Serenoa Repens (Saw Palmetto) for Lower Urinary Tract Symptoms (LUTS): The Evidence for Efficacy and Safety of Lipidosterolic Extracts. Part II
Abstract
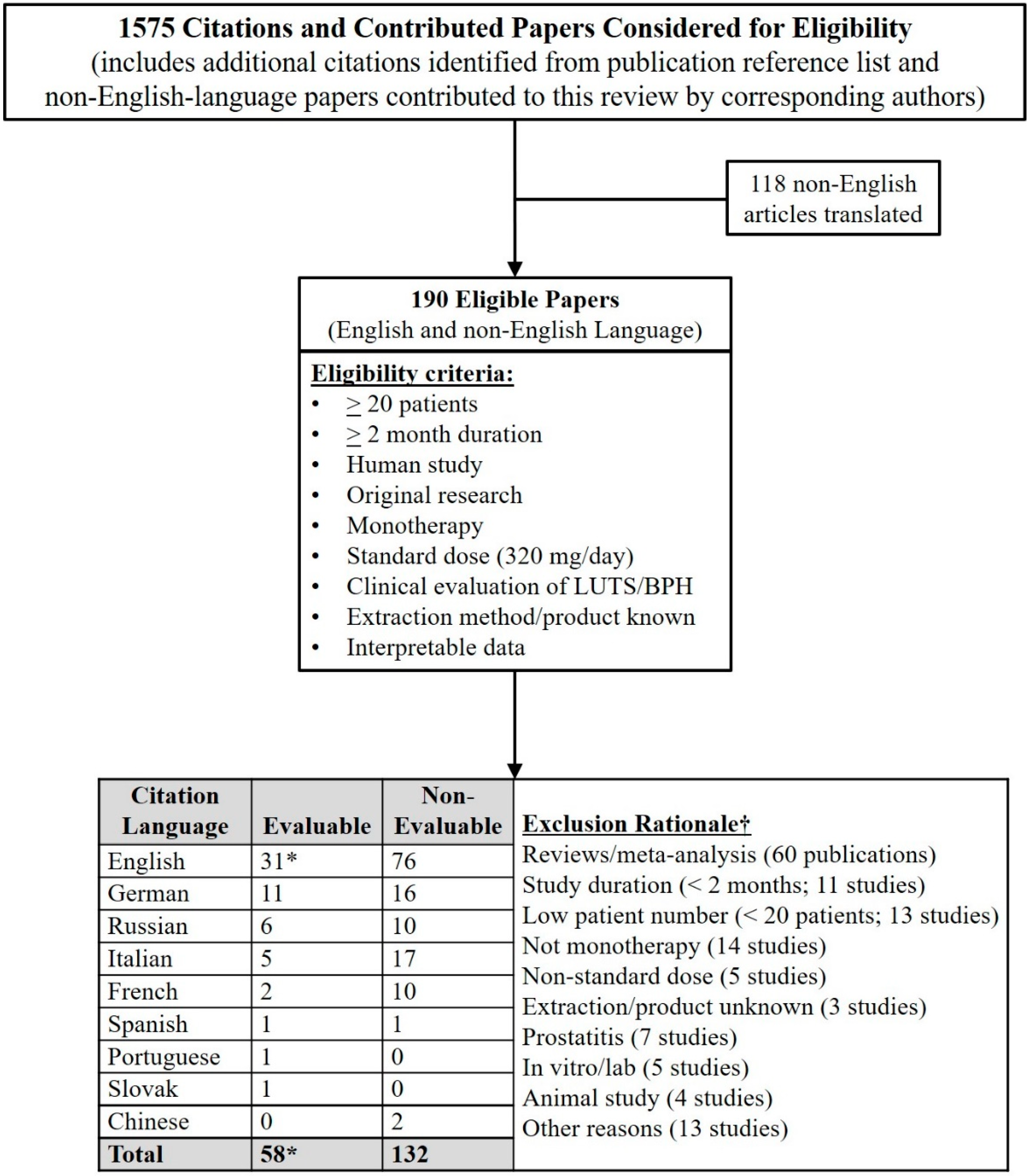
1. Search Strategy and Selection Criteria Were Pivotal to Perspective on LSESr vs. LUTS
2. LSESr Has a High Safety Profile in Contrast to Counterpart Prescription Drugs
3. An Anti-Inflammatory Effect Appears to Be the Major Mechanism of Action of LSESr
3.1. 5α-Reductase Inhibition
3.2. Anti-Inflammatory Effect
4. The Onset of LUTS Response to LSESr Occurs as Early as 4 Weeks
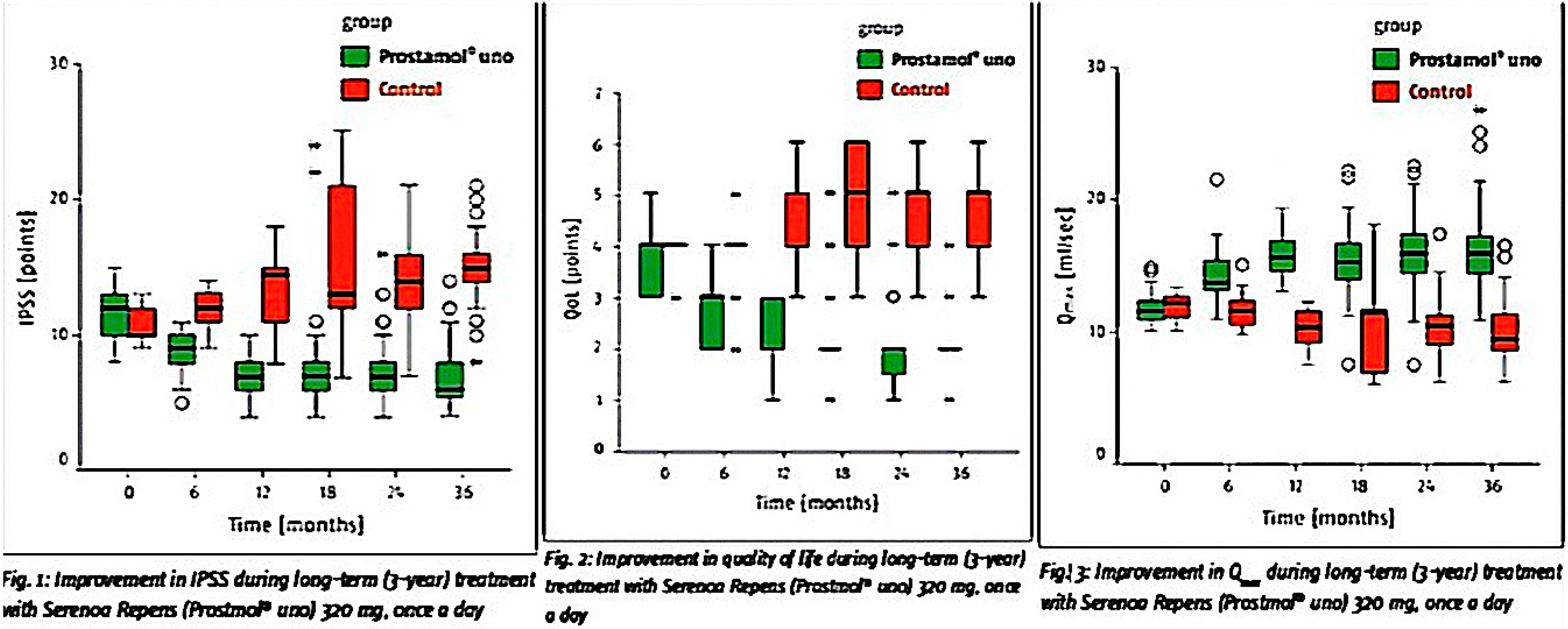
5. The Response to LSESr vs. LUTS Is Durable
6. The Early Use of LSESr Delays the Progression of LUTS/BPH
- The patient’s subjective evaluation of the aggravation of their condition, as determined by the IPSS, is a relevant sign of BPH progression since it helps a doctor choose the most appropriate treatment.
- When an active supervision method is applied, the medicinal preparations are prescribed as soon as BPH symptoms start impacting the patient’s quality of life. Therefore, the study of long-term pharmacological therapy of patients with minimal subjective manifestations of BPH and the risk of its progression is of utmost relevance.
- Serenoa’s complex pathogenetic effects are aimed at both inhibiting the process of BPH development and eliminating the symptoms of chronic prostatitis.
- Because Serenoa does not decrease the PSA level it does not conceal the development of prostate cancer.
- The presence of the risk of BPH progression was a necessary criterion for admission of patients into the observation group. The reason for this is that the presence of men not subject to the risk of BPH progression would have complicated our ability to prove efficacy using a multi-year continuous administration of the extract of Serenoa repens to prevent BPH progression.
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethical Approval/Patient Consent
References
- Alken, C.E. Leitfaden Der Urologie, 6th ed.; Thieme: Stuttgart, Germany, 1973; pp. 180–182. [Google Scholar]
- Vahlensieck, W. Konservative behandlung von prostatadenomen. Urol. B 1972, 12, 182. [Google Scholar]
- Vahlensieck, W. Konservative behandlung von prostatadenomen. Urol. B 1973, 13, 176. [Google Scholar]
- Robert, G.Y. Comparison of the Effects of Hexanic extract of Serenoa repens(Permixon) and tamsulosin on inflammatory biomarkers in the treatment of benign prostatic hyperplasia-related lower urinary tract symptoms. Eur. Urol. Suppl. 2015, 14, e1470–e1474. [Google Scholar] [CrossRef]
- Latil, A.; Petrissans, M.T.; Rouquet, J.; Robert, G.; de la Taille, A. Effects of hexanic extract of Serenoa repens (Permixon(R) 160 mg) on inflammation biomarkers in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Prostate 2015, 75, 1857–1867. [Google Scholar] [CrossRef]
- Cirillo-Marucco, E.; Pagliarulo, A.; Tritto, G.; Piccinno, A.; Di Rienzo, U. Serenoa repens extract (Permixon®) in the early treatment of prostatic hypertrophy. Urol. J. 1983, 50, 1269–1277. (In Italian) [Google Scholar] [CrossRef]
- Cukier; Ducassou; Guillou, L. Permixon versus placebo. CR Ther. Pharmacol. Clin. 1985, 4, 15–21. (In French) [Google Scholar]
- Tosto, A.; Rovereto, B.; Paoletti, M.C.; Rizzo, M.; Nicolucci, A.; Costantini, A. Serenoa Repens extract in the treatment of functional disorders secondary to adenoma of the prostate: Considerations on 20 cases. Urol. J. 1985, 52, 536–542. (In Italian) [Google Scholar] [CrossRef]
- Pannunzio, E.; D’Ascenzo, R.; Giardinetti, F.; Civili, P.; Persichelli, E. Serenoa Repens vs. Gestonorone Caproato in the treatment of benign prostatic hypertrophy: Randomized study. Urol. J. 1986, 53, 696–705. (In Italian) [Google Scholar] [CrossRef]
- Pescatore, D.; Calvi, P.; Michelotti, P. Urodynamic assessment of treatment in patients with prostatic adenoma with Serenoa repens extract. Urol. J. 1986, 53, 894–897. (In Italian) [Google Scholar] [CrossRef]
- Authie, D.; Cauquil, J. Assessment of the effectiveness of Permixon * in daily practice multicentric study. CR Ther. Pharmacol. Clin. 1987, 5, 3–13. (In French) [Google Scholar]
- Carreras, O.J. Our experience with hexane extract from Serenoa repens in the treatment of benign prostatic hypertrophy. Arch. Esp. Urol. 1987, 40, 310–313. (In Spanish) [Google Scholar]
- Orfei, S.; Grumelli, B.; Galetti, G. Clinical and uroflowimetric evaluation of Permixon® in geriatrics. Urol. J. 1988, 55, 373–381. (In Italian) [Google Scholar] [CrossRef]
- Mattei, F.M.; Capone, M.; Acconcia, A. Medicamentous therapy of benign prostatic hyperplasia with an extract of the sagebrush. TW Urol. Nephrol. 1990, 2, 346–350. (In German) [Google Scholar]
- Dathe, G.; Schmid, H. Phytotherapy for benign prostatic hyperplasia (BPH) with extractum Serenoa repens (Permixon). Urol. Ausg. B 1991, 31, 223–330. (In German) [Google Scholar]
- Vahlensieck, W., Jr.; Volp, A.; Lubos, W.; Kuntze, M. Benign prostatic hyperplasia--treatment with sabal fruit extract. A treatment study of 1,334 patients. Fortschr. Med. 1993, 111, 323–326. (In German) [Google Scholar]
- Vahlensieck, W.; Völp, A.; Kuntze, M.; Lubos, W. Changes in micturition in patients with benign prostatic hyperplasia under sabal fruit treatment. Urol. Ausg. B 1993, 33, 380–383. (In German) [Google Scholar]
- Fabricius, P.G.; Vahlensieck Jr., W. Therapy for benign prostatic hyperplasia: Sabal fruit extract: One dose is enough! Therapiewoche 1993, 43, 1616–1620. (In German) [Google Scholar]
- Derakhshani, P.; Geerke, H.; Böhnert, K.J.; Engelmann, U. Influencing the international prostate symptom score during therapy with saw palmetto fruit extract with a single daily dose. Der. Urol. B 1997, 37, 384–391. (In German) [Google Scholar] [CrossRef]
- Eickenberg, H.U. Treatment of benign prostatic hyperplasia with a lipophilic extract from saw palmetto fruits (Sita). Der. Urol. B 1997, 37, 130–133. (In German) [Google Scholar] [CrossRef]
- Foroutan, F. Effectiveness and tolerability of Permixon in a larger patient population (592 patients) under practical conditions. J. Urol. Urogynäkol. 1997, 2, 17–21. (In German) [Google Scholar]
- Redecker, K.D.; Funk, P. Sabal-Extrakt WS 1473 bei benigner Prostatahyperplasie. Extracta. Urol. 1998, 21, 23–25. [Google Scholar]
- Ziegler, H.; Holscher, U. Efficacy of saw palmetto fruit special extract WS 1473 in patients with Alken stage I-II benign prostatic hyperplasia-open multicentre study. Jatros. Urol. 1998, 14, 34–43. (In German) [Google Scholar]
- Bauer, H.W.; Casarosa, C.; Cosci, M.; Fratta, M.; Blessmann, G. Saw palmetto fruit extract for treatment of benign prostatic hyperplasia. Results of a placebo-controlled double-blind study. MMW Fortschr. Med. 1999, 141, 62. (In German) [Google Scholar] [PubMed]
- Medeiros, A.S.; Verona, C.B.M.; Mattos, D., Jr.; Silva, E.G.; Fonseca, G.N.; Begliomini, H.; Pous, J.H.; Cury, J.; Costa, M.M.; Prado, M.J.; et al. Efficacy and tolerability of the extract of Serenoa repens in a multicentric study in patients with symptomatic benign prostatic hyperplasia. Rev. Bras. Med. 2000, 57, 321–324. (In Portuguese) [Google Scholar]
- Aliaev, G.; Vinarov, A.Z.; Lokshin, K.L.; Spivak, L.G. Five-year experience in treating patients with prostatic hyperplasia patients with permixone (Serenoa repens Pierre Fabre Medicament). Urology 2002, 1, 23–25. (In Russian) [Google Scholar]
- Breza, J.; Kliment, J.; Valansky, L.; Capova, G. Prostamol uno (alcohol extract of the fruits of Serenoa repens) in the treatment of symptomatic benign prostatic hyperplasia. Lek. Obz. 2005, 54, 139–144. (In Slovakian) [Google Scholar]
- Aliaev, Y.G.; Apolikhin, O.I.; Mazo, E.B.; Vinarov, A.Z.; Lokshin, K.L.; Medvedev, A.A.; Permyakova, O.V.; Spivak, L.G.; Shkol’nikov, M.E. First results of a clinical trial of the efficacy and safety of Prostamol® Uno in patients with the early signs of prostatic hyperplasia. Eff. Pharm. Urol 2007, 8, 11. (In Russian) [Google Scholar]
- Razumov, S.V.; Egorov, A.A. Prostamol Uno and α-1-blockers in combination therapy for benign prostatic hyperplasia and the transition to monotherapy with Prostamol Uno. Eff Pharm. Urol 2007, 1, 28–30. (In Russian) [Google Scholar]
- Aliaev, G.; Apolikhin, O.I.; Mazo, E.B.; Vinarov, A.Z.; Lokshin, K.L.; Medvedev, A.A.; Permiakova, O.V.; Spivak, L.G.; Shkol’nikov, M.E. Efficacy and safety of Prostamol-UNO in the treatment of patients with initial symptoms of prostatic adenoma and risk of progression: 2 years of investigations. Urology 2009, 4, 36–40. (In Russian) [Google Scholar]
- Vinarov, A.Z.; Aliaev Yu, G.; Apolikhin, O.I.; Mazo, E.B.; Darenkov, S.P.; Iu, D.L.; Lokshin, K.L.; Medvedev, A.A.; Permiakova, O.V.; Spivak, L.G.; et al. Results of three-year clinical study of Prostamol Uno efficacy and safety in patients with initial symptoms of prostatic adenoma and risk of its progression. Urology 2010, 6, 3–10. (In Russian) [Google Scholar]
- Aliaev, G.; Vinarov, A.Z.; Iu, D.L.; Spivak, L.G. The results of the 10-year study of efficacy and safety of Serenoa repens extract in patients at risk of progression of benign prostatic hyperplasia. Urology 2013, 4, 32–36. (In Russian) [Google Scholar]
- Breza, J.; Dzurny, O.; Borowka, A.; Hanus, T.; Petrik, R.; Blane, G.; Chadha-Boreham, H. Efficacy and acceptability of tadenan (Pygeum africanum extract) in the treatment of benign prostatic hyperplasia (BPH): A multicentre trial in central Europe. Curr. Med. Res. Opin. 1998, 14, 127–139. [Google Scholar] [CrossRef]
- Bach, D.; Ebeling, L. Long-term drug treatment of benign prostatic hyperplasia - results of a prospective 3-year multicenter study using Sabal extract IDS 89. Phytomedicine 1996, 3, 105–111. (In German) [Google Scholar] [CrossRef]
- Djavan, B.; Fong, Y.K.; Chaudry, A.; Reissigl, A.; Anagnostou, T.; Bagheri, F.; Waldert, M.; Fajkovic, H.; Marihart, S.; Harik, M.; et al. Progression delay in men with mild symptoms of bladder outlet obstruction: A comparative study of phytotherapy and watchful waiting. World J. Urol. 2005, 23, 253–256. [Google Scholar] [CrossRef]
- Pytel, Y.A.; Vinarov, A.; Lopatkin, N.; Sivkov, A.; Gorilovsky, L.; Raynaud, J.P. Long-term clinical and biologic effects of the lipidosterolic extract of Serenoa repens in patients with symptomatic benign prostatic hyperplasia. Adv. Ther. 2002, 19, 297–306. [Google Scholar] [CrossRef]
- Sinescu, I.; Geavlete, P.; Multescu, R.; Gangu, C.; Miclea, F.; Coman, I.; Ioiart, I.; Ambert, V.; Constantin, T.; Petrut, B.; et al. Long-term efficacy of Serenoa repens treatment in patients with mild and moderate symptomatic benign prostatic hyperplasia. Urol. Int. 2011, 86, 284–289. [Google Scholar] [CrossRef]
- Vinarov, A.Z.; Spivak, L.G.; Platonova, D.V.; Rapoport, L.M.; Korolev, D.O. 15 years’ survey of safety and efficacy of Serenoa repens extract in benign prostatic hyperplasia patients with risk of progression. Urology 2019, 86, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Descotes, J.L.; Rambeaud, J.J.; Deschaseaux, P.; Faure, G. Placebo-Controlled evaluation of the efficacy and. tolerability of Permixon® in benign prostatic hyperplasia after exclusion of placebo responders. Clin. Drug Investig. 1995, 9, 291–297. [Google Scholar] [CrossRef]
- Laekeman, G.; Vlietinck, A. Assessment Report on Serenoa Repens (W. Bartram) Small, Fructus. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2014/12/WC500179593.pdf (accessed on 14 November 2020).
- MacDonald, R.; Tacklind, J.W.; Rutks, I.; Wilt, T.J. Serenoa repens monotherapy for benign prostatic hyperplasia (BPH): An updated Cochrane systematic review. BJU Int. 2012, 109, 1756–1761. [Google Scholar] [CrossRef]
- Novara, G.; Giannarini, G.; Alcaraz, A.; Cozar-Olmo, J.M.; Descazeaud, A.; Montorsi, F.; Ficarra, V. Efficacy and Safety of hexanic lipidosterolic extract of Serenoa repens (Permixon) in the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia: Systematic review and meta-analysis of randomized controlled trials. Eur. Urol. Focus 2016, 2, 553–561. [Google Scholar] [CrossRef]
- Stepanov, V.N.; Siniakova, L.A.; Sarrazin, B.; Raynaud, J.P. Efficacy and tolerability of the lipidosterolic extract of Serenoa repens (Permixon) in benign prostatic hyperplasia: A double-blind comparison of two dosage regimens. Adv. Ther. 1999, 16, 231–241. [Google Scholar] [PubMed]
- Tacklind, J.; Macdonald, R.; Rutks, I.; Stanke, J.U.; Wilt, T.J. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst. Rev. 2012, 12, CD001423. [Google Scholar] [CrossRef]
- Vela-Navarrete, R.; Alcaraz, A.; Rodriguez-Antolin, A.; Minana Lopez, B.; Fernandez-Gomez, J.M.; Angulo, J.C.; Castro Diaz, D.; Romero-Otero, J.; Brenes, F.J.; Carballido, J.; et al. Efficacy and safety of a hexanic extract of Serenoa repens (Permixon((R)) ) for the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS/BPH): Systematic review and meta-analysis of randomised controlled trials and observational studies. BJU Int. 2018, 122, 1049–1065. [Google Scholar]
- Wilt, T.; Ishani, A.; Mac Donald, R. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst. Rev. 2002. [Google Scholar] [CrossRef]
- Avins, A.L.; Bent, S.; Staccone, S.; Badua, E.; Padula, A.; Goldberg, H.; Neuhaus, J.; Hudes, E.; Shinohara, K.; Kane, C. A detailed safety assessment of a saw palmetto extract. Complement Ther. Med. 2008, 16, 147–154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schulman, C.C.; Cortvriend, J.; Jonas, U.; Lock, T.M.; Vaage, S.; Speakman, M.J. Tamsulosin: 3-year long-term efficacy and safety in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction: Analysis of a European, multinational, multicenter, open-label study. European tamsulosin study group. Eur. Urol. 1999, 36, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Huang, J.; Zhou, L.; Chen, S.; Wang, Z.; Ma, L.; Wang, D.; Wang, G.; Wang, S.; Liang, C.; et al. Efficacy and safety of Serenoa repens extract among patients with benign prostatic hyperplasia in china: A multicenter, randomized, double-blind, placebo-controlled trial. Urology 2019, 129, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Avins, A.L.; Lee, J.Y.; Meyers, C.M.; Barry, M.J.; Group, C.S. Safety and toxicity of saw palmetto in the CAMUS trial. J. Urol. 2013, 189, 1415–1420. [Google Scholar] [CrossRef]
- Giuliano, F. Impact of medical treatments for benign prostatic hyperplasia on sexual function. BJU Int. 2006, 97 (Suppl. 2), 34–38. [Google Scholar] [CrossRef]
- Carraro, J.C.; Raynaud, J.P.; Koch, G.; Chisholm, G.D.; Di Silverio, F.; Teillac, P.; Da Silva, F.C.; Cauquil, J.; Chopin, D.K.; Hamdy, F.C.; et al. Comparison of phytotherapy (Permixon) with finasteride in the treatment of benign prostate hyperplasia: A randomized international study of 1,098 patients. Prostate 1996, 29, 231–240. [Google Scholar] [CrossRef]
- Zlotta, A.R.; Teillac, P.; Raynaud, J.P.; Schulman, C.C. Evaluation of male sexual function in patients with lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH) treated with a phytotherapeutic agent (Permixon), Tamsulosin or Finasteride. Eur. Urol. 2005, 48, 269–276. [Google Scholar] [CrossRef]
- Razumov, S.V.; Egorov, A.A. Expediency of switching from combined therapy with prostamol Uno and alpha-1-adrenoblockers to monotherapy with prostamol Uno in patients with prostatic adenoma. Urology 2007, 3, 47–50. (In Russian) [Google Scholar]
- Buck, A.C. Is there a scientific basis for the therapeutic effects of Serenoa repens in benign prostatic hyperplasia? Mechanisms of action. J. Urol. 2004, 172, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- de la Taille, A. Therapeutic approach: The Importance of controlling prostatic inflammation. Eur. Urol. Suppl. 2013, 12, 116–122. [Google Scholar] [CrossRef]
- Ficarra, V.; Rossanese, M.; Zazzara, M.; Giannarini, G.; Abbinante, M.; Bartoletti, R.; Mirone, V.; Scaglione, F. The role of inflammation in lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH) and its potential impact on medical therapy. Curr. Urol. Rep. 2014, 15, 463. [Google Scholar] [CrossRef] [PubMed]
- Habib, F.K. Serenoa repens: The Scientific basis for the treatment of benign prostatic hyperplasia. Eur. Urol. Suppl. 2009, 8, 887–893. [Google Scholar] [CrossRef]
- Wang, K.; Fan, D.D.; Jin, S.; Xing, N.Z.; Niu, Y.N. Differential expression of 5-alpha reductase isozymes in the prostate and its clinical implications. Asian J. Androl. 2014, 16, 274–279. [Google Scholar] [PubMed]
- Yamana, K.; Labrie, F.; Luu-The, V. Human type 3 5alpha-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride. Horm. Mol. Biol. Clin. Investig. 2010, 2, 293–299. [Google Scholar] [PubMed]
- Scaglione, F.; Lucini, V.; Pannacci, M.; Dugnani, S.; Leone, C. Comparison of the potency of 10 different brands of Serenoa repens extracts. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 569–574. [Google Scholar]
- Di Silverio, F.; Monti, S.; Sciarra, A.; Varasano, P.A.; Martini, C.; Lanzara, S.; D’Eramo, G.; Di Nicola, S.; Toscano, V. Effects of long-term treatment with Serenoa repens (Permixon) on the concentrations and regional distribution of androgens and epidermal growth factor in benign prostatic hyperplasia. Prostate 1998, 37, 77–83. [Google Scholar] [CrossRef]
- Marks, L.S.; Hess, D.L.; Dorey, F.J.; Luz Macairan, M.; Cruz Santos, P.B.; Tyler, V.E. Tissue effects of saw palmetto and finasteride: Use of biopsy cores for in situ quantification of prostatic androgens. Urology 2001, 57, 999–1005. [Google Scholar] [CrossRef]
- Rhodes, L.; Primka, R.L.; Berman, C.; Vergult, G.; Gabriel, M.; Pierre-Malice, M.; Gibelin, B. Comparison of finasteride (Proscar), a 5α reductase inhibitor, and various commercial plant extracts in vitro and in vivo 5α reductase inhibition. Prostate 1993, 22, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Strauch, G.; Perles, P.; Vergult, G.; Gabriel, M.; Gibelin, B.; Cummings, S.; Malbecq, W.; Malice, M.P. Comparison of finasteride (Proscar®) and Serenoa repens (Permixon®) in the inhibition of 5-Alpha reductase in healthy male volunteers. Eur. Urol. 1994, 26, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Ekman, P. Finasteride in the treatment of benign prostatic hypertrophy: An update. New indications for finasteride therapy. Scand. J. Urol. Nephrol. 1999, 203, 15–20. [Google Scholar] [CrossRef]
- Stuart, J.D.; Lee, F.W.; Simpson Noel, D.; Kadwell, S.H.; Overton, L.K.; Hoffman, C.R.; Kost, T.A.; Tippin, T.K.; Yeager, R.L.; Batchelor, K.W.; et al. Pharmacokinetic parameters and mechanisms of inhibition of rat type 1 and 2 steroid 5alpha-reductases: Determinants for different in vivo activities of GI198745 and finasteride in the rat. Biochem. Pharm. 2001, 62, 933–942. [Google Scholar] [CrossRef]
- Kim, S.H.; Jung, K.I.; Koh, J.S.; Min, K.O.; Cho, S.Y.; Kim, H.W. Lower urinary tract symptoms in benign prostatic hyperplasia patients: Orchestrated by chronic prostatic inflammation and prostatic calculi? Urol. Int. 2013, 90, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Nickel, J.C.; Roehrborn, C.G.; Castro-Santamaria, R.; Freedland, S.J.; Moreira, D.M. Chronic Prostate inflammation is associated with severity and progression of benign prostatic hyperplasia, lower urinary tract symptoms and risk of acute urinary retention. J. Urol. 2016, 196, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Nickel, J.C.; Roehrborn, C.G.; O’Leary, M.P.; Bostwick, D.G.; Somerville, M.C.; Rittmaster, R.S. The relationship between prostate inflammation and lower urinary tract symptoms: Examination of baseline data from the REDUCE trial. Eur. Urol. 2008, 54, 1379–1384. [Google Scholar] [CrossRef]
- Robert, G.; Descazeaud, A.; Allory, Y.; Vacherot, F.; De La Taille, A. Should we investigate prostatic inflammation for the management of benign prostatic hyperplasia? Eur. Urol. Suppl. 2009, 8, 879–886. [Google Scholar] [CrossRef]
- Song, Q.; Abrams, P.; Sun, Y. Beyond prostate, beyond surgery and beyond urology: The “3Bs” of managing non-neurogenic male lower urinary tract symptoms. Asian J. Urol. 2019, 6, 169–173. [Google Scholar] [CrossRef]
- Lee, C.L.; Kuo, H.C. Pathophysiology of benign prostate enlargement and lower urinary tract symptoms: Current concepts. Tzu-Chi Med. J. 2017, 29, 79–83. [Google Scholar]
- Lin, P.H.; Freedland, S.J. Lifestyle and lower urinary tract symptoms: What is the correlation in men? Curr. Opin. Urol. 2015, 25, 1–5. [Google Scholar] [CrossRef]
- Raheem, O.A.; Parsons, J.K. Associations of obesity, physical activity and diet with benign prostatic hyperplasia and lower urinary tract symptoms. Curr. Opin. Urol. 2014, 24, 10–14. [Google Scholar] [CrossRef][Green Version]
- Sanford, M.T.; Rodriguez, L.V. The role of environmental stress on lower urinary tract symptoms. Curr. Opin. Urol. 2017, 27, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.G.; Chen, J.; Meng, J.L.; Zhang, Y.; Liu, Y.; Zhan, C.S.; Chen, X.G.; Zhang, L.; Liang, C.Z. Effect of alcohol on chronic pelvic pain and prostatic inflammation in a mouse model of experimental autoimmune prostatitis. Prostate 2019, 79, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Saez, C.; Gonzalez-Baena, A.C.; Japon, M.A.; Giraldez, J.; Segura, D.I.; Rodriguez-Vallejo, J.M.; Gonzalez-Esteban, J.; Miranda, G.; Torrubia, F. Expression of basic fibroblast growth factor and its receptors FGFR1 and FGFR2 in human benign prostatic hyperplasia treated with finasteride. Prostate 1999, 40, 83–88. [Google Scholar] [CrossRef]
- Vela Navarrete, R.; Garcia Cardoso, J.V.; Barat, A.; Manzarbeitia, F.; López Farré, A. BPH and inflammation: Pharmacological Effects of permixon on histological and molecular inflammatory markers. Results of a double blind pilot clinical assay. Eur. Urol. 2003, 44, 549–555. [Google Scholar] [CrossRef]
- Giulianelli, R.; Pecoraro, S.; Sepe, G.; Leonardi, R.; Gentile, B.C.; Albanesi, L.; Brunori, S.; Mavilla, L.; Pisanti, F.; Giannella, R.; et al. Multicentre study on the efficacy and tolerability of an extract of Serenoa repens in patients with chronic benign prostate conditions associated with inflammation. Arch. Ital. Urol. Androl. 2012, 84, 94–98. (In Italian) [Google Scholar] [PubMed]
- Bucala, R. MIF rediscovered: Cytokine, pituitary hormone and glucocorticoid-induced regulator of the immune response. FASEB J. 1996, 10, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Bernichtein, S.; Pigat, N.; Camparo, P.; Latil, A.; Viltard, M.; Friedlander, G.; Goffin, V. Anti-inflammatory properties of Lipidosterolic extract of Serenoa repens (Permixon(R)) in a mouse model of prostate hyperplasia. Prostate 2015, 75, 706–722. [Google Scholar] [CrossRef]
- Gravas, S.; Samarinas, M.; Zacharouli, K.; Karatzas, A.; Tzortzis, V.; Koukoulis, G.; Melekos, M. The effect of hexanic extract of Serenoa repens on prostatic inflammation: Results from a randomized biopsy study. World J. Urol. 2019, 37, 539–544. [Google Scholar] [CrossRef]
- Latil, A.; Libon, C.; Templier, M.; Junquero, D.; Lantoine-Adam, F.; Nguyen, T. Hexanic lipidosterolic extract of Serenoa repens inhibits the expression of two key inflammatory mediators, MCP-1/CCL2 and VCAM-1, in vitro. BJU Int. 2012, 110, E301–E307. [Google Scholar] [CrossRef] [PubMed]
- Sirab, N.; Robert, G.; Fasolo, V.; Descazeaud, A.; Vacherot, F.; de la Taille, A.; Terry, S. Lipidosterolic extract of Serenoa repens modulates the expression of inflammation related-genes in benign prostatic hyperplasia epithelial and stromal cells. Int. J. Mol. Sci. 2013, 14, 14301–14320. [Google Scholar] [CrossRef]
- Bartoletti, R. Chronic Inflammatory infiltrate and benign prostatic hyperplasia: What do we know? Eur. Urol. Suppl. 2013, 12, 99–102. [Google Scholar] [CrossRef]
- De Nunzio, C.; Salonia, A.; Gacci, M.; Ficarra, V. Inflammation is a target of medical treatment for lower urinary tract symptoms associated with benign prostatic hyperplasia. World J. Urol. 2020, 38, 2771–2779. [Google Scholar] [CrossRef] [PubMed]
- Kramer, G.; Mitteregger, D.; Marberger, M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur. Urol. 2007, 51, 1202–1216. [Google Scholar] [CrossRef]
- Robert, G.; Descazeaud, A.; Nicolaiew, N.; Terry, S.; Sirab, N.; Vacherot, F.; Maille, P.; Allory, Y.; de la Taille, A. Inflammation in benign prostatic hyperplasia: A 282 patients’ immunohistochemical analysis. Prostate 2009, 69, 1774–1780. [Google Scholar] [CrossRef]
- Sciarra, A.; Di Silverio, F.; Salciccia, S.; Autran Gomez, A.M.; Gentilucci, A.; Gentile, V. Inflammation and chronic prostatic diseases: Evidence for a link? Eur. Urol. 2007, 52, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Aliaev Yu, G.; Vinarov, A.Z.; Demidko Iu, L.; Spivak, L.G. Treatment of chronic prostatitis in prophylaxis of prostatic adenoma. Urologiia 2012, 39–40, 42–43. [Google Scholar]
- Aliaev Yu, G.; Vinarov, A.Z.; Lokshin, K.L.; Spivak, L.G. Efficiency and safety of prostamol-Uno in patients with chronic abacterial prostatitis. Urologiia 2006, 1, 47–50. (In Russian) [Google Scholar]
- Aliaev Yu, G.; Vinarov, A.Z.; Lokshin, K.L.; Spivak, L.G. Extracts Serenoa repens in the treatment of prostatic adenoma and chronic abacterial prostatitis: Results of short-term (3-month courses) therapy. Urologiia 2007, 2, 80–82. [Google Scholar]
- Lopatkin, N.A.; Apolikhin, O.I.; Sivkov, A.V.; Alyaev Yu, G.; Komiakov, B.K.; Zhuravlev, V.N.; Oshchepkov, V.N.; Vinarov, A.Z.; Bazhenov, I.V.; Medvedev, A.A.; et al. Results of a multicenter trial of Serenoa repens extract (permixon) in patients with chronic abacterial prostatitis. Urologiia 2007, 5, 3–7. (In Russian) [Google Scholar]
- Mazo, E.B.; Dmitriev, D.G. Clinical effect of the drug "Prostamol-Uno" in patients with benign prostatic hyperplasia and chronic prostatitis. Urologiia 2001, 5, 38–41. [Google Scholar]
- Razumov, S.V.; Medvedev, A.A.; Chirun, N.V.; Sivkov, A.V.; Oshchepkov, V.N.; Siniukhin, V.N. Role of cytokines in the diagnosis of chronic prostatitis. Urologiia 2003, 6, 25–28. [Google Scholar]
- Seregin, S.P.; Bratchikov, O.I.; Konoplia, A.I.; Shestakov, S.G.; Dolzhenkov, S.D.; Novikov, A.V.; Shatokhin, M.N.; Kotov, A.V. Effect of prostamol-Uno on oxidative and local immune status in patients with benign prostatic hyperplasia and chronic prostatitis. Urologiia 2002, 4, 14–16. (In Russian) [Google Scholar]
- Wu, T.; Zhang, X.; Wu, R.; Liu, X. Effects of prostadyn sabale capsules on chronic prostatitis. Zhonghua Nan Ke Xue Natl. J. Androl. 2004, 10, 337–339. [Google Scholar]
- Zhang, K.; Guo, R.Q.; Chen, S.W.; Chen, B.; Xue, X.B.; Chen, S.; Huang, J.; Liu, M.; Tian, Y.; Zuo, L.; et al. The efficacy and safety of Serenoa repens extract for the treatment of patients with chronic prostatitis/chronic pelvic pain syndrome: A multicenter, randomized, double-blind, placebo-controlled trial. World J. Urol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Hou, S.; Kang, Z.; Lin, Q. Serenoa repens induces growth arrest and apoptosis of human multiple myeloma cells via inactivation of STAT 3 signaling. Oncol. Rep. 2009, 22, 377–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silvestri, I.; Cattarino, S.; Agliano, A.; Nicolazzo, C.; Scarpa, S.; Salciccia, S.; Frati, L.; Gentile, V.; Sciarra, A. Effect of Serenoa repens (Permixon(R)) on the expression of inflammation-related genes: Analysis in primary cell cultures of human prostate carcinoma. J Inflamm. 2013, 10, 11. [Google Scholar] [CrossRef]
- Goldmann, W.H.; Sharma, A.L.; Currier, S.J.; Johnston, P.D.; Rana, A.; Sharma, C.P. Saw palmetto berry extract inhibits cell growth and COX-2 expression in prostatic cancer cells. Cell Biol. Int. 2001, 25, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Petrangeli, E.; Lenti, L.; Buchetti, B.; Chinzari, P.; Sale, P.; Salvatori, L.; Ravenna, L.; Lococo, E.; Morgante, E.; Russo, A.; et al. Lipido-sterolic extract of Serenoa repens (LSESr, Permixon) treatment affects human prostate cancer cell membrane organization. J. Cell. Physiol. 2009, 219, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hui, L.; Yuqin, C.; Jie, L.; Shuai, H.; Tiezhu, Z.; Wei, W. Effect of saw palmetto extract on PI3K cell signaling transduction in human glioma. Exp. Ther. Med. 2014, 8, 563–566. [Google Scholar] [CrossRef]
- Ding, H.; Shen, J.; Yang, Y.; Che, Y. Saw Palmetto extract inhibits metastasis and antiangiogenesis through STAT3 signal pathway in glioma cell. Evid. Based Complementary Altern. Med. 2015. [Google Scholar] [CrossRef][Green Version]
- Zhou, T.; Yang, Y.; Zhang, H.; Che, Y.; Wang, W.; Lv, H.; Li, J.; Wang, Y.; Hou, S. Serenoa Repens induces growth arrest, apoptosis and inactivation of STAT3 signaling in human glioma cells. Technol. Cancer. Res. Treat. 2015, 14, 729–736. [Google Scholar] [CrossRef]
- Ishii, K.; Usui, S.; Sugimura, Y.; Yamamoto, H.; Yoshikawa, K.; Hiran, K. Extract from Serenoa repens suppresses the invasion activity of human urological cancer cells by inhibiting urokinase-type plasminogen activator. Biol. Pharm. Bull. 2001, 24, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Baron, A.; Mancini, M.; Caldwell, E.; Cabrelle, A.; Bernardi, P.; Pagano, F. Serenoa repens extract targets mitochondria and activates the intrinsic apoptotic pathway in human prostate cancer cells. BJU Int. 2009, 103, 1275–1283. [Google Scholar] [CrossRef]
- Debruyne, F.; Koch, G.; Boyle, P.; da Silva, F.C.; Gillenwater, J.G.; Hamdy, F.C.; Perrin, P.; Teillac, P.; Vela-Navarrete, R.; Raynaud, J.P. Comparison of a phytotherapeutic agent (Permixon) with an alpha-blocker (Tamsulosin) in the treatment of benign prostatic hyperplasia: A 1-year randomized international study. Eur. Urol. 2002, 41, 497–506. [Google Scholar] [CrossRef]
- Barry, M.J.; Meleth, S.; Lee, J.Y.; Kreder, K.J.; Avins, A.L.; Nickel, J.C.; Roehrborn, C.G.; Crawford, E.D.; Foster, H.E., Jr.; Kaplan, S.A.; et al. Effect of increasing doses of saw palmetto extract on lower urinary tract symptoms: A randomized trial. JAMA 2011, 306, 1344–1351. [Google Scholar] [CrossRef]
- Braeckman, J. The extract of Serenoa repens in the treatment of benign prostatic hyperplasia: A multicenter open study. Curr. Ther. Res. 1994, 55, 776–785. [Google Scholar] [CrossRef]
- Al-Shukri, S.H.; Deschaseaux, P.; Kuzmin, I.V.; Amdiy, R.R. Early urodynamic effects of the lipido-sterolic extract of Serenoa repens (Permixon(R)) in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2000, 3, 195–199. [Google Scholar] [CrossRef]
- Ju, X.B.; Gu, X.J.; Zhang, Z.Y.; Wei, Z.Q.; Xu, Z.Q.; Miao, H.D.; Zhou, W.M.; Xu, R.F.; Cheng, B.; Ma, J.G.; et al. Efficacy and safety of Saw Palmetto Extract Capsules in the treatment of benign prostatic hyperplasia. Zhonghua Nan Ke Xue Natl. J. Androl. 2015, 21, 1098–1101. (In Chinese) [Google Scholar]
- Bent, S.; Kane, C.; Shinohara, K.; Neuhaus, J.; Hudes, E.S.; Goldberg, H.; Avins, A.L. Saw palmetto for benign prostatic hyperplasia. N. Engl. J. Med. 2006, 354, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Braeckman, J.; Bruhwyler, J.; Vanderkerckhove, K.; Géczy, J. Efficacy and safety of the extract of Serenoa repens in the treatment of benign prostatic hyperplasia: Therapeutic equivalence between twice and once daily dosage forms. Phytother. Res. 1997, 11, 558–563. [Google Scholar] [CrossRef]
- Debruyne, F.; Boyle, P.; da Silva, C.F.; Gillenwater, J.G.; Hamdy, F.C.; Perrin, P.; Teillac, P.; Vela-Navarrete, R.; Raynaud, J.P.; Schulman, C.C. Evaluation of the clinical benefit of permixon and tamsulosin in severe BPH patients-PERMAL study subset analysis. Eur. Urol. 2004, 45, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Romics, I.; Schmitz, H.; Frang, D. Experience in treating benign prostatic hypertrophy with Sabal serrulata for one year. Int. Urol. Nephrol. 1993, 25, 565–569. [Google Scholar]
- Saidi, S.; Stavridis, S.; Stankov, O.; Dohcev, S.; Panov, S. Effects of Serenoa repens Alcohol extract on benign prostate hyperplasia. Pril Makedon Akad Nauk Umet Odd Med Nauk. 2017, 38, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Ball, A.J.; Feneley, R.C.; Abrams, P.H. The natural history of untreated “prostatism”. Br. J. Urol. 1981, 53, 613–616. [Google Scholar] [CrossRef]
- Birkhoff, J.D.; Wiederhorn, A.R.; Hamilton, M.L.; Zinsser, H.H. Natural history of benign prostatic hypertrophy and acute urinary retention. Urology 1976, 7, 48–52. [Google Scholar] [CrossRef]
- Craigen, A.A.; Hickling, J.B.; Saunders, C.R.; Carpenter, R.G. Natural history of prostatic obstruction. A prospective survey. J. R. Coll. Gen. Pract. 1969, 18, 226–232. [Google Scholar]
- Vinarov, A.Z. Long-term treatment of BPH symptoms with Serenoa Repens. Eur. Urol. Today 2011, 23, 9. [Google Scholar]
| First Author | Ref # | Year | Extraction Method | Serenoa Patients (#) a | Study Duration (mos) | IPSS | QoL | Qmax | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Δ | % | Δ | % | Δ | % | ||||||
| Cirillo-Marucco ε | [6] | 1983 | Hexane | 47 | 4 | 56 | +4.6 | 50 | |||
| Cukierψλ | [7] | 1985 | Hexane | 73 | 2 | 33 | |||||
| TostoΩ | [8] | 1985 | Hexane | 20 | 3 | −5.0 | 28 | ||||
| Pannunzio | [9] | 1986 | Hexane | 30 | 2 | +5.0 | 74 | ||||
| Pescatore | [10] | 1986 | Hexane | 30 | 3 | +2.5 | 27 | ||||
| Authieπ | [11] | 1987 | Hexane | 500 | 3 | 78 | |||||
| Ollé Carrerasφ | [12] | 1987 | Hexane | 40 | 2 | 68 | |||||
| Orfeiχ | [13] | 1988 | Hexane | 30 | 3 | 50 | −2.2 | +0.0 | 0.2 | ||
| Matteiψω | [14] | 1990 | CO2 | 20 | 3 | 55 | |||||
| Dathe | [15] | 1991 | Hexane | 49 | 6 | +5.9 | 49 | ||||
| VahlensieckϷ | [16] | 1993 | CO2 | 1334 | 4 | 47 | |||||
| Vahlensieck | [17] | 1993 | CO2 | 312 | 3 | +5.8 | 52 | ||||
| Fabriciusδ | [18] | 1993 | CO2 | 176 | 6 | 39;59 | |||||
| Derakhshani | [19] | 1997 | Ethanol | 1047 | 3 | −7.4 | 40 | −1.6 | 46 | +3.7 | 31 |
| Eickenberg | [20] | 1997 | Ethanol 96% | 6967 | 6 | −8.0 | 44 | −1.8 | 38 | +3.0 | 23 |
| Foroutan | [21] | 1997 | Hexane | 592 | 3 | −6.5 | 38 | −1.5 | 45 | +5.9 | 66 |
| Redeckerν | [22] | 1998 | Ethanol 90% | 50 | 3 | 48 | +3.4 | 24 | |||
| ZieglerΘ | [23] | 1998 | Ethanol 90% | 109 | 3 | 36 | +3.7 | 29 | |||
| Bauer γ ψ | [24] | 1999 | CO2 | 101 | 6 | 37 | 16 | ||||
| Medeiros † | [25] | 2000 | Hexane | 130 | 3 | −6.5 | 37 | −1.4 | 39 | +2.0 | 22 |
| Aliaev | [26] | 2002 | Hexane | 26 | 60 | −8.8 | 76 | −1.3 | 53 | +4.3 | 35 |
| Breza | [27] | 2005 | Ethanol | 596 | 12 | −5.9 | 36 | −1.7 | 54 | + 2.3 | 19 |
| Aliaev | [28] | 2007 | Ethanol | 50 | 6 | −2.9 | 26 | −1.8 | 43 | + 1.7 | 14 |
| Razumov | [29] | 2007 | Ethanol | 30 | 6 | −6.9 | 43 | -2.7 | 68 | +2.8 | 23 |
| Aliaev∞ | [30] | 2009 | Ethanol | 50 | 24 | −4.2 | 37 | −2.2 | 52 | + 2.7 | 21 |
| Vinarov | [31] | 2010 | Ethanol | 50 | 36 | −6.0 | 50 | −2.0 | 50 | + 4.5 | 39 |
| Aliaev | [32] | 2013 | Ethanol | 38 | 120 | −1.3 | 12 | −1.1 | 35 | + 3.3 | 26 |
| Mean Across All 27 Studies | 463 | 12 | −5.8 | 40–41 b | −1.8 | 47 | +3.5 | 31 | |||
| Hexane extraction n = 12 Ethanol extraction n = 10 Carbon dioxide extraction n = 5 | |||||||||||
| Senior Author | Ref. (#) | Year | Extraction | Serenoa Patients (#) a | Study Duration (mos) | IPSS * | QoL | Qmax | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Δ | % | Δ | % | Δ | % | ||||||
| Bent | [114] | 2006 | CO2 | 102 | 12 | −0.7 | 4 | +0.4 | 4 | ||
| Braeckman π | [115] | 1997 | CO2 | 67 | 12 | −10.2 | 60 | −1.5 | 42 | +2.6 | 24 |
| Aliaev Ω | [30] | 2009 | Ethanol | 50 | 24 | −4.2 | 37 | −2.2 | 52 | +2.7 | 21 |
| Aliaev | [32] | 2013 | Ethanol | 38 | 120 | −1.3 | 12 | −1.1 | 35 | +3.3 | 26 |
| Bach | [34] | 1996 | Ethanol | 315 | 36 | 73 | +6.1 | 46 | |||
| Barry π | [110] | 2011 | Ethanol | 151 | 18 | −2.2 | 15 | ||||
| Breza | [27] | 2005 | Ethanol | 596 | 12 | −5.9 | 36 | −1.7 | 54 | +2.3 | 19 |
| Romics | [117] | 1993 | Ethanol | 31 | 12 | +4.3 | 39 | ||||
| Saidi | [118] | 2019 | Ethanol | 40 | 12 | −2.1 | 18 | +0.8 | 6 | ||
| Sinescu | [37] | 2011 | Ethanol | 120 | 24 | −5.5 | 40 | −1.8 | 50 | +5.6 | 54 |
| Vinarov | [31] | 2010 | Ethanol | 50 | 36 | −6.0 | 50 | −2.0 | 50 | +4.5 | 39 |
| Vinarov | [38] | 2019 | Ethanol | 30 | 180 | −6.0 | 50 | −3.0 | 60 | +5.0 | 45 |
| Aliaev | [26] | 2002 | Hexane | 26 | 60 | −8.8 | 76 | −1.3 | 53 | +4.1 | 35 |
| Debruyne | [109] | 2002 | Hexane | 350 | 12 | −4.4 | 28 | +1.9 | 17 | ||
| Debruyne | [116] | 2004 | Hexane | 124 | 12 | −7.8 | 35 | −1.2 | 29 | +1.2 | 11 |
| Djavan δ | [35] | 2005 | Hexane | 88 | 24 | −1.0 | 17 | −0.4 | 19 | +1.8 | 15 |
| Pytel | [36] | 2002 | Hexane | 116 | 24 | −5.3 | 42 | −1.3 | 40 | +1.2 | 10 |
| Averages of 17 Studies 15 positive studies 2 negative studies | Ethanol (10) Hexane (5) CO2 (2) | 135 | 37 | −4.8 | 37 | −1.6 | 44 | + 3.0 | 26 | ||
| Study Group | Cumulative Progression | Changes * in IPSS, QoL, Qmax at 2-Years (%) | p Value | Changes ** in IPSS, QoL, Qmax at 3-Years | p Value |
|---|---|---|---|---|---|
| Watchful Waiting | IPSS: −0.3 (+5%) QoL: −0.2 (−9%) Qmax: 0.10 (−8%) | p = 0.03 at 2-years | IPSS: +5% QoL: −10% Qmax: −9% | p = 0.001 at 3-years | |
| At 6 months | 6% | ||||
| At 12 months | 13% | ||||
| At 18 months | 15% | ||||
| At 24 months | 24% | ||||
| At 36 months | 31% | ||||
| Permixon | IPSS: −1.0 (−17%) QoL: −0.4 (−19%) Qmax: +1.8 (+15%) | IPSS: −22% QoL: −24% Qmax: +14% | |||
| At 6 months | 1% | ||||
| At 12 months | 7% | ||||
| At 18 months | 9% | ||||
| At 24 months | 16% | ||||
| At 36 months | 19% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strum, S.B. Serenoa Repens (Saw Palmetto) for Lower Urinary Tract Symptoms (LUTS): The Evidence for Efficacy and Safety of Lipidosterolic Extracts. Part II. Uro 2021, 1, 139-154. https://doi.org/10.3390/uro1030016
Strum SB. Serenoa Repens (Saw Palmetto) for Lower Urinary Tract Symptoms (LUTS): The Evidence for Efficacy and Safety of Lipidosterolic Extracts. Part II. Uro. 2021; 1(3):139-154. https://doi.org/10.3390/uro1030016
Chicago/Turabian StyleStrum, Stephen B. 2021. "Serenoa Repens (Saw Palmetto) for Lower Urinary Tract Symptoms (LUTS): The Evidence for Efficacy and Safety of Lipidosterolic Extracts. Part II" Uro 1, no. 3: 139-154. https://doi.org/10.3390/uro1030016
APA StyleStrum, S. B. (2021). Serenoa Repens (Saw Palmetto) for Lower Urinary Tract Symptoms (LUTS): The Evidence for Efficacy and Safety of Lipidosterolic Extracts. Part II. Uro, 1(3), 139-154. https://doi.org/10.3390/uro1030016






