification caused an increase in the surface area of the SOH biomass attributive SS. There is a growing interaction with the adsorbent–adsorbate corresponding to the surface area, increasing the consecutively upgrading biomass adsorptive capacity.
According to the XRD analysis in
Figure 2, it is can be observed that both biomasses present a typical signal of amorphous cellulose composite at 2θ = 20° and 26° [
23,
28,
29]. The lowest intensity peaks between 2θ = 35° and 45° may refer to inorganic substances (ash) [
30].
In work by Souza and Carvalho [
31], the pretreatment using organosolve in the residual eucalyptus biomass revealed an increase in the characteristic peak intensity of the chemically modified biomass compared to
in nature. This was justified during the treatment with acid in the most susceptible regions to suffer an attack, which are the amorphous regions composed of hemicellulose and lignin. Given the result, an increase in peak intensity at 2θ = 26° for SOH indicates that
Salvinia sp., because it also has lignocellulosic biomass in its composition, may increase peak intensity due to the chemical treatment.
Other physicochemical characterization tests for the biomasses of this study were previously performed by Ferreira et al. [
21], in which it was possible to verify the change in the structure of SOH compared to SS, as well as the removal of lignin and cellulose. The results presented in the present study support the hypothesis that chemical modification favors oil adsorption in emulsions.
3.2. Batch Adsorption Tests
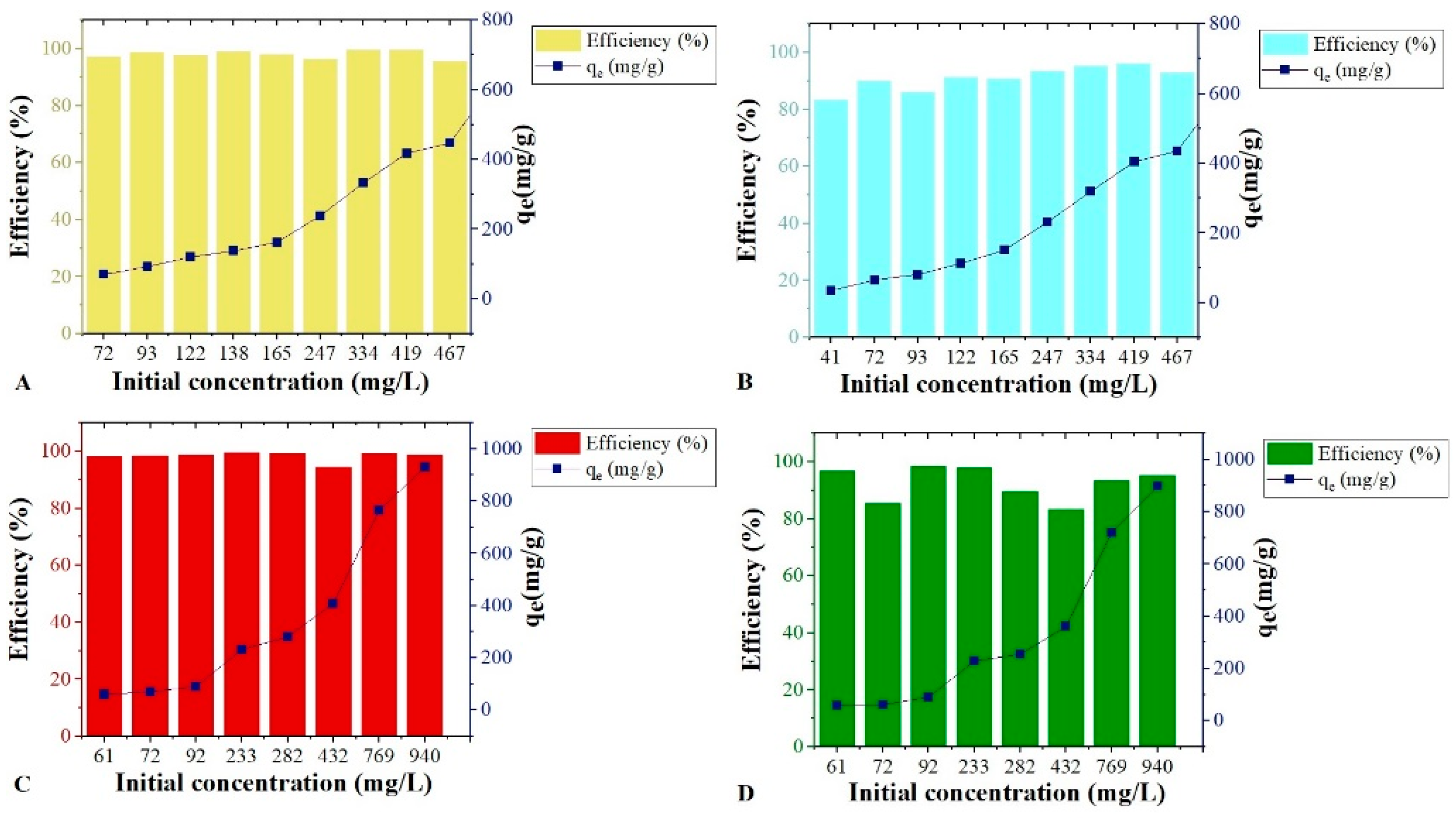
The four isotherms were obtained with the biosorbents SS and SOH for the emulsions with oil in distilled and salt water. As shown in
Figure 3, both biomasses had good responses to the oil concentration variation in the two emulsion types and efficiency that varied from 83 to 99% of oil removal for all tests. In
Figure 3A, the SS, with q
max of 846.51 mg g
−1 tended to equilibrium at the points of highest concentration. SOH in
Figure 3B, with q
max (836.42 mg g
−1) close to SS biomass, obtained an increasing linear response in the adsorptive capacity as the initial oil concentration increased. However, there was no statistically significant evidence that the adsorption of SS was greater than SOH for the isotherm with the emulsion in distilled water with ship oil.
On the other hand, emulsions with saltwater tended to saturate at higher concentrations. The SS in
Figure 3C obtained the highest qmax of all isotherms, 930.59 mg g
−1, and the lowest saturation point. Although the SS obtained the best response, it presented high instability. Also, SOH in
Figure 3D was saturated, but more mildly, and had a higher qmax (898.31 mg g
−1) than its emulsion in distilled water. Higher adsorption of SS concerning SOH in the isotherm of emulsion in saltwater with oil was statistically proven.
For emulsions in saltwater, biomass adsorption was higher, and according to the literature, electrolytes tend to influence the solubilization of emulsions. In the case of the monovalent cation, Na
+ competes for the water of hydration of the emulsifier, thus causing a decrease in the solubility of the emulsion (salting out), which increases the adsorption of oil on the surface of the adsorbents [
18,
32]. Therefore, the adsorption response was higher for both biomasses. Adsorption research in oil emulsion with fresh and saltwater for varied materials obtained better results for adsorption in a salt–oil emulsion. A study on adsorption and desorption in an oil emulsion with a membrane composed of thermo-responsive polyvinylidene fluoride and silica showed a considerable increase in adsorption with increasing NaCl concentration in the emulsion [
32]. In the case of adsorption on biochar, the maximum amount adsorbed, measured experimentally, was 80% higher in the salty medium than in freshwater, showing that the presence of salt in the emulsion increased the oil adsorption [
33].
A review study by Dai et al. [
33] verified oil adsorption by biochar. The adsorption mechanism to remove organic pollutants depends on the contaminant’s nature and the chemical properties of the adsorbent surface. Differences in organic structure, electrical surface properties, and surface functional groups of the adsorbent material are essential attributes for different adsorption mechanisms. In the case of plant-origin adsorbents, organic contaminants’ adsorption mechanisms can be divided into electrostatic attraction, pore filling, π-π electron interaction with donor and acceptor, hydrogen bonding, adsorption complexes, and hydrophobic interactions. The adsorption capacity of the material increases with the increase in functional groups containing oxygen, which in part, occurs due to interactions of π-π electrons. Also, according to the study, the carboxylic acid, nitro, and ketone groups on the surface of the biosorbent function as electron acceptors and from the interaction of the π-π electron donor-acceptor with aromatic molecules. Several hydroxyl and amine groups on biosorbent can be π electron donor sites. Like SOH, the surface of biochar is electronegative, which causes electrostatic attraction between the adsorbent and positively charged organic compounds. The magnitude of electrostatic attraction depends on the size of each atomic charge and the distance between two atoms. The biosorbent with low surface oxidation exhibits hydrophobicity and indicates that adsorption occurs by hydrophobic interaction, pore filling, the noncarbonized fraction of partition, and π-π interaction. When the surface of the biosorbent has a negative charge, it is easy to attract the cationic organic compound electrostatically [
33].
As biochar and SOH are of plant origin and have similar surface groups, it is possible to infer that the SOH adsorption mechanism occurs similarly. The decrease in hydrophilic compounds, such as cellulose and hemicellulose, removed with chemical modification, confer more hydrophobic character than SOH to SS. In the same way, the decrease in the average pore size can favor the oil adherence on the biomass’s surface.
The results of Langmuir and Freundlich’s nonlinear models from the isotherms above are in
Table 3. The Freundlich model was the best fit for biomasses, which have heterogeneous adsorption sites and favorable adsorption, with
n values more significant than one for both biosorbents [
34].
Based on the results of the physicochemical characterization of the biomasses in the BET analysis, it was observed that the biomasses do not necessarily present complete adsorption of the first layer and have slit-shaped pores, highlighting an irregular structure. As for the Langmuir model, the R
2 was lower, which corroborates the postulates for this model, which assumes monolayer adsorption and equally distributed active sites [
35].
When comparing the results obtained in
Table 4 with those described in the literature, the biomasses of this study are among the best results, especially for oil sorption in oil emulsions, whose maximum adsorption capacities ranged from 75.1 mg g
−1 to 118.50 g g
−1.
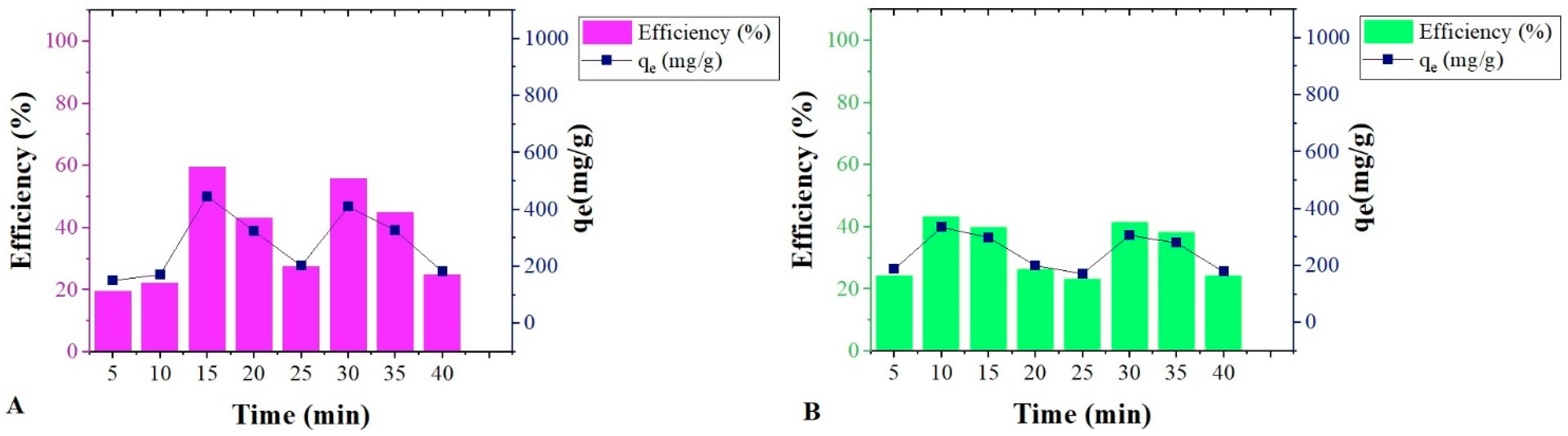
The adsorption kinetics of both biomasses did not show thermodynamic stability and showed desorption points over time for both biomasses, as shown in
Figure 4. This result supports the hypothesis that this adsorption variability over time is due to emulsion instability [
40].
SS performed better when compared to SOH with qmax in 15 min. After this period, it obtained desorption points two times, adsorbed again in 30 min, and desorbed in 35 and 40 min. However, the adsorption of SS higher than SOH at 298 K for emulsion was not statistically significant.
SOH presented the fastest adsorption kinetics, with its apex in 10 min. It followed the same behavior as the SS, with subsequent desorption points, again, the adsorption process in 30 min and desorption in 35 and 40 min.
This type of fast adsorption for oils was also verified by Barthlott et al. [
41]. The surface of
Salvinia sp. is in contact with a film of supernatant oil, with complete adsorption of the oil taking place in about 50 s. According to the research, this high speed of adsorption and transport was due to the surface structure, which has a hydrophobic and oleophilic surface. Likewise, the oil transport speed by the leaf occurs due to the high density of trichomes because the average distance of the hairs was 244.29 ± 38.27 µm in
Salvinia. According to the authors, the vital prerequisite for a high capillary interaction between leaf hairs is the separation distance; smaller trichomes close together and transport oil much faster.
After adding these results to the Freundlich adsorption models, the adsorption process of both biomasses may be reversible, with the possibility of reusing them to treat effluents.
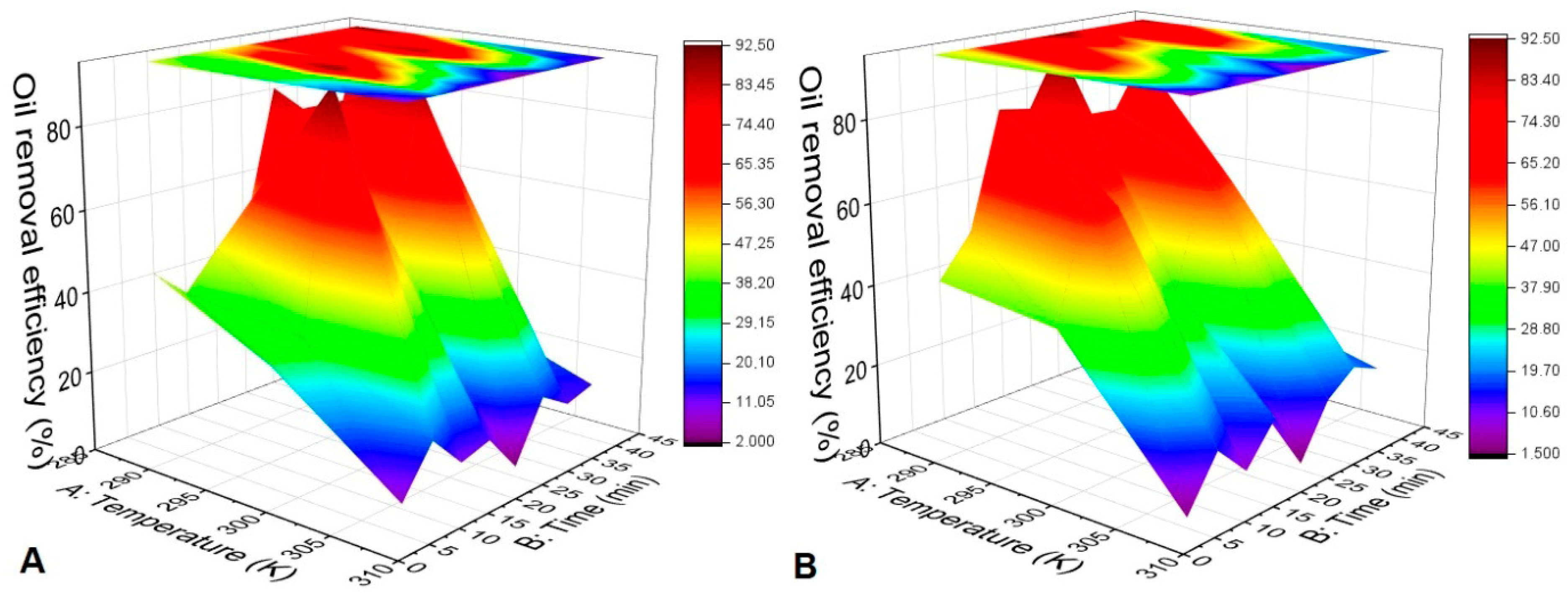
At temperatures of 288, 298, and 308 K, SS performed better than SOH. However, the adsorption of SS results greater than SOH for the temperatures of 288 and 308 K in the emulsion were not statistically significant.
The temperature variation for the SS in
Figure 5A had no direct relationship with the adsorptive capacity, whose temperature relationship with adsorption was 298 K > 288 K > 308 K. However, it showed efficiency of up to 92% for 298 K. The higher temperature had a lower performance for all points but showed strong stability in its curve. There was a statistical difference in oil adsorption between temperatures 398 and 308 K. On the other hand, the temperature variation from 288 to 298 K in oil adsorption was not statistically significant for SS.
All temperatures showed similar behavior in the first 15 min. Adsorption increased, and in subsequent times, there was desorption, followed by new adsorption. Thus, the equilibrium was not reached within 40 min. This equilibrium would occur if the experiments’ time ranges were increased to verify the time required. However, the focus of this study was to verify short periods of adsorption for the application of biosorbents in a fixed-bed column.
The SS performed better than SOH with q
max in 15 min. After this period, desorption points in the SOH also showed similar behavior to SS with temperature variation,
Figure 5B shows the relationship between temperature and adsorption equaling 298 K > 288 K > 308 K. There was no statistically significant difference in oil adsorption between 288 K and 298 K for SOH. In contrast, the difference in oil adsorption for SOH between 298 and 308 K reached statistical significance.
The temperature variation curves with time were concave downward at all evaluated temperatures. However, the maximum adsorption points differed at 10, 25, and 35 min for 298, 288, and 398 K, respectively. The adsorption of hydrocarbons by activated carbon from Casuarina equiseitifolia was also not linear, and the adsorption was lower at higher temperatures [
15].
In all tests, the biomasses showed instability. However, observing adsorption in all tests within 5 min is interesting, and the biomasses obtained rapid kinetics regardless of temperature.
Nonlinear kinetic models assessed with the three temperatures are shown in
Table 5. The intraparticle diffusion model was the most adjusted at all temperatures and biomasses. Thus, it confirmed the adsorption interaction between these biomasses and oil emulsions in more than one step.
Table 6 presents the thermodynamic calculations from the temperature variation. Both biomasses showed positive and low enthalpy values (less than 40 kJ mol
−1), showing that the adsorption process is physical and endothermic [
42]. The entropy energy of the system was reduced and negative, signaling a minimal change in the system, which demonstrates that the adsorption with oil emulsion may not be fully reversible for the biomasses of this study [
43].
The Gibbs energy obtained was positive for the three temperatures with the two biomasses, as described in
Table 7, proving that the adsorption process is not spontaneous. ΔG varied little with temperature, especially for SS.
Although the thermodynamic results were unfavorable for SOH, they had good adsorption for different concentrations, times, and temperature variations. Studies have shown that nonspontaneous endothermic processes do not prevent the potential for application of the material as an adsorbent [
15,
44,
45].
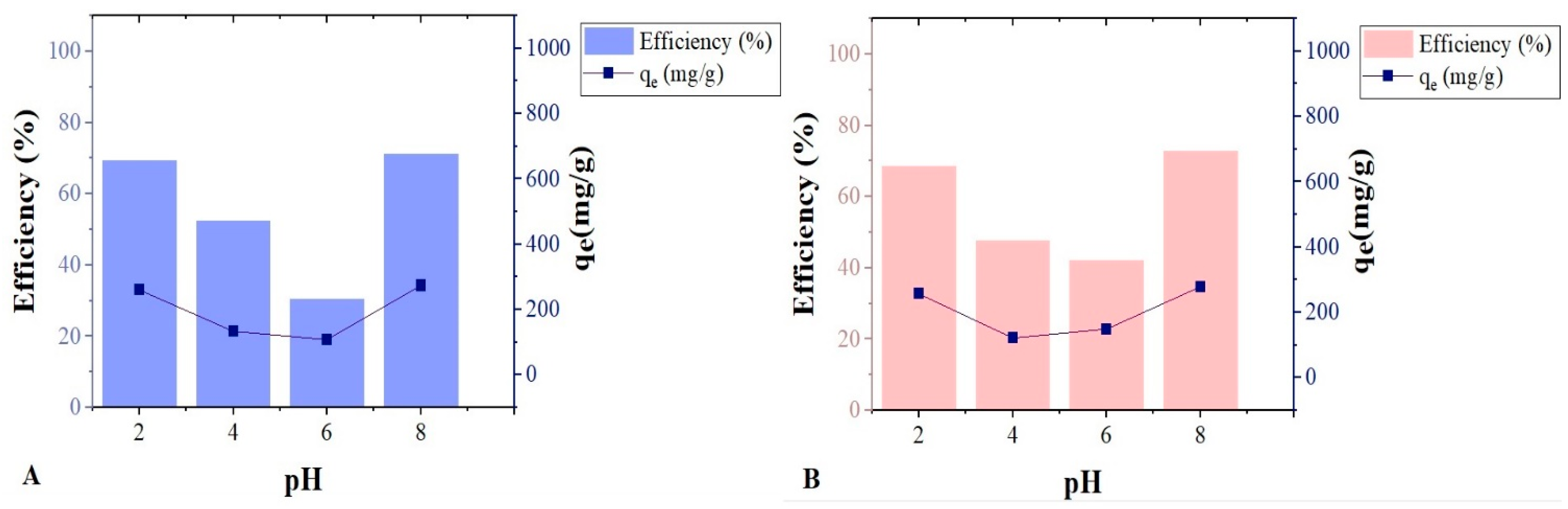
The effect of pH variation on oil adsorption for the two biomasses was unusual, as extreme pHs showed higher results (
Figure 6); this reaffirms the hypothesis that excess ions in the medium, negative or positive, displace the electron cloud from the long chains of oil hydrocarbons. In this way, they promote a dipole moment and thus facilitate the dipole–dipole interaction with the active sites of the biomasses.
In this way, they promote a dipole moment and thus facilitate the dipole–dipole interaction with the active sites of the biomasses. Statistically, there were no significant differences in oil adsorption between the biomasses for the four evaluated pHs.
Figure 7 illustrates our hypothesis of the dipole moment formation in oil molecules and their interaction with the biomass surface. The pH in the solution also affects the charged distribution of the biomass surface. As discussed by Ferreira et al., in the physicochemical characterization of the points of zero charge (PZC), the medium can increase or decrease the negatively charged or positively charged biosorbent.
Inverse behavior was obtained in the study of nano-silica adsorption in oil, in which the adsorption was higher at pH < pHpzc. This fact was attributed to increased dispersion forces at pH < pHpzc, even as electrostatic repulsive forces dominated at pH > pHpzc [
17].
3.3. Fixed-Bed Column Adsorption Tests
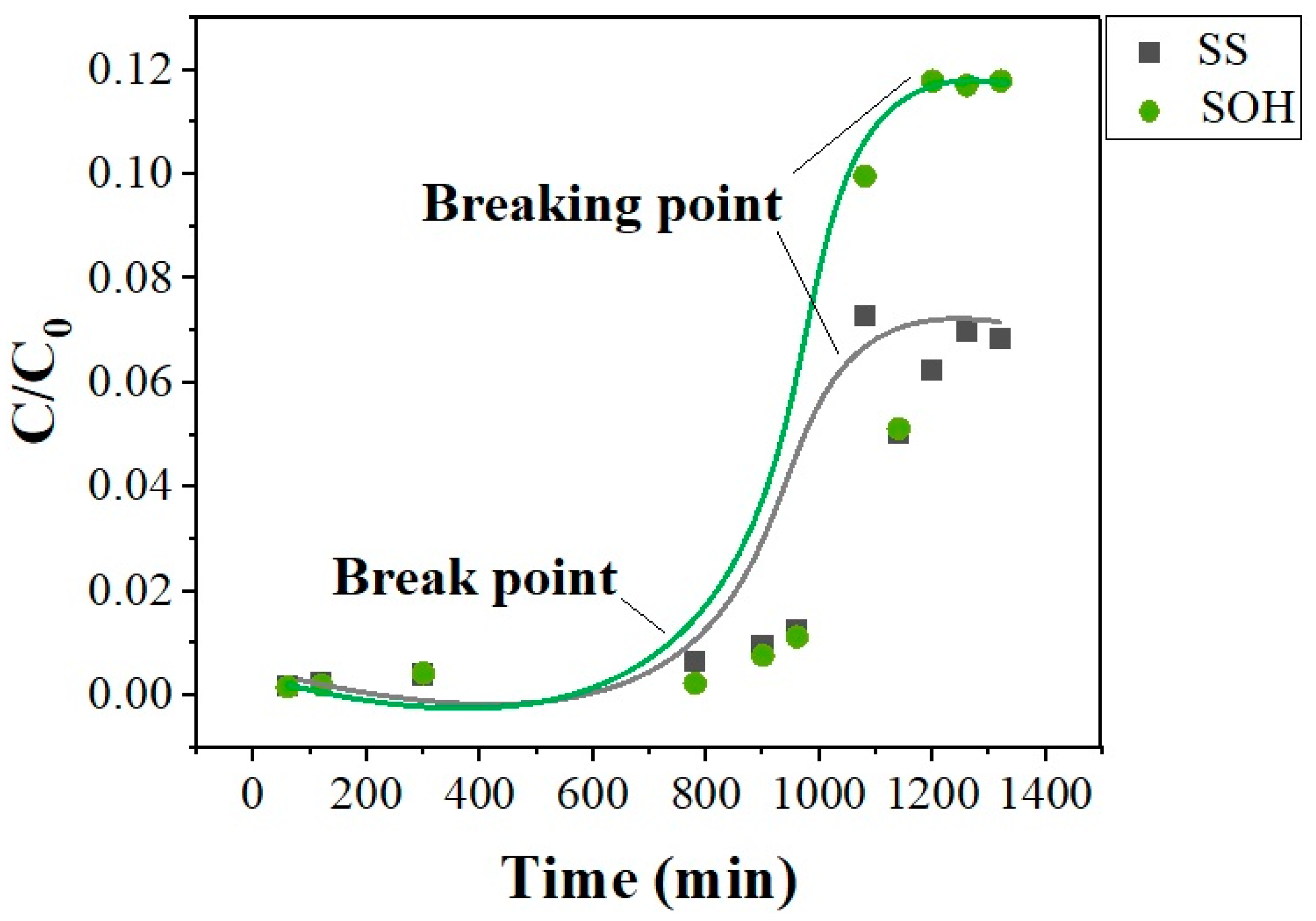
Column failure curves for SS and SOH biomass reached a breakpoint at 960 min with both biosorbents,
Figure 8. The breakpoint occurred together, 1200 min, for the two biomasses, but at different concentrations. According to the curves obtained, SS showed lower oil adsorption capacity due to a smaller mass transfer zone when compared to SOH, whose breakpoint was higher than SS.
The parameters for both biomasses are in
Table 8. It is possible to observe that the parameters calculated for the biosorbents are similar. The biomasses presented a longer reasonable time and high saturation capacity. It is noted that these values are theoretical and are based on the oily emulsion’s constant concentration, even as it is not held to the system walls. Comparing both biomasses was conducted according to the oil retained real value. After that column adsorption, the qe obtained for SOH was 3.49 g g
−1, while 2.99 g g
−1 was reached for SS. Therefore, it was verified that SOH presented better adsorptive capacity, as previously observed, due to the more significant mass transfer zone.
A study separated condensed oil from produced water using a thin film of amorphous carbon prepared with oil palm leaves using fixed-bed adsorption techniques. As a result, the adsorption was 132.77 mg g
−1 in 6 h at room temperature [
28].
In another study, toluene, benzene, and σ-Xylene adsorption was conducted in a fixed-bed system filled with organoclay. The useful removal quantities were 0.012, 0.030, and 0.140 mmol g
−1 for benzene, toluene, and p-xylene, respectively [
46].
In another proposal, removing emulsified and dissolved diesel oil in high-salinity wastewater with graphene oxide as adsorbent obtained a maximum adsorption capacity of 1335 mg g
−1 using 200 mg L
−1 of diesel and 1 mg of graphene oxide [
18].
The adsorption model for Bohart–Adams columns was applied, and their respective constants for the SS and SOH biomasses are represented in
Table 9.
The Bohart–Adams model was the one that best fitted the experimental data of this study. Following this presumptions model, lateral interactions affect the adsorption process, just as the nearest neighboring adsorption site affects the diffusion of the adsorbate in the solution to the surface of the adsorbent. K
AB constant measures the transfer rate from the adsorbate to the adsorbent, which depends on solute–solute, solute–adsorbent, and solute–neighborhood interactions [
32].