Cutaneous Manifestations of Liver Cirrhosis: Clinical Significance and Diagnostic Implications
Abstract
1. Introduction
2. Overview of Liver Cirrhosis and Systemic Manifestations
3. Cutaneous Manifestations: Clinical Features
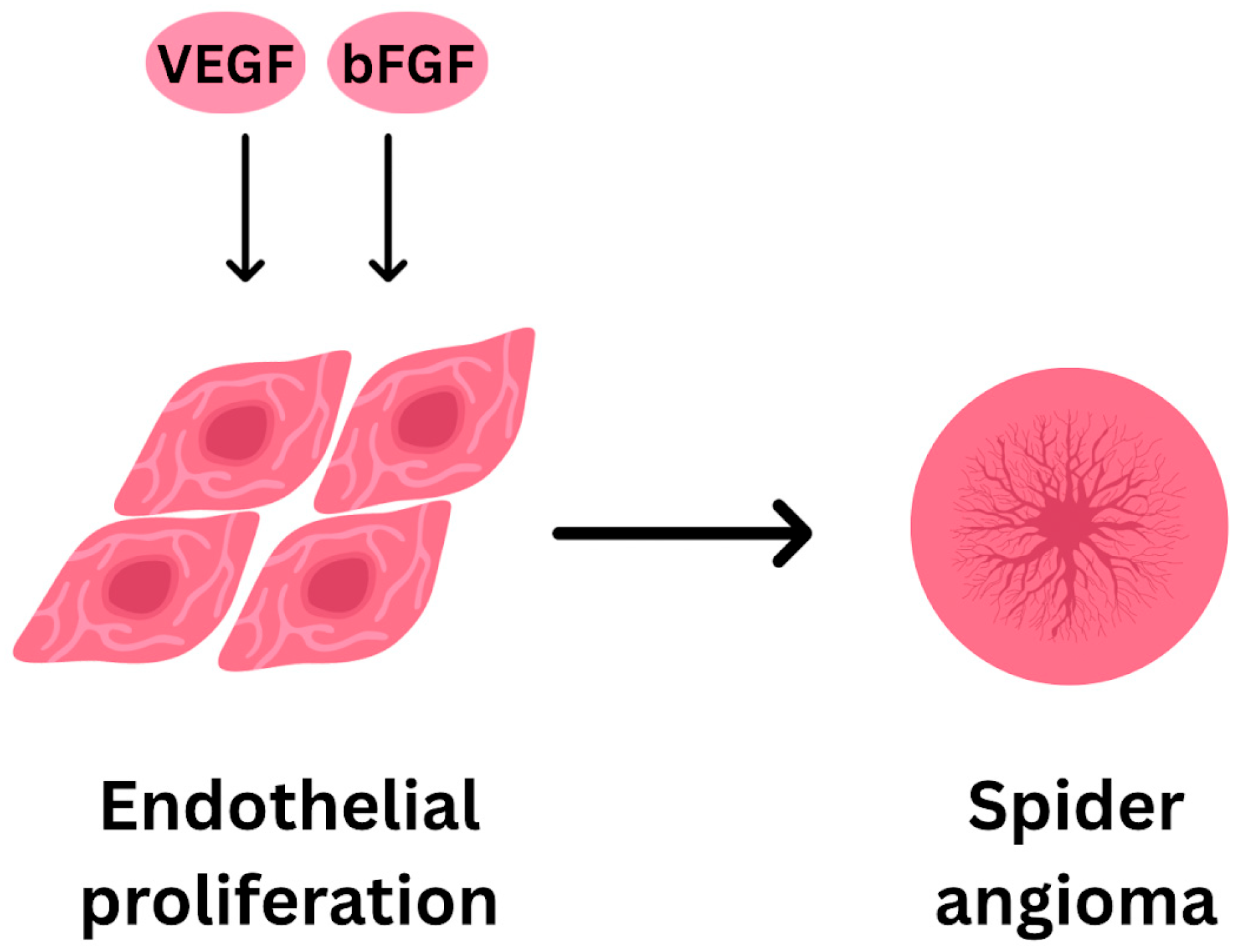
- Spider Angiomas/Telangiectasias
- Palmar Erythema
- Jaundice
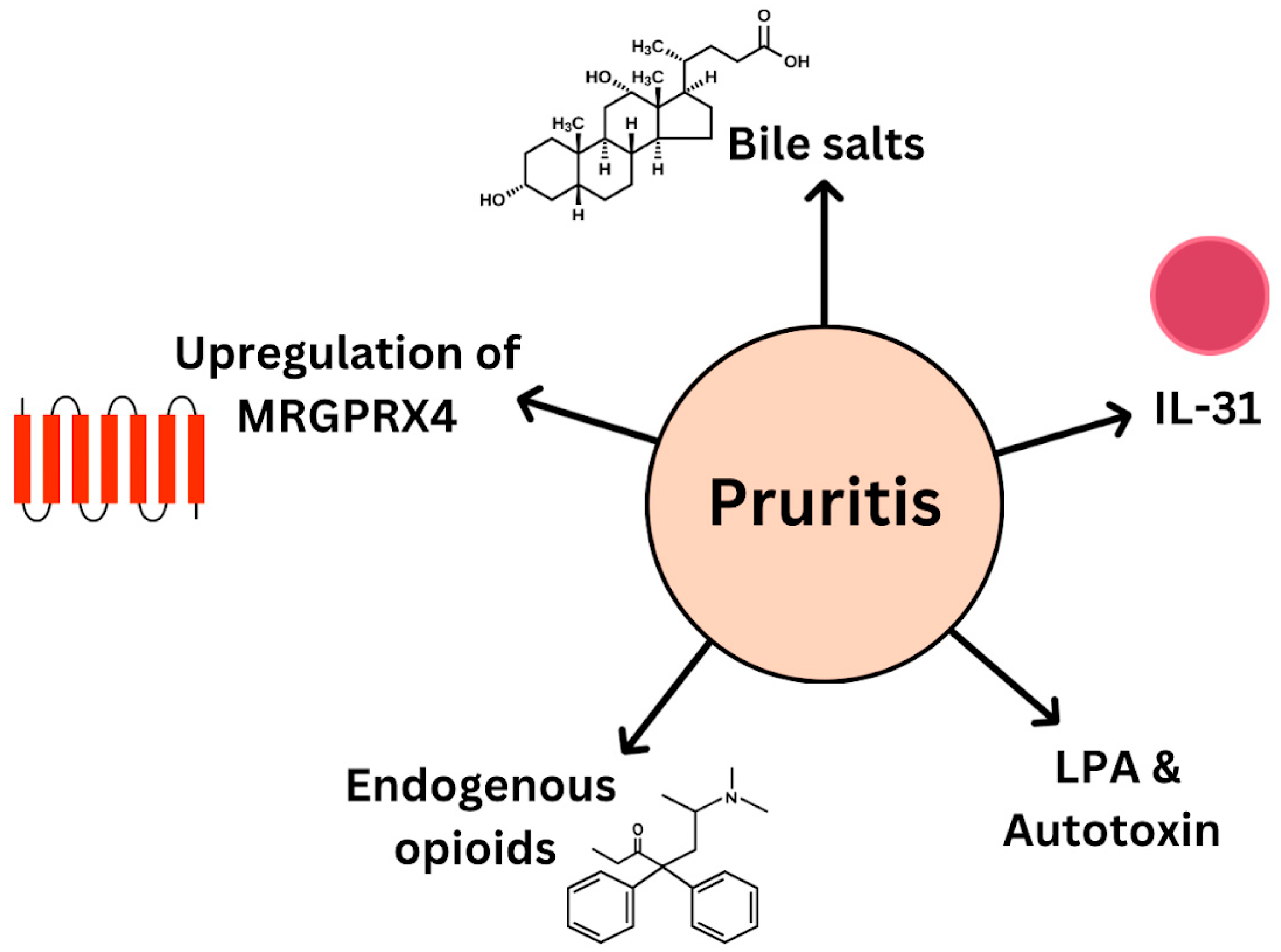
- Pruritus
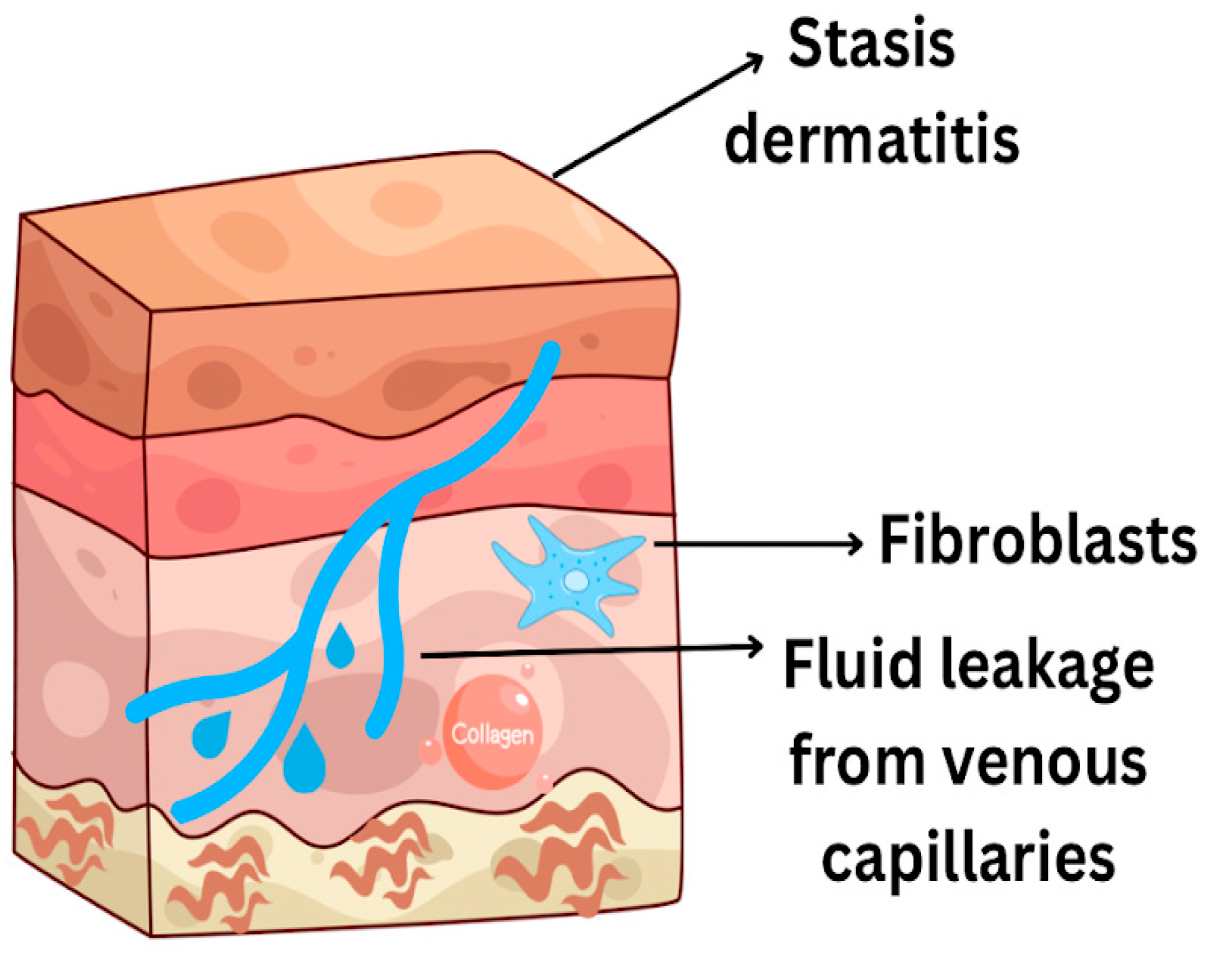
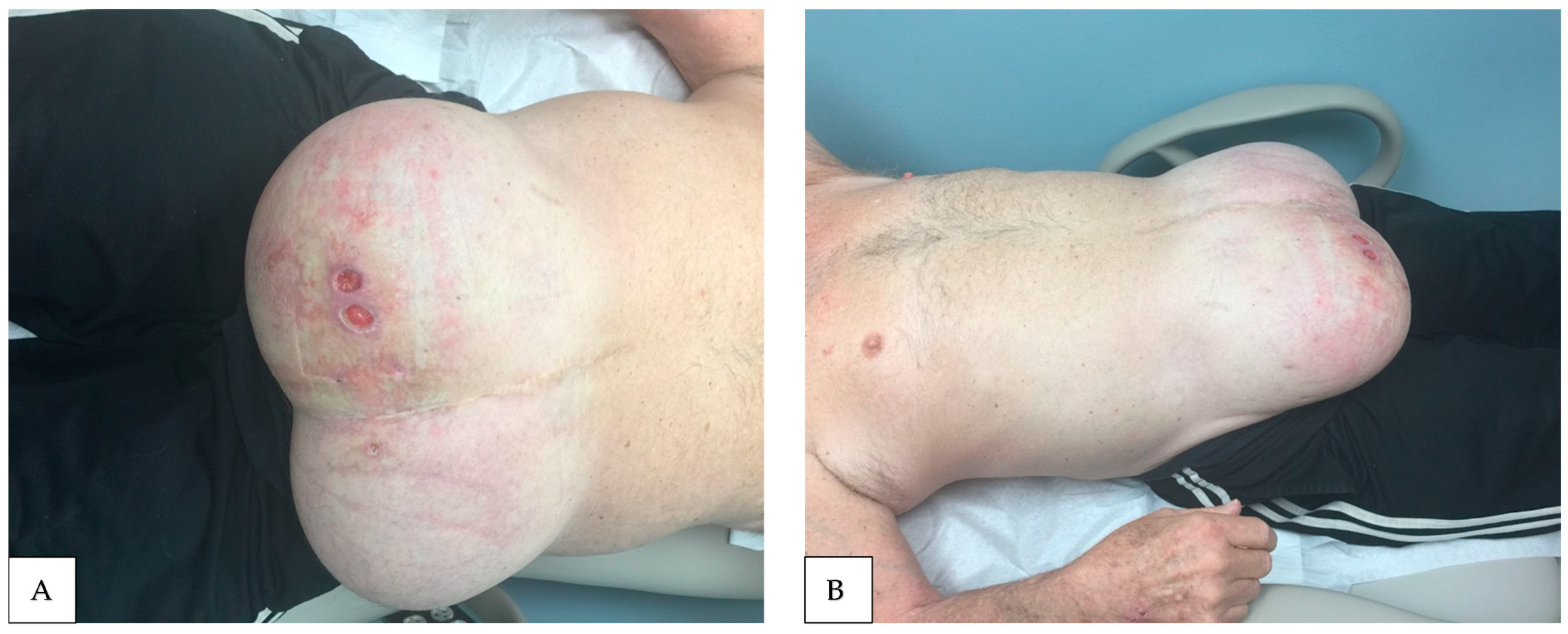
- Stasis ulcers
- Other Skin and Nails Findings:
4. Pathophysiological Mechanisms Linking Liver Dysfunction to Skin Changes
5. Diagnostic and Prognostic Value of Skin Signs
6. Management Implications
7. Overall Importance of Recognizing and Addressing Cutaneous Manifestations of Liver Cirrhosis
8. Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef] [PubMed]
- Schuppan, D.; Afdhal, N.H. Liver cirrhosis. Lancet 2008, 371, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Horvatits, T.; Drolz, A.; Trauner, M.; Fuhrmann, V. Liver Injury and Failure in Critical Illness. Hepatology 2019, 70, 2204–2215. [Google Scholar] [CrossRef] [PubMed]
- Martínez Jiménez, S. Cutaneous Manifestations of Liver Disease: A Narrative Review. Cureus 2024, 16, e70357. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Y.; Gao, X.; Liu, J.; Ji, F.; Hsu, Y.-C.; Li, Z.; Nguyen, M.H. Recognizing skin conditions in patients with cirrhosis: A narrative review. Ann. Med. 2022, 54, 3016–3028. [Google Scholar] [CrossRef] [PubMed]
- Niederau, C.; Lange, S.; Frühauf, M.; Thiel, A. Cutaneous signs of liver disease: Value for prognosis of severe fibrosis and cirrhosis. Liver Int. 2008, 28, 659–666. [Google Scholar] [CrossRef]
- Runyon, B.A. A Primer on Detecting Cirrhosis and Caring for These Patients without Causing Harm. Int. J. Hepatol. 2011, 2011, 801983. [Google Scholar] [CrossRef]
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef]
- Somnay, K.; Wadgaonkar, P.; Sridhar, N.; Roshni, P.; Rao, N.; Wadgaonkar, R. Liver Fibrosis Leading to Cirrhosis: Basic Mechanisms and Clinical Perspectives. Biomedicines 2024, 12, 2229. [Google Scholar] [CrossRef]
- Jagdish, R.K.; Roy, A.; Kumar, K.; Premkumar, M.; Sharma, M.; Rao, P.N.; Reddy, D.N.; Kulkarni, A.V. Pathophysiology and management of liver cirrhosis: From portal hypertension to acute-on-chronic liver failure. Front. Med. 2023, 10, 1060073. [Google Scholar] [CrossRef]
- Fallahzadeh, M.A.; Rahimi, R.S. Hepatic Encephalopathy: Current and Emerging Treatment Modalities. Clin. Gastroenterol. Hepatol. 2022, 20, S9–S19. [Google Scholar] [CrossRef]
- Samant, H.; Kothadia, J.P. Spider Angioma. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Li, C.P. Spider angiomas in patients with liver cirrhosis: Role of vascular endothelial growth factor and basic fibroblast growth factor. World J. Gastroenterol. 2003, 9, 2832. [Google Scholar] [CrossRef]
- Li, C.P.; Lee, F.Y.; Hwang, S.J.; Chang, F.-Y.; Lin, H.-C.; Lu, R.-H.; Hou, M.-C.; Chu, C.-J.; Chan, C.-C.; Luo, J.-C.; et al. Role of Substance P in The Pathogenesis of Spider Angiomas in Patients with Nonalcoholic Liver Cirrhosis. Am. J. Gastroenterol. 1999, 94, 502–507. [Google Scholar] [CrossRef]
- Bhandari, A.; Mahajan, R. Skin Changes in Cirrhosis. J. Clin. Exp. Hepatol. 2022, 12, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Terp, K.; Izquierdo-Pretel, G. Spider Angioma Number and Location as Potential Prognostic Indicators in Chronic Liver Disease: A Case Report. Cureus 2023, 15, e34193. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-W.; Shin, K.; Kim, T.-W.; Kim, T.-W.; You, H.-S.; Jin, H.-J.; Shim, W.-H.; Kim, H.-S.; Ko, H.-C.; Kim, B.-S.; et al. The importance of dermoscopy for the diagnosis of acquired bilateral telangiectatic macules: The angioid streak pattern reveals underlying chronic liver disease. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1597–1601. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Desai, A.; Brinster, N.; Marmon, S. Telangiectasia macularis eruptiva perstans in the presence of liver cirrhosis. JAAD Case Rep. 2020, 6, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Serrao, R.; Zirwas, M.; English, J.C. Palmar Erythema. Am. J. Clin. Dermatol. 2007, 8, 347–356. [Google Scholar] [CrossRef]
- Nautiyal, A.; Chopra, K.B. Liver Palms (Palmar Erythema). Am. J. Med. 2010, 123, 596–597. [Google Scholar] [CrossRef]
- Milam, P.; Berger, M.; Ramaswamy, B.; Reinbolt, R.; Wesolowski, R.; Kaffenberger, B.H. Spider Telangiectases and Palmar Erythema as Harbingers of Structural Liver Changes in Three Breast Cancer Patients on Ado-trastuzumab Emtansine. J. Clin. Aesthet Dermatol. 2019, 12, 23. [Google Scholar]
- Gustot, T.; Stadlbauer, V.; Laleman, W.; Alessandria, C.; Thursz, M. Transition to decompensation and acute-on-chronic liver failure: Role of predisposing factors and precipitating events. J. Hepatol. 2021, 75, S36–S48. [Google Scholar] [CrossRef]
- Harrison, P.M. Management of patients with decompensated cirrhosis. Clin. Med. 2015, 15, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Selim, R.; Ahn, J. Pruritus in Chronic Liver Disease. Clin. Liver Dis. 2023, 27, 47–55. [Google Scholar] [CrossRef]
- Bassari, R. Jaundice associated pruritis: A review of pathophysiology and treatment. World J. Gastroenterol. 2015, 21, 1404. [Google Scholar] [CrossRef]
- Düll, M.M.; Kremer, A.E. Newer Approaches to the Management of Pruritus in Cholestatic Liver Disease. Curr. Hepatol. Rep. 2020, 19, 86–95. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Khoshdeli, M.; Peach, M.; Chuang, J.; Lin, J.; Tsai, W.; Mahadevan, S.; Minto, W.; Diehl, L.; et al. IL-31 levels correlate with pruritus in patients with cholestatic and metabolic liver diseases and is farnesoid X receptor responsive in NASH. Hepatology 2023, 77, 20–32. [Google Scholar] [CrossRef]
- Terg, R.; Coronel, E.; Sordá, J.; Muñoz, A.E.; Findor, J. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J. Hepatol. 2002, 37, 717–722. [Google Scholar] [CrossRef]
- Bergasa, N.V.; Talbot, T.L.; Alling, D.W.; Schmitt, J.M.; Walker, E.C.; Baker, B.L.; Korenman, J.C.; Park, Y.; Hoofnagle, J.H.; Jones, E. A controlled trial of naloxone infusions for the pruritus of chronic cholestasis. Gastroenterology 1992, 102, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Meixiong, J.; Vasavda, C.; Snyder, S.H.; Dong, X. MRGPRX4 is a G protein-coupled receptor activated by bile acids that may contribute to cholestatic pruritus. Proc. Natl. Acad. Sci. USA 2019, 116, 10525–10530. [Google Scholar] [CrossRef] [PubMed]
- Naschitz, J.E. Stasis Ulcers—The Unifying Concept. J. Clin. Res. Rep. 2021, 9, 1–5. [Google Scholar] [CrossRef]
- El Younis, C.M.; Bergasa, N.V. Healing of leg ulcers associated with transjugular intrahepatic portosystemic shunt in decompensated cirrhosis: Case series of a possible hepatodermal syndrome. Gastroenterol Hepatol. 2008, 4, 211. [Google Scholar]
- Terry, R. White nails in hepatic cirrhosis. Lancet 1954, 263, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Nia, A.M.; Ederer, S.; Dahlem, K.M.; Gassanov, N.; Er, F. Terry’s Nails: A Window to Systemic Diseases. Am. J. Med. 2011, 124, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Sack, J.S.; Liu, A.F.; Gray, M.; Roat, J.; Zucker, S.D. Association of Terry Nails with Liver Cirrhosis. Am. J. Gastroenterol. 2021, 116, 2455–2458. [Google Scholar] [CrossRef]
- Fernandez-Somoza, J.-M.; Ginarte, M.; Otero, E.; Tomé, S.; Soutullo, C.; Martínez-Ulloa, A.; Gonzalez-Quintela, A.; Sepehrimanesh, M. Clinical and capillaroscopic findings in patients with liver disease and proximal apparent leukonychia (Terry nails and its variants). Medicine 2021, 100, e26207. [Google Scholar] [CrossRef]
- Salem, A.; Al Mokadem, S.; Attwa, E.; Abd El Raoof, S.; Ebrahim, H.; Faheem, K. Nail changes in chronic renal failure patients under haemodialysis. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Hojo, S.; Fujita, J.; Yamadori, I.; Ezaki, T.; Watanabe, S.; Yamanouchi, H.; Miyawaki, H.; Yamaji, Y.; Nishioka, M.; Takahara, J. Hepatocyte Growth Factor and Digital Clubbing. Intern. Med. 1997, 36, 44–46. [Google Scholar] [CrossRef][Green Version]
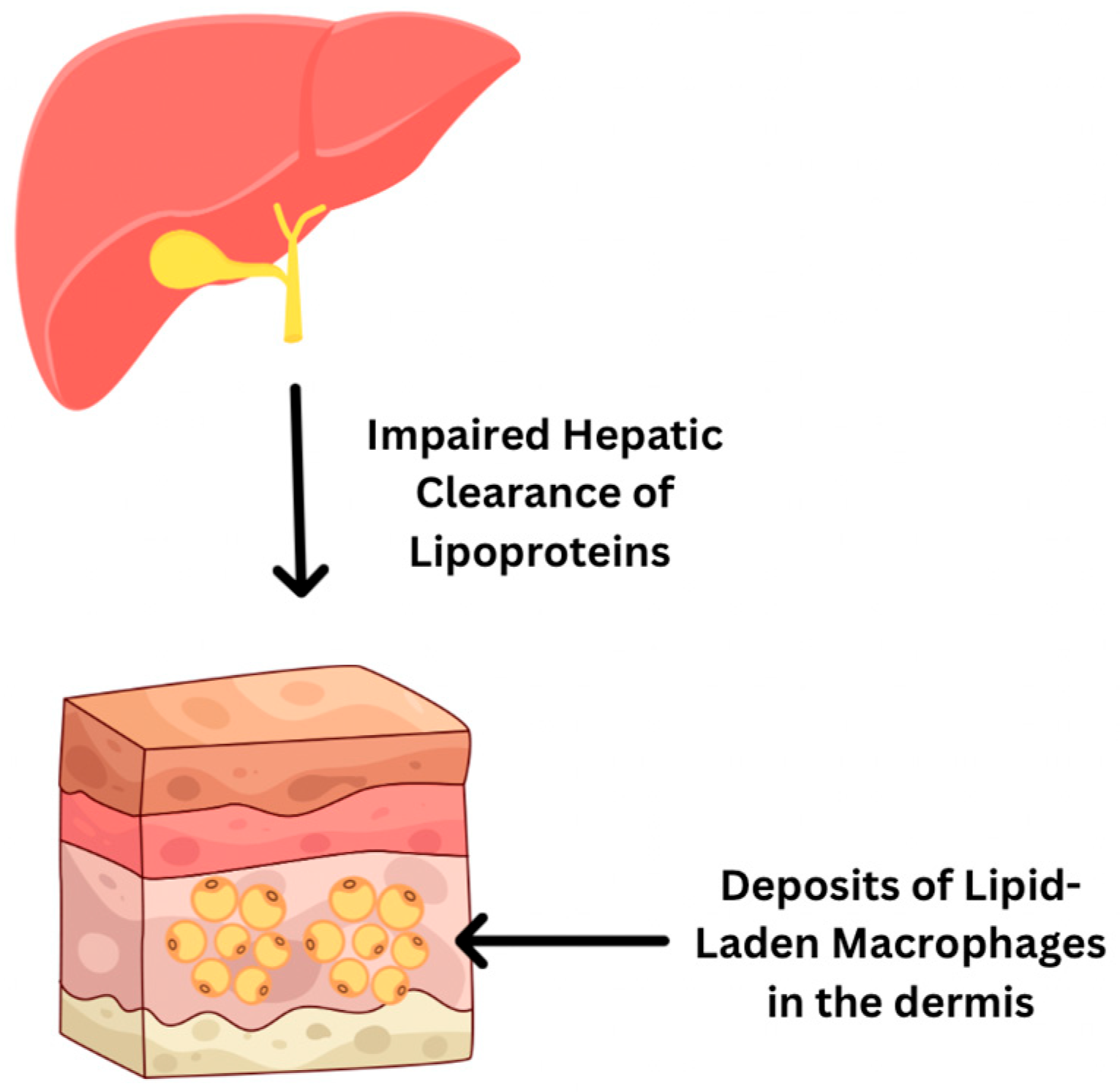
- Baila-Rueda, L.; Mateo-Gallego, R.; Lamiquiz-Moneo, I.; Cenarro, A.; Civeira, F. Severe hypercholesterolemia and phytosterolemia with extensive xanthomas in primary biliary cirrhosis: Role of biliary excretion on sterol homeostasis. J. Clin. Lipidol. 2014, 8, 520–524. [Google Scholar] [CrossRef]
- Al Aboud, A.M.; Shah, S.S.; Blair, K.; Al Aboud, D.M. Xanthelasma Palpebrarum. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK531501/ (accessed on 1 July 2025).
- Sexton, F.; Shanahan, W.; Ryan, J.D. Xanthoma striatum palmare: Getting a handle on hyperlipidaemia in primary biliary cholangitis. Clin. Res. Hepatol. Gastroenterol. 2024, 48, 102255. [Google Scholar] [CrossRef]
- Suzuki, L.; Hirayama, S.; Fukui, M.; Sasaki, M.; Hiroi, S.; Ayaori, M.; Terai, S.; Tozuka, M.; Watada, H.; Miida, T. Lipoprotein-X in cholestatic patients causes xanthomas and promotes foam cell formation in human macrophages. J. Clin. Lipidol. 2017, 11, 110–118. [Google Scholar] [CrossRef]
- Hazin, R.; Tamimi, T.I.A.R.; Abuzetun, J.Y.; Zein, N.N. Recognizing and treating cutaneous signs of liver disease. Cleve Clin. J. Med. 2009, 76, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Van Alfen, B.; Hatch, J.; Conley, M.; Seeliyur Duraiswamy, S. A Case Report of Xanthelasma and the Associated Differential Diagnosis. Cureus 2025, 17, e81971. [Google Scholar] [CrossRef]
- Saunders Elsevier. Evidence-Based Physical Diagnosis; Saunders Elsevier: Philadelphia, PA, USA, 2012. [Google Scholar]
- Wilson, R.; Williams, D.M. Cirrhosis. Med. Clin. N. Am. 2022, 106, 437–446. [Google Scholar] [CrossRef]
- Abraldes, J.G.; Caraceni, P.; Ghabril, M.; Garcia-Tsao, G. Update in the Treatment of the Complications of Cirrhosis. Clin. Gastroenterol. Hepatol. 2023, 21, 2100–2109. [Google Scholar] [CrossRef]
- Simonetto, D.A.; Liu, M.; Kamath, P.S. Portal Hypertension and Related Complications: Diagnosis and Management. Mayo Clin. Proc. 2019, 94, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.R.; Bulun, S.E. Estrogen production and action. J. Am. Acad. Dermatol. 2001, 45, S116–S124. [Google Scholar] [CrossRef] [PubMed]
- Neong, S.F.; Billington, E.O.; Congly, S.E. Sexual Dysfunction and Sex Hormone Abnormalities in Patients with Cirrhosis: Review of Pathogenesis and Management. Hepatology 2019, 69, 2683–2695. [Google Scholar] [CrossRef]
- Sinclair, M.; Grossmann, M.; Gow, P.J.; Angus, P.W. Testosterone in men with advanced liver disease: Abnormalities and implications. J. Gastroenterol. Hepatol. 2015, 30, 244–251. [Google Scholar] [CrossRef]
- Patel, A.D.; Katz, K.; Gordon, K.B. Cutaneous Manifestations of Chronic Liver Disease. Clin. Liver Dis. 2020, 24, 351–360. [Google Scholar] [CrossRef]
- Roche, S.P.; Kobos, R. Jaundice in the adult patient. Am. Fam. Phys. 2004, 69, 299–304. [Google Scholar]
- Berumen, J.; Baglieri, J.; Kisseleva, T.; Mekeel, K. Liver fibrosis: Pathophysiology and clinical implications. WIREs Mech Dis. 2021, 13, e1499. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Jindal, R. Cutaneous Manifestations of Common Liver Diseases. J. Clin. Exp. Hepatol. 2011, 1, 177–184. [Google Scholar] [CrossRef][Green Version]
- Roche, F.C.; Harris-Tryon, T.A. Illuminating the Role of Vitamin A in Skin Innate Immunity and the Skin Microbiome: A Narrative Review. Nutrients. 2021, 13, 302. [Google Scholar] [CrossRef]
- Igarashi, A.; Uzuka, M.; Nakajima, K. The effects of vitamin E deficiency on rat skin. Br. J. Dermatol. 1989, 121, 43–49. [Google Scholar] [CrossRef]
- Ballal, S.; Prathibha, J.P.; Pinto, A.V.; Srinivasa, S.; Augustine, M. Unusual Manifestation of Vitamin K Deficiency, Nodular Purpura: A Case Series. Indian Dermatol. Online J. 2025, 16, 132–136. [Google Scholar] [CrossRef]
- Mayo, M.J.; Carey, E.; Smith, H.T.; Mospan, A.R.; McLaughlin, M.; Thompson, A.; Morris, H.L.; Sandefur, R.; Kim, W.R.; Bowlus, C.; et al. Impact of Pruritus on Quality of Life and Current Treatment Patterns in Patients with Primary Biliary Cholangitis. Dig. Dis. Sci. 2023, 68, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.; Schwarz, C.; Burghart, L.; Pfisterer, N.; Bauer, D.; Hübl, W.; Mandorfer, M.; Gschwantler, M.; Reiberger, T.; Elbahrawy, A. Late-stage presentation with decompensated cirrhosis is alarmingly common but successful etiologic therapy allows for favorable clinical outcomes. PLoS ONE 2023, 18, e0290352. [Google Scholar] [CrossRef] [PubMed]
- Jindal, A.; Gupta, A.; Bhadoria, A.S. Resolution of Multiple Large Spider Angiomas After Liver Transplantation in Severe Alcoholic Hepatitis. Indian J. Dermatol. 2022, 67, 837. [Google Scholar] [CrossRef]
- Fadlallah, H.; El Masri, D.; Bahmad, H.F.; Abou-Kheir, W.; El Masri, J. Update on the Complications and Management of Liver Cirrhosis. Med. Sci. 2025, 13, 13. [Google Scholar] [CrossRef]
- Ebhohon, E.; Chung, R.T. Systematic review: Efficacy of therapies for cholestatic pruritus. Therap. Adv. Gastroenterol. 2023, 16. [Google Scholar] [CrossRef]
- Bolier, R.; de Vries, E.S.; Parés, A.; Helder, J.; Kemper, E.M.; Zwinderman, K.; Elferink, R.P.O.; Beuers, U. Fibrates for the treatment of cholestatic itch (FITCH): Study protocol for a randomized controlled trial. Trials 2017, 18, 230. [Google Scholar] [CrossRef]
- Udell, J.A.; Wang, C.S.; Tinmouth, J.; FitzGerald, J.M.; Ayas, N.T.; Simel, D.L.; Schulzer, M.; Mak, E.; Yoshida, E.M. Does This Patient with Liver Disease Have Cirrhosis? JAMA 2012, 307, 832. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, R.; Méndez-Sánchez, N.; Peng, Y.; Guo, X.; Qi, X. Impact of spider nevus and subcutaneous collateral vessel of chest/abdominal wall on outcomes of liver cirrhosis. Arch. Med. Sci. 2019, 15, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Ginès, P.; Castera, L.; Lammert, F.; Graupera, I.; Serra-Burriel, M.; Allen, A.M.; Wong, V.W.; Hartmann, P.; Thiele, M.; Caballeria, L.; et al. Population screening for liver fibrosis: Toward early diagnosis and intervention for chronic liver diseases. Hepatology 2022, 75, 219–228. [Google Scholar] [CrossRef] [PubMed]



| Skin Manifestation | Prevalence in Cirrhosis | Clinical Relevance |
|---|---|---|
| Spider Angiomas | 33–40% | More specific for cirrhosis; associated with advanced disease and portal hypertension |
| Palmar Erythema | 23–30% | More specific for cirrhosis; reflects hyperestrogenism and advanced disease |
| Jaundice | 28–47% | Indicates impaired bilirubin metabolism; maker of decompensation |
| Pruritus | 39% | Nonspecific; common in cholestatic liver disease; impacts quality of life |
| Stasis Ulcers | <5% | Nonspecific; may indicate severe hypoalbuminemia or venous insufficiency |
| Terry’s Nails | 25.6% | Highly specific for cirrhosis; associated with advanced disease |
| Clubbing | 5–15% | Nonspecific |
| Xanthomas | <5% | Nonspecific |
| Caput Medusae | 1–5% | More specific for portal hypertension and advanced cirrhosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamoua, R.; Reese, R.; Annamraju, R.; Chen, T.; Doyle, C.; Parella, A.; Liu, A.; Abboud, Y.; Rohan, C.; Travers, J.B. Cutaneous Manifestations of Liver Cirrhosis: Clinical Significance and Diagnostic Implications. Livers 2025, 5, 37. https://doi.org/10.3390/livers5030037
Kamoua R, Reese R, Annamraju R, Chen T, Doyle C, Parella A, Liu A, Abboud Y, Rohan C, Travers JB. Cutaneous Manifestations of Liver Cirrhosis: Clinical Significance and Diagnostic Implications. Livers. 2025; 5(3):37. https://doi.org/10.3390/livers5030037
Chicago/Turabian StyleKamoua, Rita, Rebecca Reese, Risha Annamraju, Tian Chen, Colleen Doyle, Adriana Parella, Amelia Liu, Yazan Abboud, Craig Rohan, and Jeffrey B. Travers. 2025. "Cutaneous Manifestations of Liver Cirrhosis: Clinical Significance and Diagnostic Implications" Livers 5, no. 3: 37. https://doi.org/10.3390/livers5030037
APA StyleKamoua, R., Reese, R., Annamraju, R., Chen, T., Doyle, C., Parella, A., Liu, A., Abboud, Y., Rohan, C., & Travers, J. B. (2025). Cutaneous Manifestations of Liver Cirrhosis: Clinical Significance and Diagnostic Implications. Livers, 5(3), 37. https://doi.org/10.3390/livers5030037








