Clinical Experience with a Medical Device Containing Xyloglucan, Hibiscus, and Propolis for the Control of Acute Uncomplicated Urinary Tract Infection-like Symptoms
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Medical Device Characteristics and Doses
2.3. Data Collection and Variables
3. Results
3.1. Study Population
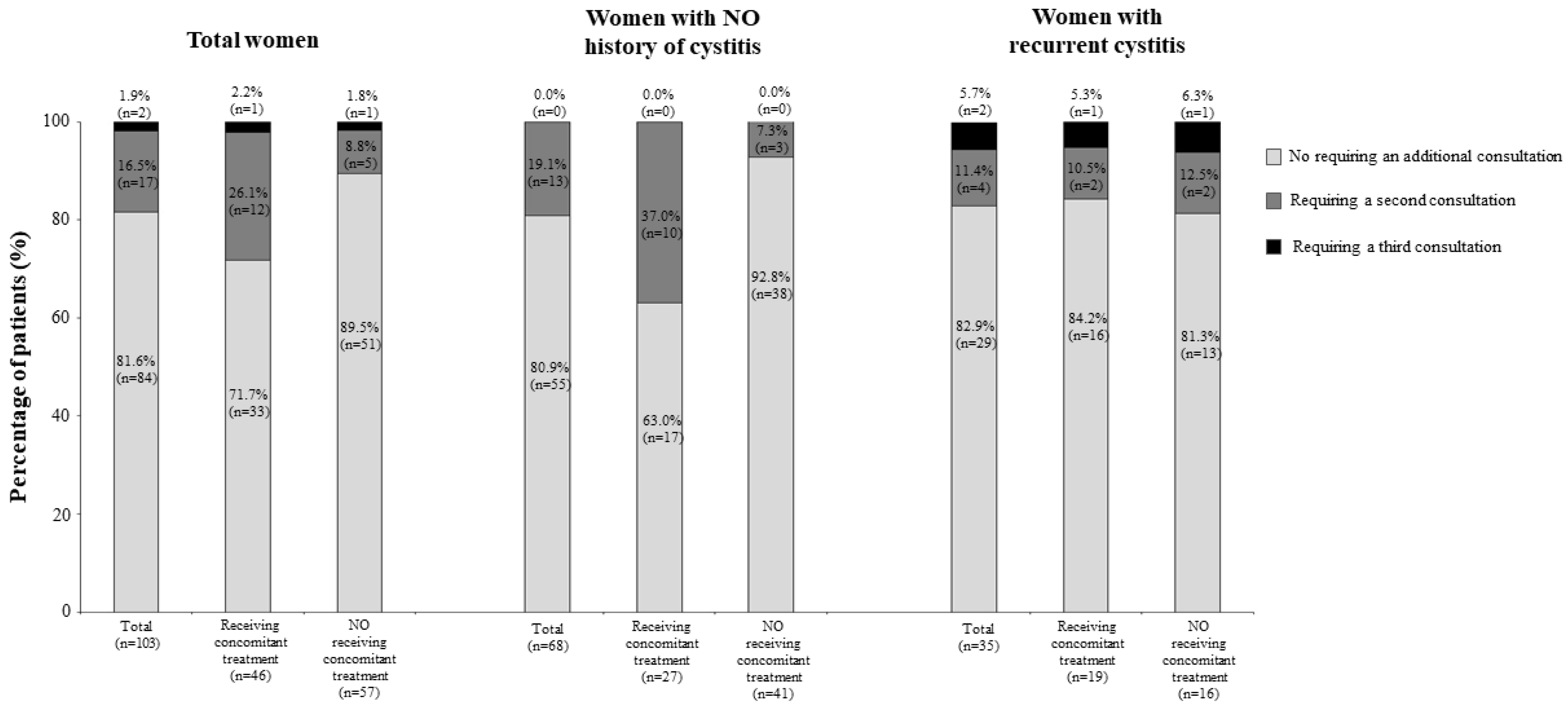
3.2. Therapeutic Management
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Medina, M.; Castillo-Pino, E. An introduction to the epidemiology and burden of urinary tract infections. Ther. Adv. Urol. 2019, 11, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.D.; Hultgren, S.J. Urinary tract infections: Microbial pathogenesis, host-pathogen interactions and new treatment strategies. Nat. Rev. Microbiol. 2020, 18, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Desouky, E. SARS-CoV-2 tropism: What urologists need to know. Afr. J. Urol. 2021, 27, 23. [Google Scholar] [CrossRef] [PubMed]
- Van Laethem, J.; Wuyts, S.C.M.; Pierreux, J.; Seyler, L.; Verschelden, G.; Depondt, T.; Meuwissen, A.; Lacor, P.; Piérard, D.; Allard, S.D. Presumed Urinary Tract Infection in Patients Admitted with COVID-19: Are We Treating Too Much? Antibiotics 2021, 10, 1493. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Tascini, C.; Novelli, A.; Anceschi, U.; Bonkat, G.; Wagenlehner, F.; Bjerklund Johansen, T.E. The Management of Urinary Tract Infections during the COVID-19 Pandemic: What Do We Need to Know? Uro 2022, 2, 55–64. [Google Scholar] [CrossRef]
- Foxman, B. Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13. [Google Scholar] [CrossRef]
- Martin, S.; Vincent, A.; Taylor, A.W.; Atlantis, E.; Jenkins, A.; Januszewski, A.; O’Loughlin, P.; Wittert, G. Lower Urinary Tract Symptoms, Depression, Anxiety and Systemic Inflammatory Factors in Men: A Population-Based Cohort Study. PLoS ONE 2015, 10, e0137903. [Google Scholar] [CrossRef]
- Kranz, J.; Schmidt, S.; Lebert, C.; Schneidewind, L.; Mandraka, F.; Kunze, M.; Helbig, S.; Vahlensieck, W.; Naber, K.; Schmiemann, G.; et al. The 2017 Update of the German Clinical Guideline on Epidemiology, Diagnostics, Therapy, Prevention, and Management of Uncomplicated Urinary Tract Infections in Adult Patients: Part 1. Urol. Int. 2018, 100, 263–270. [Google Scholar] [CrossRef]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, 103–120. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.; Bartoletti, R.; Cek, M.; Grabe, M.; Kahlmeter, G.; Pickard, R.; Bjerklund-Johansen, T.E. Antibiotic Stewardship: A Call for Action by the Urologic Community. Eur. Urol. 2013, 64, 358–360. [Google Scholar] [CrossRef]
- Bonkat, G.; Bartoletti, R.; Bruyère, F.; Cai, T.; Geerlings, S.E.; Köves, B.; Schubert, S.; Pilatz, A.; Veeratterapillay, R.; Wagenlehner, F. EAU Guidelines on Urological Infections. Available online: https://uroweb.org/guideline/urological-infections (accessed on 9 September 2022).
- Noventure. Utipro® Plus Leaflet. Available online: https://noventure.com/sites/default/files/utiproplus_utiproplusaf_leaflets.pdf (accessed on 9 September 2022).
- Piqué, N.; Gómez-Guillén, M.D.C.; Montero, M.P. Xyloglucan, a Plant Polymer with Barrier Protective Properties over the Mucous Membranes: An Overview. Int. J. Mol. Sci. 2018, 19, 673. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, S.; Wiklund, N.; Engstrand, L.; Weitzberg, E.; Lundberg, J. Effects of pH, Nitrite, and Ascorbic Acid on Nonenzymatic Nitric Oxide Generation and Bacterial Growth in Urine. Nitric Oxide 2001, 5, 580–586. [Google Scholar] [CrossRef] [PubMed]
- de Servi, B.; Ranzini, F.; Piqué, N. Effect of Utipro(®) (containing gelatin-xyloglucan) against Escherichia coli invasion of intestinal epithelial cells: Results of an in vitro study. Future Microbiol. 2016, 11, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Fraile, B.; Alcover, J.; Royuela, M.; Rodríguez, D.; Chaves, C.; Palacios, R.; Piqué, N. Xyloglucan, hibiscus and propolis for the prevention of urinary tract infections: Results of in vitro studies. Future Microbiol. 2017, 12, 721–731. [Google Scholar] [CrossRef]
- Olier, M.; Sekkal, S.; Harkat, C.; Eutamene, H.; Theodorou, V. Evaluation of reticulated gelatin-hibiscus-propolis against intestinal commensal species commonly associated with urinary tract infections. Future Microbiol. 2017, 12, 505–513. [Google Scholar] [CrossRef]
- Esposito, E.; Campolo, M.; Casili, G.; Lanza, M.; Franco, D.; Fazio, E.; Filippone, A.; Paterniti, I.; Cuzzocrea, S. Efficacy of Xyloglucan against Escherichia coli Extraintestinal Urinary Tract Infection: An in vivo Study. Microb. Physiol. 2020, 30, 50–60. [Google Scholar] [CrossRef]
- García-Larrosa, A.; Alexe, O. Efficacy and Safety of a Medical Device versus Placebo in the Early Treatment of Patients with Symptoms of Urinary Tract Infection: A Randomized Controlled Trial. Clin. Microbiol. Open Access 2016, 5, 233. [Google Scholar] [CrossRef]
- Salvatorelli, N.; García-Larrosa, A.; Allegrini, A.; Pavone, D. A New Approach to the Treatment of Uncomplicated Cystitis: Results of a Randomized Placebo-Controlled Clinical Trial. Urol. Int. 2016, 97, 347–351. [Google Scholar] [CrossRef]
- Cai, T.; Tamanini, I.; Cocci, A.; Di Maida, F.; Caciagli, P.; Migno, S.; Mereu, L.; Tateo, S.; Malossini, G.; Palmieri, A.; et al. Xyloglucan, hibiscus and propolis to reduce symptoms and antibiotics use in recurrent UTIs: A prospective study. Future Microbiol. 2019, 14, 1013–1021. [Google Scholar] [CrossRef]
- Costache, R.C.; Novac, B.; Bardan, T.R.; Agapie, D.N.; Edu, A. Xyloglucan + Gelose Combination versus Placebo as Adjuvant Therapy to First-Line Antimicrobials for Uncomplicated Urinary Tract Infection in Adults. Urol. Int. 2019, 102, 468–475. [Google Scholar] [CrossRef]
- Betschart, C.; Albrich, W.C.; Brandner, S.; Faltin, D.; Kuhn, A.; Surbek, D.; Geissbuehler, V. Guideline of the Swiss Society of Gynaecology and Obstetrics (SSGO) on acute and recurrent urinary tract infections in women, including pregnancy. Swiss. Med. Wkly. 2020, 150, w20236. [Google Scholar] [CrossRef] [PubMed]
- Storme, O.; Tirán Saucedo, J.T.; Garcia-Mora, A.; Dehesa-Dávila, M.; Naber, K.G. Risk factors and predisposing conditions for urinary tract infection. Ther. Adv. Urol. 2019, 11, 1756287218814382. [Google Scholar] [CrossRef] [PubMed]
- Renard, J.; Ballarini, S.; Mascarenhas, T.; Zahran, M.; Quimper, E.; Choucair, J.; Iselin, C.E. Recurrent Lower Urinary Tract Infections Have a Detrimental Effect on Patient Quality of Life: A Prospective, Observational Study. Infect. Dis. Ther. 2014, 4, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.; Wullt, B.; Ballarini, S.; Zingg, D.; Naber, K.G. Social and economic burden of recurrent urinary tract infections and quality of life: A patient web-based study (GESPRIT). Expert Rev. Pharm. Outcomes Res. 2018, 18, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Beerepoot, M.A.J.; Geerlings, S.E.; Van Haarst, E.P.; Van Charante, N.M.; ter Riet, G. Nonantibiotic Prophylaxis for Recurrent Urinary Tract Infections: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Urol. 2013, 190, 1981–1989. [Google Scholar] [CrossRef]
- Darouiche, R.O.; Green, B.G.; Donovan, W.H.; Chen, D.; Schwartz, M.; Merritt, J.; Mendez, M.; Hull, R.A. Multicenter Randomized Controlled Trial of Bacterial Interference for Prevention of Urinary Tract Infection in Patients With Neurogenic Bladder. Urology 2011, 78, 341–346. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.; Abramov-Sommariva, D.; Höller, M.; Steindl, H.; Naber, K.G. Non-Antibiotic Herbal Therapy (BNO 1045) versus Antibiotic Therapy (Fosfomycin Trometamol) for the Treatment of Acute Lower Uncomplicated Urinary Tract Infections in Women: A Double-Blind, Parallel-Group, Randomized, Multicentre, Non-Inferiority Phase III Trial. Urol. Int. 2018, 101, 327–336. [Google Scholar] [CrossRef]
- Cai, T.; Anceschi, U.; Tamanini, I.; Migno, S.; Rizzo, M.; Liguori, G.; Garcia-Larrosa, A.; Palmieri, A.; Verze, P.; Mirone, V.; et al. Xyloglucan, Hibiscus and Propolis in the Management of Uncomplicated Lower Urinary Tract Infections: A Systematic Review and Meta-Analysis. Antibiotics 2021, 11, 14. [Google Scholar] [CrossRef]
- Bader, M.S.; Loeb, M.; Leto, D.; Brooks, A.A. Treatment of urinary tract infections in the era of antimicrobial resistance and new antimicrobial agents. Postgrad. Med. 2020, 132, 234–250. [Google Scholar] [CrossRef]
- Butler, A.M.; Durkin, M.J.; Keller, M.R.; Ma, Y.; Powderly, W.G.; A Olsen, M. Association of Adverse Events With Antibiotic Treatment for Urinary Tract Infection. Clin. Infect. Dis. 2022, 74, 1408–1418. [Google Scholar] [CrossRef]
- Cai, T.; Konstantinidis, C.; Ward, S. A non-pharmacological approach to the treatment of urinary tract infections: Case reports with Utipro®Plus. Drugs Context 2021, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
| Total Women (N = 103) | |
|---|---|
| Age, median years (range) | 47 (19–87) |
| Diagnosis of cystitis, n (%) | |
| First episode | 68 (66.0) |
| Recurrent | 35 (34.0) |
| Number of recurrent episodes, median (range) | |
| In the last 6 months | 3 (0–5) |
| In the last 12 months | 5 (1–10) |
| Time since the last episode, median days (range) | 44.5 (12–416) |
| Comorbidities, n (%) | 13 (12.6) |
| Hypertension | 4 (30.8) |
| Type 2 diabetes | 3 (23.1) |
| Cystocele | 1 (7.7) |
| Rectocele | 1 (7.7) |
| Abdominal complaints | 1 (7.7) |
| Depression | 1 (7.7) |
| Dementia | 1 (7.7) |
| Others | 1 (7.7) |
| Factors potentially associated with UTI-like symptoms, n (%) | 75 (72.3) |
| Post-menopausal/climacteric syndrome | 32 (42.7) |
| Frequent sexual activity | 18 (24.0) |
| Premenstrual problems | 6 (8.0) |
| Diabetes | 5 (6.7) |
| Others | 12 (16) |
| Not specified | 4 (33.4) |
| Irritable bowel syndrome | 2 (16.8) |
| Vaginal dryness | 1 (8.3) |
| Obesity | 1 (8.3) |
| Laboratory results, n (%) | |
| Nitrites in urine, n available | 100 |
| Positive | 63 (63.0) |
| Leukocyte-esterase in urine, n available | 90 |
| Positive | 75 (83.3) |
| Microbiological findings in urine culture, n available | 43 |
| Positive | 24 (55.8) |
| Escherichia coli | 11 (25.6) |
| Klebsiella pneumoniae | 4 (9.3) |
| Staphylococci spp. | 1 (2.3) |
| Mixed culture | 1 (2.3) |
| Not specified, ≥10,000 CFU | 4 (9.3) |
| Not specified, ≥1000 CFU | 3 (7.0) |
| Total Women (N = 103) | |
|---|---|
| The onset of symptoms, days before consultation (%) | |
| ≥3 days | 55 (53.4) |
| 2 days | 29 (28.2) |
| 1 day | 19 (18.4) |
| Fever (body temperature ≥ 37 °C) | 2 (1.9) |
| Urinary frequency, small amounts; n (%) | |
| No frequent (up to 4 times a day) | 6 (5.8) |
| Somewhat more frequent than usual (5–6 times a day) | 20 (19.4) |
| Noticeably more frequent (7–8 times a day) | 37 (35.9) |
| Very frequent (9–10 times a day) | 40 (38.8) |
| Strong, involuntary urge to urinate, n (%) | |
| No | 11 (10.7) |
| Yes, a little | 16 (15.5) |
| Yes, moderate | 35 (34.0) |
| Yes, strong | 41 (39.8) |
| Pain and burning when urinating | |
| No | 9 (8.7) |
| Yes, a little | 20 (19.4) |
| Yes, moderate | 35 (34.0) |
| Yes, strong | 39 (37.9) |
| Feeling of not emptying the bladder fully | |
| No | 8 (7.8) |
| Yes, a little | 25 (24.3) |
| Yes, moderate | 31 (30.1) |
| Yes, strong | 39 (37.9) |
| Suprapubic pain, n (%) | |
| No | 11 (10.7) |
| Yes, a little | 27 (26.2) |
| Yes, moderate | 28 (27.2) |
| Yes, strong | 37 (35.9) |
| Blood in the urine (reddish color the urine) | |
| No | 1 (1.0) |
| Yes, a little | 4 (3.9) |
| Yes, moderate | 23 (22.3) |
| Yes, strong | 75 (72.8) |
| Total (N = 103) | Women with NO History of Cystitis (N = 68) | Women with Recurrent Cystitis (N = 35) | |
|---|---|---|---|
| Medical device | |||
| Median capsules per day (range) | 2 (2–3) | 2 (2–3) | 2 (2–3) |
| Duration of treatment, median days (range) | 5 (3–15) | 5 (3–15) | 5 (5–15) |
| Receiving concomitant medication, n (%) | 46 (44.7) | 27 (39.7) | 19 (54.3) |
| Antibiotics | 23 (50.0) | 11 (40.7) | 12 (63.2) |
| NSAIDs | 14 (30.4) | 11 (40.7) | 3 (15.8) |
| Lactobacilli/estrogen (Gynoflor®) | 7 (15.2) | 5 (18.5) | 2 (10.5) |
| Antiseptic | 6 (13.0) | 5 (18.5) | 1 (5.3) |
| Parasympatholytics | 3 (6.5) | 3 (11.1) | 0 (0.0) |
| Spasmolytics | 2 (4.3) | 1 (3.7) | 1 (5.3) |
| Antifungal | 1 (2.2) | 1 (3.7) | 0 (0.0) |
| Beta-3 adrenoceptor agonist | 1 (2.2) | 1 (3.7) | 0 (0.0) |
| Other | 1 (2.2) | 0 (0.0) | 1 (5.3) |
| Virostatic | 1 (2.2) | 1 (3.7) | 0 (0.0) |
| Sugar (D Mannose) | 1 (2.2) | 1 (3.7) | 0 (0.0) |
| Homeopathy | 1 (2.2) | 1 (3.7) | 1 (5.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, P.; Benito, E.; Berrocal, F. Clinical Experience with a Medical Device Containing Xyloglucan, Hibiscus, and Propolis for the Control of Acute Uncomplicated Urinary Tract Infection-like Symptoms. Uro 2022, 2, 245-253. https://doi.org/10.3390/uro2040027
Ortega P, Benito E, Berrocal F. Clinical Experience with a Medical Device Containing Xyloglucan, Hibiscus, and Propolis for the Control of Acute Uncomplicated Urinary Tract Infection-like Symptoms. Uro. 2022; 2(4):245-253. https://doi.org/10.3390/uro2040027
Chicago/Turabian StyleOrtega, Patricia, Esther Benito, and Félix Berrocal. 2022. "Clinical Experience with a Medical Device Containing Xyloglucan, Hibiscus, and Propolis for the Control of Acute Uncomplicated Urinary Tract Infection-like Symptoms" Uro 2, no. 4: 245-253. https://doi.org/10.3390/uro2040027
APA StyleOrtega, P., Benito, E., & Berrocal, F. (2022). Clinical Experience with a Medical Device Containing Xyloglucan, Hibiscus, and Propolis for the Control of Acute Uncomplicated Urinary Tract Infection-like Symptoms. Uro, 2(4), 245-253. https://doi.org/10.3390/uro2040027






