Exploring the Classic and Novel Pathogenetic Insights of Plastic Exposure in the Genesis and Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
Abstract
1. Background
1.1. Introducing the “World” of Plastics
1.1.1. Principal Definitions
1.1.2. Main Social Sources of Exposure, Absorption, and Accumulation of Plastics
1.1.3. General Toxicological Properties of MNPs Contributing to Human Disorders
1.2. Plastics and Liver: A Consolidated Physiological and Pathological Binomial
2. Plastic Exposure Influences Hepatic Lipid Metabolism Driving Steatosis Progression
2.1. Plastic Exposure Contributes to Hepatic Fat Accumulation via Impacting Lipophagy
2.2. Plastic Exposure Contributes to Hepatic Fat Accumulation via Mitochondrial and ER Dysfunction
3. Plastic Exposure and the Gut–Liver Axis in the Pathogenesis of Steatotic Liver Disease
3.1. Gut Microbiota and Intestinal Permeability Status in Hepatic Steatosis: An Overview
3.2. Micro(nano)plastic Exposure Impacts the Gut–Liver Axis by Influencing Gut Microbiota Composition and Functioning
3.2.1. Micro(nano)plastic Exposure Alters Gut Microbiota Composition: In Vitro Evidence
3.2.2. Micro(nano)plastics Exposure Alters Gut Microbiota Composition: In-Animal Evidence
3.2.3. Micro(nano)plastics Exposure Alters Gut Microbiota Composition: In-Human Evidence
3.2.4. Micro(nano)plastics Exposure Impacts the Gut–Liver Axis by Altering Gut Microbiota Functioning and Impairing Intestinal Permeability
4. Inflammation, Oxidative Stress, and Innate Immune Dysfunction as Mutually Influenced Drivers in Steatotic Liver Disease: Is Micro(nano)plastic Exposure a Potential Pathogenetic Deus Ex Machina?
4.1. Inflammation and Oxidative Stress in Steatotic Liver Disease: A Consolidated Binomial
4.2. Micro(nano)plastic Influences Hepatic Lipid Metabolism via Inflammation and Oxidative Stress
4.3. Micro(nano)plastics and Related Additives as Activators of the Innate Immune Response: Investigating the Novel Frontier of Steatotic Liver Disease Pathogenesis
5. Micro(nano)plastic Exposure and Steatotic Liver Disease: From Basic Evidence to Clinical Bench-Side: An Urgent Need to Translate and Apply This Evidence. How Far Are We?
5.1. Main Research Challenges in the Field of Plastics and Hepatic Steatosis
5.2. Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| MPs | microplastics |
| NPs | nanoplastics |
| MNPs | micro(nano)plastics |
| PS-MPs | polystyrene microplastics |
| PE | polyethylene |
| PS | polystyrene |
| PE-MPs | polyethylene microplastics |
| PVC | polyvinyl chloride |
| PCB | polychlorinated biphenyl |
| PLA | polylactic acid |
| PLC | polycaprolactone |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| BPA | bisphenol A |
| EDCs | endocrine-disrupting chemicals |
| MASLD | Metabolic associated Steatotic Liver Disease |
| EASL | European Association for the Study of Liver |
| GSH | glutathione |
| TNF-α | tumor necrosis factor alpha |
| IL-1β | interleukin-1 beta |
| IL-1 | interleukin-1 |
| IL-6 | interleukin-6 |
| IL-8 | interleukin-8 |
| AR | androgen receptor |
| GPER | G protein-coupled estrogen receptor |
| TI | trained immunity |
| LPS | lipopolysaccharide |
| HFD | high-fat diet |
| PCL | polycaprolactone |
| PLA | polylactic acid |
| M-ARCOL | Mucosal Artificial Colon |
| FFAs | free fatty acids |
| CYP | cytochrome P450 |
| PRRs | pattern recognition receptors |
| NLRs | nucleotide-binding oligomerization domain-like receptors |
| TLRs | toll-like receptors |
| PAMPs | pathogen-associated molecular patterns |
| DAMPs | damage-associated molecular patterns |
| KCs | Kupffer cells |
| HIF-1α | hypoxia-inducible factor 1-alpha |
| PPAR | peroxisome proliferator-activated receptor |
| NaPs | Plastic-derived nanoparticles |
| TCRβ | T cell receptor beta |
| TCRδ | T cell receptor delta |
References
- Hirt, N.; Body-Malapel, M. Immunotoxicity and Intestinal Effects of Nano- and Microplastics: A Review of the Literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef]
- Pelegrini, K.; Pereira, T.C.B.; Maraschin, T.G.; Teodoro, L.D.S.; Basso, N.R.D.S.; De Galland, G.L.B.; Ligabue, R.A.; Bogo, M.R. Micro- and Nanoplastic Toxicity: A Review on Size, Type, Source, and Test-Organism Implications. Sci. Total Environ. 2023, 878, 162954. [Google Scholar] [CrossRef] [PubMed]
- Gigault, J.; Halle, A.T.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current Opinion: What Is a Nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, H.; Xie, B.; Dionysiou, D.D.; Zhao, Y. Microplastics as Both a Sink and a Source of Bisphenol A in the Marine Environment. Environ. Sci. Technol. 2019, 53, 10188–10196. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human Exposure to Bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R. Bisphenol A and Human Health: A Review of the Literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Zhang, M.; Elsamahy, T.; Abdelkarim, E.A.; Jiao, H.; Sun, S.; Sun, J. Environmental and Human Health Impact of Disposable Face Masks During the COVID-19 Pandemic: Wood-Feeding Termites as a Model for Plastic Biodegradation. Appl. Biochem. Biotechnol. 2023, 195, 2093–2113. [Google Scholar] [CrossRef]
- Cimmino, I.; Fiory, F.; Perruolo, G.; Miele, C.; Beguinot, F.; Formisano, P.; Oriente, F. Potential Mechanisms of Bisphenol A (BPA) Contributing to Human Disease. Int. J. Mol. Sci. 2020, 21, 5761. [Google Scholar] [CrossRef]
- Konieczna, A.; Rutkowska, A.; Rachoń, D. Health Risk of Exposure to Bisphenol A (BPA). Rocz. Panstw. Zakl. Hig. 2015, 66, 5–11. [Google Scholar]
- Melzer, D.; Rice, N.E.; Lewis, C.; Henley, W.E.; Galloway, T.S. Association of Urinary Bisphenol a Concentration with Heart Disease: Evidence from NHANES 2003/06. PLoS ONE 2010, 5, e8673. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of Microplastics in Human Lung Tissue Using μFTIR Spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef]
- Abbasi, S.; Turner, A. Human Exposure to Microplastics: A Study in Iran. J. Hazard. Mater. 2021, 403, 123799. [Google Scholar] [CrossRef]
- Leslie, H.A.; Van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-H.; Kim, J.-W.; Pham, T.D.; Tarafdar, A.; Hong, S.; Chun, S.-H.; Lee, S.-H.; Kang, D.-Y.; Kim, J.-Y.; Kim, S.-B.; et al. Microplastics in Food: A Review on Analytical Methods and Challenges. Int. J. Environ. Res. Public Health 2020, 17, 6710. [Google Scholar] [CrossRef] [PubMed]
- Kutralam-Muniasamy, G.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Shruti, V.C. Branded Milks—Are They Immune from Microplastics Contamination? Sci. Total Environ. 2020, 714, 136823. [Google Scholar] [CrossRef]
- Liebezeit, G.; Liebezeit, E. Non-Pollen Particulates in Honey and Sugar. Food Addit. Contam. Part A 2013, 30, 2136–2140. [Google Scholar] [CrossRef]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthetic Polymer Contamination in Bottled Water. Front. Chem. 2018, 6, 407. [Google Scholar] [CrossRef]
- Buyukunal, S.K.; Koluman, A.; Muratoglu, K. Microplastic Pollution of Drinking Water in a Metropolis. J. Water Health 2023, 21, 687–701. [Google Scholar] [CrossRef]
- Hartmann, C.; Lomako, I.; Schachner, C.; El Said, E.; Abert, J.; Satrapa, V.; Kaiser, A.-M.; Walch, H.; Köppel, S. Assessment of Microplastics in Human Stool: A Pilot Study Investigating the Potential Impact of Diet-Associated Scenarios on Oral Microplastics Exposure. Sci. Total Environ. 2024, 951, 175825. [Google Scholar] [CrossRef]
- Bastyans, S.; Jackson, S.; Fejer, G. Micro and Nano-Plastics, a Threat to Human Health? Emerg. Top. Life Sci. 2022, 6, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Tiwari, N.; Singh, D.; Tripathi, P.; Sharma, S. Toxicological Impacts of Microplastics on Human Health: A Bibliometric Analysis. Environ. Sci. Pollut. Res. Int. 2024, 31, 57417–57429. [Google Scholar] [CrossRef]
- Winiarska, E.; Jutel, M.; Zemelka-Wiacek, M. The Potential Impact of Nano- and Microplastics on Human Health: Understanding Human Health Risks. Environ. Res. 2024, 251, 118535. [Google Scholar] [CrossRef]
- Wu, B.; Wu, X.; Liu, S.; Wang, Z.; Chen, L. Size-Dependent Effects of Polystyrene Microplastics on Cytotoxicity and Efflux Pump Inhibition in Human Caco-2 cells. Chemosphere 2019, 221, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Poma, A.; Vecchiotti, G.; Colafarina, S.; Zarivi, O.; Aloisi, M.; Arrizza, L.; Chichiriccò, G.; Di Carlo, P. In Vitro Genotoxicity of Polystyrene Nanoparticles on the Human Fibroblast Hs27 Cell Line. Nanomaterials 2019, 9, 1299. [Google Scholar] [CrossRef]
- Mattioda, V.; Benedetti, V.; Tessarolo, C.; Oberto, F.; Favole, A.; Gallo, M.; Martelli, W.; Crescio, M.I.; Berio, E.; Masoero, L.; et al. Pro-Inflammatory and Cytotoxic Effects of Polystyrene Microplastics on Human and Murine Intestinal Cell Lines. Biomolecules 2023, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Brito, W.A.; Mutter, F.; Wende, K.; Cecchini, A.L.; Schmidt, A.; Bekeschus, S. Consequences of Nano and Microplastic Exposure in Rodent Models: The Known and Unknown. Part. Fibre Toxicol. 2022, 19, 28. [Google Scholar] [CrossRef]
- Dallio, M.; Diano, N.; Masarone, M.; Gravina, A.G.; Patanè, V.; Romeo, M.; Di Sarno, R.; Errico, S.; Nicolucci, C.; Abenavoli, L.; et al. Chemical Effect of Bisphenol A on Non-Alcoholic Fatty Liver Disease. Int. J. Environ. Res. Public Health 2019, 16, 3134. [Google Scholar] [CrossRef]
- Nicolucci, C.; Errico, S.; Federico, A.; Dallio, M.; Loguercio, C.; Diano, N. Human Exposure to Bisphenol A and Liver Health Status: Quantification of Urinary and Circulating Levels by LC-MS/MS. J. Pharm. Biomed. Anal. 2017, 140, 105–112. [Google Scholar] [CrossRef]
- Dallio, M.; Masarone, M.; Errico, S.; Gravina, A.G.; Nicolucci, C.; Di Sarno, R.; Gionti, L.; Tuccillo, C.; Persico, M.; Stiuso, P.; et al. Role of Bisphenol A as Environmental Factor in the Promotion of Non-Alcoholic Fatty Liver Disease: In Vitro and Clinical Study. Aliment. Pharmacol. Ther. 2018, 47, 826–837. [Google Scholar] [CrossRef]
- Savastano, S.; Tarantino, G.; D’Esposito, V.; Passaretti, F.; Cabaro, S.; Liotti, A.; Liguoro, D.; Perruolo, G.; Ariemma, F.; Finelli, C.; et al. Bisphenol-A Plasma Levels Are Related to Inflammatory Markers, Visceral Obesity and Insulin-Resistance: A Cross-Sectional Study on Adult Male Population. J. Transl. Med. 2015, 13, 169. [Google Scholar] [CrossRef]
- Croom, E. Metabolism of Xenobiotics of Human Environments. Prog. Mol. Biol. Transl. Sci. 2012, 112, 31–88. [Google Scholar] [CrossRef]
- Lu, K.; Song, Y.; Zeng, R. The Role of Cytochrome P450-Mediated Detoxification in Insect Adaptation to Xenobiotics. Curr. Opin. Insect Sci. 2021, 43, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Raps, H.; Cropper, M.; Bald, C.; Brunner, M.; Canonizado, E.M.; Charles, D.; Chiles, T.C.; Donohue, M.J.; Enck, J.; et al. The Minderoo-Monaco Commission on Plastics and Human Health. Ann. Glob. Health 2023, 89, 23. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Dallio, M.; Romeo, M.; Gravina, A.G.; Masarone, M.; Larussa, T.; Abenavoli, L.; Persico, M.; Loguercio, C.; Federico, A. Nutrigenomics and Nutrigenetics in Metabolic- (Dysfunction) Associated Fatty Liver Disease: Novel Insights and Future Perspectives. Nutrients 2021, 13, 1679. [Google Scholar] [CrossRef] [PubMed]
- Dallio, M.; Sangineto, M.; Romeo, M.; Cipullo, M.; Coppola, A.; Mammone, S.; Di Gioia, G.; Masarone, M.; Persico, M.; Serviddio, G.; et al. The Influence of Acute Lifestyle Changes on NAFLD Evolution in a Multicentre Cohort: A Matter of Body Composition. Nutr. Diabetes 2024, 14, 33. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M.; Rustichelli, A.; Dongiovanni, P. Nutrition and Genetics in NAFLD: The Perfect Binomium. Int. J. Mol. Sci. 2020, 21, 2986. [Google Scholar] [CrossRef]
- Dallio, M.; Masarone, M.; Romeo, M.; Tuccillo, C.; Morisco, F.; Persico, M.; Loguercio, C.; Federico, A. PNPLA3, TM6SF2, and MBOAT7 Influence on Nutraceutical Therapy Response for Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Front. Med. 2021, 8, 734847. [Google Scholar] [CrossRef]
- Gravina, A.G.; Romeo, M.; Pellegrino, R.; Tuccillo, C.; Federico, A.; Loguercio, C. Just Drink a Glass of Water? Effects of Bicarbonate-Sulfate-Calcium-Magnesium Water on the Gut-Liver Axis. Front. Pharmacol. 2022, 13, 869446. [Google Scholar] [CrossRef]
- Zhao, Y.; Bao, Z.; Wan, Z.; Fu, Z.; Jin, Y. Polystyrene Microplastic Exposure Disturbs Hepatic Glycolipid Metabolism at the Physiological, Biochemical, and Transcriptomic Levels in Adult Zebrafish. Sci. Total Environ. 2020, 710, 136279. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Li, X.; Zhou, Y.; Yu, H.; Xie, Y.; Guo, H.; Wang, H.; Li, Y.; Feng, Y.; Wang, Y. Polystyrene Microplastics Induce Hepatotoxicity and Disrupt Lipid Metabolism in the Liver Organoids. Sci. Total Environ. 2022, 806, 150328. [Google Scholar] [CrossRef] [PubMed]
- Guraka, A.; Souch, G.; Duff, R.; Brown, D.; Moritz, W.; Kermanizadeh, A. Microplastic-Induced Hepatic Adverse Effects Evaluated in Advanced Quadruple Cell Human Primary Models Following Three Weeks of Repeated Exposure. Chemosphere 2024, 364, 143032. [Google Scholar] [CrossRef]
- Yang, T.; Poenisch, M.; Khanal, R.; Hu, Q.; Dai, Z.; Li, R.; Song, G.; Yuan, Q.; Yao, Q.; Shen, X.; et al. Therapeutic HNF4A mRNA Attenuates Liver Fibrosis in a Preclinical Model. J. Hepatol. 2021, 75, 1420–1433. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zhang, Y.; Fang, Y.; Zhong, H.; Wei, T.; Akhtar, H.; Zhang, J.; Yang, M.; Li, Y.; Zhou, X.; et al. Polystyrene Nanoplastics Induce Lipophagy via the AMPK/ULK1 Pathway and Block Lipophagic Flux Leading to Lipid Accumulation in Hepatocytes. J. Hazard. Mater. 2024, 476, 134878. [Google Scholar] [CrossRef]
- Chiu, H.-W.; Chu, C.-W.; Huang, C.-C.; Chia, Z.-C.; Wang, Y.-L.; Lee, Y.-H. Polystyrene Microplastics Induce Hepatic Lipid Metabolism and Energy Disorder by Upregulating the NR4A1-AMPK Signaling Pathway. Environ. Pollut. (Barking Essex 1987) 2025, 369, 125850. [Google Scholar] [CrossRef]
- Prasun, P.; Ginevic, I.; Oishi, K. Mitochondrial Dysfunction in Nonalcoholic Fatty Liver Disease and Alcohol Related Liver Disease. Transl. Gastroenterol. Hepatol. 2021, 6, 4. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, H.; Wang, C.; Su, X.-L.; Song, Y.; Wu, P.; Yang, Z.; Wong, M.-H.; Cai, Z.; Zheng, C. Metabolomics Reveal Nanoplastic-Induced Mitochondrial Damage in Human Liver and Lung Cells. Environ. Sci. Technol. 2022, 56, 12483–12493. [Google Scholar] [CrossRef]
- Shen, R.; Yang, K.; Cheng, X.; Guo, C.; Xing, X.; Sun, H.; Liu, D.; Liu, X.; Wang, D. Accumulation of Polystyrene Microplastics Induces Liver Fibrosis by Activating cGAS/STING Pathway. Environ. Pollut. (Barking Essex 1987) 2022, 300, 118986. [Google Scholar] [CrossRef]
- Beaulant, A.; Dia, M.; Pillot, B.; Chauvin, M.-A.; Ji-Cao, J.; Durand, C.; Bendridi, N.; Chanon, S.; Vieille-Marchiset, A.; Da Silva, C.C.; et al. Endoplasmic Reticulum-Mitochondria Miscommunication Is an Early and Causal Trigger of Hepatic Insulin Resistance and Steatosis. J. Hepatol. 2022, 77, 710–722. [Google Scholar] [CrossRef]
- Wei, J.; Liu, J.; Wang, H.; Wen, K.; Ni, X.; Lin, Y.; Huang, J.; You, X.; Lei, Z.; Li, J.; et al. Nanoplastic Propels Diet-Induced NAFL to NASH via ER-Mitochondrial Tether-Controlled Redox Switch. J. Hazard. Mater. 2024, 465, 133142. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Qu, S. Non-Alcoholic Fatty Liver Disease and Gut Microbial Dysbiosis- Underlying Mechanisms and Gut Microbiota Mediated Treatment Strategies. Rev. Endocr. Metab. Disord. 2023, 24, 1189–1204. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Schoeler, M.; Ellero-Simatos, S.; Birkner, T.; Mayneris-Perxachs, J.; Olsson, L.; Brolin, H.; Loeber, U.; Kraft, J.D.; Polizzi, A.; Martí-Navas, M.; et al. The Interplay between Dietary Fatty Acids and Gut Microbiota Influences Host Metabolism and Hepatic Steatosis. Nat. Commun. 2023, 14, 5329. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pérez, A.M.; Ruiz-Limón, P.; Salas-Salvadó, J.; Vioque, J.; Corella, D.; Fitó, M.; Vidal, J.; Atzeni, A.; Torres-Collado, L.; Álvarez-Sala, A.; et al. Gut Microbiota in Nonalcoholic Fatty Liver Disease: A PREDIMED-Plus Trial Sub Analysis. Gut Microbes 2023, 15, 2223339. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tripathi, M.; Sinha, R.A.; Singh, B.K.; Yen, P.M. Gut Microbiota and Their Metabolites in the Progression of Non-Alcoholic Fatty Liver Disease. Hepatoma Res. 2021, 7, 11. [Google Scholar] [CrossRef]
- Hoyles, L.; Fernández-Real, J.-M.; Federici, M.; Serino, M.; Abbott, J.; Charpentier, J.; Heymes, C.; Luque, J.L.; Anthony, E.; Barton, R.H.; et al. Molecular Phenomics and Metagenomics of Hepatic Steatosis in Non-Diabetic Obese Women. Nat. Med. 2018, 24, 1070–1080. [Google Scholar] [CrossRef]
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut Microbiome-Based Metagenomic Signature for Non-Invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017, 25, 1054–1062.e5. [Google Scholar] [CrossRef]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of Gut Microbiomes in Nonalcoholic Steatohepatitis (NASH) Patients: A Connection between Endogenous Alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef]
- Fang, J.; Yu, C.-H.; Li, X.-J.; Yao, J.-M.; Fang, Z.-Y.; Yoon, S.-H.; Yu, W.-Y. Gut Dysbiosis in Nonalcoholic Fatty Liver Disease: Pathogenesis, Diagnosis, and Therapeutic Implications. Front. Cell. Infect. Microbiol. 2022, 12, 997018. [Google Scholar] [CrossRef]
- Caussy, C.; Hsu, C.; Lo, M.-T.; Liu, A.; Bettencourt, R.; Ajmera, V.H.; Bassirian, S.; Hooker, J.; Sy, E.; Richards, L.; et al. Link between Gut-Microbiome Derived Metabolite and Shared Gene-Effects with Hepatic Steatosis and Fibrosis in NAFLD. Hepatology 2018, 68, 918–932. [Google Scholar] [CrossRef] [PubMed]
- Pettinelli, P.; Arendt, B.M.; Schwenger, K.J.P.; Sivaraj, S.; Bhat, M.; Comelli, E.M.; Lou, W.; Allard, J.P. Relationship Between Hepatic Gene Expression, Intestinal Microbiota, and Inferred Functional Metagenomic Analysis in NAFLD. Clin. Transl. Gastroenterol. 2022, 13, e00466. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Erkosar, B.; Storelli, G.; Defaye, A.; Leulier, F. Host-Intestinal Microbiota Mutualism: “Learning on the Fly”. Cell Host Microbe 2013, 13, 8–14. [Google Scholar] [CrossRef]
- Cabreiro, F.; Gems, D. Worms Need Microbes Too: Microbiota, Health and Aging in Caenorhabditis Elegans. EMBO Mol. Med. 2013, 5, 1300–1310. [Google Scholar] [CrossRef]
- Qiao, J.; Chen, R.; Wang, M.; Bai, R.; Cui, X.; Liu, Y.; Wu, C.; Chen, C. Perturbation of Gut Microbiota Plays an Important Role in Micro/Nanoplastics-Induced Gut Barrier Dysfunction. Nanoscale 2021, 13, 8806–8816. [Google Scholar] [CrossRef]
- Huang, Z.; Weng, Y.; Shen, Q.; Zhao, Y.; Jin, Y. Microplastic: A Potential Threat to Human and Animal Health by Interfering with the Intestinal Barrier Function and Changing the Intestinal Microenvironment. Sci. Total Environ. 2021, 785, 147365. [Google Scholar] [CrossRef]
- Sofield, C.E.; Anderton, R.S.; Gorecki, A.M. Mind over Microplastics: Exploring Microplastic-Induced Gut Disruption and Gut-Brain-Axis Consequences. Curr. Issues Mol. Biol. 2024, 46, 4186–4202. [Google Scholar] [CrossRef]
- Karami, A.; Groman, D.B.; Wilson, S.P.; Ismail, P.; Neela, V.K. Biomarker Responses in Zebrafish (Danio Rerio) Larvae Exposed to Pristine Low-Density Polyethylene Fragments. Environ. Pollut. (Barking Essex 1987) 2017, 223, 466–475. [Google Scholar] [CrossRef]
- Güven, O.; Gökdağ, K.; Jovanović, B.; Kıdeyş, A.E. Microplastic Litter Composition of the Turkish Territorial Waters of the Mediterranean Sea, and Its Occurrence in the Gastrointestinal Tract of Fish. Environ. Pollut. (Barking Essex 1987) 2017, 223, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Deng, K.; Zhang, P.; Chen, Q.; Magnuson, J.T.; Qiu, W.; Zhou, Y. Microplastic-Mediated New Mechanism of Liver Damage: From the Perspective of the Gut-Liver Axis. Sci. Total Environ. 2024, 919, 170962. [Google Scholar] [CrossRef]
- Fackelmann, G.; Sommer, S. Microplastics and the Gut Microbiome: How Chronically Exposed Species May Suffer from Gut Dysbiosis. Mar. Pollut. Bull. 2019, 143, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Quiñonez, C.A.; Louis, V.R.; Horstick, O.; Velayudhan, R.; Dambach, P.; Runge-Ranzinger, S. Corrigendum to: Interventions against Aedes/Dengue at the Household Level: A Systematic Review and Meta-Analysis. eBioMedicine 2023, 93, 104660, corrigendum to eBioMedicine 2024, 107, 105292. https://doi.org/10.1016/j.ebiom.2024.105292. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Lu, J.; Fan, L.; Dong, W.; Jiang, M. Simulated Gastrointestinal Digestion of Two Different Sources of Biodegradable Microplastics and the Influence on Gut Microbiota. Food Chem. Toxicol. 2024, 185, 114474. [Google Scholar] [CrossRef]
- Fournier, E.; Leveque, M.; Ruiz, P.; Ratel, J.; Durif, C.; Chalancon, S.; Amiard, F.; Edely, M.; Bezirard, V.; Gaultier, E.; et al. Microplastics: What Happens in the Human Digestive Tract? First Evidences in Adults Using in Vitro Gut Models. J. Hazard. Mater. 2023, 442, 130010. [Google Scholar] [CrossRef]
- de Souza-Silva, T.G.; Oliveira, I.A.; da Silva, G.G.; Giusti, F.C.V.; Novaes, R.D.; de Almeida Paula, H.A. Impact of Microplastics on the Intestinal Microbiota: A Systematic Review of Preclinical Evidence. Life Sci. 2022, 294, 120366. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of Polystyrene Microplastic on the Gut Barrier, Microbiota and Metabolism of Mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Jin, Y.; Xia, J.; Pan, Z.; Yang, J.; Wang, W.; Fu, Z. Polystyrene Microplastics Induce Microbiota Dysbiosis and Inflammation in the Gut of Adult Zebrafish. Environ. Pollut. (Barking Essex 1987) 2018, 235, 322–329. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, Z.; Huang, Z.; Bao, Z.; Luo, T.; Jin, Y. Effects of Polyethylene Microplastics on the Microbiome and Metabolism in Larval Zebrafish. Environ. Pollut. (Barking Essex 1987) 2021, 282, 117039. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, C.; Zhou, J.; Shen, M.; Wang, X.; Fu, Z.; Jin, Y. Effects of Polystyrene Microplastics on the Composition of the Microbiome and Metabolism in Larval Zebrafish. Chemosphere 2019, 217, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics Induce Intestinal Inflammation, Oxidative Stress, and Disorders of Metabolome and Microbiome in Zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Bautista-Puig, N.; Barreiro-Gen, M.; Statulevičiūtė, G.; Stančiauskas, V.; Dikmener, G.; Akylbekova, D.; Lozano, R. Unraveling Public Perceptions of the Sustainable Development Goals for Better Policy Implementation. Sci. Total Environ. 2024, 912, 169114, corrigendum to Sci. Total Environ. 2024, 916, 170450. https://doi.org/10.1016/j.scitotenv.2024.170450. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Lv, M.; Li, J.; Ding, J.; Wang, Y.; Fu, L.; Sun, X.; Han, X.; Chen, L. The Distinct Toxicity Effects between Commercial and Realistic Polystyrene Microplastics on Microbiome and Histopathology of Gut in Zebrafish. J. Hazard. Mater. 2022, 434, 128874. [Google Scholar] [CrossRef]
- Xu, R.; Cao, J.-W.; Lv, H.-L.; Geng, Y.; Guo, M.-Y. Polyethylene Microplastics Induced Gut Microbiota Dysbiosis Leading to Liver Injury via the TLR2/NF-κB/NLRP3 Pathway in Mice. Sci. Total Environ. 2024, 917, 170518. [Google Scholar] [CrossRef]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene Microplastics Affect the Distribution of Gut Microbiota and Inflammation Development in Mice. Chemosphere 2020, 244, 125492. [Google Scholar] [CrossRef]
- Chen, X.; Zhuang, J.; Chen, Q.; Xu, L.; Yue, X.; Qiao, D. Polyvinyl Chloride Microplastics Induced Gut Barrier Dysfunction, Microbiota Dysbiosis and Metabolism Disorder in Adult Mice. Ecotoxicol. Environ. Saf. 2022, 241, 113809. [Google Scholar] [CrossRef] [PubMed]
- Djouina, M.; Vignal, C.; Dehaut, A.; Caboche, S.; Hirt, N.; Waxin, C.; Himber, C.; Beury, D.; Hot, D.; Dubuquoy, L.; et al. Oral Exposure to Polyethylene Microplastics Alters Gut Morphology, Immune Response, and Microbiota Composition in Mice. Environ. Res. 2022, 212, 113230. [Google Scholar] [CrossRef]
- Caruso, G.; Cristina, P.; Cappello, S.; Leonardi, M.; La Ferla, R.; Lo Giudice, A.; Maricchiolo, G.; Rizzo, C.; Maimone, G.; Rappazzo, A.C.; et al. Effects of Microplastics on Trophic Parameters, Abundance and Metabolic Activities of Seawater and Fish Gut Bacteria in Mesocosm Conditions. Environ. Sci. Pollut. Res. Int. 2018, 25, 30067–30083. [Google Scholar] [CrossRef]
- Horton, A.A.; Newbold, L.K.; Palacio-Cortés, A.M.; Spurgeon, D.J.; Pereira, M.G.; Carter, H.; Gweon, H.S.; Vijver, M.G.; van Bodegom, P.M.; Navarro da Silva, M.A.; et al. Accumulation of Polybrominated Diphenyl Ethers and Microbiome Response in the Great Pond Snail Lymnaea Stagnalis with Exposure to Nylon (Polyamide) Microplastics. Ecotoxicol. Environ. Saf. 2020, 188, 109882. [Google Scholar] [CrossRef]
- Ju, H.; Zhu, D.; Qiao, M. Effects of Polyethylene Microplastics on the Gut Microbial Community, Reproduction and Avoidance Behaviors of the Soil Springtail, Folsomia Candida. Environ. Pollut. (Barking Essex 1987) 2019, 247, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene Microplastics Induce Gut Microbiota Dysbiosis and Hepatic Lipid Metabolism Disorder in Mice. Sci. Total Environ. 2018, 631–632, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.-K.; Fang, Y.-M.; Zhu, D.; Christie, P.; Ke, X.; Zhu, Y.-G. Exposure to Nanoplastics Disturbs the Gut Microbiome in the Soil Oligochaete Enchytraeus Crypticus. Environ. Pollut. (Barking Essex 1987) 2018, 239, 408–415. [Google Scholar] [CrossRef]
- Li, L.-L.; Amara, R.; Souissi, S.; Dehaut, A.; Duflos, G.; Monchy, S. Impacts of Microplastics Exposure on Mussel (Mytilus Edulis) Gut Microbiota. Sci. Total Environ. 2020, 745, 141018. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Xiong, D.; Wang, Y.; Zhang, Z.; Li, H.; Dong, H.; Zhang, J. Toxicological Effects of Microplastics in Litopenaeus Vannamei as Indicated by an Integrated Microbiome, Proteomic and Metabolomic Approach. Sci. Total Environ. 2021, 761, 143311. [Google Scholar] [CrossRef]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of Different Shapes of Microplastics Initiates Intestinal Injury and Gut Microbiota Dysbiosis in the Gut of Zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef]
- Luo, T.; Wang, C.; Pan, Z.; Jin, C.; Fu, Z.; Jin, Y. Maternal Polystyrene Microplastic Exposure during Gestation and Lactation Altered Metabolic Homeostasis in the Dams and Their F1 and F2 Offspring. Environ. Sci. Technol. 2019, 53, 10978–10992. [Google Scholar] [CrossRef]
- O’Hara, S.P.; Karlsen, T.H.; LaRusso, N.F. Cholangiocytes and the Environment in Primary Sclerosing Cholangitis: Where Is the Link? Gut 2017, 66, 1873–1877. [Google Scholar] [CrossRef]
- Hsu, C.L.; Schnabl, B. The Gut-Liver Axis and Gut Microbiota in Health and Liver Disease. Nat. Rev. Microbiol. 2023, 21, 719–733. [Google Scholar] [CrossRef]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The Gut-Liver Axis and the Intersection with the Microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Trauner, M. Gut-Liver Axis: Pathophysiological Concepts and Clinical Implications. Cell Metab. 2022, 34, 1700–1718. [Google Scholar] [CrossRef] [PubMed]
- Suk, K.T.; Kim, D.J. Gut Microbiota: Novel Therapeutic Target for Nonalcoholic Fatty Liver Disease. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Palma, R.; Pronio, A.; Romeo, M.; Scognamiglio, F.; Ventriglia, L.; Ormando, V.M.; Lamazza, A.; Pontone, S.; Federico, A.; Dallio, M. The Role of Insulin Resistance in Fueling NAFLD Pathogenesis: From Molecular Mechanisms to Clinical Implications. J. Clin. Med. 2022, 11, 3649. [Google Scholar] [CrossRef]
- Dallio, M.; Sangineto, M.; Romeo, M.; Villani, R.; Romano, A.D.; Loguercio, C.; Serviddio, G.; Federico, A. Immunity as Cornerstone of Non-Alcoholic Fatty Liver Disease: The Contribution of Oxidative Stress in the Disease Progression. Int. J. Mol. Sci. 2021, 22, 436. [Google Scholar] [CrossRef] [PubMed]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular Mechanisms of Hepatic Lipid Accumulation in Non-Alcoholic Fatty Liver Disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef]
- LeFort, K.R.; Rungratanawanich, W.; Song, B.-J. Contributing Roles of Mitochondrial Dysfunction and Hepatocyte Apoptosis in Liver Diseases through Oxidative Stress, Post-Translational Modifications, Inflammation, and Intestinal Barrier Dysfunction. Cell. Mol. Life Sci. 2024, 81, 34. [Google Scholar] [CrossRef]
- Cicchinelli, S.; Pignataro, G.; Gemma, S.; Piccioni, A.; Picozzi, D.; Ojetti, V.; Franceschi, F.; Candelli, M. PAMPs and DAMPs in Sepsis: A Review of Their Molecular Features and Potential Clinical Implications. Int. J. Mol. Sci. 2024, 25, 962. [Google Scholar] [CrossRef]
- Sharma, M.; Mitnala, S.; Vishnubhotla, R.K.; Mukherjee, R.; Reddy, D.N.; Rao, P.N. The Riddle of Nonalcoholic Fatty Liver Disease: Progression from Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis. J. Clin. Exp. Hepatol. 2015, 5, 147–158. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Wang, W.; Mao, X.; Zhang, R.; Zhou, X.-X.; Liu, Y.; Zhou, H.; Jia, J.; Yan, B. Nanoplastic Exposure at Environmental Concentrations Disrupts Hepatic Lipid Metabolism through Oxidative Stress Induction and Endoplasmic Reticulum Homeostasis Perturbation. Environ. Sci. Technol. 2023, 57, 14127–14137. [Google Scholar] [CrossRef]
- Powell, J.J.; Faria, N.; Thomas-McKay, E.; Pele, L.C. Origin and Fate of Dietary Nanoparticles and Microparticles in the Gastrointestinal Tract. J. Autoimmun. 2010, 34, J226–J233. [Google Scholar] [CrossRef] [PubMed]
- Brandts, I.; Teles, M.; Gonçalves, A.P.; Barreto, A.; Franco-Martinez, L.; Tvarijonaviciute, A.; Martins, M.A.; Soares, A.M.V.M.; Tort, L.; Oliveira, M. Effects of Nanoplastics on Mytilus Galloprovincialis after Individual and Combined Exposure with Carbamazepine. Sci. Total Environ. 2018, 643, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Sadler, D.E.; Brunner, F.S.; Plaistow, S.J. Temperature and Clone-Dependent Effects of Microplastics on Immunity and Life History in Daphnia Magna. Environ. Pollut. (Barking Essex 1987) 2019, 255, 113178. [Google Scholar] [CrossRef] [PubMed]
- Lamas, B.; Martins Breyner, N.; Houdeau, E. Impacts of Foodborne Inorganic Nanoparticles on the Gut Microbiota-Immune Axis: Potential Consequences for Host Health. Part. Fibre Toxicol. 2020, 17, 19. [Google Scholar] [CrossRef]
- Mancia, A.; Chenet, T.; Bono, G.; Geraci, M.L.; Vaccaro, C.; Munari, C.; Mistri, M.; Cavazzini, A.; Pasti, L. Adverse Effects of Plastic Ingestion on the Mediterranean Small-Spotted Catshark (Scyliorhinus canicula). Mar. Environ. Res. 2020, 155, 104876. [Google Scholar] [CrossRef]
- Zha, H.; Tang, R.; Li, S.; Zhuge, A.; Xia, J.; Lv, J.; Wang, S.; Wang, K.; Zhang, H.; Li, L. Effects of Partial Reduction of Polystyrene Micro-Nanoplastics on the Immunity, Gut Microbiota and Metabolome of Mice. Chemosphere 2024, 349, 140940. [Google Scholar] [CrossRef]
- Pei, X.; Heng, X.; Chu, W. Polystyrene Nano/Microplastics Induce Microbiota Dysbiosis, Oxidative Damage, and Innate Immune Disruption in Zebrafish. Microb. Pathog. 2022, 163, 105387. [Google Scholar] [CrossRef]
- Li, R.; Nie, J.; Qiu, D.; Li, S.; Sun, Y.; Wang, C. Toxic Effect of Chronic Exposure to Polyethylene Nano/Microplastics on Oxidative Stress, Neurotoxicity and Gut Microbiota of Adult Zebrafish (Danio rerio). Chemosphere 2023, 339, 139774. [Google Scholar] [CrossRef]
- Burgos-Aceves, M.A.; Abo-Al-Ela, H.G.; Faggio, C. Physiological and Metabolic Approach of Plastic Additive Effects: Immune Cells Responses. J. Hazard. Mater. 2021, 404, 124114. [Google Scholar] [CrossRef]
- Banaee, M.; Soltanian, S.; Sureda, A.; Gholamhosseini, A.; Haghi, B.N.; Akhlaghi, M.; Derikvandy, A. Evaluation of Single and Combined Effects of Cadmium and Micro-Plastic Particles on Biochemical and Immunological Parameters of Common Carp (Cyprinus Carpio). Chemosphere 2019, 236, 124335. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, W.; Sun, S.; Du, X.; Han, Y.; Shi, W.; Liu, G. Immunotoxicity and Neurotoxicity of Bisphenol A and Microplastics Alone or in Combination to a Bivalve Species, Tegillarca granosa. Environ. Pollut. (Barking Essex 1987) 2020, 265, 115115. [Google Scholar] [CrossRef] [PubMed]
- Bowley, J.; Baker-Austin, C.; Porter, A.; Hartnell, R.; Lewis, C. Oceanic Hitchhikers—Assessing Pathogen Risks from Marine Microplastic. Trends Microbiol. 2021, 29, 107–116. [Google Scholar] [CrossRef]
- Bekkering, S.; Domínguez-Andrés, J.; Joosten, L.A.B.; Riksen, N.P.; Netea, M.G. Trained Immunity: Reprogramming Innate Immunity in Health and Disease. Annu. Rev. Immunol. 2021, 39, 667–693. [Google Scholar] [CrossRef]
- Dallio, M.; Ventriglia, L.; Romeo, M.; Scognamiglio, F.; Diano, N.; Moggio, M.; Cipullo, M.; Coppola, A.; Ziogas, A.; Netea, M.G.; et al. Environmental Bisphenol A Exposure Triggers Trained Immunity-Related Pathways in Monocytes. Front. Immunol. 2023, 14, 1270391. [Google Scholar] [CrossRef] [PubMed]
- Ventriglia, L.; Scognamiglio, F.; Romeo, M.; Cipullo, M.; Moggio, M.; Niosi, M.; Diano, N.; Dallio, M.; Federico, A. T.03.5 Effect of Environmental Bisphenol A Exposure on Trained Immunity-Related Pathways in Non-Alcoholic Fatty Liver Disease: A Preliminary Observation. Dig. Liver Dis. 2023, 55, S146–S147. [Google Scholar] [CrossRef]
- Hong, T.; Zou, J.; He, Y.; Zhang, H.; Liu, H.; Mai, H.; Yang, J.; Cao, Z.; Chen, X.; Yao, J.; et al. Bisphenol A Induced Hepatic Steatosis by Disturbing Bile Acid Metabolism and FXR/TGR5 Signaling Pathways via Remodeling the Gut Microbiota in CD-1 Mice. Sci. Total Environ. 2023, 889, 164307. [Google Scholar] [CrossRef]
- Liang, J.; Xu, C.; Xu, J.; Yang, C.; Kong, W.; Xiao, Z.; Chen, X.; Liu, Q.; Weng, Z.; Wang, J.; et al. PPARα Senses Bisphenol S to Trigger EP300-Mediated Autophagy Blockage and Hepatic Steatosis. Environ. Sci. Technol. 2023, 57, 21581–21592. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, C.H.; Park, J.B.; Ko, K.; Lee, M.H.; Chung, J.; Yoo, Y.H. Hepatic STAMP2 Alleviates Polychlorinated Biphenyl-induced Steatosis and Hepatic Iron Overload in NAFLD Models. Environ. Toxicol. 2022, 37, 2223–2234. [Google Scholar] [CrossRef]
- Zhang, Q.; He, Y.; Cheng, R.; Li, Q.; Qian, Z.; Lin, X. Recent Advances in Toxicological Research and Potential Health Impact of Microplastics and Nanoplastics in Vivo. Environ. Sci. Pollut. Res. 2022, 29, 40415–40448. [Google Scholar] [CrossRef]
- Liu, C.; Chen, S.; Chu, J.; Yang, Y.; Yuan, B.; Zhang, H. Multi-Omics Analysis Reveals the Toxicity of Polyvinyl Chloride Microplastics toward BEAS-2B Cells. Toxics 2024, 12, 399. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, J.; Chen, L.; He, J.; Qu, D.; Zhang, Z.; Liu, Y.; Li, X.; Liu, J.; Li, J.; et al. Gut Microbiota Participates in Polystyrene Microplastics-Induced Hepatic Injuries by Modulating the Gut-Liver Axis. ACS Nano 2023, 17, 15125–15145. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef] [PubMed]
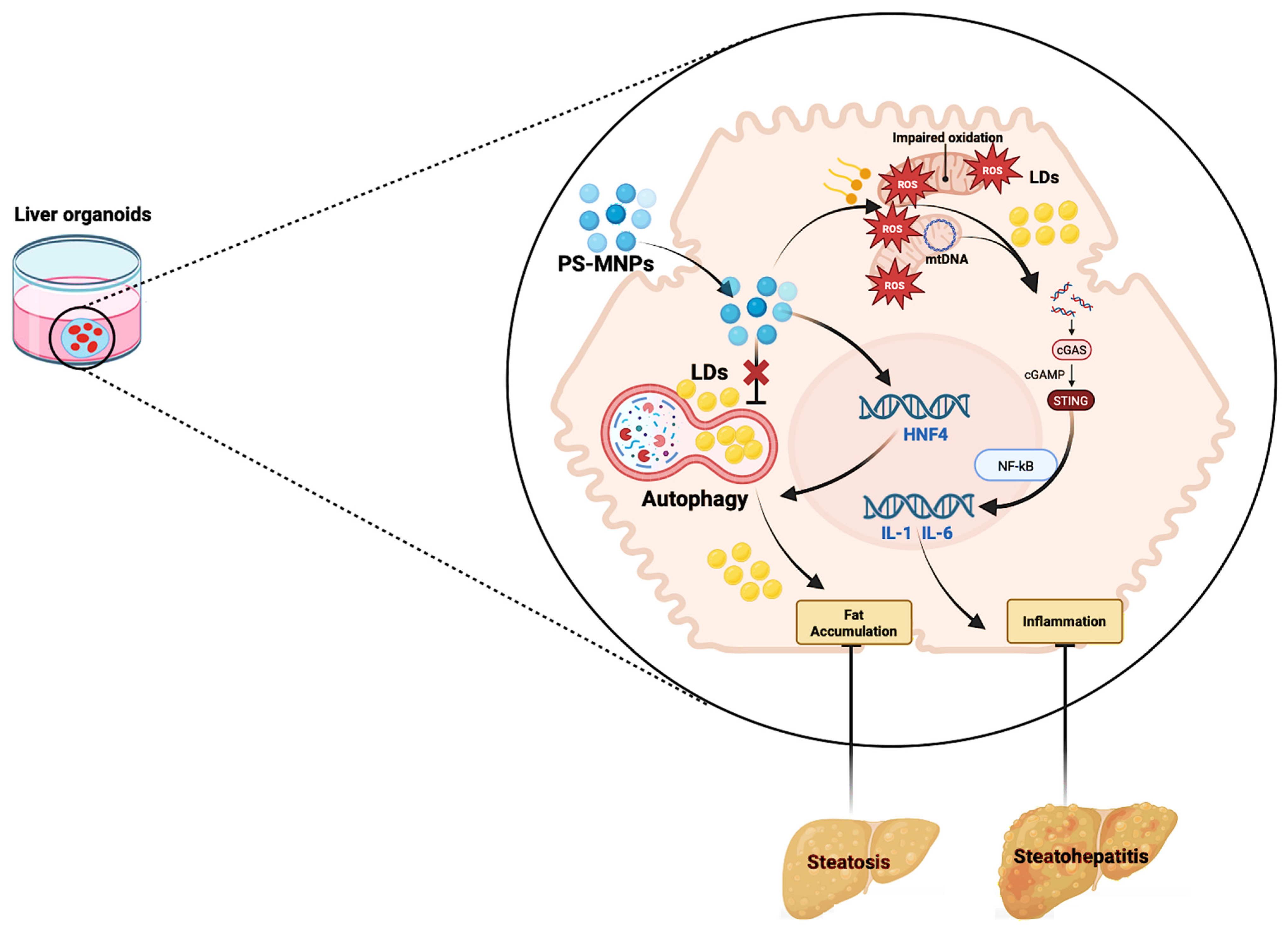
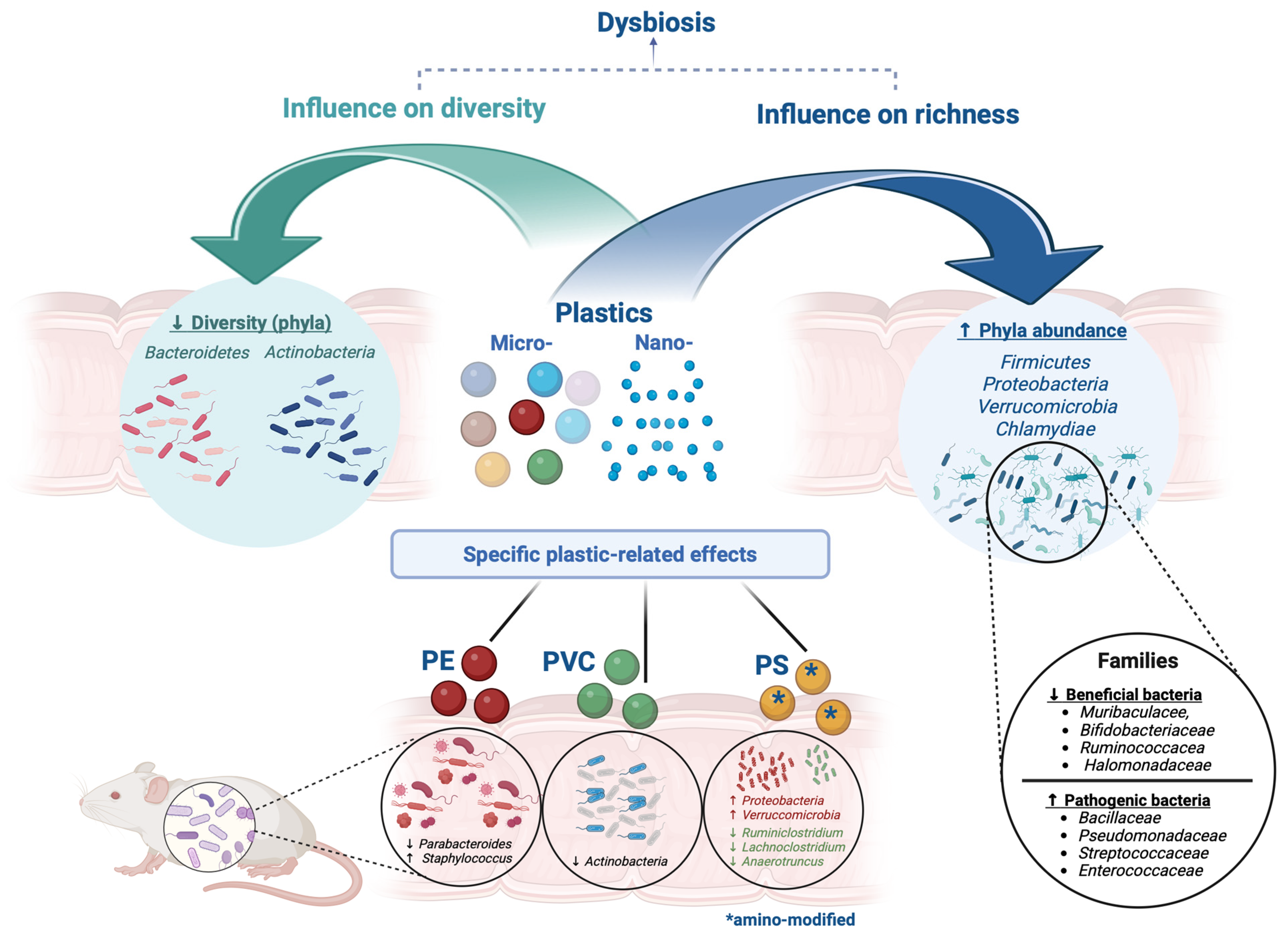
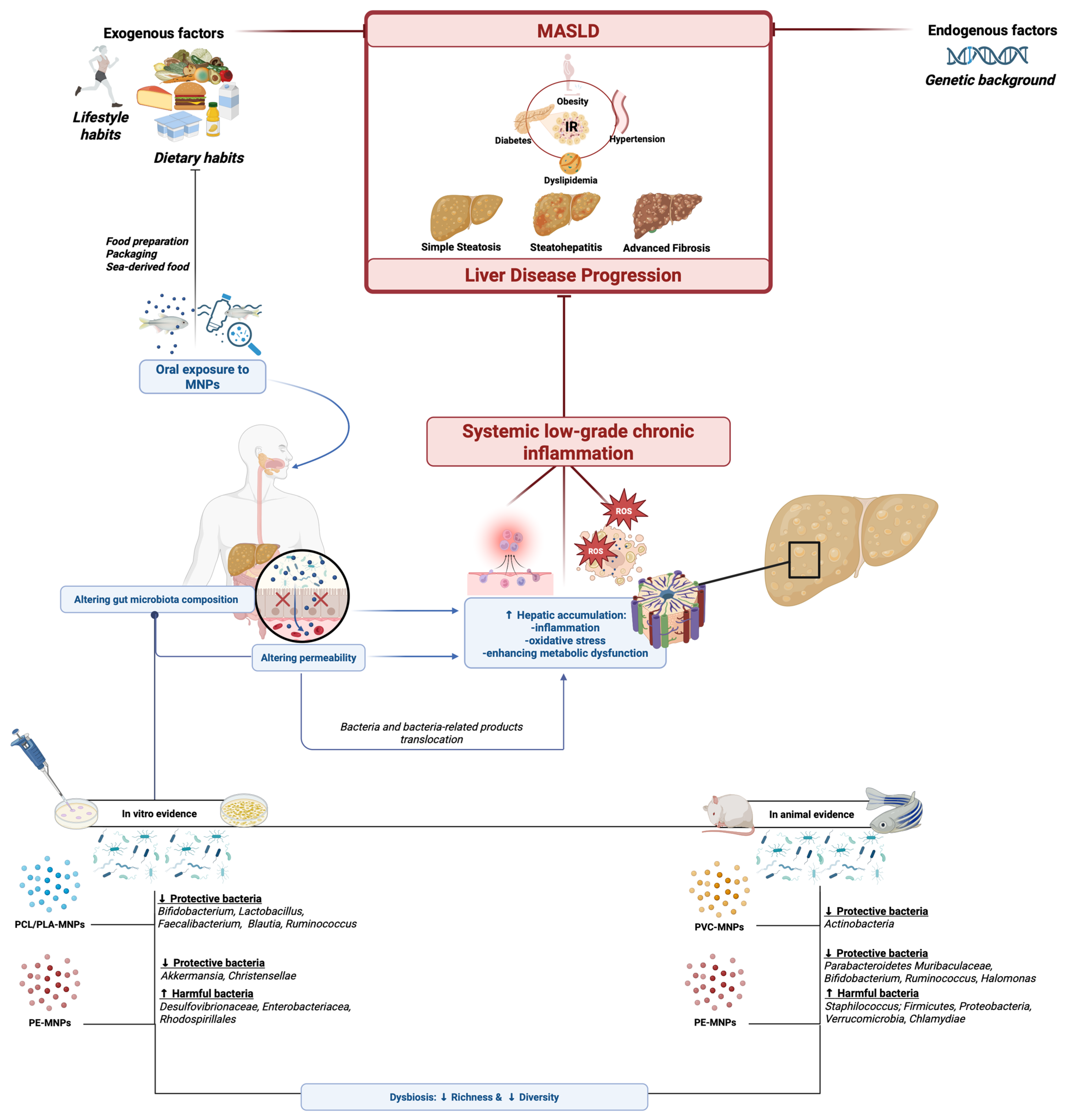
| Type of MNPs/Additive | Toxic Effects | Evidence | Pathogenetic Mechanisms/Targets | Reference |
|---|---|---|---|---|
| PS-MPs (0.1 μm–5 μm) | Cellular damage/ Oxidative stress | In vitro (Caco-2 cells) | Increased ROS production Impaired membrane integrity | [24] |
| PS-MPs (>5 μm) | Cellular damage/ Oxidative stress | In vitro (Caco-2 cells) | Mitochondrial depolarization Impaired ATP synthesis | [24] |
| PS-MPs | Inflammation/ Oxidative stress | In vitro (HRT-18 CMT-93) | Upregulated IL-8 production Altered SOD activity | [26] |
| PNP-NPs | Oxidative stress | In vitro (Hs27 cells) | Increased ROS production | [25] |
| BPA | Oxidative stress | In vitro and in human | Inhibition of CYP450 Altered SOD expression Reduction of GSH levels Increased ROS production | [30] |
| BPA | Inflammation/ Activation of endocrine pathways | In vitro and in human | “Ligand mimicking” (AR, ERα/β GPR30) MAPK/PI3K pathways activation TNF-alpha, IL-1, IL-6 production | [28] |
| Type of MNP | Principal Effects on Gut Microbiota Composition | In Vitro Model | Reference |
|---|---|---|---|
| PCL-MPs PLA-MPs | ↓ alpha diversity ↓ Protective bacteria abundance (Lactobacillus, Faecalibacterium, Blautia, Ruminococcus) | Stimulated digestion and fermentation models | [74] |
| PE-MPs (overall) | ↓ Protective families (Christensenellaceae, Akkermansiaceae) ↑ Harmful families (Desulfovibrionaceae, Enterobacteriaceae) | Mucosal Artificial Colon (M-ARCOL) | [75] |
| PE-MPs (microspheres) | ↓ Protective bacteria abundance (Lactobacillus) | Mucosal Artificial Colon (M-ARCOL) | [75] |
| PE (mixture) | ↑ Harmful bacteria abundance (Rhodospirillales) | Mucosal Artificial Colon (M-ARCOL) | [75] |
| Type of MNP/Additive | Main Role in SLD Pathogenesis | Level of Evidence | Most Common Sources | References |
|---|---|---|---|---|
| Bisphenol A (BPA) | Metabolic and immune dysfunction (TI response inducer) | Mouse, human | Food and beverage containers, receipts and tickets, water, toys, etc. | [125] |
| Bisphenol-S (BPS) | Metabolic dysfunction | Human | Food and beverage containers, receipts and tickets, water, etc. | [126] |
| Polycaprolactone (PCL), Polylactic acid (PLA) | Modulation of the gut microbiota | Human | Orthopedic fixation, sutures, tissue engineering scaffolds, food packaging, and compost bags. | [74] |
| Polyethylene (PE), Polystyrene (PS) | Modulation of the gut microbiota, immune dysfunction | Human, mouse, fish | Plastic bags, plastic bottles, food packaging, toys, and household items. | [1,75,77,78,127] |
| Polyvinyl chloride (PVC) | Modulation of the gut microbiota | Human, mouse | Plastic bottles, shrink wrap and packaging films, toys, clothing, etc. | [73,86] |
| Polychlorinated biphenyl (PCB) | Immune dysfunction, damages to intestinal mucosa. | Human | Old electrical equipment, building materials, and environment. | [1,68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeo, M.; Dallio, M.; Di Nardo, F.; Martinelli, G.; Basile, C.; Silvestrin, A.; Senese, G.; Coppola, A.; Napolitano, C.; Amoresano, A.; et al. Exploring the Classic and Novel Pathogenetic Insights of Plastic Exposure in the Genesis and Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Livers 2025, 5, 21. https://doi.org/10.3390/livers5020021
Romeo M, Dallio M, Di Nardo F, Martinelli G, Basile C, Silvestrin A, Senese G, Coppola A, Napolitano C, Amoresano A, et al. Exploring the Classic and Novel Pathogenetic Insights of Plastic Exposure in the Genesis and Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Livers. 2025; 5(2):21. https://doi.org/10.3390/livers5020021
Chicago/Turabian StyleRomeo, Mario, Marcello Dallio, Fiammetta Di Nardo, Giuseppina Martinelli, Claudio Basile, Alessia Silvestrin, Giusy Senese, Annachiara Coppola, Carmine Napolitano, Angela Amoresano, and et al. 2025. "Exploring the Classic and Novel Pathogenetic Insights of Plastic Exposure in the Genesis and Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)" Livers 5, no. 2: 21. https://doi.org/10.3390/livers5020021
APA StyleRomeo, M., Dallio, M., Di Nardo, F., Martinelli, G., Basile, C., Silvestrin, A., Senese, G., Coppola, A., Napolitano, C., Amoresano, A., Altucci, C., & Federico, A. (2025). Exploring the Classic and Novel Pathogenetic Insights of Plastic Exposure in the Genesis and Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Livers, 5(2), 21. https://doi.org/10.3390/livers5020021







