Headspace-Selected Ion Flow Tube Mass Spectrometry Workflows for Rapid Screening and Quantitation of Hazardous Volatile Impurities in Personal Care Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.1.1. SIFT-MS
2.1.2. GC-MS
2.2. Sample Preparation
2.3. Samples and Standards
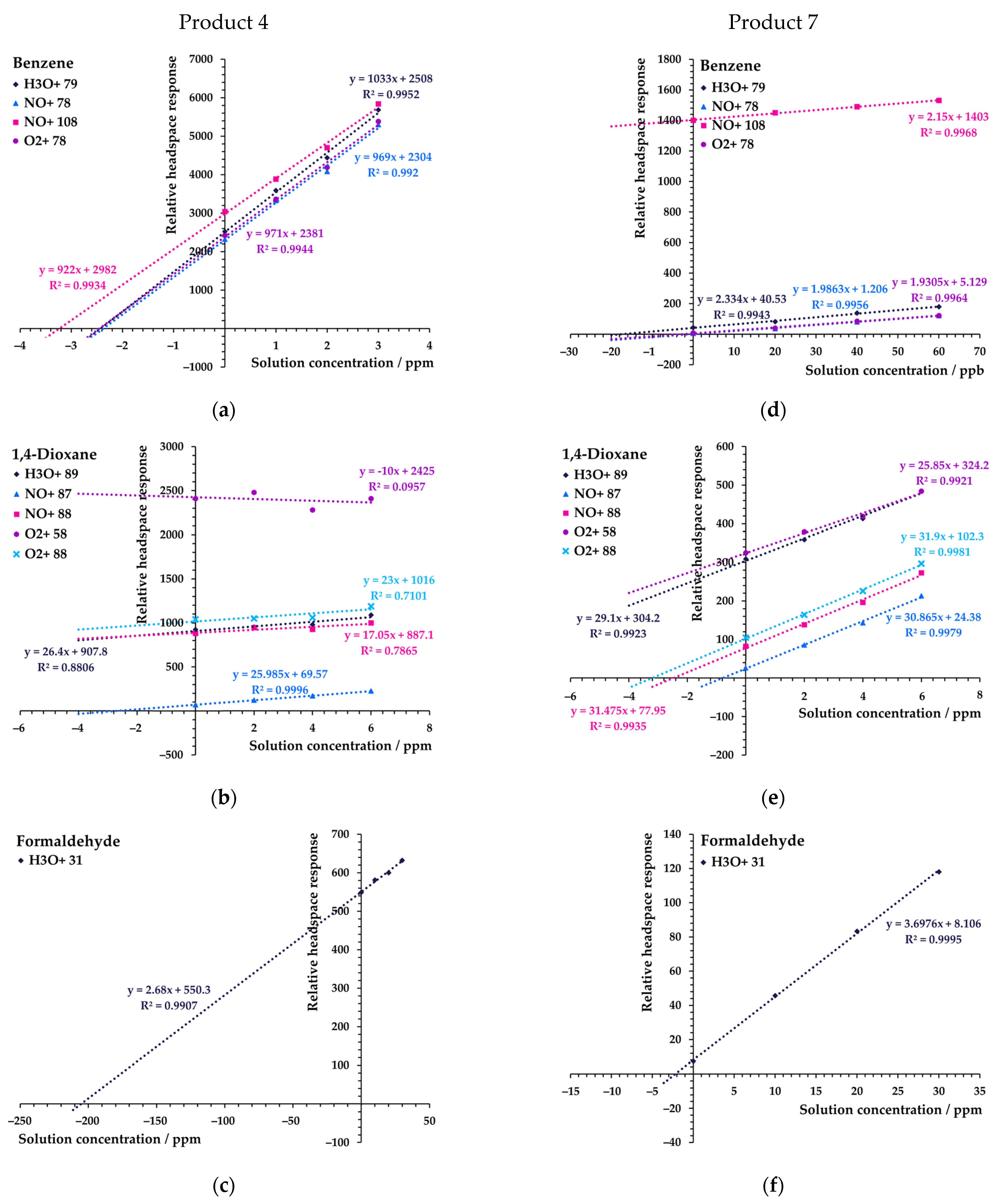
3. Results
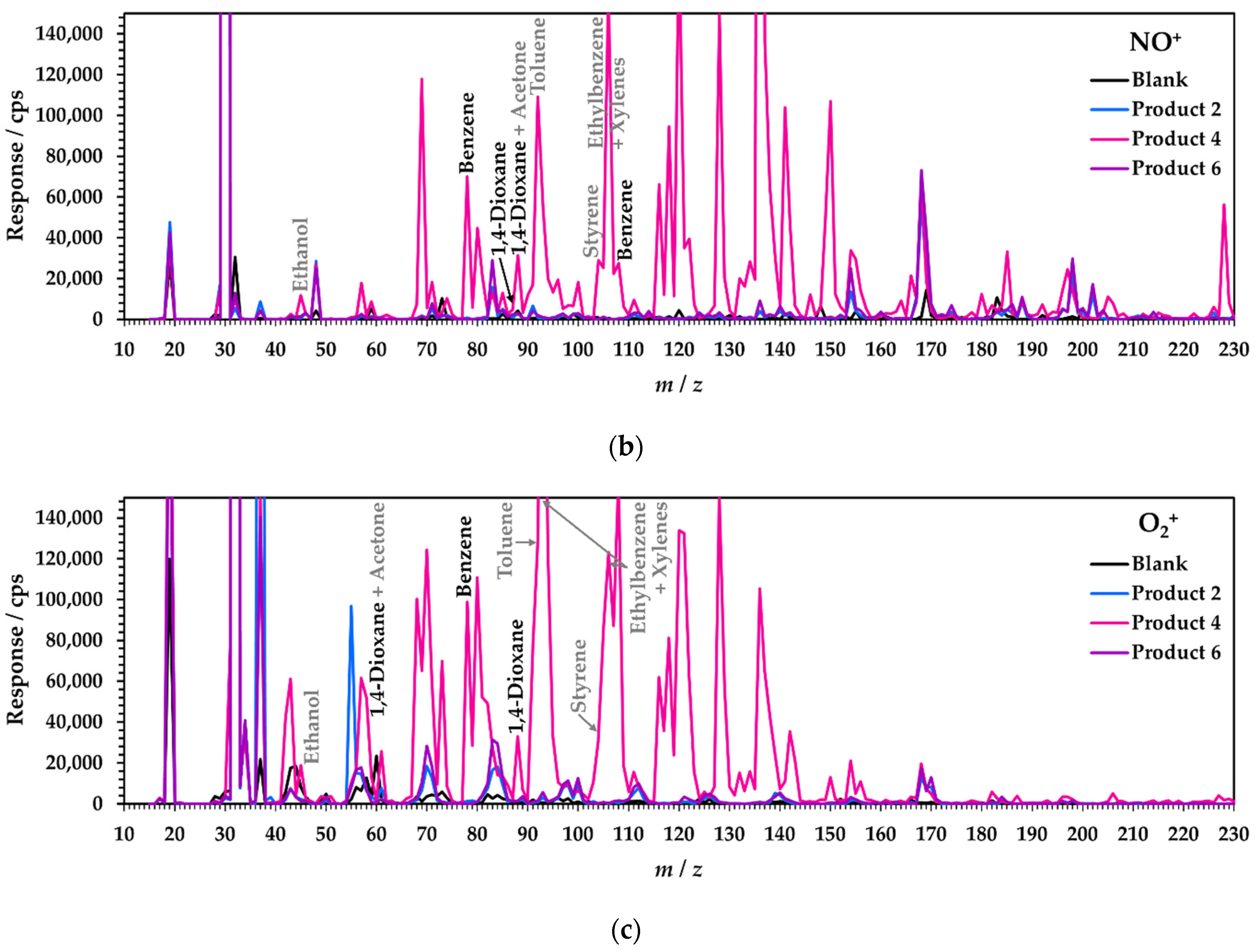
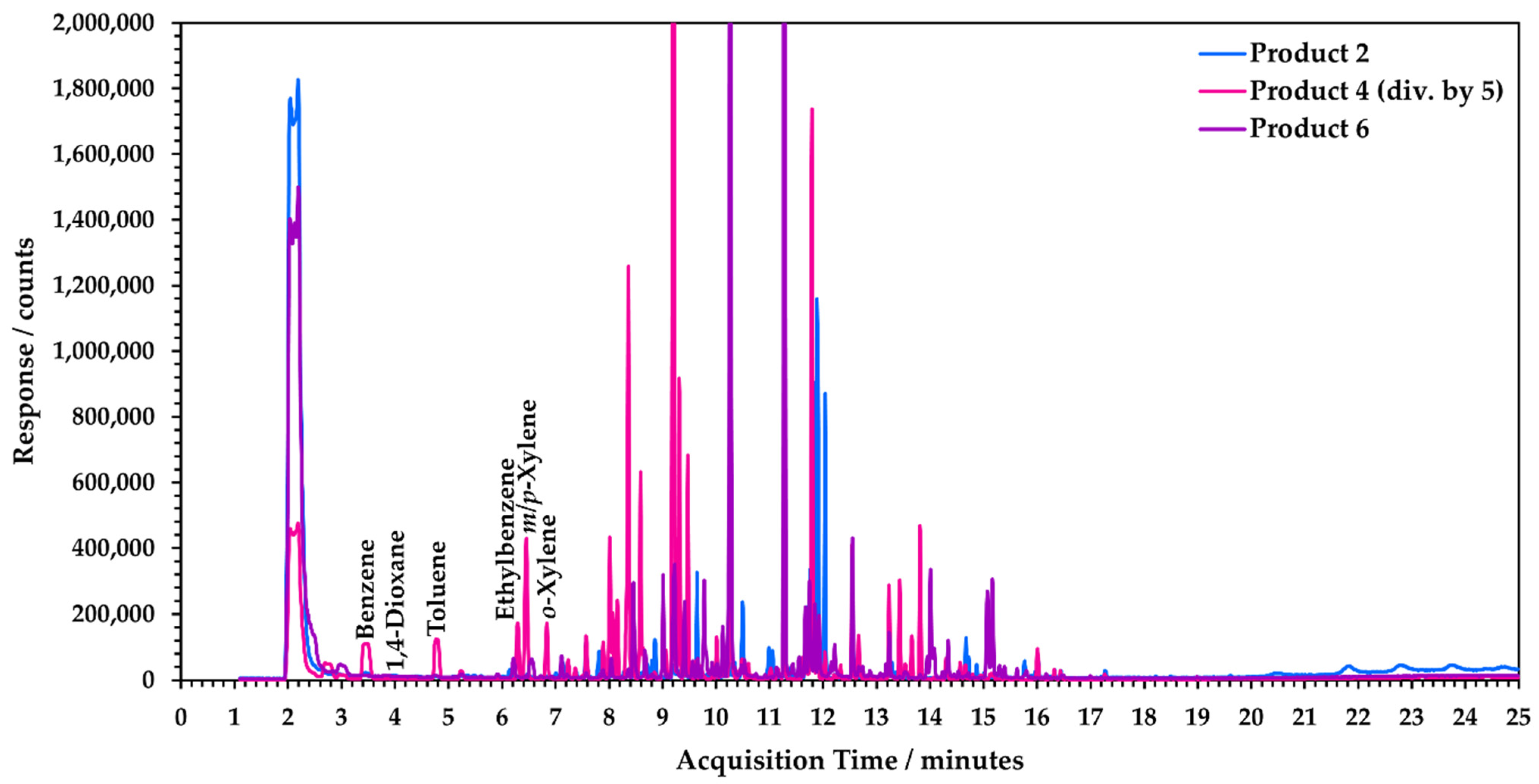
3.1. Specificity of SIFT-MS Analysis
3.1.1. Conventional Approach: Automated Interference Rejection in Static Headspace-SIFT-MS SIM Analysis
- The evaluation of the best dilution ratio for sensitivity across target compounds;
- Confirmation that interference is occurring or not (assuming different partitioning of isobars). For a given set of headspace conditions, noninterfered ions should yield very similar headspace concentration determinations.
3.1.2. Matrix Evaluation Using Full-Scan Static Headspace SIFT-MS Analysis
3.1.3. Matrix Evaluation Using Static Headspace-GC-MS Analysis
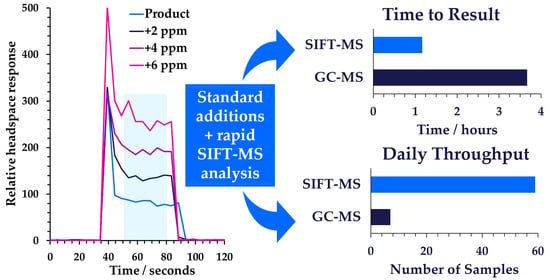
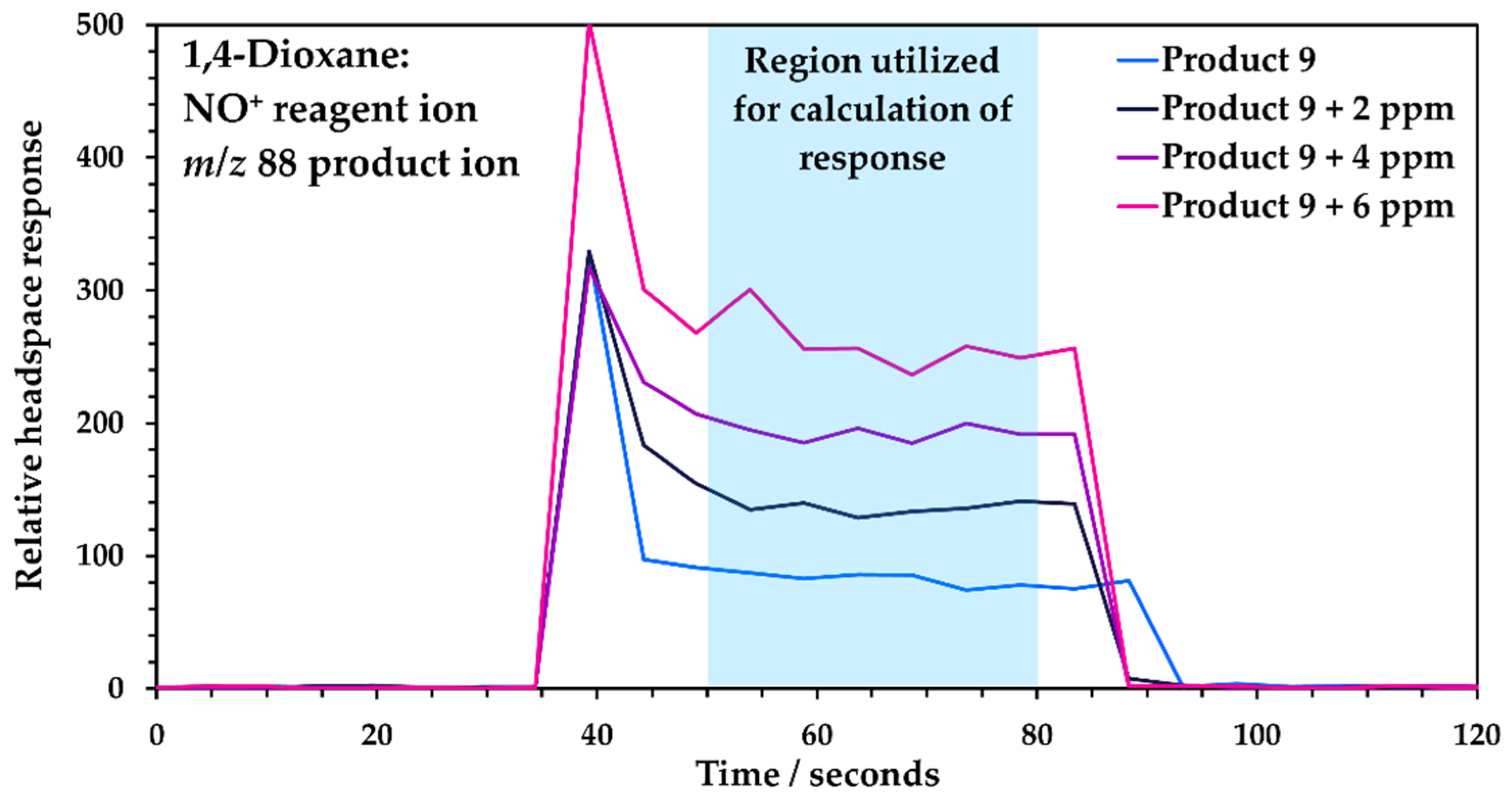
3.2. Quantitation Using the Method of Standard Additions with SIFT-MS
3.2.1. Full Standard Additions for Each Sample
3.2.2. Temporal Stability of the Standard Additions’ Curve
4. Discussion
4.1. Method Development Approach
4.1.1. Preparation: Analyte Library Entry Optimization for the SIFT-MS Instrument
- Prepare a sample of the analyte at a concentration in the low parts-per-million range (by volume in the gas phase; ppmV).
- Run a full-scan analysis to confirm that product ions are present as stated in the library.
- Create and run a targeted method (this improves the signal-to-noise ratio (S/N)), adding new m/z if necessary.
- Calculate updated reaction rate coefficients, k, and product ion branching ratios, Rb, as necessary to align the concentration data generated from each primary product ion. Note that the k for the H3O+ reaction will normally be left unchanged—for most VOCs, the rate coefficients are calculated relative to the H3O+ rate [31].
- Create an updated library entry that will be used in the workflows summarized below.
4.1.2. Workflow 1: Method Development for a Product Class (e.g., Haircare Products)
4.1.3. Workflow 2: Method Customization for a Previously Untested Product
- Acquire SIFT-MS full-scan and SIM data—the latter use the generic method as in Step 7 in Table 4.
- Identify major matrix volatiles and ensure that the product matrix does not overload the instrument (Step 2, Table 4).
- Evaluate the specificity of the individual SIFT-MS product ions (Step 4, Table 4).
- If there are concerns regarding specificity, then use full-scan GC-MS to confirm the presence of interfering compounds or not (Step 5, Table 4). Decide the appropriateness of a screening procedure versus a quantitative method for this formulation.
- Customize the method to the product by eliminating product ions that report high (Step 6, Table 4).
- Validate the method for the product (Step 8, Table 4).
4.2. Proposed Workflow for Routine Analysis
4.2.1. Workflow 3: Routine, Rapid Screening Analysis
- Calibrate for standard additions. The required frequency of calibration was determined in method development (Step 7 of Table 4) and confirmed for the product during method validation (Step 8).
- Prepare and analyze samples according to the standard operating procedure.
- Apply calibrations to individual product ions and subtract blanks.
- Is the reported concentration above the threshold for the lowest-reading ions?
- Yes: test using the standard GC-MS or LC method.
- No: product passes.
4.2.2. Workflow 4: Routine Quantitative Analysis
- Calibrate for standard additions. Calibration frequency was determined in method development (Step 7, Table 4) and validation (Step 8).
- Prepare and analyze samples according to the standard operating procedure.
- Apply calibrations to individual product ions and subtract blanks.
- Inspect data for product ion agreement observed in method development. Is behavior similar?
- Yes: process data and report results.
- No: (i) Retest and report using the standard GC-MS or LC method. (ii) Further investigate the product using SIFT-MS to understand why there was a problem and gauge whether it is an outlier or a change (e.g., to the formulation) that could cause issues for routine testing in future. If the latter, then return to Workflow 2.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Parliament. Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32009R1223 (accessed on 16 January 2024).
- Agency for Toxic Substances and Disease Registry. Toxicological Profiles. Available online: https://www.atsdr.cdc.gov/toxprofiledocs/index.html (accessed on 2 January 2024).
- Bettenhausen, C.A. Finding benzene everywhere we look. Chem. Eng. News 2022, 100, 24–26. [Google Scholar] [CrossRef]
- Pal, V.K.; Lee, S.; Kannan, K. Occurrence of and dermal exposure to benzene, toluene and styrene in sunscreen products marketed in the United States. Sci. Total Environ. 2023, 888, 164196. [Google Scholar] [CrossRef] [PubMed]
- Yeoman, A.M.; Shaw, M.; Ward, M.; Ives, L.; Andrews, S.J.; Lewis, A.C. Gas phase emissions of volatile organic compounds arising from the application of sunscreens. Int. J. Environ. Res. Public Health 2023, 20, 5944. [Google Scholar] [CrossRef] [PubMed]
- International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH). Impurities: Guideline for Residual Solvents Q3C(R8). 2021. Available online: https://database.ich.org/sites/default/files/ICH_Q3C-R8_Guideline_Step4_2021_0422_1.pdf (accessed on 16 January 2024).
- United States Pharmacopeia. Residual Solvents <467>; United States Pharmacopeia: Rockville, MD, USA, 2007. [Google Scholar]
- European Union. Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption (Recast) (Text with EEA relevance). Off. J. Eur. Union 2020, L435, 1–62. Available online: https://eur-lex.europa.eu/eli/dir/2020/2184/oj (accessed on 25 January 2024).
- United States Government. Code of Federal Regulations: Title 40, Part 141—National Primary Drinking Water Regulations (40 FR 59570). 1975. Available online: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-D/part-141 (accessed on 25 January 2024).
- European Pharmacopeia. Ethylene Oxide and Dioxan, 5th ed.; European Pharmacopeia: Strasbourg, France, 2005. [Google Scholar]
- United States Pharmacopeia. Polysorbate 80. Available online: https://www.usp.org/sites/default/files/usp/document/harmonization/excipients/polysorbate_80.pdf (accessed on 16 January 2024).
- Jairoun, A.A.; Al-Hemyari, S.S.; Shahwan, M.; Zyoud, S.H.; Ashames, A. Hidden formaldehyde content in cosmeceuticals containing preservatives that release formaldehyde and their compliance behaviors: Bridging the gap between compliance and local regulation. Cosmetics 2020, 7, 93. [Google Scholar] [CrossRef]
- Kim, S.T.; Shao, K.; Oleschkewitz, C.; Hamilton, R. Margin of exposure to free formaldehyde in personal care products containing formaldehyde-donor preservatives: Evidence for consumer safety. Regul. Toxicol. Pharmacol. 2023, 145, 105519. [Google Scholar] [CrossRef] [PubMed]
- Miralles, P.; Alonso, C.M.J.; Hernandorena, S.; Salvador, A. Determination of free formaldehyde in cosmetics containing formaldehyde-releasing preservatives by reversed-phase dispersive liquid–liquid microextraction and liquid chromatography with post-column derivatization. J. Chrom. A 2018, 1543, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Španěl, P.; Demarais, N.; Langford, V.S.; McEwan, M.J. Recent developments and applications of selected ion flow tube mass spectrometry (SIFT-MS). Mass Spec. Rev. 2023, e21835. [Google Scholar] [CrossRef] [PubMed]
- Langford, V.S. SIFT-MS: Quantifying the volatiles you smell… and the toxics you don’t. Chemosensors 2023, 11, 111. [Google Scholar] [CrossRef]
- Langford, V.S.; Dryahina, K.; Španěl, P. Robust automated SIFT-MS quantitation of volatile compounds in air using a multicomponent gas standard. J. Am. Soc. Mass Spectrom. 2023, 34, 2630–2645. [Google Scholar] [CrossRef] [PubMed]
- Perkins, M.J.; Silva, L.P.; Langford, V.S. Evaluation of solvent compatibilities for headspace-SIFT-MS analysis of pharmaceutical products. Analytica 2023, 4, 313–335. [Google Scholar] [CrossRef]
- Kolb, B.; Ettre, L.S. Static Headspace-Gas Chromatography—Theory and Practice; Wiley-VCH: New York, NY, USA, 1997; pp. 171–173. [Google Scholar]
- Perkins, M.J.; Langford, V.S. Application of routine analysis procedures to a direct mass spectrometry technique: Selected ion flow tube mass spectrometry (SIFT-MS). Rev. Sep. Sci. 2021, 3, e21003. [Google Scholar] [CrossRef]
- Perkins, M.J.; Langford, V.S. High-Throughput SIFT-MS Analysis of Formaldehyde in Fragrances Using the Method of Standard Additions. Syft Technologies Application Note. 2023. Available online: https://bit.ly/3Y9a0Ka (accessed on 16 January 2024).
- Perkins, M.J.; Langford, V.S. High-Throughput SIFT-MS Quantitative Analysis of Beer Using the Method of Standard Additions. Syft Technologies Application Note. 2023. Available online: https://bit.ly/3O51Ezr (accessed on 16 January 2024).
- Silva, L.; Langford, V. High-Throughput, Quantitative Analysis of Benzene in Personal Care Products Using Headspace-SIFT-MS. Syft Technologies Application Note. 2022. Available online: http://bit.ly/3Xv3Cew (accessed on 25 January 2024).
- Španěl, P.; Smith, D. SIFT studies of the reactions of H3O+, NO+ and O2+• with several aromatic and aliphatic hydrocarbons. Int. J. Mass Spectrom. 1998, 181, 1–10. [Google Scholar] [CrossRef]
- Wang, T.; Španěl, P.; Smith, D. A selected ion flow tube, SIFT, study of the reactions of H3O+, NO+ and O2+• ions with several N- and O-containing heterocyclic compounds in support of SIFT-MS. Int. J. Mass Spectrom. 2004, 237, 167–174. [Google Scholar] [CrossRef]
- Španěl, P.; Smith, D. Quantification of trace levels of the potential cancer biomarkers formaldehyde, acetaldehyde and propanol in breath by SIFT-MS. J. Breath Res. 2008, 2, 046003. [Google Scholar] [CrossRef] [PubMed]
- Langford, V.S.; Perkins, M.J. Untargeted SIFT-MS headspace analysis: High-throughput differentiation of virgin and recycled polyethylene pellets. Rapid Commun. Mass Spectrom. 2022, 36, e9230. [Google Scholar] [CrossRef] [PubMed]
- Langford, V.S.; Perkins, M.J.; Milligan, D.B.; Prince, B.J.; McEwan, M.J. High-speed formaldehyde analysis for the process-line and laboratory: SIFT-MS. In Proceedings of the International Society of Automation (ISA) 62nd Analysis Division Symposium, Pasadena, CA, USA, 23–27 April 2017; Available online: http://bit.ly/3INcYyh (accessed on 25 January 2024).
- Perkins, M.J.; Langford, V.S. Multiple headspace extraction-selected ion flow tube mass spectrometry (MHE-SIFT-MS). Part 1: A protocol for method development and transfer to routine analysis. Rev. Sep. Sci. 2022, 4, e22001. [Google Scholar] [CrossRef]
- Swift, S.J.; Španěl, P.; Sixtová, N.; Demarais, N. How to use ion-molecule reaction data previously obtained in helium at 300 K in the new generation of selected ion flow tube mass spectrometry instruments operating in nitrogen at 393 K. Anal. Chem. 2023, 95, 11157–11163. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Španěl, P. Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis. Mass Spec. Rev. 2005, 24, 661–700. [Google Scholar] [CrossRef]
- Perkins, M.J.; Langford, V.S. Standard validation protocol for selected ion flow tube mass spectrometry methods applied to direct headspace analysis of aqueous volatile organic compounds. Anal. Chem. 2021, 93, 8386–8392. [Google Scholar] [CrossRef]



| Compound, Molecular Formula | Reagent Ion | Product Ion Formula | Product Ion m/z | Branching Ratios | Reference |
|---|---|---|---|---|---|
| Benzene, C6H6 | H3O+ | C6H6∙H+ | 79 | 100% | [24] |
| NO+ | C6H6+• | 78 | 76% | ||
| NO+ | C6H6∙NO+ | 108 | 24% | ||
| O2+• | C6H6+• | 78 | 100% | ||
| 1,4-Dioxane, C4H8O2 | H3O+ | C4H8O2∙H+ | 89 | 100% | [25] |
| NO+ | C4H7O2+ | 87 | 55% | ||
| NO+ | C4H8O2+• | 88 | 45% | ||
| O2+• | C3H6O+• | 58 | 30% | ||
| O2+• | C4H8O2+• | 88 | 65% | ||
| Formaldehyde, CH2O | H3O+ | CH2O∙H+ | 31 (49) | 100% | [26] |
| Product Number | Product Type/Description | Ingredients |
|---|---|---|
| 1 | Micellar cleansing water | Hexylene glycol, glycerin, Poloxamer 184, disodium cocoamphodiacetate, sodium EDTA, myrtrimonium bromide |
| 2 | Shower gel | Sodium laureth sulfate, sodium chloride, cocamidopropyl betaine, glycerin, sodium benzoate, citric acid, sodium lactate, polyquarternium-7, panthenol, sodium EDTA, menthyl lactate, dipropylene glycol, propanediol, Cucumis sativus fruit extract |
| 3 | Anti-dandruff shampoo | Sodium laureth sulfate, cocamidopropyl betaine, salicylic acid, sodium coco-sulfate, glycerin, sodium chloride, sodium citrate, parfum, DMDM hydantoin, citric acid, sodium hydroxide, sodium benzoate, caramel, linalool, maltodextrin, hexylene glycol |
| 4 | Medicated shampoo | Macrogol lauryl ether, sodium lauryl ether sulfate, cocodiethanolamine, cocamidopropyl betaine, imidazolidinyl urea, sodium EDTA, citric acid, perfume, sodium chloride, solubilized coal tar extract (20 mg/mL) |
| 5 | Body wash (children) | Sodium laureth sulfate, cocamidopropyl betaine, sodium chloride, glycerin, parfum, disodium cocoamphodiacetate, citric acid, sodium benzoate, potassium sorbate, PEG-150 distearate, sodium glutamate diacetate, hexylene glycol, sodium hydroxide, ascorbyl palmitate |
| 6 | Shower cream | Sodium laureth sulfate, glycerin, sodium chloride, cocamidopropyl betaine, sodium benzoate, citric acid, glycol distearate, laureth-4, sodium lactate, panthenol, hydrolyzed wheat protein, sodium EDTA, bisabolol, dipropylene glycol, tocopherol acetate, geranium oil |
| 7 * | Shampoo (baby) | Cocamidopropyl betaine, decyl glucoside, sodium cocoyl isethionate, polyquartonium-10, coconut oil, glycerin, sodium methyl cocoyl taurate, PEG-80, sorbitan laurate, PEG-150 distearate, sodium chloride, sodium EDTA, citric acid, sodium benzoate, parfum |
| 8 | Charcoal facial scrub | Kaolin, glycerin, starch, decyl glucoside, iron oxides, sodium laureth sulfate, PEG-7 glyceryl cocoate, perlite, PEG-30 dipolyhydroxystearate, zinc gluconate, trideceth-6, sodium hydroxide, pumice, charcoal powder, sodium EDTA, citric acid, xanthan gum, polyglycerin-10, polyglyceryl-10 myristate, polyglyceryl-10 stearate, acrylates (various), sodium dehydroacetate, salicylic acid, phenoxyethanol, parfum |
| 9 | Shower gel | Sodium laureth sulfate, sodium chloride, cocamidopropyl betaine, citric acid, parfum, sodium benzoate, sodium EDTA, potassium sorbate, benzophenone-4, sodium hydroxide, hexylene glycol |
| Product Number or Calibration | Benzene | 1,4-Dioxane | Formaldehyde | |||||
|---|---|---|---|---|---|---|---|---|
| Concentration in Product/ng g−1 | Quantitation Ion(s) | Linearity (R2) | Concentration in Product/μg g−1 | Quantitation Ion(s) | Linearity (R2) | Concentration in Product/μg g−1 | Linearity (R2) | |
| 1 | <LOQ | N78 | >0.992 (4 ions) | 0.40 | N87 | >0.998 (H89, N87, N88) | <LOQ | 0.992 |
| 2 | 4.44 | N78, O78 | >0.995 (4 ions) | 0.66 | N87 | >0.995 (5 ions) | <LOQ | 1.000 |
| 3 | <LOQ | N78 | >0.991 (4 ions) | 1.63 | N87 | >0.991 (5 ions) | 31.8 | 0.995 |
| 4 | 1052 | H79, N78, O78 | >0.992 (4 ions) | 1.34 | N87 | 0.9996 (N87) | 103 | 0.991 |
| 5 | <LOQ | N78 | >0.994 (4 ions) | 0.57 | N87 | >0.996 (except O58) | <LOQ | 0.999 |
| 6 | <LOQ | O78 | >0.990 (4 ions) | 0.67 | N87 | >0.992 (5 ions) | <LOQ | 0.997 |
| 7 | <LOQ | N78 | >0.994 (4 ions) | 0.39 | N87 | >0.992 (5 ions) | <LOQ | 0.999 |
| 8 | 3.39 | N78 | >0.992 (except N108) | 0.56 | N87 | >0.990 (except O58) | <LOQ | 0.996 |
| 9 | <LOQ | N78 | >0.996 (4 ions) | 0.83 | H89 | >0.994 (except O58) | <LOQ | 1.000 |
| Water cal. | >0.993 (4 ions) | >0.992 (5 ions) | 0.9939 | |||||
| Water cal.: High benzene | >0.997 (4 ions) | |||||||
| Step | Sub-Step | Commentary |
|---|---|---|
| 1. Identify products for method development | A. Select a variety of formulations from different manufacturers. | Evaluation of a variety of product formulations helps ensure broad applicability. |
| B. Prepare samples for static headspace analysis. 1 | Initial analysis is qualitative. | |
| 2. Untargeted analysis of products | A. Acquire full-scan data using SIFT-MS and GC-MS. | From these data, identify the most significant matrix volatiles. |
| B. Additional data checks for SIFT-MS. | Check matrix volatiles for (1) total load on the instrument (i.e., how much reagent ion signal do they consume?), and (2) the extent of secondary chemistry. | |
| 3. SIFT-MS method development, part I | A. Create your “draft” targeted (SIM) SIFT-MS method in software tool. | Be sure to include major matrix volatiles in the method (including relevant secondary chemistry). Most often, this will be solvent, but volatiles could also be important. |
| B. Reprocess the full-scan SIFT-MS data in software tool. | This extracts indicative concentration data for each volatile from the full-scan spectra acquired in Step 2. | |
| 4. SIFT-MS data evaluation 2 [17] | A. Is this product ion in good agreement with one or more other ions at the lower end of the range? | This often indicates that the SIFT-MS technique is specific for the compound. However, this can sometimes be fortuitous, e.g., a compound with product ions lies at one m/z less and agreement is due to isotopologue interference (subtraction is usually straightforward in this case). |
| B. Does the concentration appear significantly lower than expected? | If it does, then: (1) Are relevant secondary product ions included in the method? (2) Did this ion appear reliable when you updated the library parameters for the instrument? (Consider especially the different conditions between samples used for library and matrix.) | |
| C. For higher-reading ions, is there any indication of interference arising from the matrix or other high-concentration volatiles in the sample? | Some of these may not be flagged by software tools: (1) Isotopologues (most commonly 13C). (2) Minor primary product ions, including those of very little or no significance at trace concentrations. (3) Secondary product ions. These may differ from the library if concentrations are sufficiently elevated. Watch for their isotopologues, too. | |
| 5. Confirm SIFT-MS specificity for product | A. Does the GC-MS analysis confirm that the analyte is present? | If GC-MS does not confirm the presence of analyte, then the SIFT-MS analysis may not be specific. Can SIFT-MS be applied as a rapid screening tool? |
| B. Does the GC-MS analysis confirm the identity of suspected interfering species or reveal unsuspected interference in SIFT-MS? | If GC-MS confirms the interferent’s identity, can this be used to facilitate a quantitative analysis using SIFT-MS (e.g., through a subtraction approach)? | |
| 6. SIFT-MS method development, part II | A. Refine the SIFT-MS method using findings from Steps 4 and 5. | This primarily involves adjusting product ion selections. |
| B. Re-extract data from scans or reanalyze samples with the SIM method. | At this point, decide on data quality required for decision making. SIM data acquisition will give better insights for quantitation (especially for Step 7). | |
| C. Reevaluate the data. | See step 4 above. If acceptable, move to Step 7, otherwise repeat Steps 4 and 6. | |
| 7. Determine quantitation approach | A. Reassess suitability of the headspace approach for the matrix. | Confirm that the analysis is best served by the method of standard additions. Or is the matrix suited to static headspace analysis (simple calibration) or multiple headspace extraction (MHE), for example? |
| B. Evaluate calibration stability. | For each product, assess the calibration stability over days to weeks to determine the frequency of recalibration. | |
| 8. Validation | A. Method should be validated using standard protocols for each product. | Validate on individual product ahead of routine sample analysis because performance is likely to vary by formulation. 3 See [32] for general guidance for headspace methods. Additionally, for standard additions, these should be optimized to the specific product. 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perkins, M.J.; Hastie, C.J.; Langford, V.S. Headspace-Selected Ion Flow Tube Mass Spectrometry Workflows for Rapid Screening and Quantitation of Hazardous Volatile Impurities in Personal Care Products. Analytica 2024, 5, 153-169. https://doi.org/10.3390/analytica5020010
Perkins MJ, Hastie CJ, Langford VS. Headspace-Selected Ion Flow Tube Mass Spectrometry Workflows for Rapid Screening and Quantitation of Hazardous Volatile Impurities in Personal Care Products. Analytica. 2024; 5(2):153-169. https://doi.org/10.3390/analytica5020010
Chicago/Turabian StylePerkins, Mark J., Colin J. Hastie, and Vaughan S. Langford. 2024. "Headspace-Selected Ion Flow Tube Mass Spectrometry Workflows for Rapid Screening and Quantitation of Hazardous Volatile Impurities in Personal Care Products" Analytica 5, no. 2: 153-169. https://doi.org/10.3390/analytica5020010
APA StylePerkins, M. J., Hastie, C. J., & Langford, V. S. (2024). Headspace-Selected Ion Flow Tube Mass Spectrometry Workflows for Rapid Screening and Quantitation of Hazardous Volatile Impurities in Personal Care Products. Analytica, 5(2), 153-169. https://doi.org/10.3390/analytica5020010