Transplantation of the Horseshoe Kidneys: A Model for Dual Adult Kidney Transplantation
Abstract
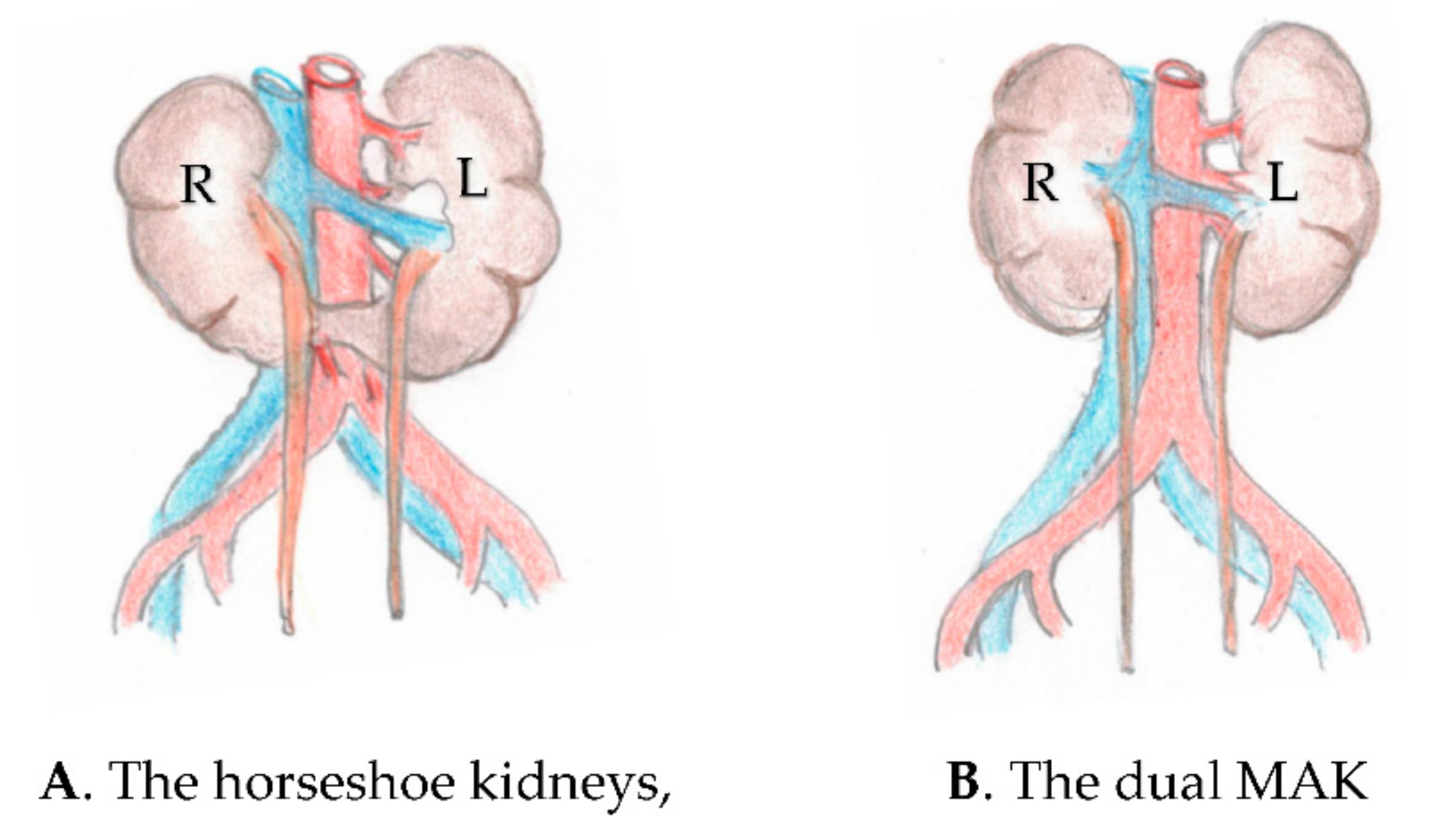
:1. Introduction
2. Material and Methods
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MAK | marginal adult kidneys. |
| UNOS | United Network for Organ Sharing. |
| SRTR | Scientific Registry of Transplant Recipients. |
| eGFR | estimated glomerular filtration rate. |
Appendix A
| En Bloc Group (n = 53) | Split Group (n = 131) | |
|---|---|---|
| Donor age (years) | 29 (1–65) | 30 (12–59) |
| Recipient age (years) | 44 (3–59) | 44 (7–69) |
| Cold ischemia time (h) | 24 (9–48) | 22 (2–61) |
| Primary nonfunction (%) | 4.3 | 13.4 |
| Delayed graft function (%) | 43 | 43 |
| Follow-up (months) | 14 (1–26) | 24 (0–166) |
| Complications rate (%) | 13 | 11.3 |
| Serum creatinine (millimole/L) | 90 (53–256) | 150 (65–400) |
| Graft survival at 22 months (%) | 87 | 87 |
References
- United Network of Organ Sharing Reports (UNOS). 2021 Data Registry. Available online: https://unos.org/data/ (accessed on 1 April 2022).
- Cooper, D.K.C.; Hara, H.; Iwase, H.; Yamamoto, T.; Jagdale, A.; Kumar, V.; Mannon, R.B.; Hanaway, M.J.; Anderson, D.J.; Eckhoff, D.E. Clinical Pig Xenotransplantation: How Close Are We? J. Am. Soc. Nephrol. 2020, 31, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Scandling, J.D.; Shen, G.K.; Salvatierra, O.; Dafoe, D.C.; Alfrey, E.J. The Kidneys that nobody wanted: Support for utilization of expanded criteria donors. Transplantation 1996, 62, 1832–1841. [Google Scholar] [CrossRef]
- Masson, D.; Hefty, T. A technique for transplantation of two adult cadaver kidneys into one recipient. J. Urol. 1998, 160, 1779–1780. [Google Scholar] [CrossRef]
- Gaber, A.O.; Shokouh-Amiri, H.; Nezakatgoo, N.; Gaber, L.W.; Saharia, A.; Shimizu, A.; Mehrazin, R.; Moore, L.W. Ipsilateral placement in double-kidney transplantation. Transplantation 2007, 84, 929–931. [Google Scholar] [CrossRef]
- Ekser, B.; Baldan, N.; Margani, G.; Furian, L.; Frison, L.; Valente, M.; Rigotti, P. Monolateral placement of both kidneys in dual kidney transplantation: Low surgical complications rate and short operating time. Transplant. Int. 2006, 19, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.C.; Dela Torre, A.; Johnson, L.B.; Schweitzer, E.J.; Bartlett, S.T. Bilateral retroperitoneal allografts: Technique for placement through a midline incision. J. Am. Coll. Surg. 1996, 183, 529–530. [Google Scholar]
- Johnson, L.B.; Kuo, P.C.; Dafoe, D.C.; Drachenberg, C.B.; Schweitzer, E.J.; Alfrey, E.J.; Ridge, L.A.; Salvatierra, P.; Papadimitriou, J.C.; Mergner, W.J.; et al. The use of bilateral adult renal allografts—A method to optimize function from donor kidneys with suboptimal nephron mass. Transplantation 1996, 61, 1261–1263. [Google Scholar] [CrossRef]
- Nghiem, D.D. Transplantation of double adult kidneys to single recipients. In Proceedings of the Movie Session of the American College of Surgeons, New Orleans, LA, USA, 7–12 October 2001. [Google Scholar]
- Veroux, P.; Giuffrida, G.; Cappellani, A.; Caglià, P.; Palmucci, S.; Sorbello, M.; Puzzo, L.; Veroux, M. Two-as-one monolateral dual kidney transplantation. Urology 2011, 77, 227–230. [Google Scholar] [CrossRef]
- Tran, K.C.; Li, D.; Taqi, A.; Sener, A.; Mc Allister, V.; Luke, P.P. Dual en bloc technique for adult renal transplantation. Clin. Transplant. 2017, 31, e13017. [Google Scholar] [CrossRef]
- Seth, A.; Sharma, A.; Singh, S.; Pandey, G.H.; Kenwar, D.B. A novel technique of dual kidney transplantation (DKT) from adult donors. Urology 2019, 130, 201–204. [Google Scholar] [CrossRef]
- Stratta, R.J.; Farney, A.C.; Orlando, G.; Farooq, U.; Al-Shraideh, Y.; Palanisamy, A.; Reeves-Daniel, A.; Doares, W.; Kaczmarski, S.; Gautreaux, M.D. Dual kidneys from Adult Marginal Donors successfully expand the limited deceased donor organ pool. Clin. Transplant. 2016, 30, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.P.; Palmer, J.M. Use of horseshoe kidney in renal transplantation. Urology 1975, 6, 357–359. [Google Scholar] [CrossRef]
- Hefty, T.R.; Olson, L.C.; Latchamsetty, K. Aortic extension for en bloc transplantation of horseshoe kidneys. Urology 2007, 69, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.B.; Lee, J.M.; Oh, C.K.; Lee, K.W.; Park, J.B.; Kim, S.J.; Lee, S.H. En bloc transplantation of horseshoe kidney in Korea. Ann. Surg. Treat. Res. 2017, 92, 168–172. [Google Scholar] [CrossRef]
- Rodriguez, S.A.; Palacios, V.H.; dos Santos, V.G.; Nicolas, V.D.; Canet, F.D.A.D.; Gordaliza, C.G.; Revilla, F.J.B. Horseshoe Kidney Transplantation: An Underpowered Source for Kidney Allografts, Review and Case Report. Nephro. Urol. Mon. 2017, 9, e61422. [Google Scholar]
- Gaillard, F.; Flamant, M.; Lemoine, S.; Baron, S.; Timsit, M.; Eladari, M.; Fournier, C.; Prot-Bertoye, C.; Bertocchio, J.; Vidal-Petiot, E.; et al. Estimated or Measured GFR in Living Kidney Donors Work-up. Am. J. Transplant. 2016, 16, 3024–3032. [Google Scholar] [CrossRef]
- Snanoudj, R.; Rabant, M.; Timsit, M.O.; Karras, A.; Savoye, E.; Tricot, L.; Loupy, A.; Hiesse, C.; Zuber, J.; Kreis, H.; et al. Donor-Estimated GFR as an Appropriate Criterion for Allocation of ECD Kidneys into Single or Dual Kidney Transplantation. Am. J. Transplant. 2009, 9, 2542–2651. [Google Scholar] [CrossRef] [PubMed]
- Butler-Peres, K.; Scalea, J.R. Horseshoe kidney in a deceased organ donor: A rare glimpse at an uncommon finding. Lancet 2018, 391, 10134. [Google Scholar] [CrossRef]
- Sengupta, B.; Khan, I.; Saighi, A.; Gaw, E.A.; Taufeeq, M.; Al Qahtani, M.S.; Obeid, M. En Bloc Transplantation of Horseshoe Kidney from Deceased Donor: An Unusual Transplantation Utilizing Kidneys with Congenital Fusion Abnormality. Case Rep. Transplant. 2021, 2021, 1–4. [Google Scholar] [CrossRef]
- Nghiem, D.D. Simultaneous double adult kidney transplantation using single arterial and venous anastomosis. Urology 2006, 67, 1076–1078. [Google Scholar] [CrossRef]
- Stroosma, O.B.; Wilhelmus, G.; Schurink, H.; Koostra, G. Current opinions in horseshoe kidney transplantation. Transplant. Int. 2002, 15, 196–199. [Google Scholar] [CrossRef]
- Stroosma, O.B.; Shurink, G.W.; Smits, J.M.; Koostra, G. Transplanting horseshoe kidneys: A worldwide survey. J. Urol. 2001, 166, 2039–2042. [Google Scholar] [CrossRef]
- Nghiem, D.D. Use of a single stent for double ureter support in transplantation. Transplant. Int. 2005, 8, 55–56. [Google Scholar] [CrossRef]
- Nemes, B.; Kanyari, Z.; Zadorri, G.; Zsom, L.; Berhes, M.; Hamar, M.; Kobor, K.; Peter, A. Horseshoe kidney transplantation. Interv. Med. Appl. Sci. 2015, 7, 85–89. [Google Scholar] [CrossRef]
- Bauer, S.B. Anomalies of the upper urinary tract. In Campbell-Walsh Urology, 9th ed.; Wein, A.J., Kavoussi, L.R., Novick, A.C., Partin, A.W., Peters, C.A., Eds.; Elsevier: Philadelphia, PA, USA, 2007; pp. 3287–3291. [Google Scholar]
- Uzzo, R.G.; Hsu, H.S.; Goldfarb, D.A.; Taylor, R.J.; Novick, A.C.; Gill, I.S. Strategies for transplantation of cadaveric kidneys with congenital fusion anomalies. J. Urol. 2001, 165, 761–765. [Google Scholar] [CrossRef]
- Galganski, L.A.; Perez, R.V.; Troppmann, C.; McVicar, J.; Santhanakrishnan, C.; Mortimer, B.; Kelly, B.; Sageshima, J. Use of Hypothermic Machine Perfusion to Identify Anatomic Variation Before Transplantation of a Pancake Kidney: A Case Report. Transplant. Direct 2019, 5, e445. [Google Scholar] [CrossRef]
- Justo-Janeiro, J.M.; Orozco, E.P.; Reyes, F.I.R.E.; de la Paredes, R.; de Lara Espinosa, A.L.; Naylor, J.M. Transplantation of a horseshoe kidney from a living donor: Case report, long term outcome and donor safety. Int. J. Surg. Case Rep. 2015, 15, 21–25. [Google Scholar] [CrossRef]
- Sozener, U. Transplantation of a horseshoe kidney from a living donor using stapler for transection. J. Surg. Case Rep. 2019, 11, rjz299. [Google Scholar] [CrossRef]
- Tan, H.; Samaniego, M.D.; Montgomery, R.A.; Burdick, J.F.; Maley, W.R.; Kraus, E.S.; Ratner, L.E. Donor Horseshoe Kidneys for Transplantation. Transplantation 2001, 72, 869–873. [Google Scholar] [CrossRef]
- Zipitis, C.S.; Augustine, T.; Tavakoli, A.; Surange, A.; Agrawal, H.N. Horseshoe kidney transplantation. Surgeon 2003, 1, 160–163. [Google Scholar] [CrossRef]
- Martinez-Mier, G.; Rayhilloye, S.C.; Katz, D.A. Cadaveric kidney transplant using horseshoe kidney: Report of two cases. Cir. Cir. 2005, 73, 211–216. [Google Scholar] [PubMed]
- Pedro, R.N.; Leitao, V.A.; Miyaoka, A.; Fregonesa, A.; Villas Boas, C.C.; Reges, R.; Matheus, W.E.; Netto, N.R. Split techniques in horseshoe kidney transplantation. Case Rep. Urol. Int. 2006, 77, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, A.T.; Shapiro, R.; Kayler, L.K. Transplantation of a Horseshoe Kidney. Transplantation 2007, 83, 828–829. [Google Scholar] [CrossRef] [PubMed]
- Salehipoor, M.; Bahador, A.; Salahi, H.; Nikeghbali, S.; Jalaeian, H.; Davari, H.R.; Hossein, S.A.M. Transplantation of a horseshoe kidney. Case Rep. Arch. Iran. Med. 2007, 10, 239–241. [Google Scholar]
- De Serres, S.A.; Caumartin, Y.; Noel, R.; Lachance, J.G.; Cote, I.; Naud, A.; Fradet, Y.; Mfarrej, B.G.; Agharazii, M.; Houde, I. Dual Kidney Transplantation as an Alternative for Very Marginal Donors: Long-Term Follow-Up in 63 patients. Transplantation 2010, 90, 1125–1130. [Google Scholar] [CrossRef]
- Ekser, B.; Furian, L.; Broggiato, A.; Silvestre, C.; Pierobon, E.S.; Baldan, N.; Rigotti, P. Technical Aspects of Unilateral Dual Kidney Transplantation from Expanded Criteria Donors: Experience of 100 Patients. Am. J. Transplant. 2010, 10, 2000–2007. [Google Scholar] [CrossRef]
- Troppmann, C.; Santhanakrishnan, C.; Fananazapir, G.; Troppmann, K.; Perez, R. Pediatric en bloc kidney transplantation from very small (</=10 kg) donation after circulatory death (versus brain death) donors: Single-center matched-pair analysis of 130 transplants. Am. J. Transplant. 2018, 11, 2811–2817. [Google Scholar] [CrossRef]
- Mangus, R.S.; Hoag, B.W. Stented vs. non stented extravesical Uretero-neo-cystostomy in Renal Transplantation, A Meta-analysis. Am. J. Transplant. 2004, 4, 1889–1896. [Google Scholar] [CrossRef]
- Nghiem, D.D. En bloc transplantation of kidneys from donors under 15 kg into adult recipients. J. Urol. 1991, 145, 14–16. [Google Scholar] [CrossRef]
- Ojo, P.; Ranga, K.V.; Brown, M.; Hull, D.; Charpentier, K.P. Transplantation of a unilateral fused kidney with inferior ectopia: Revascularization utilizing donor aorta and vena-cava. Case Rep. Conn. Med. 2008, 72, 585–588. [Google Scholar]
- Pontinen, T.; Khanmoradi, K.; Kumar, A.; Kudsi, H.; Kung, S.C.; Chewaproug, D.; Zaki, R.; Ortiz, J. Horseshoe kidneys: An underutilized resource in kidney transplant. Case Rep. Exp. Clin. Transplant. 2010, 8, 74–78. [Google Scholar]
- Hau, H.M.; Morgul, H.M.; Uhlman, D.; Thelen, A.; Fellmer, P.; Benckert, C.; Tautenhaln, H.M.; Bartels, M.; Jonas, S. Horseshoe kidney for transplantation: Technical considerations. Cases Rep. Scand. J. Urol. 2013, 47, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Ordones, F.V.; Yamamoto, H.; Paiolli, P.I.; Meduna, R.R.; Filho, F.F.G.; Guerra, R.; Amaro, J.L.; Kawano, P.R. Transplanting a horseshoe kidney: A case report and review of surgical strategies. Urol. Cases Rep. 2018, 21, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Kaabas, M.M.; Babenko, N.N.; Zokoey, A.K.; Khovrin, V.V.; Galyan, T.N. Renal Transplantation from a Living Donor with a Horseshoe Kidney. Transplant. Direct 2016, 1, e53. [Google Scholar]
- Zarrabi, A.D.; Wessels, S.G.; Viok, L.; van der Merwe, A. Successful en bloc transplantation of a horseshoe kidney without division of the isthmus: First case reported in South Africa. S. Afr. J. Surg. 2018, 56, 43–45. [Google Scholar] [CrossRef]
- Tadros, N.N. Horseshoe Kidney Treatment & Management Medscape 25 February 2020. Available online: https://emedicine.medscape.com/article/441510-treatment (accessed on 31 March 2022).
- Drug Overdose Deaths in the U.S. Top 100,000 Annually. Center for Disease Control and Prevention, as of 17 November 2021. Available online: https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2021/20211117.htm (accessed on 31 March 2022).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nghiem, D.D. Transplantation of the Horseshoe Kidneys: A Model for Dual Adult Kidney Transplantation. Uro 2022, 2, 157-165. https://doi.org/10.3390/uro2030019
Nghiem DD. Transplantation of the Horseshoe Kidneys: A Model for Dual Adult Kidney Transplantation. Uro. 2022; 2(3):157-165. https://doi.org/10.3390/uro2030019
Chicago/Turabian StyleNghiem, Dai D. 2022. "Transplantation of the Horseshoe Kidneys: A Model for Dual Adult Kidney Transplantation" Uro 2, no. 3: 157-165. https://doi.org/10.3390/uro2030019
APA StyleNghiem, D. D. (2022). Transplantation of the Horseshoe Kidneys: A Model for Dual Adult Kidney Transplantation. Uro, 2(3), 157-165. https://doi.org/10.3390/uro2030019






