Abstract
This review provides insights into the current research on pyrolytic bio-oil obtained from different feedstock regarding upgrading techniques and applications such as energy, fuels, chemicals, and carbon materials. Raw bio-oil is not appropriate for transportation and ignition due to undesired properties; therefore, several challenges have been reported regarding its suitable market application. For liquid biofuel production, thermochemical pathways, particularly hydrogenation and deoxygenation, must be carried out, and for chemical production, liquid solvents are mostly used via physical separation. The main issues related to downstream processes with environmental and economic assessment are also covered. The analysis indicates that the major bottlenecks for commercial applications of upgraded bio-oil are the initial stage (upgrading techniques), high production costs, and pilot scale production. Finally, future directions are addressed for the improvement of bio-oil upgrading.
1. Introduction
Recent agreements signed at COP26 suggest a 25% reduction in carbon emissions by 2030, which requires an immediate transition from fossil fuels to renewables [1]. The COVID-19 pandemic has impacted every aspect of the Net-Zero program from different governments worldwide. With the aim of inhibiting rapid COVID-19 spreading, more than 130 billion disposable medical face masks were consumed every month; therefore, their efficient use and recycling are required not only for public safety, but also for resource consumption and environmental protection [2]. Pyrolysis has attracted researchers’ attention since it is a straightforward process that achieves better thermochemical waste management in comparison with current incineration practices.
Pyrolysis is a thermochemical process that converts varied materials into chemicals or biofuels, often at lower temperatures (300–700 °C) than incineration and under an inert atmosphere (devoid of oxygen) [3,4]. Pyrolysis depends on operating parameters such as temperature, particle size, heating rate, and residence time, split into three distinct processes: slow pyrolysis reaches high solid yields (biochar), fast pyrolysis produces liquids (bio-oil), and flash pyrolysis targets gases [5,6,7].
Bio-oil is a condensed liquid from the pyrolysis of different feedstock, a dark brown liquid commonly named pyrolytic tar, wood liquid, liquid smoke, pyrolysis oil, pyroligneous acid, wood distillate, and wood oil; however, bio-oil, biocrude oil, pyrolytic bio-oil, and pyrolysis liquid are primarily used [5]. Bio-oil is a mixture of several different oxygenated compounds such as acids, aldehydes, ketones, aromatics, phenols, alcohols, ethers, esters, and carbohydrates. Organic acids, furfurals, and levoglucosan are the main high-value compounds found in bio-oil, based on the US Department of Energy list; therefore, the concept of value-added chemicals and biofuels from bio-oil must be correctly assessed [5,8].
There are many separation and fractionation methods for both fuel upgrading and chemical production; however, the chemical application and separation of bio-oil cannot be performed directly, as most of the compounds are found in concentrations below 1%, hindering their separation [9,10,11]. Bio-oil upgrading can be carried out through conventional technologies such as esterification, hydrogenation, steam reforming, emulsification, and cracking, and novel technologies such as vacuum and molecular distillation, supercritical fluid extraction, and membrane separation [12,13].
Several works regarding pyrolysis have shown improvements in parameters (temperature, heating rate, biomass, and so on) [14,15,16,17], reactors with varied configurations [18,19], catalysts for bio-oil upgrading [20,21,22], and mostly in biochar application [23,24,25]. However, to the best of our knowledge, review papers about the methods of separation and fractionation of bio-oil for biofuel upgrading and chemical production are scarce [25,26,27,28,29,30,31,32,33,34,35,36]. Thus, it is critical to review the updated methods regarding their advantages, drawbacks, and economics to identify the most promising methods for bio-oil upgrading. Considering these issues, this review attempts to explain the state-of-the-art knowledge on methods for bio-oil upgrading. Furthermore, the environmental and economic aspects of different methods are discussed. Finally, the impact of this review and possible approaches for future research are also assessed.
2. Bio-Oil
Bio-oil is a dark brown viscous liquid composed of highly oxygenated compounds produced from the pyrolysis of biomass, which is unstable and susceptible to aging [13,29,32,37]. In addition, low concentrations of sulfur and nitrogen can be found in bio-oils [38]. Bio-oils are produced via fast depolymerization and fragmentation of hemicellulose, cellulose, and lignin through a rapid increase in temperature (heat rate of 103–105 °C/s), followed by a fast cooling under anaerobic or limited oxygen conditions, preventing the further conversion of the intermediate products. Distinct designations for bio-oil can be found in the literature, including pyrolysis oil and pyrolysis liquid. However, the term bio-oil will be used throughout this review. The elemental composition and physicochemical properties of bio-oil are significantly different from those of petroleum-derived oils, namely due to the presence of substantial amounts of oxygen in biomass [37,39,40,41]. Table 1 depicts the chemical composition and some key physicochemical properties reported for bio-oils and petroleum-derived oils [42,43,44,45,46].

Table 1.
Typical composition and physicochemical properties reported for bio-oils and heavy petroleum-derived oils.
The HHV found in bio-oils is half of the HHV found in heavy petroleum-derived oils due to the high concentration of oxygenated compounds and moisture in bio-oil. Additionally, high oxygen content has been reported to result in bio-oil’s immiscibility with hydrocarbon fuels [39,40]. Although bio-oils are biodegradable, less toxic, and have good lubricity properties relative to petroleum-derived oils, their high viscosity, ash content, chemical instability, and corrosiveness hamper their direct use as a fuel [32]. Regarding the chemical composition of bio-oils, they are composed of a mixture of phenolics, organic and carboxylic acids, aldehydes, ketones, esters, furans, and sugars [5]. High molecular weight oligomers can be found in bio-oil due to the occurrence of depolymerization (C–O and C–C bond cleavage) followed by dehydration and repolymerization reactions of the main biomass polymers [47]. Bio-oil yield is significantly affected by pyrolysis variables (Section 3), such as heating and residence time, temperature, particle size, type of feedstock (hardwood, softwoods, and herbaceous biomasses), and catalytic and non-catalytic pyrolysis [14,17].
3. Biomass Pyrolysis Mechanism
Pyrolysis is a process that employs thermal depolymerization of biomass at moderate temperatures in an oxygen-absent setting. Temperature, pressure, and condensable gas residence time heavily influence solid, liquid, and gas yields [48]. Biomass pyrolysis is categorized according to the residence time of the solid feedstock inside the reactor: (i) slow pyrolysis, (ii) flash pyrolysis, and (iii) fast pyrolysis [49,50]. Lignocellulosic biomass pyrolysis is a complex, multistage process with a wide range of reaction mechanisms and rates that are highly dependent on thermal processing conditions and interactions between the major components found in the feedstock, leading to secondary, parallel, and concurrent reactions.
The first step in the biomass depolymerization step involves the dissolution of its macromolecules. Cellulose is a linear polysaccharide composed of β-(1,4) glycosidic bonds of glucose molecules with a (C6H10O5)n formula. A cellulosic chain structure is formed of alternating crystalline and amorphous zones; such alternation impacts the accessibility and reactivity of its functional groups [51]. Cellulose thermal degradation starts at 200 °C, and the degradation rate increases as the temperature rises [52]. Hemicellulose is a heteropolymer formed by different monomers (xylose, arabinose, galactose, mannose, among others) that can produce alcohols, organic acids (lactic acid, formic acid), and macro fragments of sugars. Hemicellulose presents a lower temperature degradation range, between 150 and 180 °C, than cellulose, as it has significantly less crystalline than cellulose due to the various monomers present in its structure. In contrast to cellulose and hemicellulose structures, lignin is an amorphous polyphenolic complex formed by the oxidative polymerization of three monomers derived from p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol; the proportions of these monomers are dependent on the source of origin and form of extraction. Unlike the linear structure exhibited in cellulose, lignin has a highly branched three-dimensional structure, allowing the various hydroxyl and polar groups present to establish strong intra- and intermolecular hydrogen bonds.
The second step is the thermal decomposition of pentoses, hexoses, and low-molecular-weight products. The major products from glucose degradation are furans (5-hydroxymethylfurfural, furfural), levoglucosan, and organic acid (acetic acid), obtained through dehydration reactions [53]. As hemicellulose structural monomeric content depends on the feedstock source, the pyrolysis behavior and products are different between biomasses; arabinose, xylose, galactose, and mannose mainly produce furanes, pyrenes, ketones, organic acids, and anhydrosugars via pyrolysis [54]. While the heating rate during lignin thermal degradation exerts no impact on product composition, pyrolysis temperature heavily affects the outcome: in lower temperatures (200 to 400 °C), products are typically derivatives from guaiacyl-type lignin (guaiacol, coniferol, coniferaldehyde, creosol, isoeugenol) and syringols due to the characteristic stability of aromatic configurations; for temperatures higher than 400 °C, hemolytic cleavage of the O–CH3 bond is observed, leading to the formation of catechols, o-creasols, phenols, and char [55].
Further increases in temperature and residence times can lead to the final step in biomass pyrolysis: repolymerization, in which highly reactive, low molecular weight intermediate compounds from the decomposition of the lignocellulosic matrix recombine into a higher molecular weight, more stable solid compound (char) [56,57]. A previous study by Chen et al. [58] identified higher biochar yields from hemicellulose and lignin due to the predominance of condensation reactions in high conversion rate settings. Inhibiting repolymerization has been an objective of several studies, as it may prevent access to the polysaccharides in the lignocellulosic biomass and decrease bio-oil yield [58,59,60,61]; the different temperatures for cellulose, hemicellulose, and lignin for the different lignocellulosic matrix compositions in each feedstock pose a significant challenge for optimum pyrolysis temperature, as bio-oil yields decrease at higher temperatures due to self-condensation reactions favoring char and tar formation.
4. Effect of Process Parameters on Bio-Oil Production
4.1. Temperature
The key role of temperature in biomass pyrolysis is to provide the necessary heat to break the wide variety of linkages present in lignocellulosic biomass. Thus, the temperature is one of the most important parameters, as it directly affects the extension of biomass decomposition, product distribution, and calorific values [3]. Several studies have been published discussing the effect of temperature on the final bio-oil yield, showing that temperatures ranging from 450 to 550 °C are required to obtain higher yields. However, final bio-oil yields are also highly affected by the feedstock and other operating conditions [62,63]. Sohaib, Muhammad, and Younas [64] investigated the effect of temperature on sugarcane bagasse fast pyrolysis. The authors reported a bio-oil yield of 60.4% at 500 °C, whereas low gas and biochar yields were obtained. Lin and Chen [65] reported similar bio-oil yields (55%) after 30 min of reaction time when sugarcane bagasse was subjected to pyrolysis at 500 °C. In addition, Tsai and Lee [66] reported an increase in bio-oil yield from 11.3% to 35.9% when the pyrolysis temperature of rice husks increased from 400 to 500 °C. Similar findings were found by Biswas and Pandey [67], who reported that the optimal pyrolysis temperature for rice husks was between 300 and 450 °C in a fixed bed reactor. The same authors mentioned that temperatures below 300 °C were not high enough to pyrolyze rice husks, while temperatures above 450 °C quickly decreased bio-oil yield.
It is worth mentioning that, under similar reaction temperatures, the type of reactor has an extensive influence on the final bio-oil yields. Alvarez et al. [68] reported excellent bio-oil yields of 70 wt. % at 450 °C when rice husks were subjected to fast pyrolysis in a continuous pyrolysis bench-scale with a conical spouted bed reactor (CSBR). The excellent bio-oil yields were attributed to the CSBR capacity of promoting higher mass and heat transfer, as well as the reduced residence time of the volatiles. Differential scanning calorimetry (DSC) studies have been used aiming to define the temperature range required to maximize the final yield of bio-oil from several types of feedstock [69,70]. For example, Gonçalves et al. [70] found that pyrolysis must be carried out at 600 °C to maximize bio-oil production because part of the lignin present in the feedstock matrix does not decompose at lower temperatures. Additionally, Puy et al. [18] reported an excellent bio-oil yield of 59% from the pyrolysis of pine woodchips in an auger reactor at a relatively low temperature of 500 °C for 2 min of residence time. It is worth noting that auger reactors are known to provide several advantages, including lower reaction temperatures, not requiring a carrier gas, and operating as a continuous process [71,72].
In addition to bio-oil yield, the overall product distribution is also influenced by temperature. Carbon and hydrogen contents are excellent metrics to evaluate the quality of the final product. As such, high-quality bio-oil should be composed of high carbon and hydrogen contents, while the oxygen content should be as low as possible [73]. Zhao et al. [74] investigated pyrolysis temperature in varying food waste and food waste solid digestate and reported that higher temperatures promote the reduction of oxygen content, while carbon and hydrogen content increased with temperature; this is an important finding, as it seems that higher temperatures lead to the cracking of hydrocarbons present in the biomass, thus decreasing the oxygen content found in the bio-oil, which is expected and highly desirable. The influence of temperature on the final product distribution has also been investigated by Alvarez et al. [68], who reported that increasing the pyrolysis temperature of rice husks led to the formation of phenols (e.g., catechols, guaiacols, and alkyl-phenols) through the decomposition of lignin above 350 °C. A decrease in the concentration of ketones (e.g., cyclopentenones and open-chain ketones) was also observed at temperatures above 600 °C due to condensation reactions of the feedstock carbohydrates. Higher pyrolysis temperatures (>600 °C) favor cracking reactions, and the formation of lighter compounds due to such temperatures favor gasification reactions. Interestingly, the content of water, ethers, and aldehydes seemed to be unaffected by temperature [68].
4.2. Heating Rate
Another critical parameter for biomass pyrolysis is the heating rate, as it impacts the types of decomposition products formed. As mentioned above (vide supra, Section 4.1), heating rates are usually higher in fast pyrolysis relative to the conventional process [63,75]. Higher heating rates can cause quick fragmentations of biomass and promote the formation of volatiles [76]. The effect of different heating rates on the final bio-oil yields has been widely published in the literature [66]. For example, varying heating rates from 100 to 500 °C/min have been studied by Tsai et al. [66], who found that 200 °C/min is the optimal heating rate to maximize the final bio-oil yield from the pyrolysis of rice husk. Additionally, Şensöz et al. [77] investigated different heating rates in the pyrolysis of olive bagasse using a fixed bed reactor. The authors reported that increasing the heating rate from 10 to 50 °C/min led to a meager 8.4% increase in bio-oil yield. Another work from the same group reported that the bio-oil yield from hornbeam shell pyrolysis was not affected by the heating rate [78]. It is worth mentioning that increasing heating rates from 10 to 50 °C/min might not be large enough to observe a significant impact on the final product yield. Higher heating rate ranges, such as from 10 to 100–300 °C/min, are therefore recommended to overcome either or both heat and mass transfer limitations. After overcoming the heat and mass transfer limitations, no further improvements in bio-oil yields may be found when increasing heating rates. For example, Bhoi et al. [79]. observed bio-oil yield improvements when employing a higher heating rate (>1000 °C/s) and a shorter residence time (<2 s). Additionally, Xiong et al. [80] reported that a fast heating rate (200 °C/s) led to a 10% increase in the final bio-oil yield from rice husk relative to the one obtained with medium (≈20 °C/s) and slow (≈0.33 °C/s) heating rates. It is worth mentioning that the final bio-oil yields are not influenced by only a single parameter, but also by the synergetic effects of other variables, such as biomass type, composition and particle size, temperature, reactor configuration, and others. In addition, heating rates can also influence the optimal temperature to maximize final bio-oil yields [19].
4.3. Pressure (Atmospheric or Vacuum)
Pyrolysis can be carried out either in vacuum (<0.1 atm) or atmospheric (1 atm) pressure. Vacuum pyrolysis has shown advantages over atmospheric pyrolysis, as it allows the removal of the primary components produced from the decomposition of biomass; avoids the occurrence of secondary reactions, such as cracking and repolymerization; and reduces residence times. Although greater bio-oil yields have been reported, large amounts of biochar and pyrolytic water can also be produced [3]. However, high moisture content decreases the flame temperature and bio-oil energy density, which may lead to ignition issues [81]. In addition, larger particle size feedstock can be used in vacuum pyrolizers, which is a great advantage relative to slow atmospheric pyrolysis due to lower biomass milling expenses [82]. Carrier et al. [81] reported that the optimal yields of bio-oil from vacuum pyrolysis of sugarcane bagasse were similar to those obtained from slow pyrolysis at 400 °C. At temperatures above 400 °C, higher bio-oil yields were produced using vacuum pyrolysis, while slow pyrolysis promoted the formation of higher quantities of biochar. Amutio et al. [83] compared the performance of flash pyrolysis of pinewood sawdust under vacuum (0.25 atm) and atmospheric pressure by using a bench-scale conical spouted bed reactor. A slight increase in bio-oil yields was found for vacuum pyrolysis in contrast to atmospheric pyrolysis, but significant changes in product characteristics (e.g., less oxygenated compounds) and composition (e.g., greater yields of heavier compounds such as phenols and levoglucosan) were reported.
4.4. Catalyst
Catalytic pyrolysis is a well-known technology for the thermal conversion of biomass into chemicals and fuels by selectively favoring decarboxylation reactions. The bio-oils with more stability and lower oxygenated compounds (e.g., acids, ketones, and carbonyl compounds) and water content are preferable for direct use or further processing of the bio-oil [56,84,85]. In general, the development of catalysts for biomass pyrolysis requires the fine-tuning of their properties, such as acidity, basicity, porosity, and metal–support interactions, as well as the controlled formation of catalyst particle size [22,37]. Another crucial aspect to consider is their thermal stability, so that new catalysts are robust enough to resist deactivation while being easy to regenerate [4]. Microporous acidic catalysts, such as zeolites, are known to catalyze the cleavage of carbon–carbon of biomass during pyrolysis for converting heavier oxygenates to lighter ones. In addition, the bio-oils obtained via the catalytic pyrolysis of biomass with zeolites have increased the number of aromatic compounds. The mechanism responsible for aromatics production from lignocellulose through catalytic pyrolysis with zeolite catalysts has been reported and is shown in Figure 1.
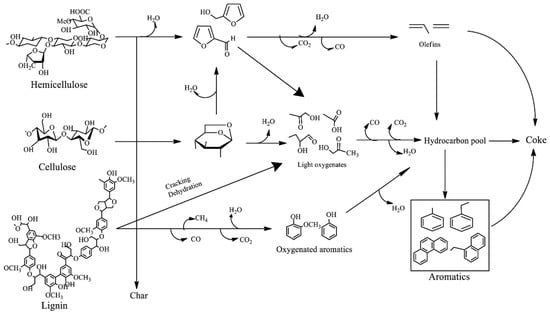
Figure 1.
Proposed mechanism for the formation of aromatics from lignocellulosic feedstock through catalytic pyrolysis [56].
Anhydrosugars and furanic compounds are the main initial products formed from the degradation of both cellulose and hemicelluloses. Acid-catalyzed dehydration reactions at the acid sites of the zeolite promote the formation of anhydrosugars, followed by the production of furans, small aldehydes, and water through dehydration and bond cleavage [56]. Then, several different reactions occur between the furfural and zeolite catalyst, including oligomerization, decarboxylation, and decarbonylation, which lead to the formation of a pool of hydrocarbons [56,86]. Aromatics from lignin can undergo depolymerization and conversion of aliphatic linkers to produce olefins. Acidic zeolite catalysts have been investigated by several research groups, and ZSM-5 has been the most-studied zeolite for pyrolytic bio-oil upgrading [87]. For example, the catalytic pyrolysis performance on pine wood was investigated by Chen et al. [88], who reported that varying proton forms of zeolite catalysts (H-Beta-25, H-Y-12, H-ZSM-5, and H-MOR-20) resulted in different bio-oil yields. H-ZSM-5-23 zeolite resulted in a bio-oil yield of 20.7 wt. %, while 15.1, 9.0, and 17.6 wt. % were obtained for H-Beta-25, H-Y-12, and H-MOR-20, respectively. Additionally, ZSM-5 led to a higher production of ketones and phenols and a lower number of acids and alcohols in the bio-oil relative to other catalysts. Sun et al. [89] investigated the effect of different zeolite structures, including ZSM-5, H-Y, and USY, in the pyrolysis of corn stalks and found that ZSM-5 resulted in the highest bio-oil yields. Interestingly, they also observed that USY resulted in a higher aromatics content, while the H-Y zeolite led to an increased content of aliphatic hydrocarbons. Li et al. [90] proposed a kinetic model that combines thermal pyrolysis followed by the catalytic upgrading of products of sawdust over H-ZSM-5 in a spouted bed reactor at 400 °C. Increasing catalyst loadings (from 4 g to 26 g of catalyst) resulted in a 19% decrease in total bio-oil yield and an approximately 65% increase in the final gas yield. The significant increase in gas yields is due to the higher formation of CO and CO2 through decarbonylation and decarboxylation of the oxygenated compounds, respectively. As expected, the bio-oil obtained using catalytic pyrolysis had less contents of oxygenated compounds and was less viscous and corrosive, in contrast to the thermal pyrolysis bio-oils [3,7,32]. Additionally, mixtures of H-ZSM-5 and silica-alumina on the product distribution of bio-oils obtained from the pyrolysis of durian rind were investigated by Tan, Abdullah, and Hammed [91], who reported that H-ZSM-5 predominantly resulted in aromatic hydrocarbons, while the mixture of H-ZSM-5 and silica-alumina led to the higher formation of aliphatic hydrocarbons. The authors also declared that the H-ZSM-5/silica-alumina mixture inhibited coke formation and increased bio-oil and gas yields.
Besides the advantages of using zeolite catalysts in the pyrolysis of lignocellulosic biomass, the increased production of gases and water and the rapid catalyst deactivation due to coke formation are critical bottlenecks of the zeolite materials [92]. Therefore, catalysts with larger pore sizes relative to zeolite materials have been investigated, as they are supposed to facilitate the diffusion, reaction, and removal of larger bio-oil molecules from the catalyst matrix and decrease the likelihood of coke deposition in the pores. For example, mesoporous acidic catalysts, such as MCM-41, MSU, and SBA-15, have been reported in the literature. MCM-41 is the major representative of the mesoporous molecular sieve of the M41S family, and it is considered a promising catalyst due to its characteristics, such as large surface area, relatively large pores, and mild-to-moderate acidity [93,94]. Adam et al. [95] investigated the performance of different Al-MCM-41 catalysts (Si/Al ratio of 20 and modified via pore enlargement and loading of copper cations into the catalyst structure) on the pyrolysis of spruce wood at 500 °C. Overall, the authors reported that larger catalyst pores resulted in a decrease in acetic acid and water yields, while larger pores coupled with copper introduction into the material structure led to a higher formation of high molecular mass products. For instance, the mesoporous mordenite framework (MFI) zeolite has been reported to exhibit the best performance, relative to H-ZSM-5 and mesoporous material from H-ZSM-5, in deoxygenation and aromatization reactions. In addition to acidic microporous and mesoporous materials, solid basic catalysts, such as ZnO, CaO and CaO-sand/silica, MgO and Fe2O3, among others, have been thoroughly investigated in the literature [85,96,97,98,99]. Zhang et al. [100] investigated the effect of different metal oxides on the catalytic pyrolysis of poplar at 600 °C, reporting that CaO significantly decreased phenol and anhydrosugar contents in the bio-oils and increased the formation of cyclopentanones, hydrocarbons, and light compounds. Cao et al. [99] compared the performance of different types of catalysts, such as metal basic oxides, metal sites, acidic metals, and zeolite catalysts, on the catalytic pyrolysis of rice straw. The highest bio-oil yield of 55.2 wt. % was found using Y-zeolite, which provided higher production of phenolic monomers in comparison with the basic (MgO), acidic (CeO2), and metal salt (MgCl2) catalysts. In addition, Stefanidis et al. [101] evaluated the performance of varying basic catalysts, including TiO2, ZrO2/TiO2, and MgO, and found that the biomass pyrolysis mechanism is distinct relative to the one known for acidic zeolites. Higher yields of CO2 along with higher production of ketones in the bio-oils were found, mostly due to significant aldol-condensation and ketonization reactions. Although promising results from catalytic pyrolysis have been reported, there is still a lack of knowledge about the mechanism of the catalytic conversion of different biomasses into bio-oil. Additionally, new catalysts must be developed to avoid sacrificing the final bio-oil yield for its quality [93,102]. An overview of the effect of catalyst types mentioned in this section on the final bio-oil yields and the bio-oil composition is presented in Table 2.

Table 2.
Overview of the influence of catalyst type on bio-oil yields and compositions obtained at different catalytic pyrolysis conditions.
5. Bio-Oil Upgrading
Considering the previous section about bio-oil, it is noticed that the undesired properties limit its application to chemicals and transportation fuels. Therefore, bio-oil upgrading is crucial to improve its quality to be used as a chemical ingredient and reach the standard of petroleum-based liquid fuels, as shown in Figure 2. In this section, different separation techniques for bio-oil upgrading, including the effect of key parameters, challenges, process fundamentals, and feasibility, are discussed in detail below (Table 3).
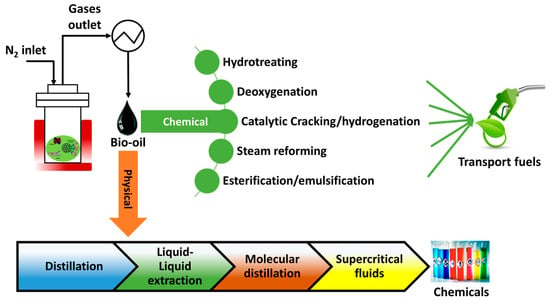
Figure 2.
Bio-oil upgrading/fractionation techniques.

Table 3.
Operation conditions, reactions, and technical feasibility of the current upgrading techniques of bio-oil.
5.1. Chemical Processes
5.1.1. Hydrotreating
It is known that high hydrogen content improves the quality of petroleum-based fuels; the process of this hydrogen addition is named hydrogenation. Hydrotreating (HDT) is used to remove oxygen from the bio-oil through hydrodeoxygenation; it is also the most common hydrogenation process used for petroleum-based product conversion [26]. HDT of the bio-oil includes noble and sulfide metals and the most-used transition metal-based catalysts [108,109,110] under atmospheric pressure to 30 MPa and temperatures between 200 and 500 °C.
HDT is performed under different systems reactors such as batch, downflow, and semi-continuous [111] in two steps: the first step consists of the stabilization of the bio-oil under temperatures between 100 and 300 °C to convert the carbonyl and carboxyl groups to alcohols in the presence of catalysts; in the second step, the stabilized bio-oil suffers cracking and deoxygenation reactions under higher temperatures (350–400 °C) and in the existence of active catalysts [31]. Reactions are commonly performed altering parameters such as pressure (hydrogen) and temperature of the reactor, flow rate, and catalyst/bio-oil ratio. These parameters are very critical to reach highly efficient bio-oil upgrading with high calorific values and carbon and less oxygen content, as well as reducing the coke formation on the catalyst to prevent its early deactivation.
Finally, HDT is recognized for its efficiency in bio-oil upgrading compared with other upgrading and separation techniques; however, HDT also produces a huge quantity of char, coke, and tar, resulting in catalyst deactivation and reactor clogging.
5.1.2. Catalytic Cracking/ Hydrocracking
Catalytic cracking (CC) is a thermal upgrading method that consists of the removal of oxygen in the form of water and carbon dioxide through the breakdown of bio-oil into light olefins and low-carbon aromatics similar to diesel in the presence of a catalyst such as zeolite, one of the most highly effective catalysts for the CC upgrading technique, at temperatures higher than 350 °C [14,28].
CC of the bio-oil can be performed in tubular and micro fixed-bed reactors [112,113]. During CC, the removal of oxygen from the bio-oil is caused by the transfer of hydrogen molecules, reaching high contents of hydrogen in the bio-oil; thus, different reactions can occur, including hydrodeoxygenation, hydrocracking, polymerization, decarboxylation, decarbonylation, and hydrogenation [112]. Hydrocracking (a variant of CC) is the combined result of CC with HDT. During hydrocracking, bio-oil vapor reacts with additional hydrogen under high temperatures (>400 °C) in the presence of a catalyst to produce large amounts of light products such as alkanes (a molecular chain that comprises high-grade hydrocarbon fuels), reduce oxygen and water content in the bio-oil to break longer molecular chains (C–C bonds), and reach high conversion yields compared with gasoline fractions (close to 20%) [28]. However, due to the intensive process requirements such as elevated temperature and hydrogen pressure to deal with acids, these routes are less cost-competitive and energy efficient.
5.1.3. Steam Reforming
Steam reforming can be a viable strategy to upgrade the bio-oil to produce H2 gas and syngas (a mixture of CO and H2). Hydrogen created from steam reforming has attracted attention due to its characteristics as a fuel and energy carrier and high energy value content (120.7 MJ/kg). Furthermore, hydrogen-fueled combustion produces water and no harmful gases, reducing the emission of greenhouse gas [29,114].
Steam reforming is an innovative route that consists of the conversion of bio-oil into hydrogen under different operating parameters, such as temperature (400–1000 °C) [31,115], steam/carbon ratio (S/C > 3) [116,117], and catalyst (noble or non-noble metals dispersed on different supports such as ZrO2, Al2O3, HZSM-5, CeO3–Al2O3, and carbon nanotubes) [31,118], which mainly affect the conversion rate of bio-oil, yield of H2, and decrease the coke formation. Rioche et al. [119] represented the overall steam reforming mechanism of different oxygen model compounds (acetic acid, acetone, ethanol, and phenol) in the following equation:
Note that the hydrogen yield is moles per mole of carbon in feed. Considering the fast production of coke, the type of reactor used influences coke removal. Therefore, the steam reforming of bio-oil is performed in reactors such as separate fixed and fluidized bed reactors (more common), combined two-stage pyrolysis-reforming reactors, tubular quartz micro-reactors (Py-GCMSs), membranes, and nozzle-fed reactors [31,120].
Catalytic and non-catalytic steam reforming processes are promising upgrading routes to produce a clean fuel gas (hydrogen or syngas), and studies about catalytic steam reforming have demonstrated significant conversion rates of bio-oil to hydrogen or even yields of more than 90% [31,116,121,122]. However, one of the main concerns of catalytic steam reforming is catalyst deactivation due to the higher temperatures that the process is performed under [120]. During catalytic steam reforming, the catalytic (Ni is the most-used catalyst in steam reforming) can be sintered, affecting the catalytic activity by occupying or blocking the active sites on the catalyst surface [31,120]. The progress of novelty catalysts with diversified behaviors that are less susceptible to deactivation and which are highly efficient is needed for the favorable conversion of bio-oil in the steam reforming route.
5.1.4. Esterification
As mentioned above, bio-oil contains a wide range of oxygenated compounds, low heating value, high viscosity, poor stability, water, and other undesired properties that limit its application into chemicals and transportation fuels. Esterification employs polar alcohol-based solvents (e.g., methanol, ethanol, and furfural) for organic acid conversion to their corresponding esters to improve bio-oil quality. Bio-oil upgraded via esterification reduces acid numbers, viscosity, and water content, whereas it increases the heating value and the stability of the bio-oil to obtain high-grade fuel [26,27,112]. Furthermore, esterification of the bio-oil improves the organic fraction and can remove char particles from bio-oil [123].
On the other hand, catalytic esterification or esterification treatment of the bio-oil reduces viscosity, acid values, and water content at a greater rate than without a catalyst. Heterogeneous catalysts are the most-used catalysts in esterification, including ionic liquid, solid acid and base, HZSM-5, and aluminum silicate catalysts. Different studies have shown that the behavior of the solid acid catalyst is prone to have high catalytic activity to convert organic acids such as acetic, propionic, and formic acid into esters [110,113].
Finally, considering that esterification is a simple unit operation (solvent addition) defined by its low cost, use of green solvents (most of them), and the aforementioned beneficial effects on the bio-oil, this route seems to be the most feasible upgrading technique for bio-oil quality improvement.
5.2. Physical Processes
Fossil fuel resources indicate uncertain availability, volatile prices, and environmental concerns; therefore, the use of biomass has stimulated the fractionation of chemicals from bio-oil. The separation routes are utilized by techniques such as atmospheric distillation, vacuum distillation, molecular distillation, supercritical fluids, and liquid–liquid extraction, among others.
5.2.1. Distillation
As mentioned above about bio-oil properties, there are more than 400 compounds produced in the bio-oil through the pyrolysis of biomass. Different distillation techniques have been researched for the recovery of these compounds, such as atmospheric, vacuum, and molecular distillation [5,32].
Atmospheric distillation is used to obtain fine chemicals from pyrolytic bio-oil and can be performed at temperatures between 80 and 250 °C with no waste generation [29]. In atmospheric distillation, with the increase in temperature to 250 °C, the reaction between the components produces water, alcohols, aldehydes, and acids that are more favorable than phenols due to their instability at high heating rates [32]. However, at higher temperatures, polymerization of reactive oxygen-containing organics (ketones, aromatics, aldehydes, etc.) can take place in atmospheric distillation [43]. Thus, pyrolyzed bio-oil cannot be completely vaporized, leaving around 40-50% of the raw pyrolytic bio-oil. Therefore, a considerable portion of the bio-oil remains as a residue.
The distillate contains water and a mixture of volatile organic compounds that requires further separation; a possible route to separate it into different fractions is by using lower temperatures. Vacuum distillation is commonly used when lowering the distillation temperature.
Although vacuum distillation is a more challenging, expensive, energy-intensive operation than atmospheric distillation, this process is operated at low temperatures (normally for liquids that have boiling points over 150 °C), avoiding the polymerization of pyrolytic bio-oil and the degradation and aging of thermally sensitive compounds [5,29]. Furthermore, in contrast to atmospheric distillation, no water is generated, and the distillate (organic fraction) contains low-oxygenated compounds [32].
Several studies show relevant results employing vacuum distillation. Lu, Yang, and Zhu [124] compared the atmospheric and vacuum distillation of pyrolytic bio-oil from rice husk under different temperatures from 50 to 250 °C. Vacuum distillation showed a significant reduction from 27.5 to 32 wt. % of the raw pyrolytic bio-oil [124]. The study performed by Zhang et al. [125] showed outstanding efficient separation of light molecules such as hydroxy acetone and formic and acetic acid [125].
Molecular distillation, also termed short path distillation, is a variation of vacuum distillation [32] in which, differently from vacuum distillation, closed boiling and thermally unstable components present on the bio-oil are fractioned under a high vacuum (pressures of 10−6 times or less atmospheric pressure) [5].
Molecular distillation is an excellent fractionate technique due to its low distillation temperature, heating rates, and high fractionation efficiency. Several studies have shown that high yields of organic molecules in bio-oil were recovered via molecular distillation into light (acids and ketones), middle (sugars and phenolics), and heavy (heavy phenols) fractions [29,43]; water can also be separated at a temperature as low as 50 °C. Multiple molecular distillations can produce a fourth fraction, mainly composed of chemicals including aldehydes, phenols, and sugars [30].
5.2.2. Supercritical Fluid Extraction
A novel route to upgrade pyrolytic bio-oil is the use of supercritical fluids. Any pure compound can be considered a supercritical fluid when its critical point is ultra-passed (temperature and pressure). Different organic solvent-based can be used for supercritical fluid extraction; however, CO2 is used due to its behavior similar to non-polar hydrocarbon solvents (hexane, methanol, acetone, petroleum ether, among others). Therefore, CO2 can be applied as an excellent solvent because of its unique advantages: it is chemically inert, non-flammable, non-toxic and environmentally friendly, liquefiable at low temperatures and pressures, has no solvent residue, is readily available, and inexpensive [5,32].
Solubility is a crucial physical property to be considered in supercritical CO2 (scCO2) extraction. Studies have shown that scCO2 has good solubility in some chemicals such as aliphatic hydrocarbons up to at least C20, aldehydes, ketones, esters, small aromatic hydrocarbons, halocarbons, and low-weight alcohols [5,9,10].
Feng and Meier [11] employed scCO2 to separate bio-oil produced from softwood under different conditions (temperature, pressure, carriers, time). The authors showed that the yields reached were 6–13 wt. % at 200 bar and 13–14 wt. % at 300 bar; additionally, the contents of the scCO2 extracts were mainly aldehydes, furanones, coniferyl alcohol, coniferyl aldehyde, and vanillin. Wang et al. [126] used scCO2 to fractionate real and simulated bio-oil, and results showed that aldehyde, ketone, and phenol functional groups increased in the scCO2 extracts. The maximum extraction yield of bio-oil using scCO2 reached 88.6 wt. %, and the extracts had almost 100% volatility.
Therefore, scCO2 extraction proved to be a promising and potential route to extract chemicals from bio-oil; however, scCO2 extraction parameters and the distribution coefficient of single compounds need to be optimized to improve the selectivity of the extraction. Furthermore, there is a high operating cost due to the high pressures required in scCO2 extraction (usually >150 bar), showing significant drawbacks when compared with other separation techniques such as distillation, membrane separation, and liquid–liquid extraction [5].
5.2.3. Liquid–Liquid Extraction
Liquid–liquid extraction (LLE) has been studied to fractionate pyrolytic bio-oil into different chemical groups using organic or novel solvents such as water, pentane, hexane, toluene, ether, dichloromethane (DCM), ethyl acetate, methanol, ethanol, acetone, and ionic liquids [5,29,32]. LLE can be performed according to its polarity and solubility for specific chemical compounds and operated under normal conditions, making it a promising technique to perform extractions. However, solvents are based on equilibrium and must satisfy some criteria: high selectivity, good solubility, low boiling point for subsequent recovery, and non-toxic if possible [5].
Water solvent extraction is normally the first step in LLE [127]. Several works have proved that the addition of 25–35% of water into the raw bio-oil produces two phases: a water-soluble phase which includes chemicals delivered from cellulose and hemicellulose such as sugars and light C1−C4 organic compounds [32], and a water-insoluble phase which contains heavy tar-like, lignin rich, compounds.
Organic solvent extraction also originates soluble and non-soluble phases; organic compounds are usually soluble in non-polar solvents (DCM, hexane, toluene, and methanol). Therefore, the polarity of the fractions is a parameter that needs to be addressed in LLE. Furthermore, the solvent’s boiling point also performs a key role in LLE to recover the solvent afterward using different methods (e.g., rotary evaporation).
Cascade LLE has been performed by several researchers to obtain different fractions of bio-oil using different organic solvents [128,129,130]. Water was added first to fractionate the bio-oil. The water-insoluble fraction consisted of phenolics, aromatics, and pyrolytic lignin compounds; it was washed with DCM and NaOH (pH = 14) to obtain a neutral fraction (inert hydrocarbons). HCl was mixed with the DCM-insoluble fraction to obtain heavy molecular weight pyrolytic lignin and solids. Alternatively, the water-soluble fraction mainly consisted of acids, sugars, ketones, aldehydes, and polar carbohydrates; this fraction was solubilized in chloroform. The chloroform-soluble fraction (organic phase) contained phenols, furans, alcohols, and ketones. The chloroform-insoluble fraction (aqueous phase) was rich in acids, sugars, alcohols, and some polar compounds. Therefore, the chloroform-insoluble fraction was solubilized in ethyl acetate to obtain acids, ketones, and some alcohols. The ethyl acetate-insoluble fraction obtained rich anhydrosugars and was mainly composed of levoglucosan [5,29,32,131].
5.2.4. Emulsification
Another route to upgrade the pyrolytic bio-oil for production as an ignition fuel in boilers and transportation is emulsification. Bio-oil can be emulsified with either petroleum-based fuels (diesel) or biodiesel and with the help of polymeric or non-polymeric surfactants such as Atlox 4914, Tween 80, Span 80, and Emarol 85. Surfactants consist of a nonpolar lipophilic ‘tail’ and a polar hydrophilic ‘head’ [30,31,132,133]; co-surfactants such as methanol, ethanol, butanol, and octanol can also be used to improve the emulsion stability [133].
A high-quality upgraded bio-oil (longer stability duration) via stable emulsion can be obtained by altering temperature, diesel-biodiesel/bio-oil, and emulsifier/surfactant ratios; mixing time; and stirring intensity, whereas unstable emulsions conduct to a poor-quality upgraded bio-oil [31].
Novel technologies have been proven effective in producing stable emulsion fuels, such as microemulsification, ultrasonic emulsification, ultrasonic mechanical-based emulsification, and pressurized emulsification [30,134]. For instance, a microemulsion is very similar to an emulsion; however, it is thermodynamically more stable and, therefore, holds less risk of phase separation at a broad temperature range.
Therefore, bio-oil upgraded via emulsification is a classic and cost-effective route providing a transient approach to dealing in diesel engines and reducing the emissions of some pollutants. Emulsions of bio-oil have potential ignition characteristics; nonetheless, there are some fuel property issues that remain unclear, e.g., the heating value, octane number, stability, and acidity (corrosivity) of the fuel system. The design, production, and testing of injectors, fuel pumps made from stainless steel, pretreatment of bio-oil, and optimization of the use of base oil would be some strategies to improve this process [26,112].
6. Environmental and Economic Aspects of Bio-Oil
Upgradation improves the properties of bio-oil, making it a potential and preferred alternative to conventional petroleum-derived liquid fuels [13]. There are different approaches to bio-oil upgrading, but each method possesses techno-economic obstacles hindering the industrial deployment of its production [12,135]. Yet, even if a perfect liquid substitute for conventional petroleum is developed, the production and utilization of bio-oil should offer fewer negative environmental consequences than conventional petroleum fuels [136]. Life-cycle assessment (LCA) has been foremostly adopted by past researchers as a tool to check the environmental impacts of bio-oil production via the pyrolysis process [136]. The majority of LCA studies for the pyrolysis process were performed by considering 1 MJ of bio-oil produced as a functional unit, cradle-to-grave as the boundary limit, and with prime emphasis on global warming potential in the impact category [136,137,138,139]. Some of the prior research studies on LCA are presented in Table 4 (dry feed rate reflects less than 10 wt. % of moisture content).

Table 4.
LCA of hydro-upgraded bio-oil production via fast pyrolysis.
For bio-oil upgradation, majorly conducted via hydro-upgrading (hydrotreatment followed by hydrocracking), the source of hydrogen plays a pivotal role in contributing to detrimental environmental impacts [136,137,138,139,140,141,142]. Usually, either natural gas or aqueous bio-oil fraction is used to produce hydrogen, and utilizing an aqueous fraction of bio-oil for steam reforming to produce hydrogen could reduce environmental impacts, but at the cost of decrement in the upgraded bio-oil yield [138]. The bio-oil upgradation sub-section, on average, contributed about 22 to 25% of positive global warming potential [141,142]. For bio-oil application in engines, the use of the cradle-to-gate boundary limit led to negative net global warming potential, whereas the cradle-to-grave approach resulted in a positive value, as emissions from the combustion of bio-oil are also considered. The literature lacks studies on the life-cycle assessment of co-pyrolysis of biomass with hydrogen-rich feedstocks such as plastics and tires, one of the promising approaches to produce upgraded bio-oil.
The shortage of crude oil in several countries and the inevitable upheaval due to its increasing and fluctuating market price create attraction toward cost-competitive alternatives [144]. For bio-oil production through pyrolysis, techno-economic analysis (TEA) has been performed to evaluate its potential to be commercialized [135]. Aspen Plus is the most widely used software for process modeling and simulation to assess insights on factors influencing the economic viability of the pyrolysis process, and a few of them are presented in Table 5 (dry feed rate reflects less than 10 wt. % of moisture content) [145,146,147,148,149].

Table 5.
TEA studies on fast pyrolysis to produce hydro-upgraded bio-oil.
Uncertainty analysis, majorly conducted via the Monte-Carlo method, reflected that the less costly option provides lower energy efficiency than the expensive one, and sensitivity analysis reflected capital cost, biomass cost, bio-oil yield, operating cost (including catalysts), and hydrogen cost used for upgradation as sensitive parameters [146,148,149]. However, the bio-oil upgradation subsection contributes remarkably to the value of the minimum fuel selling price (MFSP) that lies within the range of USD/GGE 6 to USD/GGE 8 [145,146,147,148,149]. Extensive research on the production of low-cost and stable catalysts to produce upgraded bio-oil is being conducted, which would play a significant role in reducing liquid fuel production costs [148]. There is a need to explore the techno-economic analysis of the co-pyrolysis of biomass with hydrogen-rich feedstocks that could potentially replace the demand for external hydrogen derived via the steam reforming of natural gas or the bio-oil itself for bio-oil upgradation [21]. Thus, advances in bio-oil upgradation technologies would consequently result in dwindling economic hurdles in bio-oil commercialization.
7. Applications of Upgraded Bio-Oil
One of the most known applications of upgraded bio-oil is as a biofuel, and it can either be used in boilers directly or blended with conventional fuels before injecting it into the boiler/I.C. engines for heat or power generation [29]. Bio-oil use as biofuel produces relatively less NOx, SOx, and CO2 emissions than conventional fossil fuel burning [142,144,145]. However, co-feeding 10–30 vol.% of bio-oil in engines is preferable. Therefore, advancements in the design and materials of the conventional engine (incur costs) are needed that impede its large-scale application in the long-term perspective [6].
Besides the notorious application of upgraded bio-oil as biofuel, it has the potential to be used diversely as a renewable feedstock to produce chemicals, hydrogen, carbonaceous products, binders, bioplastics, and polyurethane foam, which face several obstacles while raw bio-oil is used [143,147,148,149,150]. Hydrogen production via steam reforming of bio-oil still involves several technological (such as the absence of robust, low-cost catalyst) and economical (20 to 40 USD /GJ) challenges that inhibit its commercialization [151]. Bio-oil could also be used as a precursor for chemical production via distillation (atmospheric/vacuum/molecular) or solvent extraction; however, reactive oxygenated compounds present in it (that polymerize to form coke) and the high cost of solvents creates techno-economic barriers [29]. Polyurethane foam could also be produced using bio-oil, but the high-water content in bio-oil is responsible for the decrement in the mechanical strength of polyurethane produced from it, which limits its fraction in the feed with conventional petroleum to produce polyurethane [29,149]. Additionally, bio-oil could be used to produce bio-binders to replace petroleum-derived binders, but the high fraction of oxygen, phenolics, and low carbon content in the bio-binder create difficulties (enhanced aging) in its application, and if mixing it with conventional binders, there is need to explore the optimum ratio of blending [29,152]. Some of the studies on the possible applications of bio-oil are discussed in Error! Reference source not found. Thus, technological barriers in the path of potential applications of bio-oil that are in the early stage could be resolved with advancements in the production and upgradation techniques that still require extensive research to make them technically and economically practicable (Table 6).

Table 6.
Studies of possible applications of bio-oil.
8. Future Directions
In summary, bio-oil is not considered a drop-in fuel yet. The reason is mainly related to the cost-competitiveness of bio-oil under any coupling upgrading technique. Thus, it is clear that there is an essential need for dedicated policies to decrease the operating and capital costs to further the commercialization and development of bio-oil. Some directions and recommendations are described as follows:
- Enhancing the properties and yield of pyrolytic bio-oil by handling biomasses and different operating conditions.
- Exploring novel catalyst synthesis mainly in the field of bi-functional, multifunctional, and biochar-based catalysts to be used as upgrading catalysts of the bio-oil in an integrated system process.
- Understanding the behavior and kinetics of the mechanism reaction, including hydrotreating, catalytic cracking, steam reforming, and esterification.
- Improving economically and environmentally suitable technologies for valuable chemical extraction from bio-oil.
- Exploring spectral and chemical characterization of bio-oil, upgrading, and fractionation products using novel advances to understand several complexities of each upgrading technique.
9. Conclusions
This review presented a detailed analysis on the available upgrading techniques to produce valuable chemicals and biofuels. In particular, novel upgrading techniques lead to the production of bio-oil as biofuel, and the fractionation techniques lead to the production of valuable chemicals. Based on the technical, environmental, and economic aspects, the review shows that hydrotreating looks like the most promising upgrading technique for biofuel application, whereas LLE is a more cost-effective route to obtain chemicals. Most of the current upgrading techniques have the potential to produce bio-oil as a preferred alternative to conventional petroleum-derived chemical and liquid fuels.
Author Contributions
D.L.-P.: Writing and review and editing; J.C.M.-V.: Writing and review and editing; J.M.: Writing; K.A.: Writing; O.K.S.: Writing; S.K.T.: Writing and review and editing; A.R.C.M.: Writing and review and editing; E.H.T.: Writing and review and editing; D.A.B.: Writing and review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
D.L.-P. and D.A.B. are grateful for the financial support and scholarships of the Human Resources Program of the National Agency for Petroleum, Natural Gas and Biofuels—PRH-ANP through the Human Resources Training Program for Petroleum and Biofuels Processing (PRH 52.1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are given in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lennan, M.; Morgera, E. The Glasgow Climate Conference (COP26). Int. J. Mar. Coast. Law 2022, 37, 137–151. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Shi, L.; Liu, Q. Pyrolysis of COVID-19 disposable masks and catalytic cracking of the volatiles. J. Anal. Appl. Pyrolysis 2022, 163, 105481. [Google Scholar] [CrossRef] [PubMed]
- Toscano Miranda, N.; Lopes Motta, I.; Maciel Filho, R.; Wolf Maciel, M.R. Sugarcane bagasse pyrolysis: A review of operating conditions and products properties. Renew. Sustain. Energy Rev. 2021, 149, 111394. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; César Torres-Mayanga, P.; Abaide, E.R.; Zabot, G.L.; De Castilhos, F. Hydrothermal carbonization and Liquefaction: Differences, progress, challenges, and opportunities. Bioresour. Technol. 2022, 343, 126084. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y. Fractionation of Pyrolysis Liquids with Supercritical Carbon Dioxide. Ph.D. Thesis, Staats-und Universitätsbibliothek Hamburg Carl von Ossietzky, Hamburg, Germany, 2018. [Google Scholar]
- Lehto, J.; Oasmaa, A.; Solantausta, Y.; Kytö, M.; Chiaramonti, D. Review of fuel oil quality and combustion of fast pyrolysis bio-oils from lignocellulosic biomass. Appl. Energy 2014, 116, 178–190. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.K.; Sivashanmugam, P.; Kavitha, S.; Kannah, Y.; Varjani, S.; AdishKumar, S.; Kumar, G.B. Lignocellulosic biomass-based pyrolysis: A comprehensive review. Chemosphere 2022, 286, 131824. [Google Scholar] [CrossRef]
- De Jong, E.; Higson, A.; Walsh, P.; Wellisch, M. Task 42 Biobased Chemicals—Value Added Products from Biorefineries. 2011. Available online: https://www.ieabioenergy.com/blog/publications/bio-based-chemicals-value-added-products-from-biorefineries/ (accessed on 7 December 2022).
- Feng, Y.; Meier, D. Comparison of supercritical CO2, liquid CO2, and solvent extraction of chemicals from a commercial slow pyrolysis liquid of beech wood. Biomass Bioenergy 2016, 85, 346–354. [Google Scholar] [CrossRef]
- Feng, Y.; Meier, D. Extraction of value-added chemicals from pyrolysis liquids with supercritical carbon dioxide. J. Anal. Appl. Pyrolysis 2015, 113, 174–185. [Google Scholar] [CrossRef]
- Feng, Y.; Meier, D. Supercritical carbon dioxide extraction of fast pyrolysis oil from softwood. J. Supercrit. Fluids 2017, 128, 6–17. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Julson, J.; Rabnawaz, M. Upgrading pyrolysis bio-oil through hydrodeoxygenation (HDO) using non-sulfided Fe-Co/SiO2 catalyst. Energy Convers. Manag. 2017, 150, 331–342. [Google Scholar] [CrossRef]
- Panwar, N.L.; Paul, A.S. An overview of recent development in bio-oil upgrading and separation techniques. Environ. Eng. Res. 2021, 26, 200382. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, Y.; Liu, S.; Zhou, N.; Chen, P.; Cheng, Y.; Addy, M.; Lu, Q.; Omar, M.M.; Liu, Y.; et al. Bio-oil from fast pyrolysis of lignin: Effects of process and upgrading parameters. Bioresour. Technol. 2017, 241, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Saleem, M.; Shahzad, K.; Hussain, S.; Chughtai, A. Effect of operating parameters on production of bio-oil from fast pyrolysis of maize stalk in bubbling fluidized bed reactor. Polish J. Chem. Technol. 2016, 18, 88–96. [Google Scholar] [CrossRef]
- Choudhury, N.D.; Chutia, R.S.; Bhaskar, T.; Kataki, R. Pyrolysis of jute dust: Effect of reaction parameters and analysis of products. J. Mater. Cycles Waste Manag. 2014, 16, 449–459. [Google Scholar] [CrossRef]
- Varma, A.K.; Thakur, L.S.; Shankar, R.; Mondal, P. Pyrolysis of wood sawdust: Effects of process parameters on products yield and characterization of products. Waste Manag. 2019, 89, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Puy, N.; Murillo, R.; Navarro, M.V.; López, J.M.; Rieradevall, J.; Fowler, G.; Aranguren, I.; García, T.; Bartrolí, J.; Mastral, A.M. Valorisation of forestry waste by pyrolysis in an auger reactor. Waste Manag. 2011, 31, 1339–1349. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Z.; Tian, X.; Dai, L.; Jiang, L.; Zhang, S.; Wu, Q.; Wen, P.; Fu, G.; Liu, Y.; et al. Production of bio-oil from agricultural waste by using a continuous fast microwave pyrolysis system. Bioresour. Technol. 2018, 269, 162–168. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Zhou, N.; Dai, L.; Deng, W.; Liu, C.; Cheng, Y.; Liu, Y.; Cobb, K.; Chen, P.; et al. Applications of calcium oxide–based catalysts in biomass pyrolysis/gasification—A review. J. Clean. Prod. 2021, 291, 125826. [Google Scholar] [CrossRef]
- Singh, M.; Salaudeen, S.A.; Gilroyed, B.H.; Al-Salem, S.M.; Dutta, A. A review on co-pyrolysis of biomass with plastics and tires: Recent progress, catalyst development, and scaling up potential. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Ryu, H.W.; Kim, D.H.; Jae, J.; Lam, S.S.; Park, E.D.; Park, Y.K. Recent advances in catalytic co-pyrolysis of biomass and plastic waste for the production of petroleum-like hydrocarbons. Bioresour. Technol. 2020, 310, 123473. [Google Scholar] [CrossRef]
- Missau, J.; Bertuol, D.A.; Tanabe, E.H. Charcoal Briquetting: An Environmentally Friendly Destination for Waste Materials. Environ. Eng. Sci. 2021, 38, 841–853. [Google Scholar] [CrossRef]
- Missau, J.; Bertuol, D.A.; Tanabe, E.H. Highly efficient adsorbent for removal of Crystal Violet Dye from Aqueous Solution by CaAl/LDH supported on Biochar. Appl. Clay Sci. 2021, 214, 106297. [Google Scholar] [CrossRef]
- Kamali, M.; Sweygers, N.; Al-Salem, S.; Appels, L.; Aminabhavi, T.M.; Dewil, R. Biochar for soil applications-sustainability aspects, challenges and future prospects. Chem. Eng. J. 2022, 428, 131189. [Google Scholar] [CrossRef]
- Drugkar, K.; Rathod, W.; Sharma, T.; Sharma, A.; Joshi, J.; Pareek, V.K.; Ledwani, L.; Diwekar, U. Advanced separation strategies for up-gradation of bio-oil into value-added chemicals: A comprehensive review. Sep. Purif. Technol. 2022, 283, 120149. [Google Scholar] [CrossRef]
- Kumar, R.; Strezov, V. Thermochemical production of bio-oil: A review of downstream processing technologies for bio-oil upgrading, production of hydrogen and high value-added products. Renew. Sustain. Energy Rev. 2021, 135, 110152. [Google Scholar] [CrossRef]
- Pinheiro Pires, A.P.; Arauzo, J.; Fonts, I.; Domine, M.E.; Fernández Arroyo, A.; Garcia-Perez, M.E.; Montoya, J.; Chejne, F.; Pfromm, P.; Garcia-Perez, M. Challenges and opportunities for bio-oil refining: A review. Energy Fuels 2019, 33, 4683–4720. [Google Scholar] [CrossRef]
- Gupta, S.; Mondal, P.; Borugadda, V.B.; Dalai, A.K. Advances in upgradation of pyrolysis bio-oil and biochar towards improvement in bio-refinery economics: A comprehensive review. Environ. Technol. Innov. 2021, 21, 101276. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Progress of the applications of bio-oil. Renew. Sustain. Energy Rev. 2020, 134, 110124. [Google Scholar] [CrossRef]
- Hansen, S.; Mirkouei, A.; Diaz, L.A. A comprehensive state-of-technology review for upgrading bio-oil to renewable or blended hydrocarbon fuels. Renew. Sustain. Energy Rev. 2020, 118, 109548. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Reddy, M.; Subramanyam, M.D.; Kishore, N. A review on the upgradation techniques of pyrolysis oil. Renew. Sustain. Energy Rev. 2016, 58, 1543–1568. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A. Bio-oil production and upgrading research: A review. Renew. Sustain. Energy Rev. 2012, 16, 4406–4414. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Reyes, C.D.G. Acidic ionic liquid catalyzed liquefactions of corn cobs and switchgrass in acetone: Analysis of bio-oils using LC-MS and GC-MS. J. Anal. Appl. Pyrolysis 2020, 145, 104752. [Google Scholar] [CrossRef]
- Chan, Y.H.; Loh, S.K.; Chin, B.L.F.; Yiin, C.L.; How, B.S.; Cheah, K.W.; Wong, M.K.; Loy, A.C.M.; Gwee, Y.L.; Lo, S.L.Y.; et al. Fractionation and extraction of bio-oil for production of greener fuel and value-added chemicals: Recent advances and future prospects. Chem. Eng. J. 2020, 397, 125406. [Google Scholar] [CrossRef]
- Zhou, Y.; Remón, J.; Gracia, J.; Jiang, Z.; Pinilla, J.L.; Hu, C.; Suelves, I. Toward developing more sustainable marine biorefineries: A novel ‘sea-thermal’ process for biofuels production from microalgae. Energy Convers. Manag. 2022, 270, 116201. [Google Scholar] [CrossRef]
- Su, G.; Ong, H.C.; Gan, Y.Y.; Chen, W.H.; Chong, C.T.; Ok, Y.S. Co-pyrolysis of microalgae and other biomass wastes for the production of high-quality bio-oil: Progress and prospective. Bioresour. Technol. 2022, 344, 126096. [Google Scholar] [CrossRef]
- Lyu, G.; Wu, S.; Zhang, H. Estimation and comparison of bio-oil components from different pyrolysis conditions. Front. Energy Res. 2015, 3, 28. [Google Scholar] [CrossRef]
- Seo, M.W.; Lee, S.H.; Nam, H.; Lee, D.; Tokmurzin, D.; Wang, S.; Park, Y.K. Recent advances of thermochemical conversieon processes for biorefinery. Bioresour. Technol. 2022, 343, 126109. [Google Scholar] [CrossRef]
- Oh, S.; Lee, J.; Lam, S.S.; Kwon, E.E.; Ha, J.M.; Tsang, D.C.W.; Ok, Y.S.; Chen, W.H.; Park, Y.K. Fast hydropyrolysis of biomass Conversion: A comparative review. Bioresour. Technol. 2021, 342, 126067. [Google Scholar] [CrossRef]
- Lee, D.J.; Lu, J.S.; Chang, J.S. Pyrolysis synergy of municipal solid waste (MSW): A review. Bioresour. Technol. 2020, 318, 123912. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, J.; Wang, T.; Xu, Y. Review of biomass pyrolysis oil properties and upgrading research. Energy Convers. Manag. 2007, 48, 87–92. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A.V. Overview of applications of biomass fast pyrolysis oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Agblevor, F.A.; Mante, O.; McClung, R.; Oyama, S.T. Co-processing of standard gas oil and biocrude oil to hydrocarbon fuels. Biomass Bioenergy 2012, 45, 130–137. [Google Scholar] [CrossRef]
- Peacocke, G.V.C.; Bridgwater, A.V. Ablative plate pyrolysis of biomass for liquids. Biomass Bioenergy 1994, 7, 147–154. [Google Scholar] [CrossRef]
- Peacocke, G.V.C.; Russell, P.A.; Jenkins, J.D.; Bridgwater, A.V. Physical properties of flash pyrolysis liquids. Biomass Bioenergy 1994, 7, 169–177. [Google Scholar] [CrossRef]
- Zerva, C.; Karakoulia, S.A.; Kalogiannis, K.G.; Margellou, A.; Iliopoulou, E.F.; Lappas, A.A.; Papayannakos, N.; Triantafyllidis, K.S. Hydrodeoxygenation of phenol and biomass fast pyrolysis oil (bio-oil) over Ni/WO3-ZrO2 catalyst. Catal. Today 2021, 366, 57–67. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification and Pyrolysis: Practical design and theory; Academic press: Cambridge, MA, USA, 2012; Volume 5, ISBN 9780080878737. [Google Scholar]
- Zhang, J.; Zhang, X. The thermochemical conversion of biomass into biofuels. In Biomass, Biopolymer-Based Materials, and Bioenergy; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 327–368. ISBN 9780081024263. [Google Scholar]
- Fahmy, T.Y.A.; Fahmy, Y.; Mobarak, F.; El-Sakhawy, M.; Abou-Zeid, R.E. Biomass pyrolysis: Past, present, and future. Environ. Dev. Sustain. 2020, 22, 17–32. [Google Scholar] [CrossRef]
- Tursi, A. A review on biomass: Importance, chemistry, classification, and conversion. Biofuel Res. J. 2019, 6, 962–979. [Google Scholar] [CrossRef]
- Gagić, T.; Perva-Uzunalić, A.; Knez, Ž.; Škerget, M. Hydrothermal Degradation of Cellulose at Temperature from 200 to 300 °C. Ind. Eng. Chem. Res. 2018, 57, 6576–6584. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Räisänen, U.; Pitkänen, I.; Halttunen, H.; Hurtta, M. Formation of the main degradation compounds from arabinose, xylose, mannose and arabinitol during pyrolysis. J. Therm. Anal. Calorim. 2003, 72, 481–488. [Google Scholar] [CrossRef]
- Kawamoto, H. Lignin pyrolysis reactions. J. Wood Sci. 2017, 63, 117–132. [Google Scholar] [CrossRef]
- Wang, Y.; Akbarzadeh, A.; Chong, L.; Du, J.; Tahir, N.; Awasthi, M.K. Catalytic pyrolysis of lignocellulosic biomass for bio-oil production: A review. Chemosphere 2022, 297, 134181. [Google Scholar] [CrossRef] [PubMed]
- Martins-Vieira, J.C.; Torres-Mayanga, P.C.; Lachos-Perez, D. Hydrothermal Processing of Lignocellulosic Biomass: An Overview of Subcritical and Supercritical Water Hydrolysis. Bioenergy Res. 2022. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Zhuang, X.; Gan, Z.; Zhou, J.; Zhang, Y.; Zhang, H. Insight into biomass pyrolysis mechanism based on cellulose, hemicellulose, and lignin: Evolution of volatiles and kinetics, elucidation of reaction pathways, and characterization of gas, biochar and bio-oil. Combust. Flame 2022, 242, 112142. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. In situ fast pyrolysis of biomass with zeolite catalysts for bioaromatics/gasoline production: A review. Energy Convers. Manag. 2015, 105, 338–354. [Google Scholar] [CrossRef]
- Hoang, A.T.; Ong, H.C.; Fattah, I.M.R.; Chong, C.T.; Cheng, C.K.; Sakthivel, R.; Ok, Y.S. Progress on the lignocellulosic biomass pyrolysis for biofuel production toward environmental sustainability. Fuel Process. Technol. 2021, 223, 106997. [Google Scholar] [CrossRef]
- Madadi, M.; Bakr, M.M.A.; Abdulkhani, A.; Zahoor; Asadollahi, M.A.; Sun, C.; Sun, F.; Abomohra, A.E.F. Alleviating lignin repolymerization by carbocation scavenger for effective production of fermentable sugars from combined liquid hot water and green-liquor pretreated softwood biomass. Energy Convers. Manag. 2022, 251, 114956. [Google Scholar] [CrossRef]
- Zhao, A.; Liu, S.; Yao, J.; Huang, F.; He, Z.; Liu, J. Characteristics of bio-oil and biochar from cotton stalk pyrolysis: Effects of torrefaction temperature and duration in an ammonia environment. Bioresour. Technol. 2022, 343, 126145. [Google Scholar] [CrossRef]
- Fu, J.; Liu, J.; Xu, W.; Chen, Z.; Evrendilek, F.; Sun, S. Torrefaction, temperature, and heating rate dependencies of pyrolysis of coffee grounds: Its performances, bio-oils, and emissions. Bioresour. Technol. 2022, 345, 126346. [Google Scholar] [CrossRef]
- Sohaib, Q.; Muhammad, A.; Younas, M. Fast pyrolysis of sugarcane bagasse: Effect of pyrolysis conditions on final product distribution and properties. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 184–190. [Google Scholar] [CrossRef]
- Lin, B.J.; Chen, W.H. Sugarcane bagasse pyrolysis in a carbon dioxide atmosphere with conventional and microwave-assisted heating. Front. Energy Res. 2015, 3, 4. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lee, M.K.; Chang, Y.M. Fast pyrolysis of rice husk: Product yields and compositions. Bioresour. Technol. 2007, 98, 22–28. [Google Scholar] [CrossRef]
- Biswas, B.; Pandey, N.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Pyrolysis of agricultural biomass residues: Comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour. Technol. 2017, 237, 57–63. [Google Scholar] [CrossRef]
- Alvarez, J.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Bio-oil production from rice husk fast pyrolysis in a conical spouted bed reactor. Fuel 2014, 128, 162–169. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, R.; Zhao, B.; Li, Y.; Wang, S.; Wu, H.; Zhuo, Y.; Chen, C. Investigation of heat of biomass pyrolysis and secondary reactions by simultaneous thermogravimetry and differential scanning calorimetry. Fuel 2014, 134, 467–476. [Google Scholar] [CrossRef]
- Gonçalves, E.V.; Seixas, F.L.; de Souza Scandiuzzi Santana, L.R.; Scaliante, M.H.N.O.; Gimenes, M.L. Economic trends for temperature of sugarcane bagasse pyrolysis. Can. J. Chem. Eng. 2017, 95, 1269–1279. [Google Scholar] [CrossRef]
- Veses, A.; Aznar, M.; Martínez, I.; Martínez, J.D.; López, J.M.; Navarro, M.V.; Callén, M.S.; Murillo, R.; García, T. Catalytic pyrolysis of wood biomass in an auger reactor using calcium-based catalysts. Bioresour. Technol. 2014, 162, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Fakayode, O.A.; Aboagarib, E.A.A.; Zhou, C.; Ma, H. Co-pyrolysis of lignocellulosic and macroalgae biomasses for the production of biochar—A review. Bioresour. Technol. 2020, 297, 122408. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Qin, J.; He, Q.; Wen, Y.; Huang, S.; Li, B.; Hu, J.; Zhou, N.; Zhou, Z. Torrefied herb residues in nitrogen, air and oxygen atmosphere: Thermal decomposition behavior and pyrolytic products characters. Bioresour. Technol. 2021, 342, 125991. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Z.; Li, J.; Yan, B.; Chen, G. Pyrolysis of food waste and food waste solid digestate: A comparative investigation. Bioresour. Technol. 2022, 354, 127191. [Google Scholar] [CrossRef]
- Akhtar, J.; Saidina Amin, N. A review on operating parameters for optimum liquid oil yield in biomass pyrolysis. Renew. Sustain. Energy Rev. 2012, 16, 5101–5109. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Shujaa Aldeen, A.; Mwenya, S.; Cheng, H.; Xu, Z.; Zhang, H. Enhancing production of hydrocarbon-rich bio-oil from biomass via catalytic fast pyrolysis coupled with advanced oxidation process pretreatment. Bioresour. Technol. 2022, 359, 127450. [Google Scholar] [CrossRef] [PubMed]
- Şensöz, S.; Demiral, I.; Gerçel, H.F. Olive bagasse (Olea europea L.) pyrolysis. Bioresour. Technol. 2006, 97, 429–436. [Google Scholar] [CrossRef]
- Morali, U.; Şensöz, S. Pyrolysis of hornbeam shell (Carpinus betulus L.) in a fixed bed reactor: Characterization of bio-oil and bio-char. Fuel 2015, 150, 672–678. [Google Scholar] [CrossRef]
- Bhoi, P.R.; Ouedraogo, A.S.; Soloiu, V.; Quirino, R. Recent advances on catalysts for improving hydrocarbon compounds in bio-oil of biomass catalytic pyrolysis. Renew. Sustain. Energy Rev. 2020, 121, 109676. [Google Scholar] [CrossRef]
- Xiong, Z.; Wang, Y.; Syed-Hassan, S.S.A.; Hu, X.; Han, H.; Su, S.; Xu, K.; Jiang, L.; Guo, J.; Berthold, E.E.S.; et al. Effects of heating rate on the evolution of bio-oil during its pyrolysis. Energy Convers. Manag. 2018, 163, 420–427. [Google Scholar] [CrossRef]
- Carrier, M.; Hugo, T.; Gorgens, J.; Knoetze, H. Comparison of slow and vacuum pyrolysis of sugar cane bagasse. J. Anal. Appl. Pyrolysis 2011, 90, 18–26. [Google Scholar] [CrossRef]
- Wan Mahari, W.A.; Nam, W.L.; Sonne, C.; Peng, W.; Phang, X.Y.; Liew, R.K.; Yek, P.N.Y.; Lee, X.Y.; Wen, O.W.; Show, P.L.; et al. Applying microwave vacuum pyrolysis to design moisture retention and pH neutralizing palm kernel shell biochar for mushroom production. Bioresour. Technol. 2020, 312, 123572. [Google Scholar] [CrossRef]
- Amutio, M.; Lopez, G.; Aguado, R.; Artetxe, M.; Bilbao, J.; Olazar, M. Effect of vacuum on lignocellulosic biomass flash pyrolysis in a conical spouted bed reactor. Energy Fuels 2011, 25, 3950–3960. [Google Scholar] [CrossRef]
- Jae, J.; Coolman, R.; Mountziaris, T.J.; Huber, G.W. Catalytic fast pyrolysis of lignocellulosic biomass in a process development unit with continual catalyst addition and removal. Chem. Eng. Sci. 2014, 108, 33–46. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Zhong, Z.; Ding, K.; Deng, A.; Min, M.; Chen, P.; Ruan, R. Catalytic fast co-pyrolysis of bamboo residual and waste lubricating oil over an ex-situ dual catalytic beds of MgO and HZSM-5: Analytical PY-GC/MS study. Energy Convers. Manag. 2017, 139, 222–231. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Z.; Ding, K.; Deng, A.; Hao, N.; Meng, X.; Ben, H.; Ruan, R.; Ragauskas, A.J. Catalytic fast pyrolysis of bamboo sawdust via a two-step bench scale bubbling fluidized bed/fixed bed reactor: Study on synergistic effect of alkali metal oxides and HZSM-5. Energy Convers. Manag. 2018, 176, 287–298. [Google Scholar] [CrossRef]
- Lappas, A.A.; Kalogiannis, K.G.; Iliopoulou, E.F.; Triantafyllidis, K.S.; Stefanidis, S.D. Catalytic pyrolysis of biomass for transportation fuels. Wiley Interdiscip. Rev. Energy Environ. 2012, 1, 285–297. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Deng, M.; Wang, J.; Chen, M.; Yan, B.; Yuan, Q. Effect of torrefaction pretreatment and catalytic pyrolysis on the pyrolysis poly-generation of pine wood. Bioresour. Technol. 2016, 214, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, Z.; Zhang, Z.; Wang, Z.; Yang, S.; Yang, Y.; Wang, X.; Liu, S.; Zhang, Q.; Lei, T. Fast corn stalk pyrolysis and the influence of catalysts on product distribution. Bioresour. Technol. 2020, 301, 122739. [Google Scholar] [CrossRef]
- Li, Y.; Yellezuome, D.; Liu, R.; Cai, J.; Gao, Y. Investigation of product selectivity and kinetics of poplar sawdust catalytic pyrolysis over bi-metallic Iron-Nickel/ZSM-5 catalyst. Bioresour. Technol. 2022, 349, 126838. [Google Scholar] [CrossRef]
- Tan, Y.L.; Abdullah, A.Z.; Hameed, B.H. Catalytic fast pyrolysis of durian rind using silica-alumina catalyst: Effects of pyrolysis parameters. Bioresour. Technol. 2018, 264, 198–205. [Google Scholar] [CrossRef]
- Limlamthong, M.; Yip, A.C.K. Recent advances in zeolite-encapsulated metal catalysts: A suitable catalyst design for catalytic biomass conversion. Bioresour. Technol. 2020, 297, 122488. [Google Scholar] [CrossRef]
- Ahmed, M.H.M.; Batalha, N.; Mahmudul, H.M.D.; Perkins, G.; Konarova, M. A review on advanced catalytic co-pyrolysis of biomass and hydrogen-rich feedstock: Insights into synergistic effect, catalyst development and reaction mechanism. Bioresour. Technol. 2020, 310, 123457. [Google Scholar] [CrossRef]
- Che, Q.; Yang, M.; Wang, X.; Yang, Q.; Chen, Y.; Chen, X.; Chen, W.; Hu, J.; Zeng, K.; Yang, H.; et al. Preparation of mesoporous ZSM-5 catalysts using green templates and their performance in biomass catalytic pyrolysis. Bioresour. Technol. 2019, 289, 121729. [Google Scholar] [CrossRef]
- Adam, J.; Blazsó, M.; Mészáros, E.; Stöcker, M.; Nilsen, M.H.; Bouzga, A.; Hustad, J.E.; Grønli, M.; Øye, G. Pyrolysis of biomass in the presence of Al-MCM-41 type catalysts. Fuel 2005, 84, 1494–1502. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Z.F.; Dong, C.Q.; Zhu, X.F. Catalytic upgrading of biomass fast pyrolysis vapors with nano metal oxides: An analytical Py-GC/MS study. Energies 2010, 3, 1805–1820. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, J.; Xiao, R.; Jia, Y.; Shen, D.; Jin, B.; Xiao, G. Study on pyrolysis of pine sawdust with solid base and acid mixed catalysts by thermogravimetry-fourier transform infrared spectroscopy and pyrolysis-gas chromatography/mass spectrometry. Energy Fuels 2014, 28, 4294–4299. [Google Scholar] [CrossRef]
- Chang, R.; Zhu, L.; Jin, F.; Fan, M.; Liu, J.; Jia, Q.; Tang, C.; Li, Q. Production of bio-based p-xylene via catalytic pyrolysis of biomass over metal oxide-modified HZSM-5 zeolites. J. Chem. Technol. Biotechnol. 2018, 93, 3292–3301. [Google Scholar] [CrossRef]
- Cao, Z.; Niu, J.; Gu, Y.; Zhang, R.; Liu, Y.; Luo, L. Catalytic pyrolysis of rice straw: Screening of various metal salts, metal basic oxide, acidic metal oxide and zeolite catalyst on products yield and characterization. J. Clean. Prod. 2020, 269, 122079. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Zhang, L.; Li, Q.; Li, C.; Chen, G.; Zhang, S.; Liu, Q.; Hu, X. Evolution of the functionalities and structures of biochar in pyrolysis of poplar in a wide temperature range. Bioresour. Technol. 2020, 304, 123002. [Google Scholar] [CrossRef] [PubMed]
- Stefanidis, S.D.; Kalogiannis, K.G.; Iliopoulou, E.F.; Lappas, A.A.; Pilavachi, P.A. In-situ upgrading of biomass pyrolysis vapors: Catalyst screening on a fixed bed reactor. Bioresour. Technol. 2011, 102, 8261–8267. [Google Scholar] [CrossRef]
- Li, Y.; Xing, B.; Ding, Y.; Han, X.; Wang, S. A critical review of the production and advanced utilization of biochar via selective pyrolysis of lignocellulosic biomass. Bioresour. Technol. 2020, 312, 123614. [Google Scholar] [CrossRef]
- Abu Bakar, M.S.; Titiloye, J.O. Catalytic pyrolysis of rice husk for bio-oil production. J. Anal. Appl. Pyrolysis 2013, 103, 362–368. [Google Scholar] [CrossRef]
- Auta, M.; Ern, L.M.; Hameed, B.H. Fixed-bed catalytic and non-catalytic empty fruit bunch biomass pyrolysis. J. Anal. Appl. Pyrolysis 2014, 107, 67–72. [Google Scholar] [CrossRef]
- Aysu, T. Catalytic pyrolysis of Eremurus spectabilis for bio-oil production in a fixed-bed reactor: Effects of pyrolysis parameters on product yields and character. Fuel Process. Technol. 2015, 129, 24–38. [Google Scholar] [CrossRef]
- Yorgun, S.; Şimşek, Y.E. Catalytic pyrolysis of Miscanthus × giganteus over activated alumina. Bioresour. Technol. 2008, 99, 8095–8100. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, P. Pyrolysis of cellulose, xylan and lignin with the K2CO 3 and ZnCl2 addition for bio-oil production. Fuel Process. Technol. 2011, 92, 517–522. [Google Scholar] [CrossRef]
- Sanna, A.; Vispute, T.P.; Huber, G.W. Hydrodeoxygenation of the aqueous fraction of bio-oil with Ru/C and Pt/C catalysts. Appl. Catal. B Environ. 2015, 165, 446–456. [Google Scholar] [CrossRef]
- Mu, W.; Ben, H.; Du, X.; Zhang, X.; Hu, F.; Liu, W.; Ragauskas, A.J.; Deng, Y. Noble metal catalyzed aqueous phase hydrogenation and hydrodeoxygenation of lignin-derived pyrolysis oil and related model compounds. Bioresour. Technol. 2014, 173, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, H.; Agblevor, F.A. Hydrodeoxygenation of Aqueous-Phase Catalytic Pyrolysis Oil to Liquid Hydrocarbons Using Multifunctional Nickel Catalyst. Ind. Eng. Chem. Res. 2018, 57, 13257–13268. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Alsowij, M.R.; Corbin, F.; Julson, J.; Boakye, E.; Raynie, D. In situ hydrodeoxygenation upgrading of pine sawdust bio-oil to hydrocarbon biofuel using Pd/C catalyst. J. Energy Inst. 2018, 91, 163–171. [Google Scholar] [CrossRef]
- Baloch, H.A.; Nizamuddin, S.; Siddiqui, M.T.H.; Riaz, S.; Jatoi, A.S.; Dumbre, D.K.; Mubarak, N.M.; Srinivasan, M.P.; Griffin, G.J. Recent advances in production and upgrading of bio-oil from biomass: A critical overview. J. Environ. Chem. Eng. 2018, 6, 5101–5118. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Z.; Cai, Q.; Guo, L. Catalytic conversion of carboxylic acids in bio-oil for liquid hydrocarbons production. Biomass Bioenergy 2012, 45, 138–143. [Google Scholar] [CrossRef]
- Kumar, A.; Chakraborty, J.P.; Singh, R. Bio-oil: The future of hydrogen generation. Biofuels 2017, 8, 663–674. [Google Scholar] [CrossRef]
- Remiro, A.; Ochoa, A.; Arandia, A.; Castaño, P.; Bilbao, J.; Gayubo, A.G. On the dynamics and reversibility of the deactivation of a Rh/CeO2–ZrO2 catalyst in raw bio-oil steam reforming. Int. J. Hydrog. Energy 2019, 44, 2620–2632. [Google Scholar] [CrossRef]
- Valle, B.; Aramburu, B.; Benito, P.L.; Bilbao, J.; Gayubo, A.G. Biomass to hydrogen-rich gas via steam reforming of raw bio-oil over Ni/La2O3-AAl2O3 catalyst: Effect of space-time and steam-to-carbon ratio. Fuel 2018, 216, 445–455. [Google Scholar] [CrossRef]
- Arregi, A.; Lopez, G.; Amutio, M.; Artetxe, M.; Barbarias, I.; Bilbao, J.; Olazar, M. Role of operating conditions in the catalyst deactivation in the in-line steam reforming of volatiles from biomass fast pyrolysis. Fuel 2018, 216, 233–244. [Google Scholar] [CrossRef]
- Chen, J.; Sun, J.; Wang, Y. Catalysts for Steam Reforming of Bio-oil: A Review. Ind. Eng. Chem. Res. 2017, 56, 4627–4637. [Google Scholar] [CrossRef]
- Rioche, C.; Kulkarni, S.; Meunier, F.C.; Breen, J.P.; Burch, R. Steam reforming of model compounds and fast pyrolysis bio-oil on supported noble metal catalysts. Appl. Catal. B Environ. 2005, 61, 130–139. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Otoikhian, K.S.; Ighalo, J.O. Steam Reforming of Biomass Pyrolysis Oil: A Review. Int. J. Chem. React. Eng. 2019, 17, 20180328. [Google Scholar] [CrossRef]
- Valle, B.; Aramburu, B.; Olazar, M.; Bilbao, J.; Gayubo, A.G. Steam reforming of raw bio-oil over Ni/La2O3-AAl2O3: Influence of temperature on product yields and catalyst deactivation. Fuel 2018, 216, 463–474. [Google Scholar] [CrossRef]
- Bizkarra, K.; Bermudez, J.M.; Arcelus-Arrillaga, P.; Barrio, V.L.; Cambra, J.F.; Millan, M. Nickel based monometallic and bimetallic catalysts for synthetic and real bio-oil steam reforming. Int. J. Hydrog. Energy 2018, 43, 11706–11718. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Wang, K.; Luo, Z. Influence of the interaction of components on the pyrolysis behavior of biomass. J. Anal. Appl. Pyrolysis 2011, 91, 183–189. [Google Scholar] [CrossRef]
- Liang, J.; Qian, Y.; Yuan, X.; Leng, L.; Zeng, G.; Jiang, L.; Shao, J.; Luo, Y.; Ding, X.; Yang, Z.; et al. Span80/Tween80 stabilized bio-oil-in-diesel microemulsion: Formation and combustion. Renew. Energy 2018, 126, 774–782. [Google Scholar] [CrossRef]
- Leng, L.; Li, H.; Yuan, X.; Zhou, W.; Huang, H. Bio-oil upgrading by emulsification/microemulsification: A review. Energy 2018, 161, 214–232. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, S.; Wang, X. Stability mechanism investigation of emulsion fuels from biomass pyrolysis oil and diesel. Energy 2014, 66, 250–255. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, X.L.; Zhu, X.F. Analysis on chemical and physical properties of bio-oil pyrolyzed from rice husk. J. Anal. Appl. Pyrolysis 2008, 82, 191–198. [Google Scholar] [CrossRef]
- Zhang, X.S.; Yang, G.X.; Jiang, H.; Liu, W.J.; Ding, H.S. Mass production of chemicals from biomass-derived oil by directly atmospheric distillation coupled with co-pyrolysis. Sci. Rep. 2013, 3, srep01120. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, H.; Wei, S.; Zhuo, S.; Wang, L.; Li, Z.; Yi, W. Separation of Biomass Pyrolysis Oil by Supercritical CO2 Extraction. Smart Grid Renew. Energy 2010, 1, 98–107. [Google Scholar] [CrossRef]
- Heng, L.; Zhang, H.; Xiao, J.; Xiao, R. Life Cycle Assessment of Polyol Fuel from Corn Stover via Fast Pyrolysis and Upgrading. ACS Sustain. Chem. Eng. 2018, 6, 2733–2740. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, G.; Brown, R.C. Life cycle assessment of the production of hydrogen and transportation fuels from corn stover via fast pyrolysis. Environ. Res. Lett. 2013, 8, 025001. [Google Scholar] [CrossRef]
- Peters, J.F.; Iribarren, D.; Dufour, J. Simulation and life cycle assessment of biofuel production via fast pyrolysis and hydroupgrading. Fuel 2015, 139, 441–456. [Google Scholar] [CrossRef]
- Iribarren, D.; Peters, J.F.; Dufour, J. Life cycle assessment of transportation fuels from biomass pyrolysis. Fuel 2012, 97, 812–821. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Shahbazi, A. Life cycle assessment of fast pyrolysis of municipal solid waste in North Carolina of USA. J. Clean. Prod. 2015, 87, 130–138. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, G.; Brown, R.C. Life cycle assessment of commodity chemical production from forest residue via fast pyrolysis. Int. J. Life Cycle Assess. 2014, 19, 1371–1381. [Google Scholar] [CrossRef]
- Dang, Q.; Yu, C.; Luo, Z. Environmental life cycle assessment of bio-fuel production via fast pyrolysis of corn stover and hydroprocessing. Fuel 2014, 131, 36–42. [Google Scholar] [CrossRef]
- Zhang, Y.; Brown, T.R.; Hu, G.; Brown, R.C. Techno-economic analysis of two bio-oil upgrading pathways. Chem. Eng. J. 2013, 225, 895–904. [Google Scholar] [CrossRef]
- Shemfe, M.B.; Gu, S.; Ranganathan, P. Techno-economic performance analysis of biofuel production and miniature electric power generation from biomass fast pyrolysis and bio-oil upgrading. Fuel 2015, 143, 361–372. [Google Scholar] [CrossRef]
- Carrasco, J.L.; Gunukula, S.; Boateng, A.A.; Mullen, C.A.; DeSisto, W.J.; Wheeler, M.C. Pyrolysis of forest residues: An approach to techno-economics for bio-fuel production. Fuel 2017, 193, 477–484. [Google Scholar] [CrossRef]
- van Schalkwyk, D.L.; Mandegari, M.; Farzad, S.; Görgens, J.F. Techno-economic and environmental analysis of bio-oil production from forest residues via non-catalytic and catalytic pyrolysis processes. Energy Convers. Manag. 2020, 213, 112815. [Google Scholar] [CrossRef]
- Shemfe, M.; Gu, S.; Fidalgo, B. Techno-economic analysis of biofuel production via bio-oil zeolite upgrading: An evaluation of two catalyst regeneration systems. Biomass Bioenergy 2017, 98, 182–193. [Google Scholar] [CrossRef]
- Veses, A.; Martínez, J.D.; Callén, M.S.; Murillo, R.; García, T. Application of upgraded drop-in fuel obtained from biomass pyrolysis in a spark ignition engine. Energies 2020, 13, 2089. [Google Scholar] [CrossRef]
- Rashid, U.; Nizami, A.-S.; Rehan, M. Waste Biomass Utilization for Value-added Green Products. Curr. Org. Chem. 2019, 23, 1497–1498. [Google Scholar] [CrossRef]
- Yang, S.I.; Hsu, T.C.; Wu, C.Y.; Chen, K.H.; Hsu, Y.L.; Li, Y.H. Application of biomass fast pyrolysis part II: The effects that bio-pyrolysis oil has on the performance of diesel engines. Energy 2014, 66, 172–180. [Google Scholar] [CrossRef]
- Pradhan, D.; Volli, V.; Singh, R.K.; Murgun, S. Co-pyrolysis behavior, engine performance characteristics, and thermodynamics of liquid fuels from mahua seeds and waste thermocol: A comprehensive study. Chem. Eng. J. 2020, 393, 124749. [Google Scholar] [CrossRef]
- Raguraman, D.; Kumar, A.; Prasanna Raj Yadav, S.; Patil, P.Y.; Samson Isaac, J.; Sowmya Dhanalakshmi, C.; Madhu, P.; Isaac Joshuaramesh Lalvani, J. Performance and Emission Characteristics of Pyrolysis Oil Obtained from Neem de Oiled Cake and Waste Polystyrene in a Compression Ignition Engine. Adv. Mater. Sci. Eng. 2021, 2021, 3728852. [Google Scholar] [CrossRef]
- Hu, X.; Mourant, D.; Gunawan, R.; Wu, L.; Wang, Y.; Lievens, C.; Li, C.Z. Production of value-added chemicals from bio-oil via acid catalysis coupled with liquid-liquid extraction. RSC Adv. 2012, 2, 9366–9370. [Google Scholar] [CrossRef]
- Ruiz, M.P.; Mijnders, J.; Tweehuysen, R.; Warnet, L.; van Drongelen, M.; Kersten, S.R.A.; Lange, J.P. Fully Recyclable Bio-Based Thermoplastic Materials from Liquefied Wood. ChemSusChem 2019, 12, 4395–4399. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mahmood, N.; Ma, Z.; Zhu, M.; Wang, J.; Zheng, J.; Yuan, Z.; Wei, Q.; Xu, C. (Chunbao) Preparation and characterization of bio-polyol and bio-based flexible polyurethane foams from fast pyrolysis of wheat straw. Ind. Crops Prod. 2017, 103, 64–72. [Google Scholar] [CrossRef]
- Hu, X.; Nango, K.; Bao, L.; Li, T.; Mahmudul Hasan, M.D.; Li, C.Z. High yields of solid carbonaceous materials from biomass. Green Chem. 2019, 21, 1128–1140. [Google Scholar] [CrossRef]
- Sarkar, S.; Kumar, A. Large-scale biohydrogen production from bio-oil. Bioresour. Technol. 2010, 101, 7350–7361. [Google Scholar] [CrossRef]
- Yang, S.H.; Suciptan, T. Rheological behavior of Japanese cedar-based biobinder as partial replacement for bituminous binder. Constr. Build. Mater. 2016, 114, 127–133. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).