Serenoa repens (Saw palmetto) for Lower Urinary Tract Symptoms (LUTS): The Evidence for Efficacy and Safety of Lipidosterolic Extracts. Part I
Abstract
:1. Introduction: LUTS, Symptom Scores, and Treatment Options
2. The Basics about Serenoa repens
- (1)
- to critically evaluate the three major LSESr extraction techniques (hexane, ethanol, CO2) used in treating LUTS and present information foundational in reading the LUTS literature, (i.e., the European versus the American perspective of LSESr in LUTS treatment);
- (2)
- to discuss LSESr side effects in short studies involving months and in long-term studies spanning years;
- (3)
- to better understand the time to onset of action of LSESr;
- (4)
- to ascertain the durability of efficacy;
- (5)
- to investigate whether LSESr used in long-term studies can prevent the progression of LUTS and BPH; and,
- (6)
- to conclude whether or not the effects of LSESr reflect a placebo effect.
3. Perspectives on Serenoa repens Efficacy versus LUTS
3.1. The European Scientific Cooperative on Phytotherapy (ESCOP 2003)
3.2. The Cochrane Meta-Analysis (Cochrane 2012)
3.3. The American Urological Association Guidelines (AUAG 2014)
3.4. The European Medicines Agency Monograph (EMA 2014)
3.5. What Can Be Learned from ESCOP 2003, Cochrane 2012, AUAG 2014, and EMA 2014?
4. What Information Is Not Controversial?
5. Conclusions in Part I
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethical Approval/Patient Consent
References
- Lee, C.L.; Kuo, H.C. Pathophysiology of benign prostate enlargement and lower urinary tract symptoms: Current concepts. Ci Ji Yi Xue Za Zhi 2017, 29, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Abrams, P.; Sun, Y. Beyond prostate, beyond surgery and beyond urology: The "3Bs" of managing non-neurogenic male lower urinary tract symptoms. Asian J. Urol. 2019, 6, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.J.; Fowler, F.J., Jr.; O’Leary, M.P.; Bruskewitz, R.C.; Holtgrewe, H.L.; Mebust, W.K.; Cockett, A.T. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J. Urol. 1992, 148, 1549–1557. [Google Scholar] [CrossRef]
- Cockett, A.T.; Aso, Y.; Denis, L.; Khoury, S.; Barry, M.; Carlton, C.E.; Coffey, D.; Fitzpatrick, J.; Griffiths, K.; Hald, T.; et al. World Health Organization Consensus Committee recommendations concerning the diagnosis of BPH. Prog. Urol. 1991, 1, 957–972. [Google Scholar]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A.; Standardisation Sub-Committee of the International Continence Society. The standardisation of terminology in lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Urology 2003, 61, 37–49. [Google Scholar] [CrossRef]
- Chapple, C.R.; Wein, A.J.; Abrams, P.; Dmochowski, R.R.; Giuliano, F.; Kaplan, S.A.; McVary, K.T.; Roehrborn, C.G. Lower urinary tract symptoms revisited: A broader clinical perspective. Eur. Urol. 2008, 54, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Yalla, S.V.; Sullivan, M.P.; Lecamwasam, H.S.; DuBeau, C.E.; Vickers, M.A.; Cravalho, E.G. Correlation of American Urological Association symptom index with obstructive and nonobstructive prostatism. J. Urol. 1995, 153, 674–679. [Google Scholar] [CrossRef]
- McVary, K.T.; Peterson, A.; Donatucci, C.F.; Baygani, S.; Henneges, C.; Clouth, J.; Wong, D.; Oelke, M. Use of Structural Equation Modeling to Demonstrate the Differential Impact of Storage and Voiding Lower Urinary Tract Symptoms on Symptom Bother and Quality of Life during Treatment for Lower Urinary Tract Symptoms Associated with Benign Prostatic Hyperplasia. J. Urol. 2016, 196, 824–830. [Google Scholar] [CrossRef] [PubMed]
- McVary, K.T.; Roehrborn, C.G.; Avins, A.L.; Barry, M.J.; Bruskewitz, R.C.; Donnell, R.F.; Foster, H.E., Jr.; Gonzalez, C.M.; Kaplan, S.A.; Penson, D.F.; et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J. Urol. 2011, 185, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Oelke, M.; Bachmann, A.; Descazeaud, A.; Emberton, M.; Gravas, S.; Michel, M.C.; N’Dow, J.; Nordling, J.; de la Rosette, J.J.; European Association of Urology. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur. Urol. 2013, 64, 118–140. [Google Scholar] [CrossRef] [PubMed]
- Lazarou, J.; Pomeranz, B.H.; Corey, P.N. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA 1998, 279, 1200–1205. [Google Scholar] [CrossRef]
- Laekeman, G.; Vlietinck, A. Assessment Report on Serenoa Repens (W. Bartram) Small, Fructus. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2014/12/WC500179593.pdf (accessed on 14 November 2020).
- USP. Saw Palmetto Extract. Available online: https://online.uspnf.com/uspnf/document/1_GUID-53E14A4F-6F17-4CF1-852C-C6547F5A79DB_5_en-US (accessed on 1 November 2020).
- Bent, S.; Kane, C.; Shinohara, K.; Neuhaus, J.; Hudes, E.S.; Goldberg, H.; Avins, A.L. Saw palmetto for benign prostatic hyperplasia. N. Engl. J. Med. 2006, 354, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Barry, M.J.; Meleth, S.; Lee, J.Y.; Kreder, K.J.; Avins, A.L.; Nickel, J.C.; Roehrborn, C.G.; Crawford, E.D.; Foster, H.E., Jr.; Kaplan, S.A.; et al. Effect of increasing doses of saw palmetto extract on lower urinary tract symptoms: A randomized trial. JAMA 2011, 306, 1344–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Shukri, S.H.; Deschaseaux, P.; Kuzmin, I.V.; Amdiy, R.R. Early urodynamic effects of the lipido-sterolic extract of Serenoa repens (Permixon(R)) in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2000, 3, 195–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carraro, J.C.; Raynaud, J.P.; Koch, G.; Chisholm, G.D.; Di Silverio, F.; Teillac, P.; Da Silva, F.C.; Cauquil, J.; Chopin, D.K.; Hamdy, F.C.; et al. Comparison of phytotherapy (Permixon) with finasteride in the treatment of benign prostate hyperplasia: A randomized international study of 1,098 patients. Prostate 1996, 29, 231–240. [Google Scholar] [CrossRef]
- Debruyne, F.; Boyle, P.; Calais Da Silva, F.; Gillenwater, J.G.; Hamdy, F.C.; Perrin, P.; Teillac, P.; Vela-Navarrete, R.; Raynaud, J.P.; Schulman, C.C. Evaluation of the clinical benefit of permixon and tamsulosin in severe BPH patients-PERMAL study subset analysis. Eur. Urol. 2004, 45, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Debruyne, F.; Koch, G.; Boyle, P.; Da Silva, F.C.; Gillenwater, J.G.; Hamdy, F.C.; Perrin, P.; Teillac, P.; Vela-Navarrete, R.; Raynaud, J.P. Comparison of a phytotherapeutic agent (Permixon) with an alpha-blocker (Tamsulosin) in the treatment of benign prostatic hyperplasia: A 1-year randomized international study. Eur. Urol. 2002, 41, 497–506. [Google Scholar] [CrossRef]
- Djavan, B.; Fong, Y.K.; Chaudry, A.; Reissigl, A.; Anagnostou, T.; Bagheri, F.; Waldert, M.; Fajkovic, H.; Marihart, S.; Harik, M.; et al. Progression delay in men with mild symptoms of bladder outlet obstruction: A comparative study of phytotherapy and watchful waiting. World J. Urol. 2005, 23, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Giannakopoulos, X.; Baltogiannis, D.; Giannakis, D.; Tasos, A.; Sofikitis, N.; Charalabopoulos, K.; Evangelou, A. The lipidosterolic extract of Serenoa repens in the treatment of benign prostatic hyperplasia: A comparison of two dosage regimens. Adv. Ther. 2002, 19, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Hizli, F.; Uygur, M.C. A prospective study of the efficacy of Serenoa repens, tamsulosin, and Serenoa repens plus tamsulosin treatment for patients with benign prostate hyperplasia. Int. Urol. Nephrol. 2007, 39, 879–886. [Google Scholar] [CrossRef]
- Pytel, Y.A.; Vinarov, A.; Lopatkin, N.; Sivkov, A.; Gorilovsky, L.; Raynaud, J.P. Long-term clinical and biologic effects of the lipidosterolic extract of Serenoa repens in patients with symptomatic benign prostatic hyperplasia. Adv. Ther. 2002, 19, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Stepanov, V.N.; Siniakova, L.A.; Sarrazin, B.; Raynaud, J.P. Efficacy and tolerability of the lipidosterolic extract of Serenoa repens (Permixon) in benign prostatic hyperplasia: A double-blind comparison of two dosage regimens. Adv. Ther. 1999, 16, 231–241. [Google Scholar] [PubMed]
- MacDonald, R.; Tacklind, J.W.; Rutks, I.; Wilt, T.J. Serenoa repens monotherapy for benign prostatic hyperplasia (BPH): An updated Cochrane systematic review. BJU Int. 2012, 109, 1756–1761. [Google Scholar] [CrossRef]
- Tacklind, J.; Macdonald, R.; Rutks, I.; Stanke, J.U.; Wilt, T.J. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst. Rev. 2012, 12, CD001423. [Google Scholar] [CrossRef]
- Serenoae Repentis Fructus (Sabal Fructus) Saw Palmetto Fruit. ESCOP Monographs, 2nd ed.; George Thieme Verlag: New York, NY, USA, 2003. [Google Scholar]
- McVary, K.T.; Roehrborn, C.; Avins, A.L.; Barry, M.J.; Bruskewitz, R.C.; Donnell, R.F.; Foster, J.; Harris, E.; Gonzalez, C.M.; Kaplan, S.A.; et al. Management of Benign Prostatic Hyperplasia. Published 2010; Reviewed and Validity Confirmed 2014. Available online: https://www.auanet.org/guidelines/benign-prostatic-hyperplasia-(bph)-guideline/benign-prostatic-hyperplasia-(2010-reviewed-and-validity-confirmed-2014) (accessed on 4 May 2020).
- Cirillo-Marucco, E.; Pagliarulo, A.; Tritto, G.; Piccinno, A.; Di Rienzo, U. Serenoa repens extract (Permixon®) in the early treatment of prostatic hypertrophy. Urol. J. 1983, 50, 1269–1277. [Google Scholar] [CrossRef]
- Cukier; Ducassou; Guillou, L. Permixon versus placebo. CR Ther. Pharmacol. Clin. 1985, 4, 15–21. (In French) [Google Scholar]
- Pescatore, D.; Calvi, P.; Michelotti, P. Urodynamic assessment of treatment in patients with prostatic adenoma with Serenoa repens extract. Urol. J. 1986, 53, 894–897. [Google Scholar] [CrossRef]
- Authie, D.; Cauquil, J. Assessment of the effectiveness of Permixon* in daily practice multicentric study. CR Ther. Pharmacol. Clin. 1987, 5, 3–13. (In French) [Google Scholar]
- Ollé Carreras, J. Our experience with hexane extract from Serenoa repens in the treatment of benign prostatic hypertrophy. Arch. Esp. Urol. 1987, 40, 310–313. (In Spanish) [Google Scholar] [PubMed]
- Orfei, S.; Grumelli, B.; Galetti, G. Clinical and uroflowimetric evaluation of Permixon® in geriatrics. Urol. J. 1988, 55, 373–381. [Google Scholar] [CrossRef]
- Mattei, F.M.; Capone, M.; Acconcia, A. Medicamentous therapy of benign prostatic hyperplasia with an extract of the sagebrush. TW Urol. Nephrol. 1990, 2, 346–350. (In German) [Google Scholar]
- Dathe, G.; Schmid, H. Phytotherapy for benign prostatic hyperplasia (BPH) with Extractum Serenoa repens (Permixon). Urologe. Ausgabe B 1991, 31, 223–330. (In German) [Google Scholar]
- Fabricius, P.G.; Vahlensieck jr., W. Therapy for benign prostatic hyperplasia: Sabal fruit extract: One dose is enough! Therapiewoche 1993, 43, 1616–1620. (In German) [Google Scholar]
- Romics, I.; Schmitz, H.; Frang, D. Experience in treating benign prostatic hypertrophy with Sabal serrulata for one year. Int. Urol. Nephrol. 1993, 25, 565–569. [Google Scholar] [PubMed]
- Vahlensieck, W., Jr.; Volp, A.; Lubos, W.; Kuntze, M. Benign prostatic hyperplasia--treatment with sabal fruit extract. A treatment study of 1334 patients. Fortschr. Med. 1993, 111, 323–326. (In German) [Google Scholar]
- Vahlensieck, W.; Völp, A.; Kuntze, M.; Lubos, W. Changes in micturition in patients with benign prostatic hyperplasia under sabal fruit treatment. Urologe. Ausg. B 1993, 33, 380–383. (In German) [Google Scholar]
- Braeckman, J. The extract of Serenoa repens in the treatment of benign prostatic hyperplasia: A multicenter open study. Curr. Ther. Res. 1994, 55, 776–785. [Google Scholar] [CrossRef]
- Bach, D.; Ebeling, L. Long-term drug treatment of benign prostatic hyperplasia—Results of a prospective 3-year multicenter study using Sabal extract IDS 89. Phytomedicine 1996, 3, 105–111. [Google Scholar] [CrossRef]
- Kondas, J.; Philipp, V.; Dioszeghy, G. Sabal serrulata extract (Strogen forte) in the treatment of symptomatic benign prostatic hyperplasia. Int. Urol. Nephrol. 1996, 28, 767–772. [Google Scholar] [CrossRef]
- Braeckman, J.; Denis, L.; De Leval, J.; Keuppens, F.; Cornet, A.; De Bruyne, R.; De Smedt, E.; Pacco, J.; Timmermans, L.; Van Vliet, P. A double-blind, placebo-controlled study of the plant extract Serenoa repens in the treatment of benign hyperplasia of the prostate. Eur. J. Clin. Res. 1997, 9, 247–259. [Google Scholar]
- Braeckman, J.; Bruhwyler, J.; Vanderkerckhove, K.; Géczy, J. Efficacy and safety of the extract of Serenoa repens in the treatment of benign prostatic hyperplasia: Therapeutic equivalence between twice and once daily dosage forms. Phytother. Res. 1997, 11, 558–563. [Google Scholar] [CrossRef]
- Gerber, G.S.; Zagaja, G.P.; Bales, G.T.; Chodak, G.W.; Contreras, B.A. Saw palmetto (Serenoa repens) in men with lower urinary tract symptoms: Effects on urodynamic parameters and voiding symptoms. Urology 1998, 51, 1003–1007. [Google Scholar] [CrossRef]
- Derakhshani, P.; Geerke, H.; Böhnert, K.J.; Engelmann, U. Influencing the international prostate symptom score during therapy with saw palmetto fruit extract with a single daily dose. Der. Urol. B 1997, 37, 384–391. [Google Scholar] [CrossRef]
- Eickenberg, H.U. Treatment of benign prostatic hyperplasia with a lipophilic extract from saw palmetto fruits (Sita). Der. Urol. B 1997, 37, 130–133. [Google Scholar] [CrossRef]
- Redecker, K.D.; Funk, P. Sabal-Extrakt WS 1473 bei benigner Prostatahyperplasie. Extr. Urol. 1998, 21, 23–25. [Google Scholar]
- Ziegler, H.; Holscher, U. Efficacy of saw palmetto fruit special extract WS 1473 in patients with Alken stage I-II benign prostatic hyperplasia-open multicentre study. Jatros. Urol. 1998, 14, 34–43. (In German) [Google Scholar]
- Wilt, T.J.; Ishani, A.; Rutks, I.; MacDonald, R. Phytotherapy for benign prostatic hyperplasia. Public Health Nutr. 2000, 3, 459–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilt, T.; Ishani, A.; Mac Donald, R. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst. Rev. 2002, CD001423. [Google Scholar]
- Tacklind, J.; MacDonald, R.; Rutks, I.; Wilt, T.J. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst. Rev. 2009, CD001423. [Google Scholar] [CrossRef]
- Sökeland, J.; Albrecht, J. Combined Sabal and Urtica extract vs finasteride in BPH (Alken stage I–II). Der Urol. 1997, 36, 327–333. [Google Scholar] [CrossRef]
- Sokeland, J. Combined sabal and urtica extract compared with finasteride in men with benign prostatic hyperplasia: Analysis of prostate volume and therapeutic outcome. BJU Int. 2000, 86, 439–442. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, N.; Jha, R.; Dutt, C. Randomized double-blind controlled clinical trial of serenoa repens versus placebo in the management of patients with symptomatic grade I to grade II benign prostatic hyperplasia (BPH). Indian J. Urol. 1999, 15, 26–31. [Google Scholar]
- Reece Smith, H.; Memon, A.; Smart, C.J.; Dewbury, K. The value of permixon in benign prostatic hypertrophy. Br. J. Urol. 1986, 58, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Pannunzio, E.; D’Ascenzo, R.; Giardinetti, F.; Civili, P.; Persichelli, E. Serenoa Repens vs. Gestonorone Caproato in the Treatment of Benign Prostatic Hypertrophy: Randomized Study. Urol. J. 1986, 53, 696–705. [Google Scholar] [CrossRef]
- Bauer, H.W.; Casarosa, C.; Cosci, M.; Fratta, M.; Blessmann, G. Saw palmetto fruit extract for treatment of benign prostatic hyperplasia. Results of a placebo-controlled double-blind study. MMW Fortschr. Med. 1999, 141, 62. (In German) [Google Scholar]
- Gerber, G.S.; Kuznetsov, D.; Johnson, B.C.; Burstein, J.D. Randomized, double-blind, placebo-controlled trial of saw palmetto in men with lower urinary tract symptoms. Urology 2001, 58, 960–964. [Google Scholar] [CrossRef] [Green Version]
- Willetts, K.E.; Clements, M.S.; Champion, S.; Ehsman, S.; Eden, J.A. Serenoa repens extract for benign prostate hyperplasia: A randomized controlled trial. BJU Int. 2003, 92, 267–270. [Google Scholar] [CrossRef] [Green Version]
- Engelmann, U.; Walther, C.; Bondarenko, B.; Funk, P.; Schlafke, S. Efficacy and safety of a combination of sabal and urtica extract in lower urinary tract symptoms. A randomized, double-blind study versus tamsulosin. Arzneimittelforschung 2006, 56, 222–229. [Google Scholar] [PubMed]
- Lobelenz, J. Extractum Sabal fructus for benign prostatic hyperplasia (BPH). Clinical trial in stages I and II. Therapeutikon 1992, 6, 34–37. (In German) [Google Scholar]
- Descotes, J.L.; Rambeaud, J.J.; Deschaseaux, P.; Faure, G. Placebo-Controlled Evaluation of the Efficacy and.Tolerability of Permixon® in Benign Prostatic Hyperplasia after Exclusion of Placebo Responders. Clin. Drug Investig. 1995, 9, 291–297. [Google Scholar] [CrossRef]
- Boccafoschi, C.; Annoscia, S. Comparison of Serenoa repens extract and placebo in controlled clinical trial in patients with prostatic adenomatosis. Urologiia 1983, 50, 1–14. (In Italian) [Google Scholar]
- Emili, E.; Lo Cigno, M.; Petrone, U. Clinical results on a new drug in prostate hypertrophy therapy (Permixon). Nefrologia Chirurgica 1983, 50, 1042–1048. (In Italian) [Google Scholar] [CrossRef]
- Mandressi, A.; Tarallo, U.; Maggioni, A.; Tombolini, P.; Rocco, F.; Quadraccia, S. Medical treatment of benign prostatic hyperplasia: Efficacy of the extract of Serenoa repens (Permixon) compared to that of the extract of Pygeum africanum and a placebo. Urologia 1983, 50, 752–758. (In Italian) [Google Scholar] [CrossRef]
- Tasca, A.; Barulli, M.; Cavazzana, A.; Zattoni, F.; Artibani, W.; Pagano, F. Treatment of obstructive symptomatology caused by prostatic adenoma with an extract of Serenoa repens. Double-blind clinical test v. placebo. Minerva Urol. Nefrol. 1985, 37, 87–91. (In Italian) [Google Scholar]
- Champault, G.; Patel, J.C.; Bonnard, A.M. A double-blind trial of an extract of the plant Serenoa repens in benign prostatic hyperplasia. Br. J. Clin. Pharmacol. 1984, 18, 461–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachmann, C. Serenoa repens in benign prostatic hyperplasia. Schweiz. Z. Ganzheitsmed./Swiss J. Integr. Med. 2015, 27, 202–206. (In German) [Google Scholar] [CrossRef]
- Barry, M.J.; Williford, W.O.; Chang, Y.; Machi, M.; Jones, K.M.; Walker-Corkery, E.; Lepor, H. Benign prostatic hyperplasia specific health status measures in clinical research: How much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J. Urol. 1995, 154, 1770–1774. [Google Scholar] [CrossRef]
- Boeri, L.; Capogrosso, P.; Ventimiglia, E.; Cazzaniga, W.; Pederzoli, F.; Moretti, D.; Deho, F.; Montanari, E.; Montorsi, F.; Salonia, A. Clinically Meaningful Improvements in LUTS/BPH Severity in Men Treated with Silodosin Plus Hexanic Extract of Serenoa Repens or Silodosin Alone. Sci. Rep. 2017, 7, 15179. [Google Scholar] [CrossRef]
- Nickel, J.C.; Brock, G.B.; Herschorn, S.; Dickson, R.; Henneges, C.; Viktrup, L. Proportion of tadalafil-treated patients with clinically meaningful improvement in lower urinary tract symptoms associated with benign prostatic hyperplasia—Integrated data from 1499 study participants. BJU Int. 2015, 115, 815–821. [Google Scholar] [CrossRef] [Green Version]
- Shi, R.; Xie, Q.; Gang, X.; Lun, J.; Cheng, L.; Pantuck, A.; Rao, J. Effect of saw palmetto soft gel capsule on lower urinary tract symptoms associated with benign prostatic hyperplasia: A randomized trial in Shanghai, China. J. Urol. 2008, 179, 610–615. [Google Scholar] [CrossRef]
- Abrams, P.; Schulman, C.C.; Vaage, S. Tamsulosin, a selective alpha 1c-adrenoceptor antagonist: A randomized, controlled trial in patients with benign prostatic ’obstruction’ (symptomatic BPH). The European Tamsulosin Study Group. Br. J. Urol. 1995, 76, 325–336. [Google Scholar] [CrossRef]
- Barry, M.J.; Cantor, A.; Roehrborn, C.G.; Group, C.S. Relationships among participant international prostate symptom score, benign prostatic hyperplasia impact index changes and global ratings of change in a trial of phytotherapy in men with lower urinary tract symptoms. J. Urol. 2013, 189, 987–992. [Google Scholar] [CrossRef] [Green Version]
- McConnell, J.D.; Bruskewitz, R.; Walsh, P.; Andriole, G.; Lieber, M.; Holtgrewe, H.L.; Albertsen, P.; Roehrborn, C.G.; Nickel, J.C.; Wang, D.Z.; et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N. Engl. J. Med. 1998, 338, 557–563. [Google Scholar] [CrossRef]
- O’Leary, M.P. Validity of the "bother score" in the evaluation and treatment of symptomatic benign prostatic hyperplasia. Rev. Urol. 2005, 7, 1–10. [Google Scholar] [PubMed]
- Agbabiaka, T.B.; Pittler, M.H.; Wider, B.; Ernst, E. Serenoa repens (saw palmetto): A systematic review of adverse events. Drug Saf. 2009, 32, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Novara, G.; Giannarini, G.; Alcaraz, A.; Cozar-Olmo, J.M.; Descazeaud, A.; Montorsi, F.; Ficarra, V. Efficacy and Safety of Hexanic Lipidosterolic Extract of Serenoa repens (Permixon) in the Treatment of Lower Urinary Tract Symptoms Due to Benign Prostatic Hyperplasia: Systematic Review and Meta-analysis of Randomized Controlled Trials. Eur. Urol. Focus 2016, 2, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Roehrborn, C.G. Male lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH). Med. Clin. 2011, 95, 87–100. [Google Scholar] [CrossRef] [PubMed]
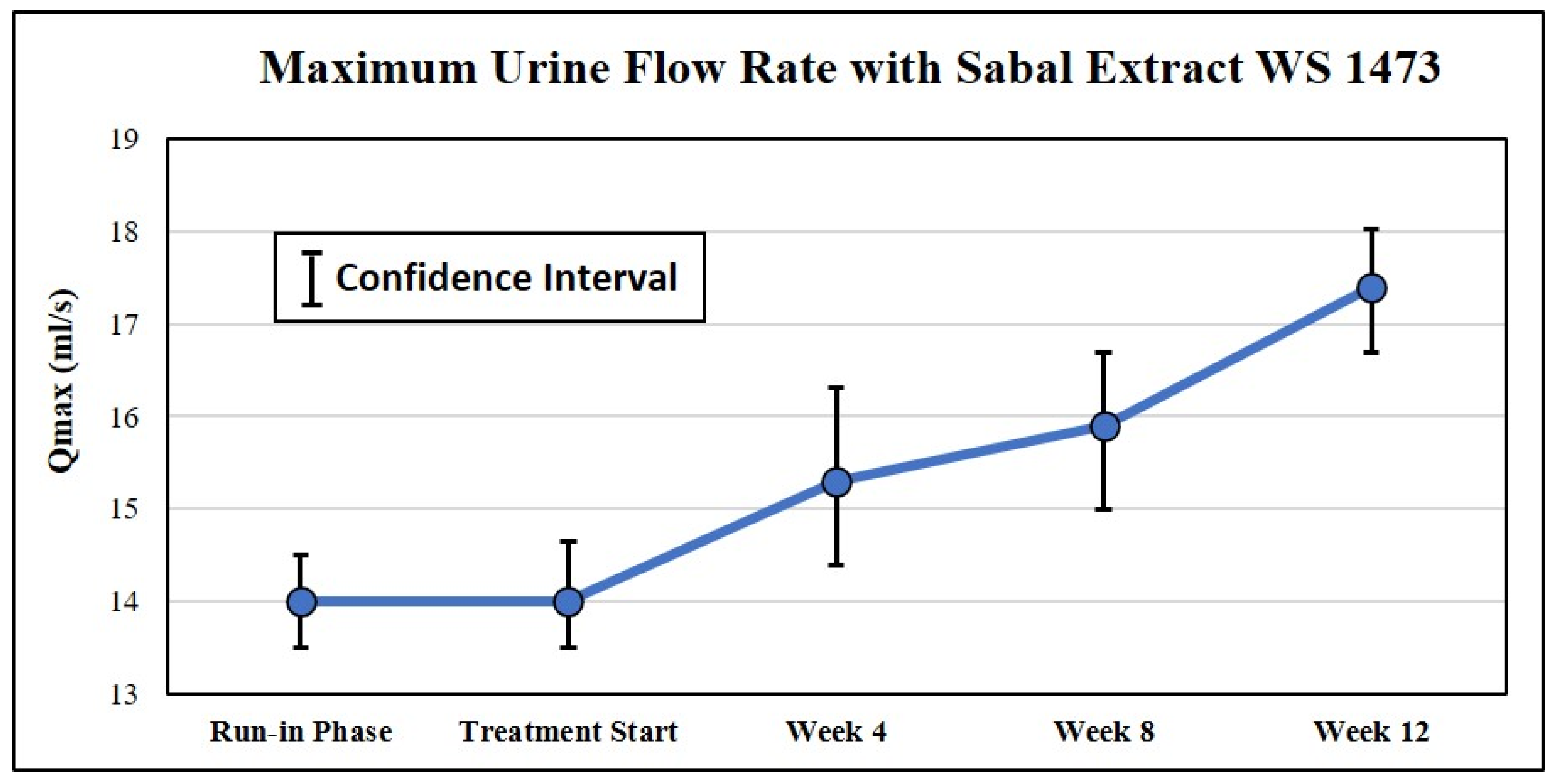
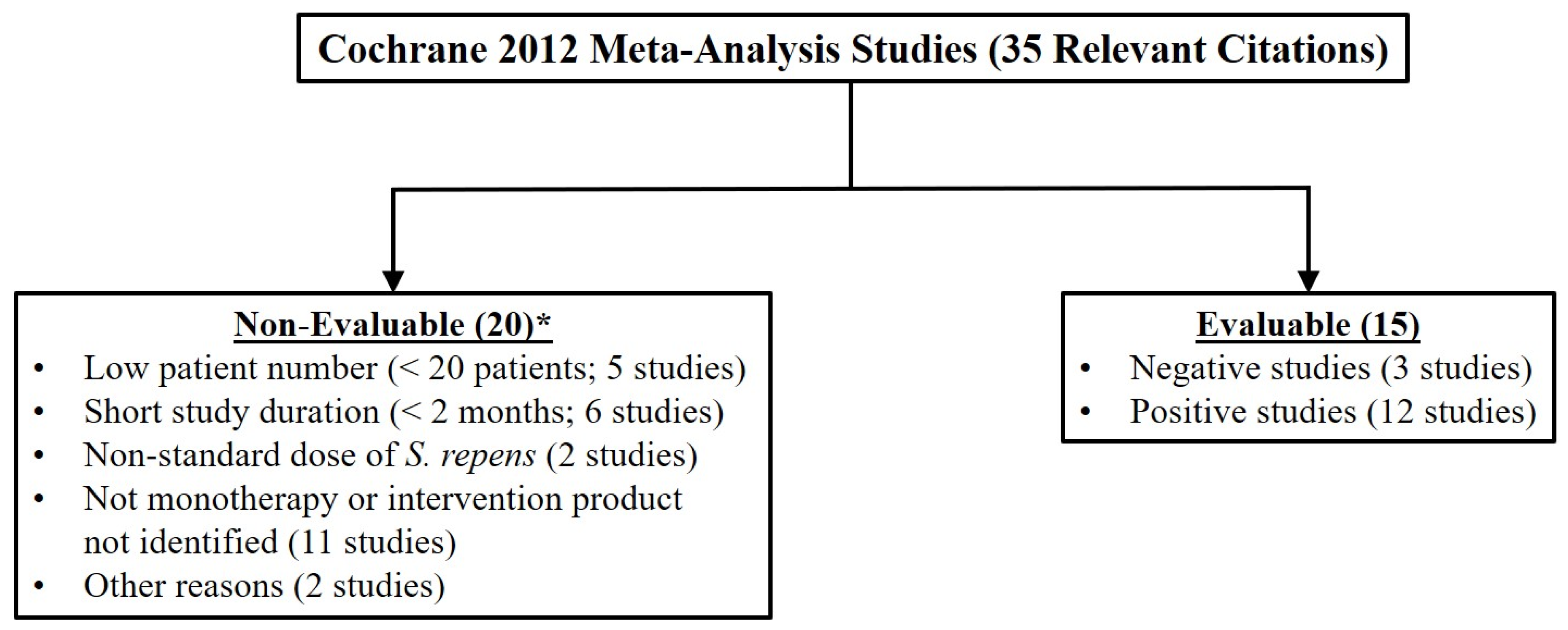
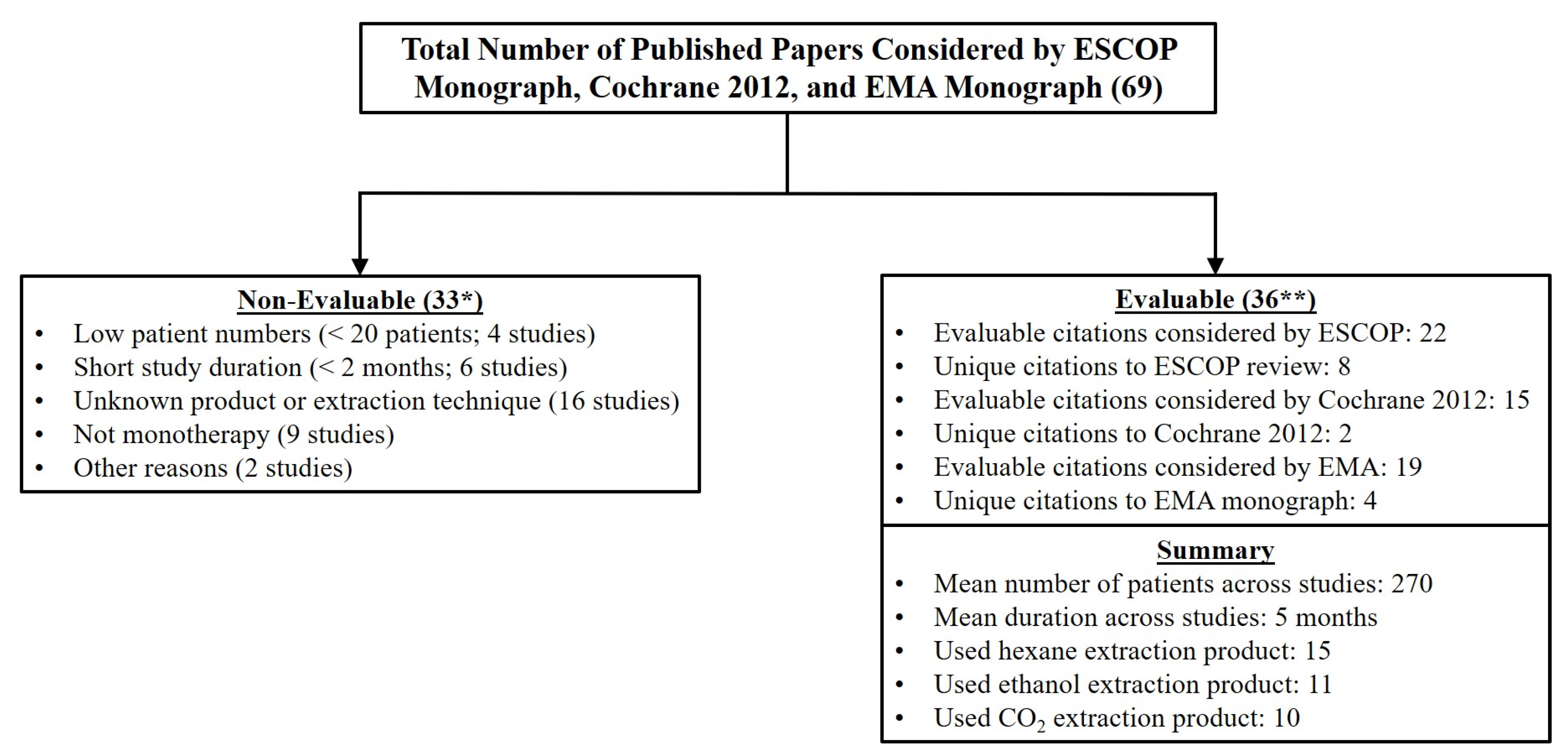
| Senior Author | Ref. (#) | Year | Extraction Method | Serenoa Patients (#) a | Study Duration (mos) | IPSS or Symptom Change | QoL | Qmax | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Δ | % | Δ | % | Δ | % | ||||||
| Cirillo-Marucco | [29] | 1983 | Hexane | 47 | 4 | 56 § | +4.55 | 50.4 § | |||
| Cukier ψ | [30] | 1985 | Hexane | 73 | 2 | 33.3 §§ | |||||
| Pescatore | [31] | 1986 | Hexane | 30 | 3 | +2.5 | 26.9 | ||||
| Authie | [32] | 1987 | Hexane | 500 | 3 | 78.1 * | |||||
| Ollé Carreras | [33] | 1987 | Hexane | 40 | 2 | 67.5 ‡‡ | |||||
| Orfei | [34] | 1988 | Hexane | 30 | 3 | 50.3 ^^ | −2.17 | +0.03 | 0.2 | ||
| Mattei ψ | [35] | 1990 | CO2 | 20 | 3 | 55.2 ^ | |||||
| Dathe | [36] | 1991 | Hexane | 49 | 6 | +5.90 | 49 | ||||
| Fabricius xx | [37] | 1993 | CO2 | 176 | 6 | 39% and 59% | |||||
| Romics | [38] | 1993 | CO2 | 31 | 12 | +4.3 | 39.0 | ||||
| Vahlensieck | [39] | 1993 | CO2 | 1334 | 4 | 46.7 §§§ | |||||
| Vahlensieck | [40] | 1993 | CO2 | 312 | 3 | + 5.8 | 51.8 | ||||
| Braeckman | [41] | 1994 | CO2 | 305 | 3 | −6.6 | 34.7 | −1.54 | 41.6 | +2.41 | 26.4 |
| Bach | [42] | 1996 | CO2 | 315 | 36 | 73.0 | + 6.1 | 45.5 | |||
| Carraro | [17] | 1996 | Hexane | 467 | 6 | −5.8 | 37.0 | −1.38 | 38.0 | +2.7 | 25.0 |
| Kondas | [43] | 1996 | Ethanol | 38 | 6 | +4.08 | 39.0 | ||||
| Braeckman | [44] | 1997b | CO2 | 125 | 3 | 64.0 | 29.8 | ||||
| Braeckman | [45] | 1997a | CO2 | 67 | 12 | −10.2 | 60.0 | −1.5 | 41.7 | +2.6 | 23.8 |
| Gerber | [46] | 1998 | Ethanol | 46 | 6 | −7.6 | 37.0 | −0.7 | −5.0 | ||
| Derakhshani | [47] | 1997 | Ethanol | 1047 | 3 | −7.4 | 40.4 | −1.61 | 45.9 | +3.7 | 30.8 |
| Eickenberg | [48] | 1997 | Ethanol | 6967 | 6 | −8.0 | 44.4 | −1.8 | 37.5 | + 3.0 | 23.1 |
| Redecker | [49] | 1998 | Ethanol | 50 | 3 | 47.7 ^^^ | + 3.4 | 24.0 | |||
| Ziegler Ж Ж | [50] | 1998 | Ethanol | 109 | 3 | 35.6 | +3.72 | 28.9 | |||
| Average Changes for All Studies | 530 | 6 | −5.3 | 25% | −1.7 | 40% | +3.4 | 27% | |||
| Senior Author | Ref. [#] | Year | Serenoa Therapy | Serenoa Patients (#) a | Study Duration (mos) | Comments on Study Data |
|---|---|---|---|---|---|---|
| Cukier | [30] | 1985 | Permixon Hexane | 73 | 2 | Frequency and nocturia significantly improved with Permixon |
| Pannunzio | [58] | 1986 | Permixon Hexane | 30 | 2 | Permixon vs. gestonorone; Qmax↑+5.1 (74%) vs. +2.2 (28%); no IPSS or QoL data |
| Mattei | [35] | 1990 | Talso CO2 | 20 | 3 | Frequency 43.8% improved vs. 7.1% for placebo; nocturia 68.9% improved vs. 4.8% for placebo; incomplete emptying 68.8% improved vs. 7.7% for placebo |
| Braeckman | [41] | 1994 | Prostaserene CO2 | 305 | 3 | Δ IPSS −6.6 (34.7%); Δ QoL −1.54 (41.6%); Δ Qmax +2.41 (26.4%) |
| Carraro | [17] | 1996 | Permixon Hexane | 467 | 6 | Permixon vs. finasteride; ↓IPSS by 37% vs. 39%; ↑QoL by 38% vs. 41%; Qmax +2.7 vs. +3.2 mL/s |
| Braeckman | [45] | 1997a | Prostaserene CO2 | 67 | 12 | IPSS −6.8, QoL −1.5, Qmax +2.6 mL/s |
| Braeckman | [44] | 1997b | Prostaserene CO2 | 125 | 3 | Δ “IPSS” −4.0 (51.3%) (scoring system per SBS); Δ Qmax +3.1 (29.8%) vs. placebo “IPSS” −1.7 (25.4%) and Δ Qmax +1.1 (10%) |
| Bauer | [59] | 1999 | Talso CO2 | 101 | 6 | IPSS↓37% at study end; no hard numbers, only % |
| Gerber | [60] | 2001 | Solaray Ethanol | 39 | 6 | IPSS −4.4 (26%) vs. −2.2 (14%); QoL −0.7 (21%) vs. −0.3 (10%); Qmax +1.0 vs. + 1.4 |
| Debruyne | [19] | 2002 | Permixon Hexane | 350 | 12 | Permixon vs. tamsulosin with IPSS improvement of 28% vs. 29%; Qmax↑1.8 vs. 1.9 mL/s |
| Willetts | [61] | 2003 | Proseren CO2 | 46 | 3 | Δ QoL −0.49 (13%) vs. placebo −0.69 (17%); Δ Qmax no significant difference, IPSS calculated from graphs −1.1 (7.8%) vs. −3.9 (21.8%) for placebo; negative study |
| Debruyne | [18] | 2004 | Permixon Hexane | 124 | 12 | Permixon vs. tamsulosin: Δ IPSS −7.8 (35%) vs. −5.8 (25%); Δ QoL −1.2 (29%) vs. −0.9 (23%); Δ Qmax + 1.2 (11%) vs. +1.7 (17%) |
| Bent | [14] | 2006 | Indena USA CO2 | 112 | 12 | Serenoa vs. placebo: Δ IPSS −0.68 (4.3%) vs. −0.72 (4.8%); Δ Qmax +0.42 (3.6%) vs. −0.01 (−0.09%); negative study |
| Hizli | [22] | 2007 | Prostagood® Ethanol * | 20 | 6 | Serenoa vs. tamsulosin vs. Serenoa plus tamsulosin; Δ IPSS −6.1 (34%) vs. −4.6 (28%) vs. −4.9 (31%); Δ QoL −2.6 (62%) vs. −2.1 (60%) vs. −2.2 (63%); Δ Qmax +3.2 (34%) vs. +3.7 (35%) vs. +4.2 (42%); no benefit with combination |
| Barry | [15] | 2011 | Prosta Urgenin Ethanol | 164 | 18 | S. repens vs. placebo: Δ AUA-SI −2.20 (15%) vs. −2.99 (20%); Δ QoL −0.34 (10.6%) vs. −0.49 (15.2%); Δ Qmax −0.18 (−1.2%) vs. −0.79 (−5.3%); negative study |
| Senior Author | Year | Ref. (#) | Serenoa Patients (#) a | Study Duration (mos) | Key Results for Serenoa vs. Placebo or Comparator |
|---|---|---|---|---|---|
| Boccafoschi | 1983 | [65] | 11 | 2 | Qmax +4.2 (42%) vs. placebo + 2.1 (20.6%) |
| Emili | 1983 | [66] | 15 | 1 | Qmax +3.56 (34.5%) vs. placebo +0.20 (2.2%) |
| Mandressi | 1983 | [67] | 19 | 1 | Serenoa vs. Pygeum vs. placebo; urgency 70% vs. 62% vs. 24%; frequency 30% vs. 22% vs. 10%; nocturia 42% vs. 38% vs. −4% |
| Champault | 1984 | [69] | 50 | 1 | Qmax +2.7 (50.5%) vs. placebo +0.25 (5%); nocturia −1.53 (49%) vs. placebo −0.48 (15%) |
| Tasca | 1985 | [68] | 14 | 2 | Qmax +3.3 (25.6%) vs. placebo −0.6 (−5%); nocturia 74.3% vs. 38.7%; urgency 60% vs. 20%; weak stream 50% vs. 16.6% |
| Descotes | 1995 | [64] | 82 | 1 | Qmax +3.4 (28.9%) vs. placebo +1.1 (8.9%) |
| Average Changes in Qmax, Nocturia and Urgency | Qmax +3.4;↓nocturia 55%;↓urgency 65% | ||||
| Senior Author Year, Ref. (#) | Study Duration (mos) | Study Arm | Patients (#) a | IPSS | QoL | Qmax | |||
|---|---|---|---|---|---|---|---|---|---|
| Δ | % | Δ | % | mL/s | % | ||||
| Debruyne 2002 [19] | 12 | LSESr | 350 | −4.5 | 29% | NR | NR | +1.8 | 17% |
| Tam | 354 | −4.4 | 29% | NR | NR | +1.8 | 16% | ||
| Hizli 2007 [22] | 6 | LSESr | 20 | −6.1 | 34% | −2.6 | 62% | +3.2 | 34% |
| Tam | 20 | −4.6 | 28% | −2.1 | 60% | +3.7 | 35% | ||
| Senior Author | Year | Evaluable y or n | Product | Extraction | Serenoa Pts (#) a | Study Duration (mos) | ESCOP | Cochrane | AUAG | EMA |
|---|---|---|---|---|---|---|---|---|---|---|
| Bach † | 1996 | y | Strogen S | CO2 | 315 | 36 | * | |||
| Bauer ‡ | 1999 | y | Talso uno | CO2 | 101 | 6 | * | |||
| Bent † | 2006 | y | Indena USA | CO2 | 112 | 12 | + | + | + | |
| Braeckman † | 1994 | y | Prostaserene | CO2 | 505 | 3 | + | + | + | |
| Braeckman1 † | 1997 | y | Prostaserene | CO2 | 67 | 12 | + | + | + | |
| Braeckman2 † | 1997 | y | Prostaserene | CO2 | 125 | 3 | + | + | ||
| Fabricius ‡ | 1993 | y | Talso | CO2 | 176 | 6 | + | + | ||
| Kondas † | 1996 | y | Strogen forte | CO2 | 38 | 6 | * | |||
| Mattei ‡ | 1988 | y | Talso | CO2 | 20 | 3 | + | + | + | |
| Romics † | 1993 | y | Strogen forte | CO2 | 31 | 12 | * | |||
| Vahlensieck ‡ | 1993 | y | Talso uno | CO2 | 1334 | 4 | + | + | ||
| Vahlensieck ‡ | 1993 | y | Talso uno | CO2 | 312 | 3 | + | + | ||
| Willetts † | 2003 | y | Proseren | CO2 | 46 | 3 | + | + | + | |
| Barry † | 2011 | y | Prosta Urgenin | ethanol | 151 | 18 | + | + | ||
| Derakhshani ‡ | 1997 | y | Prosta Urgenin | ethanol | 1047 | 3 | * | |||
| Eickenberg ‡ | 1997 | y | Sita | ethanol | 6967 | 6 | * | |||
| Gerber † | 1998 | y | Solaray | ethanol | 46 | 6 | * | |||
| Gerber † | 2001 | y | Solaray | ethanol | 39 | 6 | + | + | + | |
| Hizli † | 2007 | y | Prostagood | ethanol | 20 | 6 | + | + | + | |
| Redecker ‡ | 1998 | y | Prostagutt | ethanol | 50 | 3 | * | |||
| Ziegler ‡ | 1998 | y | Prostagutt | ethanol | 109 | 3 | * | |||
| Authie ‡ | 1987 | y | Permixon | hexane | 500 | 3 | * | |||
| Carraro † | 1996 | y | Permixon | hexane | 467 | 6 | + | + | + | + |
| Cirillo-Marucco ‡ | 1983 | y | Permixon | hexane | 47 | 4 | * | |||
| Cukier ‡ | 1985 | y | Permixon | hexane | 73 | 2 | + | + | + | |
| Dathe ‡ | 1991 | y | Permixon | hexane | 49 | 6 | + | + | ||
| Debruyne † | 2002 | y | Permixon | hexane | 350 | 12 | + | + | + | |
| Debruyne † | 2004 | y | Permixon | hexane | 124 | 12 | + | + | ||
| Giannakopoulos † | 2002 | y | Permixon | hexane | 100 | 6 | * | |||
| Giulianelli † | 2012 | y | Permixon | hexane | 591 | 6 | * | |||
| Ollé Carreras ‡ | 1987 | y | “hexane” | hexane | 40 | 2 | * | |||
| Orfei ‡ | 1988 | y | Permixon | hexane | 30 | 3 | * | |||
| Pannunzio ‡ | 1986 | y | Permixon | hexane | 30 | 2 | * | |||
| Pescatore ‡ | 1986 | y | Permixon | hexane | 30 | 3 | * | |||
| Pytel † | 2002 | y | Permixon | hexane | 116 | 24 | * | |||
| Stepanov † | 1999 | y | Permixon | hexane | 92 | 3 | * | |||
| Kaplan † | 2004 | n | nos | - | * | |||||
| Marks † | 2000 | n | Nutrilite Blend | - | 20 | 6 | + | + | ||
| Mohanty † | 1999 | n | nos | - | 36 | 2 | * | |||
| Preuss † | 2001 | n | Herbs Vitamins | - | + | + | ||||
| Roveda ‡ | 1994 | n | Serpens nos | - | 30 | 1 | * | |||
| Schneider ‡ | 1994 | n | nos | - | + | + | ||||
| Breu ‡ | 1992 | n | Talso uno | CO2 | + | + | ||||
| Hagenlocher ‡ | 1993 | n | SG 291 λ | CO2 | * | |||||
| Shi † | 2008 | n | Prostataplex | CO2 | 46 | 3 | + | + | + | |
| Carbin † | 1990 | n | Sabal serrulata + Curbicin | combo | 26 | 3 | * | |||
| Engelmann † | 2006 | n | Prostagutt forte + Urtica | combo | 56 | 15 | + | + | ||
| Lopatkin † | 2005 | n | Prostagutt + Urtica | combo | 129 | 6 | + | + | ||
| Sökeland ‡ | 1997 | n | Prostagutt + Urtica | combo | 245 | 12 | * | |||
| Gabric ‡ | 1987 | n | Prostagutt | ethanol | 15 | 1.5 | * | |||
| Helfand † | 2012 | n | Prosta Urgenin | ethanol | * | |||||
| Koch ‡ | 1994 | n | Prostagutt | ethanol | * | |||||
| Koch ‡ | 1995 | n | Prostagutt | ethanol | * | |||||
| Löbelenz ‡ | 1992 | n | Sabal | ethanol | 30 | 1.5 | + | + | ||
| Metzker ‡ | 1996 | n | Prostagutt + Urtica | ethanol | 37 | 12 | * | |||
| Boccafoschi ‡ | 1983 | n | Permixon | hexane | 11 | 2 | + | + | + | |
| Champault ‡ | 1984 | n | Permixon | hexane | 50 | 1 | + | + | + | |
| Descotes † | 1995 | n | Permixon | hexane | 82 | 1 | + | + | + | |
| Emili ‡ | 1983 | n | Permixon | hexane | 15 | 1 | + | + | + | |
| Glémain ‡ | 2002 | n | Permixon | hexane | 159 | 12 | + | + | ||
| Grasso † | 1995 | n | Permixon | hexane | + | + | ||||
| Mancuso ‡ | 1986 | n | Permixon | hexane | * | |||||
| Mandressi ‡ | 1983 | n | Permixon | hexane | 19 | 1 | * | |||
| Martorana ‡ | 1986 | n | Permixon | hexane | * | |||||
| Paoletti ‡ | 1986 | n | Permixon | hexane | 18 | 2 | * | |||
| Pytel ‡ Ψ | 2004 | n | Permixon | hexane | * | |||||
| Reece Smith † | 1986 | n | Permixon | hexane | 33 | 3 | + | + | + | |
| Tasca ‡ | 1985 | n | Permixon | hexane | 14 | 2 | + | + | + | |
| Vespasiani ‡ | 1987 | n | Permixon | hexane | 15 | 9 | * | |||
| Averages Across All Studies with Data Tabulated | 270 | 5 | E Total Citations (%) | E Total Citations (%) | E Total Citations (%) | E Total Citations (%) | ||||
| 22 of 38 (58%) | 15 of 35 (43%) | 6 of 11 (54%) | 19 of 33 (58%) | |||||||
69 Total Citations/36 Evaluable Citations
| ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strum, S.B. Serenoa repens (Saw palmetto) for Lower Urinary Tract Symptoms (LUTS): The Evidence for Efficacy and Safety of Lipidosterolic Extracts. Part I. Uro 2021, 1, 118-138. https://doi.org/10.3390/uro1030015
Strum SB. Serenoa repens (Saw palmetto) for Lower Urinary Tract Symptoms (LUTS): The Evidence for Efficacy and Safety of Lipidosterolic Extracts. Part I. Uro. 2021; 1(3):118-138. https://doi.org/10.3390/uro1030015
Chicago/Turabian StyleStrum, Stephen B. 2021. "Serenoa repens (Saw palmetto) for Lower Urinary Tract Symptoms (LUTS): The Evidence for Efficacy and Safety of Lipidosterolic Extracts. Part I" Uro 1, no. 3: 118-138. https://doi.org/10.3390/uro1030015
APA StyleStrum, S. B. (2021). Serenoa repens (Saw palmetto) for Lower Urinary Tract Symptoms (LUTS): The Evidence for Efficacy and Safety of Lipidosterolic Extracts. Part I. Uro, 1(3), 118-138. https://doi.org/10.3390/uro1030015






