Buccal Mucosa Graft in Urological Surgery: A State-of-the-Art Review and Expert Opinion
Abstract
1. Introduction
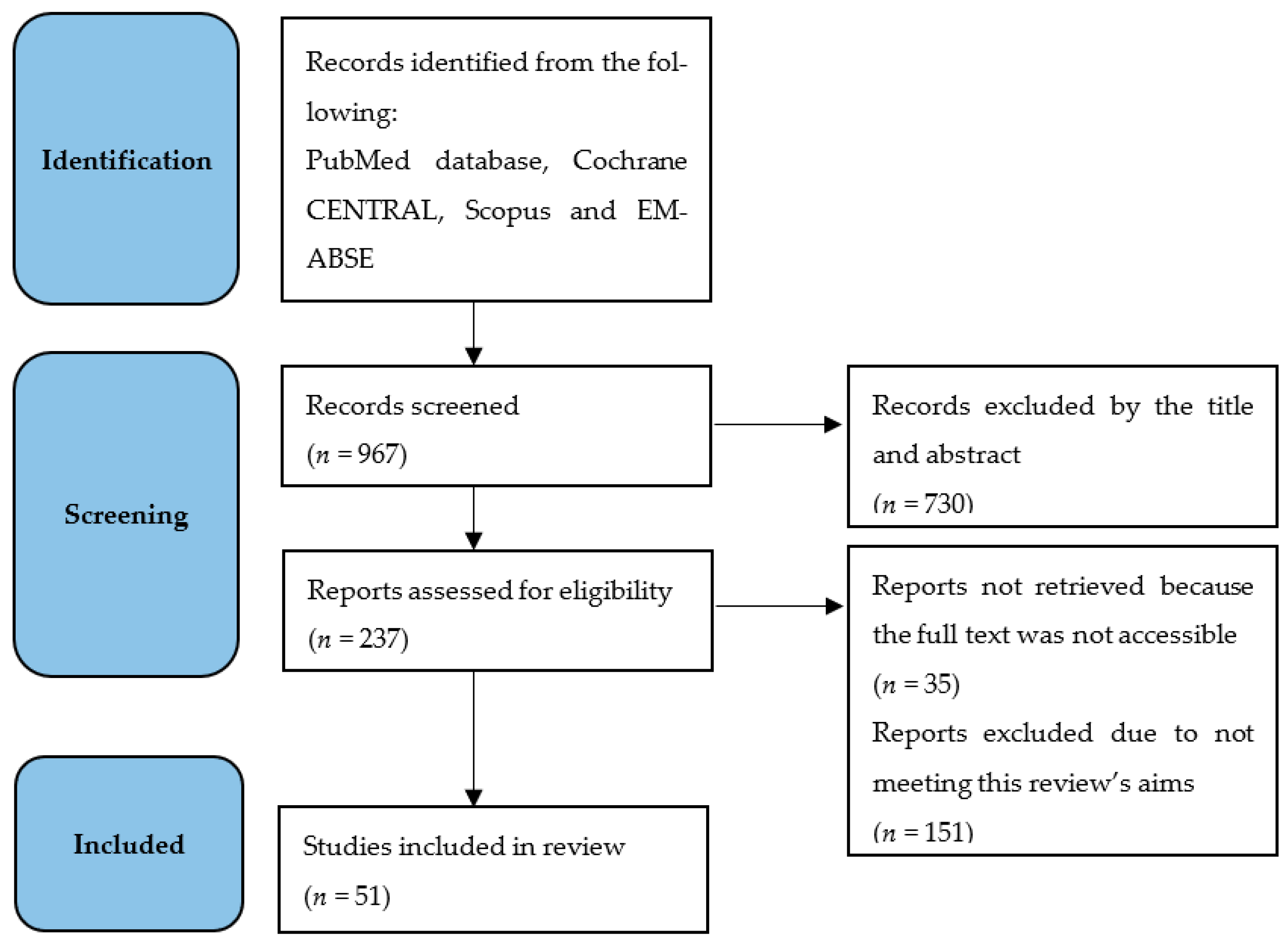
2. Materials and Methods
2.1. Research Strategy
2.2. Inclusion and Exclusion Criteria
3. Research Evidence
3.1. Buccal Mucosa Graft: The Surgical Procedure
3.2. Buccal Mucosa Graft: Characteristics
3.3. Buccal Mucosa Graft: Donor Site Complications
3.4. Tips and Tricks
- Not all patients are candidates for oral mucosal harvesting. The selection of patients is the most important part of the flow chart presented above. An accurate medical history must be obtained to eliminate patients who are heavy smokers or have a history of alcohol abuse, a diagnosis of oral lichen planus, etc. An accurate objective examination of the oral cavity must exclude the presence of dental or mucosal pathologies (oral lichen planus, leuko/erythroplakia, dysplasia, carcinomas, etc.) in the harvesting sites. These clinical conditions can lead to the malignant transformation of the oral mucosa [19].
- To reduce the risk of infection of the donor site, it is useful to perform professional oral hygiene treatment a few days before the surgical procedure [20].
- Nasotracheal intubation is recommended to facilitate the harvesting procedure, especially in bilateral sampling.
- It is important to respect and maintain a safe distance from anatomical landmarks: the labial commissure, Stensen duct orifice and attached gingiva.
- The infiltration of the donor site with local anesthesia with a vasoconstrictor allows for the hydro-dissection of the mucosa from the underlying layers, making graft harvesting easier. The vasoconstrictor prevents excessive bleeding, allowing for an easier surgical procedure.
- It is mandatory to identify wide safety margins from the described anatomical landmarks; these can be marked with a dermographic pen, or for example, the Stensen duct can be cannulated with a lacrimal probe.
3.5. Urethroplasty: Techniques and Functional Results
3.6. Ureteroplasty: Techniques and Functional Results
3.7. Ureteroplasty: Laparoscopic Robotic-Assisted Surgery
3.8. Penile Curvature Surgery: Techniques and Functional Results
3.9. Hypospadias Surgery in Adults: The Role of Buccal Mucosa Graft
3.10. Complications and Preventing Strategies for Reducing Complications
3.11. The Use of BMG in Pediatric Surgery
4. Novel Applications
5. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMG | Buccal mucosa graft |
| OMG | Oral mucosa graft |
| LMG | Lingual mucosa graft |
| tEPA | Transecting excision and primary anastomosis |
| ED | Erection disfunction |
References
- Ławkowska, K.; Rosenbaum, C.; Petrasz, P.; Kluth, L.; Koper, K.; Drewa, T.; Pokrywczynska, M.; Adamowicz, J. Trauma and Reconstructive Urology Working Party of the European Association of Urology Young Academic Urologists. Tissue engineering in reconstructive urology-The current status and critical insights to set future directions-critical review. Front. Bioeng. Biotechnol. 2023, 10, 1040987. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Markiewicz, M.R.; Lukose, M.A.; Margarone, J.E.; Barbagli, G.; Miller, K.S.; Chuang, S.K. The oral mucosa graft: A systematic review. J. Urol. 2007, 178, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Korneyev, I.; Ilyin, D.; Schultheiss, D.; Chapple, C. The first oral mucosal graft urethroplasty was carried out in the 19th century: The pioneering experience of Kirill Sapezhko (1857–1928). Eur. Urol. 2012, 62, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Humby, G.; Twistington Higgins, T. A one-stage operation for hypospadias. Br. J. Surg. 1941, 29, 84–92. [Google Scholar] [CrossRef]
- Bürger, R.A.; Müller, S.C.; el-Damanhoury, H.; Tschakaloff, A.; Riedmiller, H.; Hohenfellner, R. The buccal mucosal graft for urethral reconstruction: A preliminary report. J. Urol. 1992, 147, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Dessanti, A.; Rigamonti, W.; Merulla, V.; Falchetti, D.; Caccia, G. Autologous buccal mucosa graft for hypospadias repair: An initial report. J. Urol. 1992, 147, 1081–1083. [Google Scholar] [CrossRef] [PubMed]
- Wessells, H.; Morey, A.; Vanni, A.; Rahimi, L.; Souter, L. Urethral stricture disease guideline amendment. J. Urol. 2023, 210, 64–71. [Google Scholar] [CrossRef]
- European Association of Urology. EAU Guidelines on Urethral Stricture 2023. Uroweb. Available online: https://uroweb.org/guidelines (accessed on 22 February 2025).
- Gn, M.; Sterling, J.; Sinkin, J.; Cancian, M.; Elsamra, S. The Expanding Use of Buccal Mucosal Grafts in Urologic Surgery. Urology 2021, 156, e58–e65. [Google Scholar] [CrossRef] [PubMed]
- Naude, J.H. Buccal mucosal grafts in the treatment of ureteric lesions. BJU Int. 1999, 83, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.N.; Mishra, K.; Zhao, L.C. Buccal Mucosal Ureteroplasty for the Management of Ureteral Strictures: Patient Selection and Considerations. Res. Rep. Urol. 2022, 14, 135–140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Natsos, A.; Tatanis, V.; Kontogiannis, S.; Waisbrod, S.; Gkeka, K.; Obaidad, M.; Peteinaris, A.; Pagonis, K.; Papadopoulos, C.; Kallidonis, P.; et al. Grafts in Peyronie’s surgery without the use of prostheses: A systematic review and meta-analysis. Asian J. Androl. 2024, 26, 250–259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Santis, D.; Gelpi, F.; Castellani, R.; Palumbo, C.; Ferretti, M.; Zanotti, G.; Zotti, F.; Montagna, L.; Luciano, U.; Marconcini, S.; et al. Bi-layered collagen nano-structured membrane prototype collagen matrix CM-10826 for oral soft tissue regeneration: An in vivo ultrastructural study on 13 patients. J. Biol. Regul. Homeost. Agents 2019, 33 (Suppl. 1), 29–41. [Google Scholar] [PubMed]
- Bozkurt, İ.H.; Yalçınkaya, F.; Sertçelik, M.N.; Zengin, K. Comparison of uni-and bilateral buccal mucosa harvesting in terms of oral morbidity. Turk. J. Urol. 2013, 39, 43–47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chauhan, S.; Yadav, S.S.; Tomar, V. Outcome of buccal mucosa and lingual mucosa graft urethroplasty in the management of urethral strictures: A comparative study. Urol. Ann. 2016, 8, 36–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akyüz, M.; Güneş, M.; Koca, O.; Sertkaya, Z.; Kanberoğlu, H.; Karaman, M.İ. Evaluation of intraoral complications of buccal mucosa graft in augmentation urethroplasty. Turk. J. Urol. 2014, 40, 156–160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lumen, N.; Vierstraete-Verlinde, S.; Oosterlinck, W.; Hoebeke, P.; Palminteri, E.; Goes, C.; Maes, H.; Spinoit, A.F. Buccal Versus Lingual Mucosa Graft in Anterior Urethroplasty: A Prospective Comparison of Surgical Outcome and Donor Site Morbidity. J. Urol. 2016, 195, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Ansari, H.M.; Rao, S.; Sharma, A.; Galhotra, V. Oral Submucous Fibrosis Secondary to Buccal Mucosal Graft for Urethroplasty. Indian. J. Otolaryngol. Head. Neck Surg. 2022, 74 (Suppl 3), 5601–5603. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zotti, F.; Nocini, R.; Capocasale, G.; Fior, A.; Peretti, M.; Albanese, M. Malignant transformation evidences of Oral Lichen Planus: When the time is of the essence. Oral. Oncol. 2020, 104, 104594. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Baccini, F.; De Manzoni, R.; Viviani, M.; Brentaro, S.; Zangani, A.; Faccioni, P.; Luciano, U.; Zuffellato, N.; Signoriello, A.; et al. Air polishing therapy in supportive periodontal treatment: A systematic review. J. Appl. Cosmetol. 2023, 41, 13–24. [Google Scholar] [CrossRef]
- Barbagli, G.; Palminteri, E.; Rizzo, M. Dorsal onlay graft urethroplasty using penile skin or buccal mucosa in adult bulbourethral strictures. J. Urol. 1998, 160, 1307–1309. [Google Scholar] [CrossRef]
- Barbagli, G.; Selli, C.; Tosto, A.; Palminteri, E. Dorsal free graft urethroplasty. J. Urol. 1996, 155, 123–126. [Google Scholar] [CrossRef]
- Turner-Warwick, R. The use of buccal mucosa patch grafts in the repair of bulbar urethral strictures. J. Urol. 1977, 118, 671–674. [Google Scholar]
- Barbagli, G.; Palminteri, E.; Guazzoni, G.; Montorsi, F.; Turini, D.; Lazzeri, M. Bulbar urethroplasty using buccal mucosa grafts placed on the ventral, dorsal or lateral surface of the urethra: Are results affected by the surgical technique? J. Urol. 2005, 174, 955–957. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Guo, Q.; Zhang, X.; Xing, Q.; Ren, S.; Song, Y.; Li, C.; Hao, C.; Wang, J. The urinary and sexual outcomes of buccal mucosal graft urethroplasty versus end-to-end anastomosis: A systematic review with meta-analysis. Sex. Med. 2024, 12, qfae064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gupta, N.P.; Ansari, M.S.; Dogra, P.N.; Tandon, S. Dorsal buccal mucosal graft urethroplasty by a ventral sagittal urethrotomy and minimal-access perineal approach for anterior urethral stricture. BJU Int. 2004, 93, 1287–1290. [Google Scholar] [CrossRef] [PubMed]
- Asopa, H.S.; Garg, M.; Singhal, G.G.; Singh, L.; Asopa, J.; Nischal, A. Dorsal free graft urethroplasty for urethral stricture by ventral sagittal urethrotomy approach. Urology 2001, 58, 657–659. [Google Scholar] [CrossRef]
- Palminteri, E.; Manzoni, G.; Berdondini, E.; Di Fiore, F.; Testa, G.; Poluzzi, M.; Molon, A. Combined dorsal plus ventral double buccal mucosa graft in bulbar urethral reconstruction. Eur. Urol. 2008, 53, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Mangera, A.; Patterson, J.M.; Chapple, C.R. A systematic review of graft augmentation urethroplasty techniques for the treatment of anterior urethral strictures. Eur. Urol. 2011, 59, 797–814. [Google Scholar] [CrossRef] [PubMed]
- Alberca-Del Arco, F.; Santos-Pérez, D.L.; Blanca, R.; Amores Vergara, C.; Herrera-Imbroda, B.; Sáez-Barranquero, F. Bulbar urethroplasty techniques and stricture recurrence: Differences between end-to-end urethroplasty versus the use of graft. Minerva Urol. Nephrol. 2024, 76, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, O.J.; Holm, H.V.; Ekerhult, T.O.; Lindqvist, K.; Grabowska, B.; Persson, B.; Sairanen, J. To Transect or Not Transect: Results from the Scandinavian Urethroplasty Study, A Multicentre Randomised Study of Bulbar Urethroplasty Comparing Excision and Primary Anastomosis Versus Buccal Mucosal Grafting. Eur. Urol. 2022, 81, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Oszczudlowski, M.; Yepes, C.; Dobruch, J.; Martins, F.E. Outcomes of transecting versus non-transecting urethroplasty for bulbar urethral stricture: A meta-analysis. BJU Int. 2023, 132, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xing, Y.; Zhang, X.; Guo, Q.; Li, C.; Guo, C.; Wang, J.; Hao, C. Low risk of erectile dysfunction after nontransecting bulbar urethroplasty for urethral stricture: A systematic review and meta-analysis. J. Sex. Med. 2023, 21, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.M.; Blakely, S.A.; O’Donnell, C.I.; Nikolavsky, D.; Flynn, B.J. Primary non-transecting bulbar urethroplasty long-term success rates are similar to transecting urethroplasty. Int. Urol. Nephrol. 2017, 49, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Chapple, C.; Andrich, D.; Atala, A.; Barbagli, G.; Cavalcanti, A.; Kulkarni, S.; Mangera, A.; Nakajima, Y. SIU/ICUD Consultation on Urethral Strictures: The management of anterior urethral stricture disease using substitution urethroplasty. Urology 2014, 83 (Suppl. 3), S31–S47. [Google Scholar] [CrossRef] [PubMed]
- Jasionowska, S.; Bochinski, A.; Shiatis, V.; Singh, S.; Brunckhorst, O.; Rees, R.W.; Ahmed, K. Anterior Urethroplasty for the Management of Urethral Strictures in Males: A Systematic Review. Urology 2022, 159, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Barratt, R.; Chan, G.; La Rocca, R.; Dimitropoulos, K.; Martins, F.E.; Campos-Juanatey, F.; Greenwell, T.J.; Waterloos, M.; Riechardt, S.; Osman, N.I.; et al. Free Graft Augmentation Urethroplasty for Bulbar Urethral Strictures: Which Technique Is Best? A Systematic Review. Eur. Urol. 2021, 80, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Chua, M.; Talla, V.; Fernandez, N.; Ming, J.; Sarino, E.M.; DeLong, J.; Virasoro, R.; Tonkin, J.; McCammon, K. Lingual versus buccal mucosal graft for augmentation urethroplasty: A meta-analysis of surgical outcomes and patient-reported donor site morbidity. Int. Urol. Nephrol. 2021, 53, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, S.; Parmar, K. Buccal mucosa or penile skin for substitution urethroplasty: A systematic review and meta-analysis. Indian. J. Urol. 2020, 36, 81–88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lumen, N.; Oosterlinck, W.; Hoebeke, P. Urethral reconstruction using buccal mucosa or penile skin grafts: Systematic review and meta-analysis. Urol. Int. 2012, 89, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, A. Substitution urethroplasty using oral mucosa graft for male anterior urethral stricture disease: Current topics and reviews. Int. J. Urol. 2017, 24, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Simonato, A.; Gregori, A.; Lissiani, A.; Galli, S.; Ottaviani, F.; Rossi, R.; Zappone, A.; Carmignani, G. The tongue as an alternative donor site for graft urethroplasty: A pilot study. J. Urol. 2006, 175, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Benson, C.R.; Li, G.; Brandes, S.B. Long term outcomes of one-stage augmentation anterior urethroplasty: A systematic review and meta-analysis. Int. Braz. J. Urol. 2021, 47, 237–250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- You, Y.; Gao, X.; Chai, S.; Chen, J.; Wang, J.; Zhang, H.; Zhou, Y.; Yu, Z.; Cheng, G.; Li, B.; et al. Oral mucosal graft ureteroplasty versus ileal ureteric replacement: A meta-analysis. BJU Int. 2023, 132, 122–131. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Fan, J.; Cheng, S.; Fan, S.; Yin, L.; Li, Z.; Guan, H.; Yang, K.; Li, X. The application of the “omental wrapping” technique with autologous onlay flap/graft ureteroplasty for the management of long ureteral strictures. Transl. Androl. Urol. 2021, 10, 2871–2878. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tao, C.; Jin, X.; Zhang, H. Dorsal oral mucosa graft urethroplasty for female urethral stricture reconstruction: A narrative review. Front. Surg. 2023, 10, 1146429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, Y.; Yang, C.; Fang, L.; Chen, R.; Li, X.; Jiang, C.; Hu, W.; Chen, H.; Yu, D.; Wang, Y. The application of the “perinephric fat wrapping” technique with oral mucosal graft for the management of ureter repair and reconstruction. World J. Urol. 2024, 42, 528. [Google Scholar] [CrossRef] [PubMed]
- Chao, B.W.; Lee, M.; Eun, D.D. Robotic Multiport Buccal Mucosa Graft Ureteroplasty: Tips and Tricks. J. Endourol. 2025, 39 (Suppl. 1), S52–S59. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.C.; Yamaguchi, Y.; Bryk, D.J.; Adelstein, S.A.; Stifelman, M.D. Robot-Assisted Ureteral Reconstruction Using Buccal Mucosa. Urology 2015, 86, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Sahay, S.C.; Kesarwani, P.; Sharma, G.; Tiwari, A. Buccal mucosal graft ureteroplasty: The new normal in ureteric reconstructive surgery—Our initial experience with the laparoscopic and robotic approaches. J. Minim. Access Surg. 2024, 20, 393–396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, C.H.; Lin, Y.S.; Weng, W.C.; Lu, C.H.; Hsu, C.Y.; Tung, M.C.; Ou, Y.C. Validation of robotic-assisted ureteroplasty with buccal mucosa graft for stricture at the proximal and middle ureters: The first comparative study. J. Robot. Surg. 2022, 16, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- European Association of Urology. EAU Guidelines on Sexual and Reproductive Health. 2024. Available online: https://uroweb.org/guidelines (accessed on 22 February 2025).
- Osmonov, D.; Ragheb, A.; Ward, S.; Blecher, G.; Falcone, M.; Soave, A.; Dahlem, R.; van Renterghem, K.; Christopher, N.; Hatzichristodoulou, G.; et al. ESSM Position Statement on Surgical Treatment of Peyronie’s Disease. Sex. Med. 2022, 10, 100459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garaffa, G.; Sacca, A.; Christopher, A.N.; Ralph, D.J. Circumcision is not mandatory in penile surgery. BJU Int. 2010, 105, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Dalkin, B.L.; Carter, M.F. Venogenic impotence following dermal graft repair for Peyronie’s disease. J. Urol. 1991, 146, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.G.; Somani, B.K.; Rees, R.W. Twenty Years of Plaque Incision and Grafting for Peyronie’s Disease: A Review of Literature. Sex. Med. 2019, 7, 115–128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hatzichristodoulou, G.; Osmonov, D.; Kübler, H.; Hellstrom, W.J.G.; Yafi, F.A. Contemporary review of grafting techniques for the surgical treatment of Peyronie’s disease. Sex. Med. Rev. 2017, 5, 544–552. [Google Scholar] [CrossRef]
- Kadioglu, A.; Sanli, O.; Akman, T.; Ersay, A.; Guven, S.; Mammadov, F. Graft materials in Peyronie’s disease surgery: A comprehensive review. J. Sex. Med. 2007, 4, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, A.; Silvani, M.; Pastore, A.L.; Fioretti, F.; Fabiani, A.; Villirillo, T.; Costantini, E. Corporoplasty using buccal mucosa graft in Peyronie disease: Is it a first choice? Urology 2015, 85, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Shioshvili, T.J.; Kakonashvili, A.P. The surgical treatment of Peyronie’s disease: Replacement of plaque by free autograft of buccal mucosa. Eur. Urol. 2005, 48, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Cormio, L.; Zucchi, A.; Lorusso, F.; Selvaggio, O.; Fioretti, F.; Porena, M.; Carrieri, G. Surgical treatment of Peyronie’s disease by plaque incision and grafting with buccal mucosa. Eur. Urol. 2009, 55, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.K.; Singh, A.K.; Trivedi, S.; Dwivedi, U.S.; Ramole, Y.; Khan, F.A.; Pandey, M. Role of lingual mucosa as a graft material in the surgical treatment of Peyronie’s disease. Urol. Ann. 2024, 16, 227–232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ding, J.; Li, Q.; Li, S.; Li, F.; Zhou, C.; Zhou, Y.; Hu, J.; Xie, L.; Cao, Y.; Zhang, S. Ten years’ experience for hypospadias repair: Combined buccal mucosa graft and local flap for urethral reconstruction. Urol. Int. 2014, 93, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Berglund, R.K.; Angermeier, K.W. Combined buccal mucosa graft and genital skin flap for reconstruction of extensive anterior urethral strictures. Urology 2006, 68, 707–710; discussion 710. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, Y.; Tang, Y.; Chen, W.; Yang, Z.; Li, Q.; Zhou, C.; Li, F.; Zhou, Y. Two-stage repair with buccal mucosa for severe and complicated hypospadias in adults. Int. J. Urol. 2011, 18, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Sahin, C.; Seyhan, T. Use of buccal mucosal grafts in hypospadia-crippled adult patients. Ann. Plast. Surg. 2003, 50, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Pogorelić, Z.; Stričević, L.; Elezović Baloević, S.; Todorić, J.; Budimir, D. Safety and Effectiveness of Triclosan-Coated Polydioxanone (PDS Plus) versus Uncoated Polydioxanone (PDS II) Sutures for Prevention of Surgical Site Infection after Hypospadias Repair in Children: A 10-Year Single Center Experience with 550 Hypospadias. Biomedicines 2024, 12, 583. [Google Scholar] [CrossRef]
- Sufaru, I.G.; Macovei, G.; Stoleriu, S.; Martu, M.A.; Luchian, I.; Kappenberg-Nitescu, D.C.; Solomon, S.M. 3D Printed and Bioprinted Membranes and Scaffolds for the Periodontal Tissue Regeneration: A Narrative Review. Membranes 2022, 12, 902. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, C.; Ma, L.; Li, P.; Yang, Y.; Wang, J.; Zhou, X.; Tao, T.; Zhao, Y.; Lyu, X.; Zhuo, R.; et al. Robot-Assisted Ureteroplasty with Labial Mucosal Onlay Grafting for Long Left-Sided Proximal Ureteral Stenosis in Children and Adolescents: Technical Tips and Functional Outcomes. J. Endourol. 2024, 38, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, V.; de Jesus, L.E.; Romao, R.L.; Farhat, W.A.; Lorenzo, A.J.; Pippi Salle, J. Buccal grafts for urethroplasty in pre-pubertal boys: What happens to the neourethra after puberty? J. Pediatr. Urol. 2014, 10, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Elifranji, M.; Abbas, T.; Vallasciani, S.; Leslie, B.; Elkadhi, A.; Pippi Salle, J.L. Upper lip graft (ULG) for redo urethroplasties in children. A step by step video. J. Pediatr. Urol. 2020, 16, 510–511. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.D.; Cinà, D.P.; Gonzalez, C.M.; Hofer, M.D. Surgical Approaches and Long-Term Outcomes in Adults with Complex Reoperative Hypospadias Repair. J. Urol. 2018, 199, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, M.; Simsek, A.; Bizic, M.; Stojanovic, B.; Martins, F.; Roth, J.; Purohit, R. “Watch” Shaped Buccal Mucosa Graft for Simultaneous Correction of Severe Chordee and Urethroplasty as a One-Stage Repair of Scrotal Hypospadias. Urology 2020, 137, 205. [Google Scholar] [CrossRef] [PubMed]
- Avallone, M.A.; Quach, A.; Warncke, J.; Nikolavsky, D.; Flynn, B.J. Robotic-assisted Laparoscopic Subtrigonal Inlay of Buccal Mucosal Graft for Treatment of Refractory Bladder Neck Contracture. Urology 2019, 130, 209. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, O.; Sen, V.; Demir, O.; Esen, A. Subtrigonal Inlay Patch Technique with Buccal Mucosa Graft for Recurrent Bladder Neck Contractures. Urol. Int. 2022, 106, 256–260. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Type of Study | Aim | Description of Findings |
|---|---|---|---|---|
| Barbagli, G. [21] | 1998 | Prospective study | To report the outcome and functional results of a 1-stage correction of bulbar urethral stricture using a penile skin or buccal mucosa graft | Onlay graft urethroplasty provided excellent results in 92% of adults with bulbourethral stricture |
| Barbagli, G. [22] | 1996 | Prospective study | To evaluate the outcome and functional results of dorsal free graft urethroplasty | The use of free skin grafts for urethral reconstruction is anatomically healthier in the dorsal than in the ventral position |
| Barbagli, G. [24] | 2005 | Comparative study | To compare the outcome of 3 types of bulbar urethroplasty using buccal mucosa graft | BMG into the ventral; the dorsal or lateral surface of the bulbar urethra showed the same success rates (83% to 85%) |
| Gupta, N.P. et al. [26] | 2004 | Prospective study | To present the technique of dorsal buccal mucosal graft urethroplasty through a ventral sagittal urethrotomy and minimal access perineal approach for anterior urethral stricture | Dorsal buccal mucosal graft urethroplasty via a minimal access perineal approach is a simple technique with a good surgical outcome |
| Asopa, H.S. et al. [27] | 2001 | Prospective study | To present the technique of applying a dorsal free graft to treat urethral stricture by the ventral sagittal urethrotomy approach without mobilizing the urethra | The ventral sagittal urethrotomy approach for dorsal free graft urethroplasty is not only feasible and successful but is also easy to perform |
| Palminteri, E. et al. [28] | 2008 | Prospective study | To describe a new technique for bulbar urethral reconstruction using a combined dorsal plus ventral double BMG | The double dorsal and ventral graft may provide a simple and reliable solution to achieve an adequate urethral lumen in selected patients |
| Nilsen, O.J. et al. [31] | 2022 | RCT | To evaluate sexual dysfunction and penile complications after urethroplasty with transecting excision and primary anastomosis versus BMG | Penile problems are more common after the transection technique than after the grafting technique |
| Anderson, K.M. et al. [34] | 2017 | Retrospective comparative study | To review the long-term outcomes of transecting versus non-transecting urethroplasty to repair bulbar urethral strictures | Transecting and non-transecting primary bulbar urethroplasty resulted in a similar long-term stricture resolution rate |
| Lumen, N. et al. [17] | 2016 | Comparative study | To compare BMG and lingual mucosa graft urethroplasty with respect to donor site morbidity and urethroplasty outcome | The success of urethroplasty with lingual and buccal mucosa grafts was similar |
| Simonato, A. et al. [42] | 2006 | Prospective study | To evaluate the outcome and functional results of lingual mucosal graft for urethroplasty | Harvesting the lingual mucosal graft is feasible and easy to perform |
| Author | Year | Type of Study | Aim | Description of Findings |
|---|---|---|---|---|
| You, Y. et al. [44] | 2023 | Systematic Review and Meta-Analysis | To compare the outcomes of oral mucosal graft ureteroplasty and ileal ureter replacement | Oral mucosal graft ureteroplasty is an effective, minimally invasive and safe procedure. Oral mucosal graft ureteroplasty should be the preferred treatment for long ureteric strictures, especially obstructed ureter segments of ≤8 cm |
| Wang, J. et al. [45] | 2021 | Prospective Study | To present the “omental wrapping” technique in laparoscopic and robotic ureteroplasty using onlay flaps or grafts for the management of long proximal or middle ureteral strictures | This procedure is an efficient, safe, reproducible and simple technique |
| Jiang, Y. et al. [47] | 2024 | Prospective Study | To evaluate the effectiveness of the “perinephric fat wrapping” technique in laparoscopic ureteroplasty with oral mucosal graft | This technique is safe and effective in repairing and reconstructing the ureter using oral mucosal grafts |
| Author | Year | Type of Study | Aim | Description of Findings |
|---|---|---|---|---|
| Chao, B.W. et al. [48] | 2025 | Review (tips and tricks) | To give the readers an overview of the current experiences related to the use of the buccal mucosa graft ureteroplasty performed by using laparoscopic robotic-assisted surgery. | Robotic ureteroplasty by using BMG showed a high rate of clinical success with comparatively minimal morbidity and excellent success rates. |
| Zhao, L.C. et al. [49] | 2015 | Prospective study | To describe RU-BMG as a minimally invasive ureteral reconstruction technique for ureteral strictures. | This procedure is a flexible and feasible surgical procedure for treating long-segment strictures of the ureteropelvic junction, proximal ureter or mid-ureter. |
| Sahay, S.C. et al. [50] | 2024 | Prospective study | To present the functional outcome of 16 cases of BMG ureteroplasty performed by the laparoscopic and robotic approaches. | RU-BMG provides the benefits of improved ergonomics, easy maneuverability and precision surgery to patients. |
| You, Y. et al. [44] | 2023 | Systematic review and meta-analysis | To describe the outcomes of oral mucosal graft ureteroplasty and ileal ureter replacement and determine the relative merits of both procedures. | RU-BMG is an effective, minimally invasive and safe alternative to ileal ureter replacement for the management of long ureteric strictures. |
| Author | Year | Type of Study | Aim | Description of Findings |
|---|---|---|---|---|
| Osmonov, D. et al. [53] | 2022 | Scientific Society Position Statement | To review the evidence associated with the surgical treatment of Peyronie’s disease and provide clinical recommendations on behalf of the European Society for Sexual Medicine | Patient preoperative counseling is recommended in order to increase patients’ satisfaction. Surgical treatment should only be offered in the chronic phase of the condition |
| Garaffa, G. et al. [54] | 2010 | Comparative Study | To assess the outcome of not circumcising patients who have surgery to correct a congenital or acquired curvature | Circumcision should not be considered a routine part of penile surgery unless a significant phimosis is present or revisional surgery is contemplated |
| Hatzichristodoulou, G. et al. [57] | 2024 | Review | To provide an overview of recent studies reporting the outcomes of grafting techniques and to report advances in the development of new grafting materials for Peyronie’s disease surgery | Surgeon experience, careful patient selection, patient preference and the type of penile deformity affect the use of BMG and the surgical approach used |
| Zucchi, A. et al. [59] | 2015 | Prospective Study | To assesses the surgical and functional efficacy of corporoplasty with buccal mucosa graft, patients’ and their partners’ satisfaction and the low cost of this operation | Corporoplasty with buccal mucosa graft is easy to perform and represents a good treatment choice for most forms of Peyronie’s disease with curvature preventing penetration and sexual intercourse |
| Shioshvili, T.J. et al. [60] | 2005 | Comparative Study | To evaluate the clinical results of the use of buccal mucosa for the replacement of Peyronie’s disease plaque | BMG showed good properties of adaptation and revascularization and good anatomical and functional clinical results |
| Cormio, L. et al. [61] | 2009 | Multicenter Study | To evaluate the efficacy, safety and reproducibility of plaque incision and BMG in patients affected by Peyronie’s disease | BMG provided excellent short-term results with the fast return of spontaneous erections and prevented shrinkage |
| Author | Year | Type of Study | Aim | Description of Findings |
|---|---|---|---|---|
| Han, C. et al. [69] | 2024 | Prospective study | To evaluate the functional outcomes of robot-assisted ureteroplasty with BMG for long proximal ureteral stenosis in children and adolescents. | BMG appears to be safe and feasible for repairing long ureteral strictures in pediatric and adolescent patients. |
| Figueroa, V. et al. [70] | 2014 | Prospective study | To report the data for post-pubertal follow-up after pre-pubertal BMG urethroplasties. | Buccal mucosa grafts appear to grow proportionally to the phallus after pubertal endogenous androgen stimulation. |
| Elifranji, M. et al. [71] | 2020 | Prospective study | To evaluate the functional outcomes of the use of upper lip graft in urethroplasty. | Upper lip graft harvest is easy and a suitable alternative source of oral mucosa for urethroplasty in children. |
| Djordjevic, M. et al. [73] | 2020 | Prospective study | To present a novel and 1-stage technique in scrotal hypospadias repair. | A “watch”-shaped buccal mucosa graft for simultaneous curvature correction and urethroplasty is a viable and reliable option for the single-stage repair of scrotal hypospadias with severe chordee. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botti, S.; Ceccato, T.; Cassaro, M.; Sanna, G.; Trevisiol, L.; Cai, T. Buccal Mucosa Graft in Urological Surgery: A State-of-the-Art Review and Expert Opinion. Uro 2025, 5, 16. https://doi.org/10.3390/uro5030016
Botti S, Ceccato T, Cassaro M, Sanna G, Trevisiol L, Cai T. Buccal Mucosa Graft in Urological Surgery: A State-of-the-Art Review and Expert Opinion. Uro. 2025; 5(3):16. https://doi.org/10.3390/uro5030016
Chicago/Turabian StyleBotti, Simone, Tommaso Ceccato, Marco Cassaro, Giangiacomo Sanna, Lorenzo Trevisiol, and Tommaso Cai. 2025. "Buccal Mucosa Graft in Urological Surgery: A State-of-the-Art Review and Expert Opinion" Uro 5, no. 3: 16. https://doi.org/10.3390/uro5030016
APA StyleBotti, S., Ceccato, T., Cassaro, M., Sanna, G., Trevisiol, L., & Cai, T. (2025). Buccal Mucosa Graft in Urological Surgery: A State-of-the-Art Review and Expert Opinion. Uro, 5(3), 16. https://doi.org/10.3390/uro5030016







