Serum Level of Glypican-3 in Patients with Hepatocellular Carcinoma and Advanced Chronic Liver Disease: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Analysis of Disease Characteristics in Patients with HCC and Advanced Stages of Fibrosis and Cirrhosis
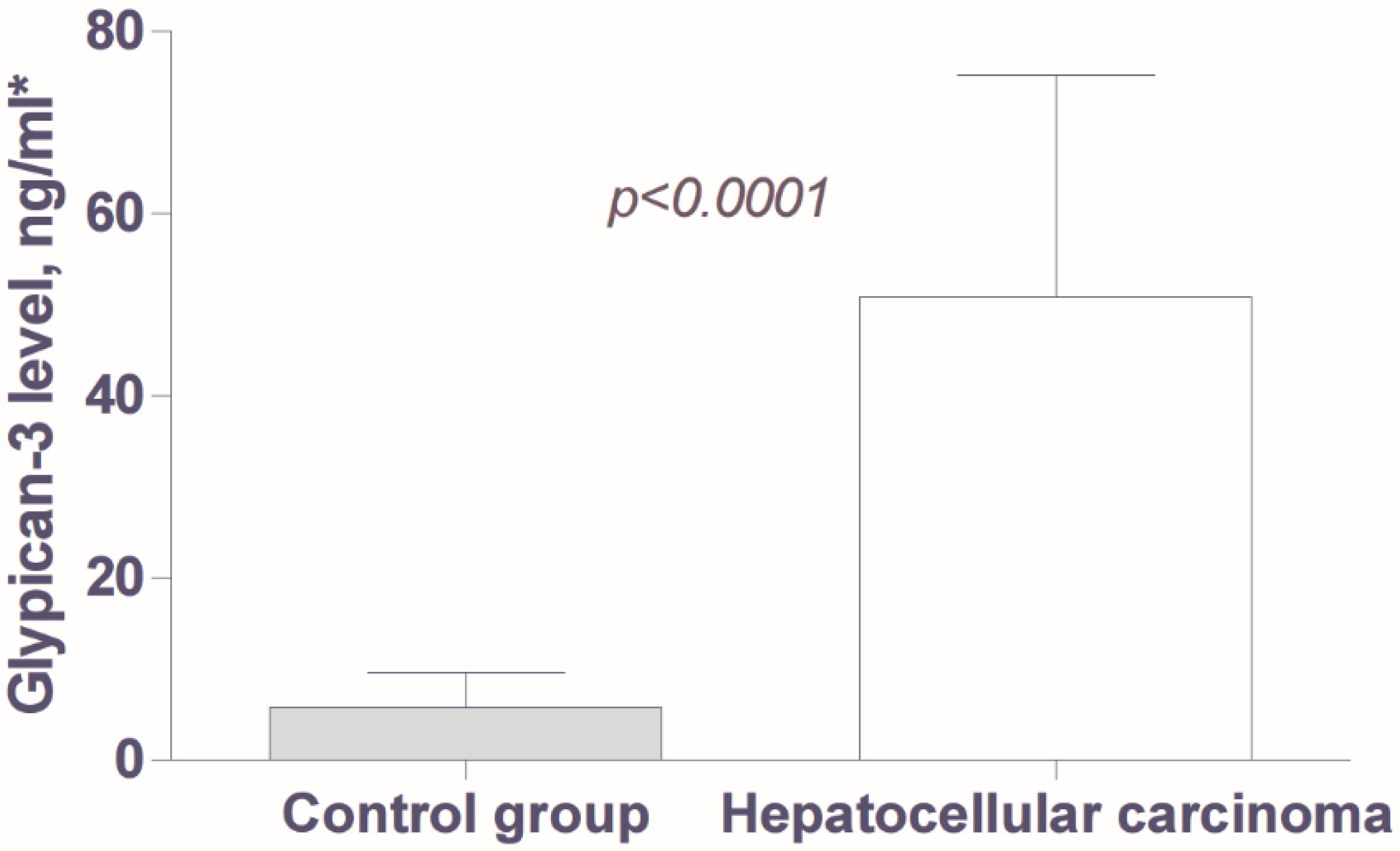
3.2. Analysis of Tumor Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| AFP | Alpha-fetoprotein |
| DCP | Des-gamma carboxyprothrombin |
| US | Ultrasound |
| CEUS | Contrast-enhanced ultrasound |
| CT | Computed tomography |
| MR | Magnetic resonance |
| EASL | European Association of Study of the Liver |
| HCV | Hepatitis C virus |
| HBV | Hepatitis B virus |
| GPC3 | Glypican-3 |
| RNA | Ribonucleic acid |
| BCLC | Barcelona Clinic Liver Cancer |
| CLIP | Cancer of the Liver Italian Program |
| ROC | Receiver operating characteristic curve |
| CTP | Child-Turcotte-Pugh classification system |
| CI | Confidence interval |
| SEM | Standard error of mean |
| SD | Standard deviation |
| SALL-4 | Spalt-like transcription factor |
| AUC | Area under the curve |
| GGT | Gamma-glutamyl transferase |
| HS-GGT | Hepatoma-specific gamma-glutamyl transferase |
| HSP70 | Heat shock protein 70 |
| GS | Glutamine synthetase |
References
- Goldberg, D.; Ditah, I.C.; Saeian, K.; Lalehzari, M.; Aronsohn, A.; Gorospe, E.C.; Charlton, M. Changes in the Prevalence of Hepatitis C Virus Infection, Nonalcoholic Steatohepatitis, and Alcoholic Liver Disease Among Patients with Cirrhosis or Liver Failure on the Waitlist for Liver Transplantation. Gastroenterology 2017, 152, 1090–1099.e1. [Google Scholar] [CrossRef]
- Makarova-Rusher, O.V.; Altekruse, S.F.; McNeel, T.S.; Ulahannan, S.; Duffy, A.G.; Graubard, B.I.; Greten, T.F.; McGlynn, K.A. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer 2016, 122, 1757–1765. [Google Scholar] [CrossRef]
- Sangro, B.; Argemi, J.; Ronot, M.; Paradis, V.; Meyer, T.; Mazzaferro, V.; Jepsen, P.; Golfieri, R.; Galle, P.; Dawson, L.; et al. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J. Hepatol. 2025, 82, 315–374. [Google Scholar] [CrossRef]
- van der Meer, A.J.; Feld, J.J.; Hofer, H.; Almasio, P.L.; Calvaruso, V.; Fernández-Rodríguez, C.M.; Aleman, S.; Ganne-Carrié, N.; D’aMbrosio, R.; Pol, S.; et al. Risk of cirrhosis-related complications in patients with advanced fibrosis following hepatitis C virus eradication. J. Hepatol. 2017, 66, 485–493. [Google Scholar] [CrossRef]
- Park, J.; Chen, M.; Colombo, M.; Roberts, L.R.; Schwartz, M.; Chen, P.; Kudo, M.; Johnson, P.; Wagner, S.; Orsini, L.S.; et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study. Liver Int. 2015, 35, 2155–2166. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, B.H.; Park, J.-W. Overview of Asian clinical practice guidelines for the management of hepatocellular carcinoma: An Asian perspective comparison. Clin. Mol. Hepatol. 2023, 29, 252–262. [Google Scholar] [CrossRef]
- Kudo, M.; Kawamura, Y.; Hasegawa, K.; Tateishi, R.; Kariyama, K.; Shiina, S.; Toyoda, H.; Imai, Y.; Hiraoka, A.; Ikeda, M.; et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 2021, 10, 181–223. [Google Scholar] [CrossRef]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1. [Google Scholar] [CrossRef]
- Calderaro, J.; Couchy, G.; Imbeaud, S.; Amaddeo, G.; Letouze, E.; Blanc, J.-F.; Laurent, C.; Hajji, Y.; Azoulay, D.; Bioulac-Sage, P.; et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J. Hepatol. 2017, 67, 727–738. [Google Scholar] [CrossRef]
- Capurro, M.; Wanless, I.R.; Sherman, M.; Deboer, G.; Shi, W.; Miyoshi, E.; Filmus, J. Glypican-3: A novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003, 125, 89–97. [Google Scholar] [CrossRef]
- Liu, J.-W.; Zuo, X.-L.; Wang, S. Diagnosis accuracy of serum Glypican-3 level in patients with hepatocellular carcinoma and liver cirrhosis: A meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3655–3673. [Google Scholar]
- Sun, B.; Huang, Z.; Wang, B.; Yu, Y.; Lin, S.; Luo, L.; Wang, Y.; Huang, Z. Significance of Glypican-3 (GPC3) Expression in Hepatocellular Cancer Diagnosis. Med. Sci. Monit. 2017, 23, 850–855. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Zhou, C.-J.; Li, J.; Zhou, L.; Li, M.-S.; Xiao, B. Value of detection of serum glypican-3 level in diagnosis and therapeutic effect evaluation of primary hepatocellular carcinoma. Nan Fang Yi Ke Da Xue Xue Bao 2017, 37, 1060–1065. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Nolsøe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.-A.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver–Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020, 46, 2579–2604. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- The Cancer of the Liver Italian Program (CLIP) Investigators. Prospective validation of the CLIP score: A new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology 2000, 31, 840–845. [Google Scholar] [CrossRef]
- Bruix, J.; Reig, M.; Sherman, M. Evidence-Based Diagnosis, Staging, and Treatment of Patients with Hepatocellular Carcinoma. Gastroenterology 2016, 150, 835–853. [Google Scholar] [CrossRef]
- Forner, A.; Vilana, R.; Ayuso, C.; Bianchi, L.; Sole, M.; Ayuso, J.R.; Boix, L.; Sala, M.; Varela, M.; Llovet, J.M.; et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008, 47, 97–104. [Google Scholar] [CrossRef]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef]
- Zhang, B.-H.; Yang, B.-H.; Tang, Z.-Y. Randomized controlled trial of screening for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2004, 130, 417–422. [Google Scholar] [CrossRef]
- Parikh, N.D.; Singal, A.G.; Hutton, D.W.; Tapper, E.B. Cost-Effectiveness of Hepatocellular Carcinoma Surveillance: An Assessment of Benefits and Harms. Am. J. Gastroenterol. 2020, 115, 1642–1649. [Google Scholar] [CrossRef]
- Choi, J.; Kim, G.; Han, S.; Lee, W.; Chun, S.; Lim, Y. Longitudinal Assessment of Three Serum Biomarkers to Detect Very Early-Stage Hepatocellular Carcinoma. Hepatology 2019, 69, 1983–1994. [Google Scholar] [CrossRef]
- Tateishi, R.; Yoshida, H.; Matsuyama, Y.; Mine, N.; Kondo, Y.; Omata, M. Diagnostic accuracy of tumor markers for hepatocellular carcinoma: A systematic review. Hepatol. Int. 2008, 2, 17–30. [Google Scholar] [CrossRef]
- Singal, A.G.; Tayob, N.; Mehta, A.; Marrero, J.A.; El-Serag, H.; Jin, Q.; de Viteri, C.S.; Fobar, A.; Parikh, N.D. GALAD demonstrates high sensitivity for HCC surveillance in a cohort of patients with cirrhosis. Hepatology 2022, 75, 541–549. [Google Scholar] [CrossRef]
- Norman, J.S.; Mehta, N. The Role of AFP-L3 and DCP Biomarkers in the Diagnosis and Management of Hepatocellular Carcinoma. Curr. Hepatol. Rep. 2025, 24, 16. [Google Scholar] [CrossRef]
- Shirakawa, H.; Kuronuma, T.; Nishimura, Y.; Hasebe, T.; Nakano, M.; Gotohda, N.; Takahashi, S.; Nakagohri, T.; Konishi, M.; Kobayashi, N.; et al. Glypican-3 is a useful diagnostic marker for a component of hepatocellular carcinoma in human liver cancer. Int. J. Oncol. 2009, 34, 649–656. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kojiro, M.; Wanless, I.R.; Alves, V.; Badve, S.; Balabaud, C.; Bedosa, P.; Bhathal, P.; Bioulac-Sage, P.; Brunt, E.M.; Burt, A.D.; et al. Pathologic diagnosis of early hepatocellular carcinoma: A report of the International Consensus Group for Hepatocellular Neoplasia. Hepatology 2009, 49, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.; Volk, M.L.; Waljee, A.; Salgia, R.; Higgins, P.; Rogers, M.A.M.; Marrero, J.A. Meta-analysis: Surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment. Pharmacol. Ther. 2009, 30, 37–47. [Google Scholar] [CrossRef]
- Hsu, H.C.; Cheng, W.; Lai, P.L. Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: Biological significance and temporospatial distribution. Cancer Res. 1997, 57, 5179–5184. [Google Scholar]
- Liu, X.-F.; Hu, Z.-D.; Liu, X.-C.; Cao, Y.; Ding, C.-M.; Hu, C.-J. Diagnostic accuracy of serum glypican-3 for hepatocellular carcinoma: A systematic review and meta-analysis. Clin. Biochem. 2014, 47, 196–200. [Google Scholar] [CrossRef]
- Xiao, W.-K.; Qi, C.-Y.; Chen, D.; Li, S.-Q.; Fu, S.-J.; Peng, B.-G.; Liang, L.-J. Prognostic significance of glypican-3 in hepatocellular carcinoma: A meta-analysis. BMC Cancer 2014, 14, 104. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Zhai, Y.; Qu, C.-F.; Zhang, L.-J.; Tan, Y.-F.; Li, N.; Ding, H.-G. Diagnostic value of glypican-3 in serum and liver for primary hepatocellular carcinoma. World J. Gastroenterol. 2010, 16, 4410–4415. [Google Scholar] [CrossRef] [PubMed]
- Ofuji, K.; Saito, K.; Suzuki, S.; Shimomura, M.; Shirakawa, H.; Nobuoka, D.; Sawada, Y.; Yoshimura, M.; Tsuchiya, N.; Takahashi, M.; et al. Perioperative plasma glypican-3 level may enable prediction of the risk of recurrence after surgery in patients with stage I hepatocellular carcinoma. Oncotarget 2017, 8, 37835–37844. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Liu, J.; Gao, Y.; Huang, Y.; Du, Z. Diagnosis accuracy of serum glypican-3 in patients with hepatocellular carcinoma: A systematic review with meta-analysis. Arch. Med. Res. 2014, 45, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Su, C.; Sun, L.; Gao, Y.; Li, Y. Performance of Serum Glypican 3 in Diagnosis of Hepatocellular Carcinoma: A meta-analysis. Ann. Hepatol. 2018, 18, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Batbaatar, B.; Gurbadam, U.; Tuvshinsaikhan, O.; Narmandakh, N.-E.; Khatanbaatar, G.; Radnaabazar, M.; Erdene-Ochir, D.; Boldbaatar, M.; Byambaragchaa, M.; Amankyeldi, Y.; et al. Evaluation of glypican-3 in patients with hepatocellular carcinoma. Mol. Clin. Oncol. 2025, 22, 1–8. [Google Scholar] [CrossRef]
- Liu, S.; Wang, M.; Zheng, C.; Zhong, Q.; Shi, Y.; Han, X. Diagnostic value of serum glypican-3 alone and in combination with AFP as an aid in the diagnosis of liver cancer. Clin. Biochem. 2020, 79, 54–60. [Google Scholar] [CrossRef]
- Malov, S.; Malov, I.; Kuvshinov, A.; Marche, P.; Decaens, T.; Macek-Jilkova, Z.; Yushchuk, N. Search for effective serum tumor markers for early diagnosis of hepatocellular carcinoma associated with hepatitis C. Sovrem. Tehnol. V Med. 2021, 13, 27–33. [Google Scholar] [CrossRef]
- Feng, J.; Chen, J.; Zhu, R.; Yu, L.; Zhang, Y.; Feng, D.; Kong, H.; Song, C.; Xia, H.; Wu, J.; et al. Prediction of early recurrence of hepatocellular carcinoma within the Milan criteria after radical resection. Oncotarget 2017, 8, 63299–63310. [Google Scholar] [CrossRef]
- Sideras, K.; Bots, S.J.; Biermann, K.; Sprengers, D.; Polak, W.G.; Ijzermans, J.N.M.; A de Man, R.; Pan, Q.; Sleijfer, S.; Bruno, M.J.; et al. Tumour antigen expression in hepatocellular carcinoma in a low-endemic western area. Br. J. Cancer 2015, 112, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Haruyama, Y.; Kataoka, H. Glypican-3 is a prognostic factor and an immunotherapeutic target in hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zhang, H.; Zheng, J.; Liu, Y. Glypican-3: A New Target for Diagnosis and Treatment of Hepatocellular Carcinoma. J. Cancer 2020, 11, 2008–2021. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, X.; Lei, Y.; Wang, G.; Liu, M. Glypican-3: A Novel and Promising Target for the Treatment of Hepatocellular Carcinoma. Front. Oncol. 2022, 12, 824208. [Google Scholar] [CrossRef]
- Moudi, B.; Heidari, Z.; Mahmoudzadeh-Sagheb, H. Meta-analysis and systematic review of prognostic significance of Glypican-3 in patients with hepatitis B-related hepatocellular carcinoma. VirusDisease 2019, 30, 193–200. [Google Scholar] [CrossRef]
- Yao, D.; Jiang, D.; Huang, Z.; Lu, J.; Tao, Q.; Yu, Z.; Meng, X. Abnormal expression of hepatoma specific γ-glutamyl transferase and alteration of γ-glutamyl transferase gene methylation status in patients with hepatocellular carcinoma. Cancer 2000, 88, 761–769. [Google Scholar] [CrossRef]

| Parameters of Clinical and Laboratory Characteristics | |
|---|---|
| Sex: male/female (n) | 30/10 |
| Age (mean +/− SD, years) | 61.5 ± 11.15 |
| Etiology of liver disease: HBV/HCV/alcohol (n) | 25/11/4 |
| Diabetes (n, %) | 9 (22.5) |
| Child–Turcotte–Pugh class: A/B (n) | 16/24 |
| Ascites: no/mild/moderate (n) | 18/15/7 |
| Esophageal varices: no/small/large (n) | 16/10/12 |
| Liver stiffness, measured by FibroScan, kPa * | 24.77 ± 15.30 (4.40–53.20) |
| Hemoglobin, g/L * | 130.5 ± 21.12 (82–165) |
| Platelets, ×109/L * | 160.5 ± 96.66 (35–502) |
| Prothrombin index, % * | 69.1 ± 17.4 (35–112) |
| Aspartate aminotransferase, U/L * | 90.63 ± 76.29 (23–289) |
| Alanine aminotransferase, U/L * | 56.10 ± 45.97 (17–253) |
| Gamma-glutamyl transferase, U/L * | 159.6 ± 180.7 (15–808) |
| Alkaline phosphatase, U/L * | 205.2 ± 262.4 (41–1685) |
| Cholinesterase, U/L * | 5270 ± 5044 (1023–31,000) |
| Bilirubin, mcg/L * | 38.95 ± 44.74 (6–267) |
| Albumin, g/L * | 36.10 ± 6.55 (25–49) |
| Alpha-fetoprotein (mean +/− SD, ng/mL) | 155.9 ± 165.1 |
| Carbohydrate antigen CA 19–9 (mean +/− SD, U/mL) | 122.4 ± 144 |
| Parameter | Category | N | Glypican-3 (Mean ± SEM), ng/mL | p |
|---|---|---|---|---|
| Age | <60 years ≥60 years | 16 24 | 44.83 ± 17.31 54.85 ± 16.61 | 0.67 |
| Sex | Males Females | 30 10 | 46.50 ± 13.69 63.84 ± 25.81 | 0.68 |
| HBsAg status | HBV infection Other etiology | 25 15 | 48.93 ± 14.19 54.01 ± 22.26 | 0.39 |
| Child–Turcotte–Pugh class | A (compensated) B (decompensated) | 16 24 | 52.59 ± 19.85 49.67 ± 15.37 | 0.79 |
| Barcelona Clinic Liver Cancer stage | Early HCC Intermediate HCC Advanced HCC | 12 13 15 | 60.37 ± 27.63 35.54 ± 11.02 56.47 ± 21.91 | 0.64 |
| Size of the tumor | ≤5 cm >5 cm | 26 14 | 61.04 ± 15.91 34.07 ± 18.25 | 0.27 |
| Number of nodules | Solitary 2–3 nodules Multiple, >3 | 20 8 12 | 61.72 ± 19.88 40.41 ± 15.89 39.65 ± 20.62 | 0.80 |
| Macrovascular invasion | No Yes | 32 8 | 56.83 ± 14.48 26.86 ± 14.22 | 0.72 |
| Extrahepatic metastasis | No Yes | 30 10 | 56.57 ± 15.22 33.63 ± 14 74 | 0.29 |
| CLIP score | 0–1 2 3–4 5–6 | 12 11 10 6 | 84.70 ± 20.82 30.01 ± 16.55 38.60 ± 22.64 30.63 ± 19.02 | 0.39 |
| Wash-out in CEUS portal venous phase | No Yes | 15 25 | 55.00 ± 19.83 55.61 ± 18.93 | 0.49 |
| HCC differentiation grade | Well-differentiated Moderately differentiated Poorly differentiated | 9 11 5 | 37.96 ± 20.52 105.5 ± 44.93 28.96 ± 15.40 | 0.32 |
| Biomarker | Cut-Off Level (ng/mL) | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|
| Glypican-3 | 2.5 | 85.0 (70.1–94.3) | 73.3 (54.1–87.7) |
| 33.7 | 30.0 (16.5–46.5) | 96.7 (82.8–99.9) | |
| Alpha-fetoprotein | 5.2 | 78.9 (62.9–90.4) | 76.7 (57.7–90.0) |
| 86.5 | 44.7 (28.6–61.7) | 96.7 (82.8–99.9) |
| Alpha-Fetoprotein (ng/mL) | N | Glypican-3 (>33.7 ng/mL) | |
|---|---|---|---|
| Positive | Negative | ||
| <6 ng/mL | 9 | 55.5% | 44.5% |
| 6–200 ng/mL | 16 | 25% | 75% |
| >200 ng/mL | 15 | 20% | 80% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, I.; Banova-Chakyrova, S.; Boykova-Vylcheva, P.; Bocheva, Y. Serum Level of Glypican-3 in Patients with Hepatocellular Carcinoma and Advanced Chronic Liver Disease: A Pilot Study. Livers 2025, 5, 36. https://doi.org/10.3390/livers5030036
Ivanova I, Banova-Chakyrova S, Boykova-Vylcheva P, Bocheva Y. Serum Level of Glypican-3 in Patients with Hepatocellular Carcinoma and Advanced Chronic Liver Disease: A Pilot Study. Livers. 2025; 5(3):36. https://doi.org/10.3390/livers5030036
Chicago/Turabian StyleIvanova, Irina, Sonya Banova-Chakyrova, Pavlina Boykova-Vylcheva, and Yana Bocheva. 2025. "Serum Level of Glypican-3 in Patients with Hepatocellular Carcinoma and Advanced Chronic Liver Disease: A Pilot Study" Livers 5, no. 3: 36. https://doi.org/10.3390/livers5030036
APA StyleIvanova, I., Banova-Chakyrova, S., Boykova-Vylcheva, P., & Bocheva, Y. (2025). Serum Level of Glypican-3 in Patients with Hepatocellular Carcinoma and Advanced Chronic Liver Disease: A Pilot Study. Livers, 5(3), 36. https://doi.org/10.3390/livers5030036






