Ketosis Suppression and Ageing (KetoSAge): The Effect of Suppressing Ketosis on GKI and Liver Biomarkers in Healthy Females
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Anthropometric Measurements
2.3. Blood Collection and Measurement
2.4. Blood Marker Analysis
2.5. Statistical Analysis
3. Results
3.1. Suppression of Ketosis Is Associated with Increases in GKI
3.2. Suppression of Ketosis Is Associated with Increases in ALT and GGT
3.3. Suppression of Ketosis Is Associated with Changes in the Ratio of Aminotransferases
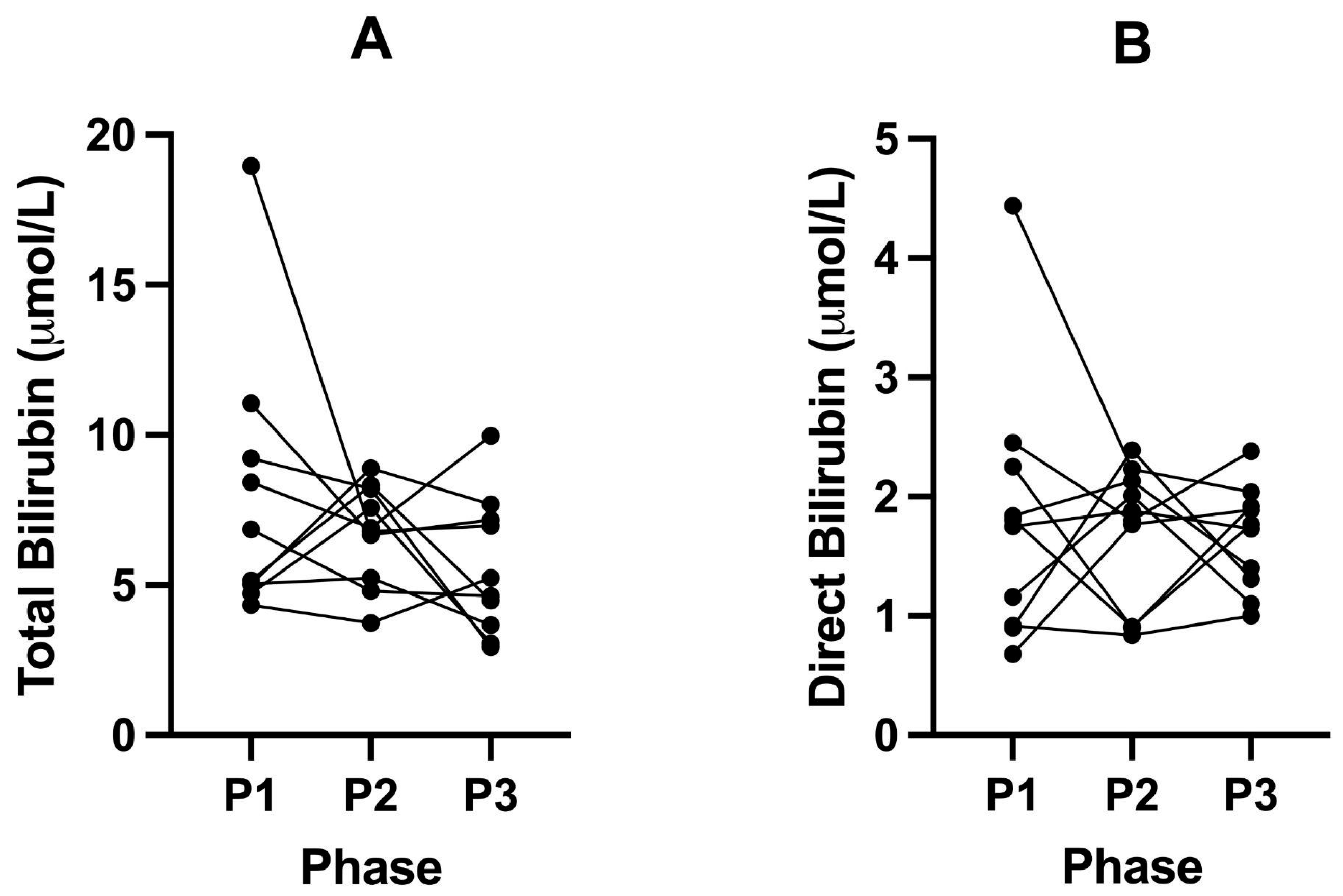
3.4. Levels of Albumin, Total Protein, Bilirubin, CK, and Iron Do Not Significantly Change
3.5. Liver Markers ALT, AST, GGT Change as Basal Insulin, HOMA-IR, and GKI Change
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ALP | alkaline phosphatase |
| ALT | alanine aminotransferase |
| AMORIS | apolipoprotein mortality risk study |
| AST | aspartate transferase |
| BDH1 | beta-hydroxybutyrate dehydrogenase-1 |
| BHB | beta-hydroxybutyrate |
| BMI | body mass index |
| Ca2+ | Calcium |
| Ch-S | cholesterol-sulphate |
| CI | Confidence interval |
| CK-NAC | creatine kinase-NAC |
| CoA | coenzyme A |
| CO2 | carbon dioxide |
| CV | Coefficient of variation |
| CVD | cardiovascular disease |
| DVT | deep vein thrombosis |
| CYP27B1 | cytochrome P450 Family 27 Subfamily B Member 1 |
| ELISA | enzyme-linked immunosorbent assay |
| eNOS | endothelial nitric oxide synthase |
| ETC | electron transport chain |
| GGT | gamma-glutamyl transferase |
| GKI | glucose ketone index |
| GLP-1 | glucagon like peptide-1 |
| GSH | reduced glutathione |
| GSSG | oxidised glutathione |
| HbA1c | haemoglobin A1c |
| HDL | high-density lipoprotein |
| HMG | 3-hydroxy-3-methylglutaryl |
| HO | haem-oxygenase |
| HOMA-IR | homeostasis model assessment for insulin resistance |
| HS | heparan-sulphate proteoglycans |
| H2O2 | hydrogen peroxide |
| Idh2 | isocitrate dehydrogenase 2 |
| IGF-1 | insulin like growth factor-1 |
| InsR | insulin receptor |
| KNHANES | Korean National Health and Nutrition Examination Surveys |
| LH | lithium heparin |
| MASLD | metabolic-dysfunction-associated steatosis liver disease |
| MCT | monocarboxylic acid transporter |
| MetS | metabolic syndrome |
| MnSOD2 | manganese superoxide dismutase 2 |
| Mt | mitochondrial |
| NAD+ | nicotinamide adenine dinucleotide |
| NADP | nicotinamide adenine dinucleotide phosphate |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| NF-kB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NK | nutritional ketosis |
| OCN | osteocalcin |
| OGTT | oral glucose tolerance test |
| O2− | superoxide |
| PAI-1 | plasminogen activator inhibitor type 1 |
| PCOS | polycystic ovarian syndrome |
| PE | pulmonary embolism |
| Pi | phosphate |
| PM | plasma membrane |
| P1 | Phase 1 |
| P2 | Phase 2 |
| P3 | Phase 3 |
| RBC | red blood cell |
| RM | repeated measures |
| ROS | reactive oxygen species |
| RQ | respiratory quotient |
| SCHI | subclinical hyperinsulinaemia |
| SIRT3 | sirtuin 3 |
| SpO2 | oxygen saturation |
| SuK | suppression of ketosis |
| SUK | Standard U.K. diet |
| SULT2B1b | sulfotransferase 2B1b |
| TATc | thrombin-antithrombin complex |
| TNF-α | Tumour necrosis factor alpha |
| T2DM | type 2 diabetes mellitus |
References
- Cooper, I.D.; Kyriakidou, Y.; Edwards, K.; Petagine, L.; Seyfried, T.N.; Duraj, T.; Soto-Mota, A.; Scarborough, A.; Jacome, S.L.; Brookler, K.; et al. Ketosis Suppression and Ageing (KetoSAge): The effects of suppressing ketosis in long term keto-adapted non-athletic females. Int. J. Mol. Sci. 2023, 24, 15621. [Google Scholar] [CrossRef]
- Cooper, I.D.; Kyriakidou, Y.; Petagine, L.; Edwards, K.; Soto-Mota, A.; Brookler, K.; Elliott, B.T. Ketosis Suppression and Ageing (KetoSAge) Part 2: The Effect of Suppressing Ketosis on Biomarkers Associated with Ageing, HOMA-IR, Leptin, Osteocalcin, and GLP-1, in Healthy Females. Biomedicines 2024, 12, 1553. [Google Scholar] [CrossRef]
- Petagine, L.; Zariwala, M.G.; Patel, V.B. Non-alcoholic fatty liver disease: Immunological mechanisms and current treatments. World J. Gastroenterol. 2023, 29, 4831–4850. [Google Scholar] [CrossRef]
- Muzica, C.M.; Sfarti, C.; Trifan, A.; Zenovia, S.; Cuciureanu, T.; Nastasa, R.; Huiban, L.; Cojocariu, C.; Singeap, A.M.; Girleanu, I.; et al. Nonalcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: A Bidirectional Relationship. Can. J. Gastroenterol. Hepatol. 2020, 2020, 6638306. [Google Scholar] [CrossRef] [PubMed]
- Huttasch, M.; Roden, M.; Kahl, S. Obesity and MASLD: Is weight loss the (only) key to treat metabolic liver disease? Metabolism 2024, 157, 155937. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hasan, M.N.; Gu, L. Bile acids regulation of cellular stress responses in liver physiology and diseases. eGastroenterology 2024, 2, e100074. [Google Scholar] [CrossRef]
- Al-Salem, A.H. Pathophysiology and Functions of the Spleen. In The Spleen; Springer Nature: Singapore, 2023; pp. 33–49. [Google Scholar] [CrossRef]
- Seneff, S.; Kyriakopoulos, A.M. Taurine prevents mitochondrial dysfunction and protects mitochondria from reactive oxygen species and deuterium toxicity. Amino Acids 2025, 57, 6. [Google Scholar] [CrossRef] [PubMed]
- Cahill, G.F.; Herrera, M.G.; Morgan, A.P.; Soeldner, J.S.; Steinke, J.; Levy, P.L.; Reichard, G.A.; Kipnis, D.M. Hormone-fuel interrelationships during fasting. J. Clin. Investig. 1966, 45, 1751–1769. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 2014, 25, 42–52. [Google Scholar] [CrossRef]
- Rojas-Morales, P.; Tapia, E.; Pedraza-Chaverri, J. β-hydroxybutyrate: A signaling metabolite in starvation response? Cell. Signal 2016, 28, 917–923. [Google Scholar] [CrossRef]
- Neudorf, H.; Little, J.P. Impact of fasting & ketogenic interventions on the NLRP3 inflammasome: A narrative review. Biomed. J. 2024, 47, 100677. [Google Scholar] [CrossRef]
- Auestad, N.; Korsak, R.A.; Morrow, J.W.; Edmond, J. Fatty acid oxidation and ketogenesis by astrocytes in primary culture. J. Neurochem. 1991, 56, 1376–1386. [Google Scholar] [CrossRef]
- Blázquez, C.; Woods, A.; De Ceballos, M.L.; Carling, D.; Guzmán, M. The AMP-activated protein kinase Is involved in the regulation of ketone body production by astrocytes. J. Neurochem. 2002, 73, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Adijanto, J.; Du, J.; Moffat, C.; Seifert, E.L.; Hurle, J.B.; Philp, N.J. The retinal pigment epithelium utilizes fatty acids for ketogenesis implications for metabolic coupling with the outer retina. J. Biol. Chem. 2014, 289, 20570–20582. [Google Scholar] [CrossRef]
- Reyes-Reveles, J.; Dhingra, A.; Alexander, D.; Bragin, A.; Philp, N.J.; Boesze-Battaglia, K. Phagocytosis-dependent ketogenesis in retinal pigment epithelium. J. Biol. Chem. 2017, 292, 8038–8047. [Google Scholar] [CrossRef]
- Grabacka, M.; Pierzchalska, M.; Dean, M.; Reiss, K. Regulation of ketone body metabolism and the role of PPARα. Int. J. Mol. Sci. 2016, 17, 2093. [Google Scholar] [CrossRef]
- Miller, V.J.; Villamena, F.A.; Volek, J.S. Nutritional ketosis and mitohormesis: Potential implications for mitochondrial function and human health. J. Nutr. Metab. 2018, 2018, 5157645. [Google Scholar] [CrossRef]
- Volek, J.S.; Freidenreich, D.J.; Saenz, C.; Kunces, L.J.; Creighton, B.C.; Bartley, J.M.; Davitt, P.M.; Munoz, C.X.; Anderson, J.M.; Maresh, C.M.; et al. Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism 2015, 65, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Cooper, I.D.; Brookler, K.H.; Kyriakidou, Y.; Elliott, B.T.; Crofts, C.A.P. Metabolic Phenotypes and Step by Step Evolution of Type 2 Diabetes: A New Paradigm. Biomedicines 2021, 9, 800. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shao, M.; Lu, L.; Zhao, C.; Qiu, L.; Liu, Z. Obesity, insulin resistance and their interaction on liver enzymes. PLoS ONE 2021, 16, e0249299. [Google Scholar] [CrossRef]
- Cooper, I.D.; Crofts, C.A.P.; DiNicolantonio, J.J.; Malhotra, A.; Elliott, B.; Kyriakidou, Y.; Brookler, K.H. Relationships between hyperinsulinaemia, magnesium, vitamin D, thrombosis and COVID-19: Rationale for clinical management. Open Hear. 2020, 7, e001356. [Google Scholar] [CrossRef]
- Santoleri, D.; Titchenell, P.M. Resolving the Paradox of Hepatic Insulin Resistance. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 447–456. [Google Scholar] [CrossRef]
- Cooper, I.D.; Kyriakidou, Y.; Petagine, L.; Edwards, K.; Elliott, B.T. Bio-Hacking Better Health—Leveraging Metabolic Biochemistry to Maximise Healthspan. Antioxidants 2023, 12, 1749. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133. [Google Scholar] [CrossRef] [PubMed]
- Mitsala, A.; Tsalikidis, C.; Romanidis, K.; Pitiakoudis, M. Non-Alcoholic Fatty Liver Disease and Extrahepatic Cancers: A Wolf in Sheep’s Clothing? Curr. Oncol. 2022, 29, 4478–4510. [Google Scholar] [CrossRef]
- Meidenbauer, J.J.; Mukherjee, P.; Seyfried, T.N. The glucose ketone index calculator: A simple tool to monitor therapeutic efficacy for metabolic management of brain cancer. Nutr. Metab. 2015, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Duraj, T.; Kalamian, M.; Zuccoli, G.; Maroon, J.C.; D’Agostino, D.P.; Scheck, A.C.; Poff, A.; Winter, S.F.; Hu, J.; Klement, R.J.; et al. Clinical research framework proposal for ketogenic metabolic therapy in glioblastoma. BMC Med. 2024, 22, 578. [Google Scholar] [CrossRef]
- Cooper, I.D.; Sanchez-Pizarro, C.; Norwitz, N.G.; Feldman, D.; Kyriakidou, Y.; Edwards, K.; Petagine, L.; Elliot, B.T.; Soto-Mota, A. Thyroid markers and body composition predict LDL-cholesterol change in lean healthy women on a ketogenic diet: Experimental support for the lipid energy model. Front. Endocrinol. 2023, 14, 1326768. [Google Scholar] [CrossRef]
- Moore, A.R.; Holland-Winkler, A.M.; Ansley, J.K.; Boone, E.D.H.; Schulte, M.K.O. Reliability and diagnostic performance of a new blood ketone and glucose meter in humans. J. Int. Soc. Sports Nutr. 2021, 18, 6. [Google Scholar] [CrossRef]
- Teshome, G.; Ambachew, S.; Fasil, A.; Abebe, M. Prevalence of Liver Function Test Abnormality and Associated Factors in Type 2 Diabetes Mellitus: A Comparative Cross-Sectional Study. EJIFCC 2019, 30, 303. [Google Scholar] [PubMed]
- Minato-Inokawa, S.; Tsuboi-Kaji, A.; Honda, M.; Takeuchi, M.; Kitaoka, K.; Kurata, M.; Wu, B.; Kazumi, T.; Fukuo, K. Associations of alanine aminotransferase/aspartate aminotransferase with insulin resistance and β-cell function in women. Sci. Rep. 2023, 13, 7853. [Google Scholar] [CrossRef]
- Abdelhameed, F.; Kite, C.; Lagojda, L.; Dallaway, A.; Chatha, K.K.; Chaggar, S.S.; Dalamaga, M.; Kassi, E.; Kyrou, I.l.; Randeva, H.S. Non-invasive Scores and Serum Biomarkers for Fatty Liver in the Era of Metabolic Dysfunction-associated Steatotic Liver Disease (MASLD): A Comprehensive Review From NAFLD to MAFLD and MASLD. Curr. Obes. Rep. 2024, 13, 510–513. [Google Scholar] [CrossRef]
- Mason, J.E.; Starke, R.D.; Van Kirk, J.E. Gamma-Glutamyl Transferase: A Novel Cardiovascular Risk BioMarker. Prev. Cardiol. 2010, 13, 36–41. [Google Scholar] [CrossRef]
- Bulusu, S.; Sharma, M. What does serum γ-glutamyltransferase tell us as a cardiometabolic risk marker? Ann. Clin. Biochem. 2016, 53, 312–332. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, F.; Ducluzeau, P.H.; Gastaldelli, A.; Laville, M.; Anderwald, C.H.; Konrad, T.; Mari, A.; Balkau, B.; RISC Study Group. Liver Enzymes Are Associated With Hepatic Insulin Resistance, Insulin Secretion, and Glucagon Concentration in Healthy Men and Women. Diabetes 2011, 60, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Fotros, D.; Sohouli, M.H.; Velu, P.; Fatahi, S.; Liu, Y. The effect of a ketogenic diet on inflammation-related markers: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2024, 83, 40–58. [Google Scholar] [CrossRef]
- Monda, A.; La Torre, M.E.; Messina, A.; Di Maio, G.; Monda, V.; Moscatelli, F.; De Stefano, M.l.; La Marra, M.; Padova, M.D.; Dipace, A.; et al. Exploring the ketogenic diet’s potential in reducing neuroinflammation and modulating immune responses. Front. Immunol. 2024, 15, 1425816. [Google Scholar] [CrossRef]
- Paoli, A.; Cerullo, G. Investigating the Link between Ketogenic Diet, NAFLD, Mitochondria, and Oxidative Stress: A Narrative Review. Antioxidants 2023, 12, 1065. [Google Scholar] [CrossRef]
- Hansen, M.E.; Tippetts, T.S.; Anderson, M.C.; Holub, Z.E.; Moulton, E.R.; Swensen, A.C.; Prince, J.T.; Bikman, B.T. Insulin increases ceramide synthesis in skeletal muscle. J. Diabetes Res. 2014, 2014, 765784. [Google Scholar] [CrossRef]
- Naidu, B.T.K.; Raju, K.S.; BhaskaraRao, J.V.; Sunil Kumar, N. Gamma-Glutamyl Transferase as a Diagnostic Marker of Metabolic Syndrome. Cureus 2023, 15, e41060. [Google Scholar] [CrossRef] [PubMed]
- Kasarala, G.; Tillmann, H.L. Standard liver tests. Clin. Liver. Dis. 2016, 8, 13. [Google Scholar] [CrossRef]
- Van Hemelrijck, M.; Jassem, W.; Walldius, G.; Fentiman, I.S.; Hammar, N.; Lambe, M.; Garmo, H.; Jungner, I.; Holmberg, L. Gamma-glutamyltransferase and risk of cancer in a cohort of 545,460 persons –The Swedish AMORIS study. Eur. J. Cancer 2011, 47, 2033–2041. [Google Scholar] [CrossRef]
- Wang, Q.; Shu, X.; Dong, Y.; Zhou, J.; Teng, R.; Shen, J.; Chen, Y.; Dong, M.; Zhang, W.; Huang, Y.; et al. Tumor and serum gamma-glutamyl transpeptidase, new prognostic and molecular interpretation of an old biomarker in gastric cancer. Oncotarget 2017, 8, 36171. [Google Scholar] [CrossRef]
- Staudigl, C.; Concin, N.; Grimm, C.; Pfeiler, G.; Nehoda, R.; Singer, C.F.; Polterauer, S. Prognostic Relevance of Pretherapeutic Gamma-Glutamyltransferase in Patients with Primary Metastatic Breast Cancer. PLoS ONE 2015, 10, e0125317. [Google Scholar] [CrossRef]
- Xiao, B.; Peng, J.; Tang, J.; Deng, Y.; Zhao, Y.; Wu, X.; Ding, P.; Lin, J.; Pan, Z. Serum Gamma Glutamyl transferase is a predictor of recurrence after R0 hepatectomy for patients with colorectal cancer liver metastases. Ther. Adv. Med. Oncol. 2020, 12, 1758835920947971. [Google Scholar] [CrossRef]
- Cicek, H. The Prognostic Role of Gama Glutamil Transferase in High Grade Glial Tumors. Cancer Ther. Oncol. Int. J. 2022, 21, 556061. [Google Scholar] [CrossRef]
- Sanna, C.; Rosso, C.; Marietti, M.; Bugianesi, E. Non-Alcoholic Fatty Liver Disease and Extra-Hepatic Cancers. Int. J. Mol. Sci. 2016, 17, 717. [Google Scholar] [CrossRef] [PubMed]
- Reiser, E.; Aust, S.; Seebacher, V.; Reinthaller, A.; von Mersi, H.; Schwameis, R.; Polterauer, S.; Grimm, C.; Helmy-Bader, S. Gamma-glutamyltransferase as a preoperative differential diagnostic marker in patients with adnexal mass. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 239, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Sayan, M.; Ozkan, D.; Kankoc, A.; Tombul, I.; Celik, A.; Kurul, I.C.; Tastepe, A.I. Is Gamma-Glutamyl Transferase a Prognostic Indicator for Early-Stage Lung Cancer Treated Surgically? Wiad. Lek. 2021, 74, 1804–1808. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Que, S.; Xu, J.; Peng, T. Alanine Aminotransferase-Old Biomarker and New Concept: A Review. Int. J. Med. Sci. 2014, 11, 925. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.M.; Brancati, F.L.; Diehl, A.M. The prevalence and etiology of elevated aminotransferase levels in the United States. Am, J. Gastroenterol. 2003, 98, 960–967. [Google Scholar] [CrossRef]
- Liu, C.; Liu, K.; Zhao, X.; Zhu, J.; Liu, Y.; Hao, L.; Gao, Y.; Liu, P. The Associations Between Alanine Aminotransferase and Other Biochemical Parameters in Lean PCOS. Reprod. Sci. 2022, 30, 633. [Google Scholar] [CrossRef]
- Khan, M.S.; Kim, H.-S.; Kim, R.; Yoon, S.H.; Kim, S.G. Dysregulated Liver Metabolism and Polycystic Ovarian Syndrome. Int. J. Mol. Sci. 2023, 24, 7454. [Google Scholar] [CrossRef]
- Kim, H.R.; Han, M.A. Association between Serum Liver Enzymes and Metabolic Syndrome in Korean Adults. Int. J. Environ. Res. Public Health 2018, 15, 1658. [Google Scholar] [CrossRef]
- Basaranoglu, M.; Basaranoglu, G.; Bugianesi, E. Carbohydrate intake and nonalcoholic fatty liver disease: Fructose as a weapon of mass destruction. Hepatobiliary Surg. Nutr. 2015, 4, 109–116. [Google Scholar] [CrossRef] [PubMed]
- De Luis, D.A.; Aller, R.; Izaola, O.; Lopez, J.J.; Gomez, E.; Torres, B.; Soto, G.D. Effect of rs6923761 gene variant of glucagon-like peptide 1 receptor on metabolic response and weight loss after a 3-month intervention with a hypocaloric diet. J. Endocrinol. Investig. 2014, 37, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.W.; Jun, D.W.; Lee, S.M.; Lee, K.N.; Lee, H.L.; Lee, O.Y.; Yoon, B.C.; Choi, H.S. Carbohydrate but not fat is associated with elevated aminotransferases. Aliment. Pharmacol. Ther. 2012, 35, 1064–1072. [Google Scholar] [CrossRef]
- Han, S.K.; Seo, M.J.; Lee, T.; Kim, M.Y. Effectiveness of the ALT/AST ratio for predicting insulin resistance in a Korean population: A large-scale, cross-sectional cohort study. PLoS ONE 2024, 19, e0303333. [Google Scholar] [CrossRef]
- Kwon, S.S.; Lee, S.G. A High Alanine Aminotransferase/Aspartate Aminotransferase Ratio Determines Insulin Resistance and Metabolically Healthy/Unhealthy Obesity in a General Adult Population in Korea: The Korean National Health and Nutritional Examination Survey 2007–2010. Exp. Clin. Endocrinol. Diabetes. 2019, 127, 677–684. [Google Scholar] [CrossRef]
- Pinnaduwage, L.; Ye, C.; Hanley, A.J.; Connelly, P.W.; Sermer, M.; Zinman, B.; Retnakaran, R. Changes Over Time in Hepatic Markers Predict Changes in Insulin Sensitivity, β-Cell Function, and Glycemia. J. Clin. Endocrinol. Metab. 2018, 103, 2651–2659. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, R.; Kohara, K.; Kusunoki, T.; Tabara, Y.l.; Abe, M.; Miki, T. Alanine aminotransferase/aspartate aminotransferase ratio is the best surrogate marker for insulin resistance in non-obese Japanese adults. Cardiovasc. Diabetol. 2012, 11, 117. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, J.; Chen, Y.; Li, Q.; Han, B.; Chen, Y.; Xia, F.; Chen, C.; Lin, D.; Yu, X.; et al. Serum alanine aminotransferase/aspartate aminotransferase ratio is one of the best markers of insulin resistance in the Chinese population. Nutr. Metab. 2017, 14, 64. [Google Scholar] [CrossRef]
- Mooli, R.G.R.; Ramakrishnan, S.K. Emerging Role of Hepatic Ketogenesis in Fatty Liver Disease. Front. Physiol. 2022, 13, 946474. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Dufour, S.; Lyu, K.; Zhang, X.M.; Hakkarainen, A.; Lehtimäki, T.E.; Cline, G.W.; Petersen, K.F.; Shulman, G.I.; Yki-Järvinen, H. Effect of a ketogenic diet on hepatic steatosis and hepatic mitochondrial metabolism in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA 2020, 117, 7347–7354. [Google Scholar] [CrossRef]
- Al-Hail, N.; Butler, A.E.; Dargham, S.R.; Abou Seif, A.; Atkin, S.L. Creatine kinase is a marker of metabolic syndrome in qatari women with and without polycystic ovarian syndrome. Front. Endocrinol. 2019, 10, 452743. [Google Scholar] [CrossRef]
- Haan, Y.C.; Oudman, I.; Diemer, F.S.; Karamat, F.A.; van Valkengoed, I.G.; van Montfrans, G.A.; Brewster, L.M. Creatine kinase as a marker of obesity in a multi-ethnic population. Mol. Cell. Endocrinol. 2017, 442, 24–31. [Google Scholar] [CrossRef]
- Bekkelund, S.I. Creatine kinase is associated with glycated haemoglobin in a nondiabetic population. The Tromsø study. PLoS ONE 2023, 18, e0281239. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, A.; Lenters-Westra, E.; De Kort, W.; Bokhorst, A.G.; Bilo, H.J.; Slingerland, R.J.; Vos, M.J. Whole Blood Donation Affects the Interpretation of Hemoglobin A1c. PLoS ONE 2017, 12, e0170802. [Google Scholar] [CrossRef]
- Sohrabi, M.; Aghapour, S.; Khoonsari, M.; Ajdarkosh, H.; Nobakht, H.; Zamani, F.; Nikkhah, M. Serum Alkaline Phosphate Level Associates with Metabolic Syndrome Components Regardless of Non-Alcoholic Fatty Liver; A Population-Based Study in Northern Iran. Middle East J. Dig. Dis. 2023, 15, 175. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, V.R.; Baird, B.C.; Wei, G.; Greene, T.; Raphael, K.; Beddhu, S. Associations of Serum Alkaline Phosphatase with Metabolic Syndrome and Mortality. Am. J. Med. 2011, 124, 566.e1–566.e7. [Google Scholar] [CrossRef] [PubMed]
- Levitt, M.D.; Hapak, S.M.; Levitt, D.G. Alkaline Phosphatase Pathophysiology with Emphasis on the Seldom-Discussed Role of Defective Elimination in Unexplained Elevations of Serum ALP—A Case Report and Literature Review. Clin. Exp. Gastroenterol. 2022, 15, 41. [Google Scholar] [CrossRef] [PubMed]
















| P1 | P2 | P3 | p Value | P1 vs. P2 | P2 vs. P3 | P1 vs. P3 | |
|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | 20.52 (±1.39) | 21.54 (±1.30) | 20.82 (±1.46) | <0.0001 | <0.0001 | <0.0001 | 0.0734 |
| Fat Mass (kg) | 14.21 (±2.55) | 15.88 (±2.23) | 14.78 (±2.20) | <0.0001 | <0.0001 | 0.0018 | 0.1102 |
| Insulin (µIU/mL) | 4.95 (±1.24) | 9.06 (±2.14) | 5.62 (±1.83) | <0.0001 | <0.0001 | 0.0001 | 0.5686 |
| Glucose (mmol/L) | 4.36 (±0.53) | 5.12 (±0.59) | 4.41 (±0.30) | 0.0063 | 0.0110 | 0.0417 | >0.9999 |
| BHB (mmol/L) | 2.43 (±1.28) | 0.18 (±0.13) | 2.31 (±0.71) | 0.0002 | 0.0012 | <0.0001 | 0.9638 |
| HOMA-IR | 0.97 (±0.32) | 2.07 (±0.61) | 1.11 (±0.41) | <0.0001 | <0.0001 | <0.0001 | 0.4074 |
| GKI (Lab Day) | 2.23 (±1.20) | 49.68 (±42.62) | 1.99 (±0.60) | 0.0001 | 0.0024 | 0.0024 | >0.9999 |
| GKI (21 Day Average) | 2.82 (±1.34) | 56.30 (±30.01) | 2.76 (±1.15) | <0.0001 | 0.0010 | 0.0052 | >0.9999 |
| Leptin (ng/mL) | 4.50 (±3.67) | 15.08 (± 8.00) | 4.57 (±3.48) | <0.0001 | 0.0010 | 0.0052 | >0.9999 |
| ALT (U/L) | 13.71 (±3.64) | 25.42 (±12.26) | 13.16 (±2.69) | <0.0001 | 0.0010 | 0.0052 | >0.9999 |
| AST (U/L) | 18.65 (±4.15) | 26.18 (±8.77) | 19.63 (±3.11) | 0.0265 | 0.0570 | 0.0915 | 0.5481 |
| GGT (U/L) | 9.60 (±3.13) | 12.40 (±2.55) | 9.70 (±2.50) | 0.0021 | 0.0044 | 0.0059 | 0.9904 |
| ALP (U/L) | 52.98 (±11.43) | 66.98 (±15.60) | 56.39 (±15.28) | 0.0160 | 0.0160 | 0.0742 | 0.7328 |
| ALT/AST | 0.74 (±0.14) | 0.96 (±0.30) | 0.69 (±0.17) | 0.0017 | 0.0266 | 0.0047 | 0.2387 |
| Albumin (g/L) | 41.82 (±3.66) | 40.49 (±2.08) | 42.37 (±2.10) | 0.1774 | 0.3881 | 0.1681 | 0.8480 |
| CK-NAC (U/L) | 55.03 (±24.15) | 77.30 (±43.75) | 61.30 (±24.24) | 0.2223 | 0.2209 | >0.9999 | >0.9999 |
| Total Protein (g/L) | 69.4 (±9.58) | 66.73 (±6.36) | 67.25 (±3.79) | 0.8302 | >0.9999 | >0.9999 | >0.9999 |
| Iron (μmol/L) | 16.62 (±7.27) | 14.40 (±8.70) | 11.76 (± 11.78) | 0.1873 | >0.9999 | 0.2209 | 0.3526 |
| Total Bilirubin (μmol/L) | 7.88 (±4.50) | 6.72 (±1.67) | 5.59 (±2.29) | 0.4362 | >0.9999 | >0.9999 | 0.5391 |
| Direct Bilirubin (μmol/L) | 1.82 (±1.10) | 1.69 (±0.59) | 1.65 (±0.44) | 0.7909 | 0.9290 | 0.9893 | 0.8352 |
| A | ||
| Model | Effect Estimate | p Value |
| ALT~Insulin | 2.0185 | 0.0017 |
| AST~Insulin | 1.3511 | 0.0028 |
| GGT~Insulin | 0.6271 | 0.0001 |
| ALP~Insulin | 1.4090 | 0.1410 |
| ALT/AST~Insulin | 0.0429 | 0.0033 |
| B Log-transformed data | ||
| Model | Effect Estimate | p Value |
| ALT~Insulin | 0.7047 | 0.0001 |
| AST~Insulin | 0.4010 | 0.0009 |
| GGT~Insulin | 0.3703 | 0.0027 |
| ALP~Insulin | 0.1498 | 0.1510 |
| ALT/AST~Insulin | 0.3008 | 0.0064 |
| A | ||
| Model | Effect Estimate | p Value |
| ALT~HOMA-IR | 8.4960 | 0.0003 |
| AST~HOMA-IR | 4.6950 | 0.0061 |
| GGT~HOMA-IR | 2.2300 | 0.0004 |
| ALP~HOMA-IR | 5.1260 | 0.1530 |
| ALT/AST~HOMA-IR | 0.1978 | 0.0001 |
| B Log-transformed data | ||
| Model | Effect Estimate | p Value |
| ALT~HOMA-IR | 0.5984 | <0.0001 |
| AST~HOMA-IR | 0.3194 | 0.0012 |
| GGT~HOMA-IR | 0.3092 | 0.0019 |
| ALP~HOMA-IR | 0.1451 | 0.0822 |
| ALT/AST~HOMA-IR | 0.2757 | 0.0016 |
| A | ||
| Model | Effect Estimate | p Value |
| ALT~GKI (Lab Day) | 0.0743 | 0.1510 |
| AST~GKI (Lab Day) | 0.0188 | 0.6140 |
| GGT~GKI (Lab Day) | 0.0423 | 0.0003 |
| ALP~GKI (Lab Day) | 0.0818 | 0.2570 |
| ALT/AST~GKI (Lab Day) | 0.0032 | 0.0030 |
| B Log-Transformed Data | ||
| Model | Effect Estimate | p Value |
| ALT~GKI (Lab Day) | 0.1593 | 0.0001 |
| AST~GKI (Lab Day) | 0.0731 | 0.0130 |
| GGT~GKI (Lab Day) | 0.0939 | 0.0002 |
| ALP~GKI (Lab Day) | 0.0593 | 0.0081 |
| ALT/AST~GKI (Lab Day) | 0.0878 | 0.0001 |
| A | ||
| Model | Effect Estimate | p Value |
| ALT~GKI (21-Day Average) | 0.1397 | 0.0077 |
| AST~GKI (21-Day Average) | 0.0807 | 0.0329 |
| GGT~GKI (21-Day Average) | 0.0400 | 0.0017 |
| ALP~GKI (21-Day Average) | 0.1482 | 0.0412 |
| ALT/AST~GKI (21-Day Average) | 0.0032 | 0.0055 |
| B Log-Transformed Data | ||
| Model | Effect Estimate | p Value |
| ALT~GKI (21-Day Average) | 0.1742 | <0.0001 |
| AST~GKI (21-Day Average) | 0.0871 | 0.0034 |
| GGT~GKI (21-Day Average) | 0.0913 | 0.0006 |
| ALP~GKI (21-Day Average) | 0.0604 | 0.0087 |
| ALT/AST~GKI (21-Day Average) | 0.0888 | 0.0002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooper, I.D.; Petagine, L.; Soto-Mota, A.; Duraj, T.; Scarborough, A.; Norwitz, N.G.; Seyfried, T.N.; Furoni, M.A.; Kyriakidou, Y. Ketosis Suppression and Ageing (KetoSAge): The Effect of Suppressing Ketosis on GKI and Liver Biomarkers in Healthy Females. Livers 2025, 5, 41. https://doi.org/10.3390/livers5030041
Cooper ID, Petagine L, Soto-Mota A, Duraj T, Scarborough A, Norwitz NG, Seyfried TN, Furoni MA, Kyriakidou Y. Ketosis Suppression and Ageing (KetoSAge): The Effect of Suppressing Ketosis on GKI and Liver Biomarkers in Healthy Females. Livers. 2025; 5(3):41. https://doi.org/10.3390/livers5030041
Chicago/Turabian StyleCooper, Isabella D., Lucy Petagine, Adrian Soto-Mota, Tomás Duraj, Andrew Scarborough, Nicolas G. Norwitz, Thomas N. Seyfried, Maricel A. Furoni, and Yvoni Kyriakidou. 2025. "Ketosis Suppression and Ageing (KetoSAge): The Effect of Suppressing Ketosis on GKI and Liver Biomarkers in Healthy Females" Livers 5, no. 3: 41. https://doi.org/10.3390/livers5030041
APA StyleCooper, I. D., Petagine, L., Soto-Mota, A., Duraj, T., Scarborough, A., Norwitz, N. G., Seyfried, T. N., Furoni, M. A., & Kyriakidou, Y. (2025). Ketosis Suppression and Ageing (KetoSAge): The Effect of Suppressing Ketosis on GKI and Liver Biomarkers in Healthy Females. Livers, 5(3), 41. https://doi.org/10.3390/livers5030041










