Comparative Analysis of the Human Proteome Profile in Visceral Adipose and Liver Tissue in Individuals with Obesity with and Without MASLD and MASH
Abstract
:1. Introduction
2. Materials and Methods
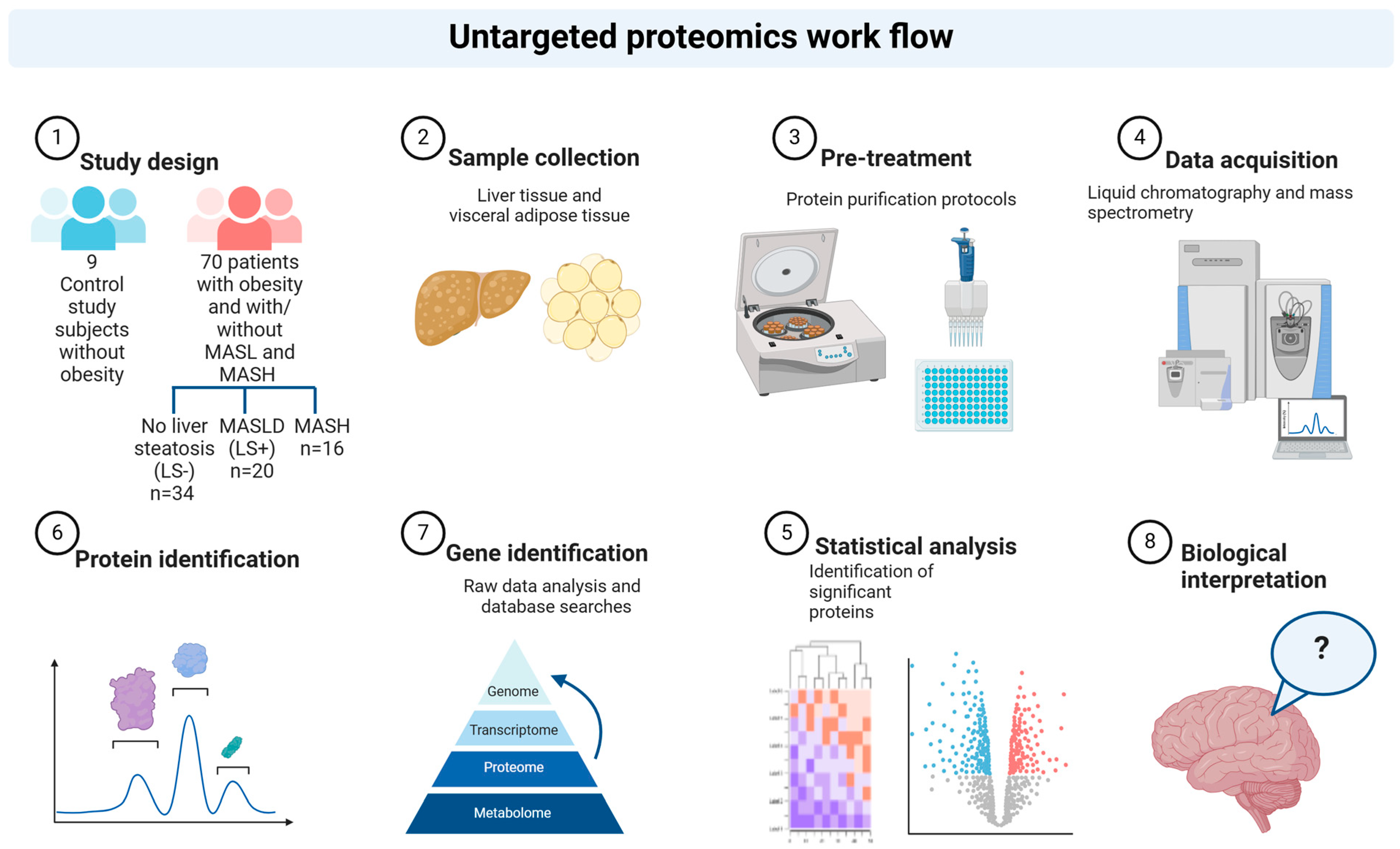
2.1. Cohort and Study Investigations
2.2. Liver Histology and Grouping of Study Subjects
- (1)
- No liver steatosis (LS−): no liver steatosis present in liver biopsies;
- (2)
- Liver steatosis present (LS+): liver steatosis present but without MASH (NAS score < 5);
- (3)
- MASH: NAS ≥ 5 with points from all subcategories (steatosis, inflammation, and ballooning).
2.3. Sample Preparation for MS Analysis
2.4. LC-MS/MS Analysis (Liquid Chromatography-Mass Spectroscopy/Mass Spectrometry Analysis)
2.5. Quantification and Statistical Analysis: Raw Data Processing and Analysis
3. Results
3.1. Liver Tissue
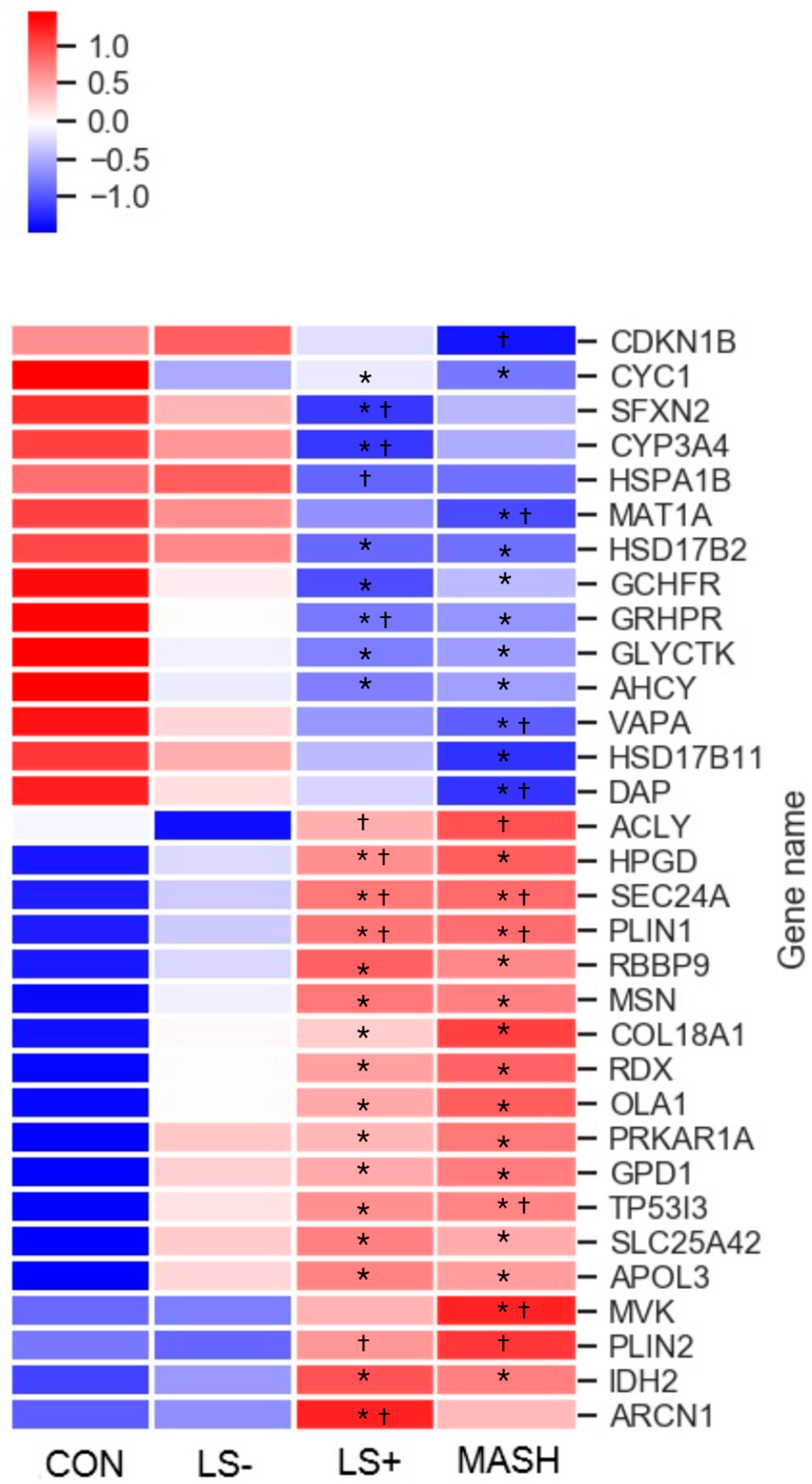
3.2. Differentially Expressed Upregulated Liver Proteins (Table 2 and Figure 2)
3.3. Differentially Expressed Downregulated Liver Proteins (Table 2 and Figure 2)
3.4. Visceral Adipose Tissue
3.5. Differentially Expressed Upregulated VAT Proteins (Table 3 and Figure 3)
3.6. Differentially Expressed Downregulated VAT Proteins (Table 3 and Figure 3)
3.7. Associations Between VAT and Liver Tissue in MASLD: Correlation Data (Table 4)
4. Discussion
4.1. No Overlapping DEPs in VAT and Liver Tissue in Subjects with Obesity, MASLD, and MASH
4.2. Changes in the VAT Proteome in Obesity, T2DM, and MASLD
4.3. Correlation Analyses Pinpoint Inflammatory and Detoxification Proteins
4.4. Changes in the Liver Proteome in Relation to Obesity and MASLD
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosen, E.D.; Spiegelman, B.M. What We Talk About When We Talk About Fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef]
- Ohlson, L.-O.; Larsson, B.; Svärdsudd, K.; Welin, L.; Eriksson, H.; Wilhelmsen, L.; Björntorp, P.; Tibblin, G. The Influence of Body Fat Distribution on the Incidence of Diabetes Mellitus: 13.5 Years of Follow-up of the Participants in the Study of Men Born in 1913. Diabetes 1985, 34, 1055–1058. [Google Scholar] [CrossRef]
- Nielsen, S.; Guo, Z.; Johnson, C.M.; Hensrud, D.D.; Jensen, M.D. Splanchnic lipolysis in human obesity. J. Clin. Investig. 2004, 113, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Eagon, J.C.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Visceral Fat Adipokine Secretion Is Associated with Systemic Inflammation in Obese Humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Vanni, E.; Bugianesi, E.; Kotronen, A.; De Minicis, S.; Yki-Järvinen, H.; Svegliati-Baroni, G. From the metabolic syndrome to NAFLD or vice versa? Dig. Liver Dis. 2010, 42, 320–330. [Google Scholar] [CrossRef]
- Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef]
- Goossens, G.H. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol. Behav. 2008, 94, 206–218. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Blissett, D.; Blissett, R.; Henry, L.; Stepanova, M.; Younossi, Y.; Racila, A.; Hunt, S.; Beckerman, R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016, 64, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Item, F.; Konrad, D. Visceral fat and metabolic inflammation: The portal theory revisited: Visceral fat and metabolic inflammation. Obes. Rev. 2012, 13, 30–39. [Google Scholar] [CrossRef]
- Arderiu, G.; Mendieta, G.; Gallinat, A.; Lambert, C.; Díez-Caballero, A.; Ballesta, C.; Badimon, L. Type 2 Diabetes in Obesity: A Systems Biology Study on Serum and Adipose Tissue Proteomic Profiles. Int. J. Mol. Sci. 2023, 24, 827. [Google Scholar] [CrossRef]
- Chen, Z.-Z.; Gao, Y.; Keyes, M.J.; Deng, S.; Mi, M.; Farrell, L.A.; Shen, D.; Tahir, U.A.; Cruz, D.E.; Ngo, D.; et al. Protein Markers of Diabetes Discovered in an African American Cohort. Diabetes 2023, 72, 532–543. [Google Scholar] [CrossRef]
- Niu, L.; Geyer, P.E.; Wewer Albrechtsen, N.J.; Gluud, L.L.; Santos, A.; Doll, S.; Treit, P.V.; Holst, J.J.; Knop, F.K.; Vilsbøll, T.; et al. Plasma proteome profiling discovers novel proteins associated with non-alcoholic fatty liver disease. Mol. Syst. Biol. 2019, 15, e8793. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Williams, S.A.; Lavine, J.E.; Neuschwander-Tetri, B.A.; Alexander, L.; Ostroff, R.; Biegel, H.; Kowdley, K.V.; Chalasani, N.; Dasarathy, S.; et al. Defining the serum proteomic signature of hepatic steatosis, inflammation, ballooning and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2023, 78, 693–703. [Google Scholar] [CrossRef]
- Bell, L.N.; Theodorakis, J.L.; Vuppalanchi, R.; Saxena, R.; Bemis, K.G.; Wang, M.; Chalasani, N. Serum proteomics and biomarker discovery across the spectrum of nonalcoholic fatty liver disease. Hepatology 2010, 51, 111–120. [Google Scholar] [CrossRef]
- Govaere, O.; Hasoon, M.; Alexander, L.; Cockell, S.; Tiniakos, D.; Ekstedt, M.; Schattenberg, J.M.; Boursier, J.; Bugianesi, E.; Ratziu, V.; et al. A proteo-transcriptomic map of non-alcoholic fatty liver disease signatures. Nat. Metab. 2023, 5, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wadhawan, S.; Greenfield, A.; Decato, B.E.; Oseini, A.M.; Collen, R.; Shevell, D.E.; Thompson, J.; Jarai, G.; Charles, E.D.; et al. SOMAscan Proteomics Identifies Serum Biomarkers Associated with Liver Fibrosis in Patients With NASH. Hepatol. Commun. 2021, 5, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Sun, Y.; Cheng, Q.; Hu, K.; Ye, J.; Zhao, Y.; Wu, J.; Shao, X.; Fang, L.; Ding, Y.; et al. Proteomic analysis to identify differentially expressed proteins between subjects with metabolic healthy obesity and non-alcoholic fatty liver disease. J. Proteomics 2020, 221, 103683. [Google Scholar] [CrossRef]
- Boel, F.; Akimov, V.; Teuchler, M.; Terkelsen, M.K.; Wernberg, C.W.; Larsen, F.T.; Hallenborg, P.; Lauridsen, M.M.; Krag, A.; Mandrup, S.; et al. Deep proteome profiling of metabolic dysfunction-associated steatotic liver disease. Commun. Med. 2025, 5, 56. [Google Scholar] [CrossRef]
- Kakehashi, A.; Stefanov, V.E.; Ishii, N.; Okuno, T.; Fujii, H.; Kawai, K.; Kawada, N.; Wanibuchi, H. Proteome Characteristics of Non-Alcoholic Steatohepatitis Liver Tissue and Associated Hepatocellular Carcinomas. Int. J. Mol. Sci. 2017, 18, 434. [Google Scholar] [CrossRef]
- Nakamura, N.; Hatano, E.; Iguchi, K.; Sato, M.; Kawaguchi, H.; Ohtsu, I.; Sakurai, T.; Aizawa, N.; Iijima, H.; Nishiguchi, S.; et al. Elevated levels of circulating ITIH4 are associated with hepatocellular carcinoma with nonalcoholic fatty liver disease: From pig model to human study. BMC Cancer 2019, 19, 621. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Karrar, A.; Pierobon, M.; Birerdinc, A.; Stepanova, M.; Abdelatif, D.; Younoszai, Z.; Jeffers, T.; Felix, S.; Jeiran, K.; et al. An exploratory study examining how nano-liquid chromatography–mass spectrometry and phosphoproteomics can differentiate patients with advanced fibrosis and higher percentage collagen in non-alcoholic fatty liver disease. BMC Med. 2018, 16, 170. [Google Scholar] [CrossRef]
- Wichmann, C.; Meier, F.; Virreira Winter, S.; Brunner, A.-D.; Cox, J.; Mann, M. MaxQuant.Live Enables Global Targeting of More Than 25,000 Peptides. Mol. Cell. Proteomics MCP 2019, 18, 982–994. [Google Scholar] [CrossRef]
- Grinfeld, D.; Aizikov, K.; Kreutzmann, A.; Damoc, E.; Makarov, A. Phase-Constrained Spectrum Deconvolution for Fourier Transform Mass Spectrometry. Anal. Chem. 2017, 89, 1202–1211. [Google Scholar] [CrossRef]
- Greenberg, A.S.; Egan, J.J.; Wek, S.A.; Garty, N.B.; Blanchette-Mackie, E.J.; Londos, C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J. Biol. Chem. 1991, 266, 11341–11346. [Google Scholar] [CrossRef]
- Karvar, S.; Ansa-Addo, E.A.; Suda, J.; Singh, S.; Zhu, L.; Li, Z.; Rockey, D.C. Moesin, an Ezrin/Radixin/Moesin Family Member, Regulates Hepatic Fibrosis. Hepatology 2019, 72, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xiao, H.; Zhou, F.; Hu, Z.; Yang, B. Study of HSPB6: Insights into the Properties of the Multifunctional Protective Agent. Cell. Physiol. Biochem. 2017, 44, 314–332. [Google Scholar] [CrossRef]
- Jackson, M.R.; Melideo, S.L.; Jorns, M.S. Human sulfide: Quinone oxidoreductase catalyzes the first step in hydrogen sulfide metabolism and produces a sulfane sulfur metabolite. Biochemistry 2012, 51, 6804–6815. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. H2S: A novel gasotransmitter that signals by sulfhydration. Trends Biochem. Sci. 2015, 40, 687. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Castañé, H.; Jiménez-Franco, A.; Hernández-Aguilera, A.; Martínez-Navidad, C.; Cambra-Cortés, V.; Onoiu, A.-I.; Jiménez-Aguilar, J.M.; París, M.; Hernández, M.; Parada, D.; et al. Multi-omics profiling reveals altered mitochondrial metabolism in adipose tissue from patients with metabolic dysfunction-associated steatohepatitis. eBioMedicine 2025, 111, 105532. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Kojima, K.; Zhou, L.; Crossman, D.K.; Mobley, J.A.; Grams, J. Analysis of the Human Proteome in Subcutaneous and Visceral Fat Depots in Diabetic and Non-diabetic Patients with Morbid Obesity. J. Proteomics Bioinform. 2015, 8, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Chae, S.; Kim, H.; Mun, D.-G.; Back, S.; Choi, H.Y.; Park, K.S.; Hwang, D.; Choi, S.H.; Lee, S.-W. A Protein Profile of Visceral Adipose Tissues Linked to Early Pathogenesis of Type 2 Diabetes Mellitus. Mol. Cell. Proteomics MCP 2014, 13, 811–822. [Google Scholar] [CrossRef]
- Gómez-Serrano, M.; Camafeita, E.; García-Santos, E.; López, J.A.; Rubio, M.A.; Sánchez-Pernaute, A.; Torres, A.; Vázquez, J.; Peral, B. Proteome-wide alterations on adipose tissue from obese patients as age-, diabetes- and gender-specific hallmarks. Sci. Rep. 2016, 6, 25756. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Masood, A.; Al-Naami, M.Y.; Chaurand, P.; Benabdelkamel, H. A Proteomics Based Approach Reveals Differential Regulation of Visceral Adipose Tissue Proteins between Metabolically Healthy and Unhealthy Obese Patients. Mol. Cells 2017, 40, 685–695. [Google Scholar] [CrossRef]
- Insenser, M.; Montes-Nieto, R.; Vilarrasa, N.; Lecube, A.; Simó, R.; Vendrell, J.; Escobar-Morreale, H.F. A nontargeted proteomic approach to the study of visceral and subcutaneous adipose tissue in human obesity. Mol. Cell. Endocrinol. 2012, 363, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Murri, M.; Insenser, M.; Bernal-Lopez, M.R.; Perez-Martinez, P.; Escobar-Morreale, H.F.; Tinahones, F.J. Proteomic analysis of visceral adipose tissue in pre-obese patients with type 2 diabetes. Mol. Cell. Endocrinol. 2013, 376, 99–106. [Google Scholar] [CrossRef]
- Shang, C.; Sun, W.; Wang, C.; Wang, X.; Zhu, H.; Wang, L.; Yang, H.; Wang, X.; Gong, F.; Pan, H. Comparative Proteomic Analysis of Visceral Adipose Tissue in Morbidly Obese and Normal Weight Chinese Women. Int. J. Endocrinol. 2019, 2019, 2302753. [Google Scholar] [CrossRef] [PubMed]
- Tansey, J.T.; Sztalryd, C.; Gruia-Gray, J.; Roush, D.L.; Zee, J.V.; Gavrilova, O.; Reitman, M.L.; Deng, C.X.; Li, C.; Kimmel, A.R.; et al. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc. Natl. Acad. Sci. USA 2001, 98, 6494–6499. [Google Scholar] [CrossRef]
- Orlicky, D.J.; Libby, A.E.; Bales, E.S.; McMahan, R.H.; Monks, J.; Rosa, F.G.; McManaman, J.L. Perilipin-2 promotes obesity and progressive fatty liver disease in mice through mechanistically distinct hepatocyte and extra-hepatocyte actions. J. Physiol. 2019, 597, 1565–1584. [Google Scholar] [CrossRef]
- Straub, B.K.; Stoeffel, P.; Heid, H.; Zimbelmann, R.; Schirmacher, P. Differential pattern of lipid droplet-associated proteins and de novo perilipin expression in hepatocyte steatogenesis. Hepatology 2008, 47, 1936–1946. [Google Scholar] [CrossRef]
- Schöttl, T.; Pachl, F.; Giesbertz, P.; Daniel, H.; Kuster, B.; Fromme, T.; Klingenspor, M. Proteomic and Metabolite Profiling Reveals Profound Structural and Metabolic Reorganization of Adipocyte Mitochondria in Obesity. Obesity 2020, 28, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef]
- She, P.; Van Horn, C.; Reid, T.; Hutson, S.M.; Cooney, R.N.; Lynch, C.J. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched chain amino acid (BCAA) metabolism. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1552–E1563. [Google Scholar] [CrossRef]
- Rochette, J.; Craig, J.E.; Thein, S.L.; Rochette, J. Fetal hemoglobin levels in adults. Blood Rev. 1994, 8, 213–224. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Transport models for secretory IgA and secretory IgM. Clin. Exp. Immunol. 1981, 44, 221–232. [Google Scholar] [PubMed]
- De Marco, M.C.; Martín-Belmonte, F.; Kremer, L.; Albar, J.P.; Correas, I.; Vaerman, J.P.; Marazuela, M.; Byrne, J.A.; Alonso, M.A. MAL2, a novel raft protein of the MAL family, is an essential component of the machinery for transcytosis in hepatoma HepG2 cells. J. Cell Biol. 2002, 159, 37–44. [Google Scholar] [CrossRef] [PubMed]
- De Marco, M.C.; Puertollano, R.; Martínez-Menárguez, J.A.; Alonso, M.A. Dynamics of MAL2 during glycosylphosphatidylinositol-anchored protein transcytotic transport to the apical surface of hepatoma HepG2 cells. Traffic Cph. Den. 2006, 7, 61–73. [Google Scholar] [CrossRef] [PubMed]

| Study Subjects with Obesity According to Histology | Subjects Without Obesity | |||
|---|---|---|---|---|
| LS− (n = 34) | LS+ (n = 20) | MASH (n = 16) | CON (n = 9) | |
| Liver histology | ||||
| MASLD activity score (NAS) | 2.3 (0.9) | 3.2 (0.7) | 5.1 (0.3) | 1.1 (0.9) |
| Steatosis | 0.0 (0.0) | 1.1 (0.3) | 1.7 (0.6) | 0.0 (0.0) |
| Inflammation | 1.1 (0.6) | 0.9 (0.3) | 1.6 (0.5) | 0.9 (0.6) |
| Ballooning | 1.2 (0.5) | 1.2 (0.5) | 1.9 (0.3) | 0.2 (0.4) |
| Fibrosis | 1.0 (0.3) | 1.2 (0.4) | 1.1 (0.5) | 0.9 (0.3) |
| Age, years | 45 (11) | 45 (8) | 45 (9) | 39 (8) |
| Female (%) | 25 (58) | 9 (21) | 9 (21) | 7 (78) |
| Diabetes, n (%) | 3 (16) | 7 (37) | 9 (47) | NA |
| Weight, kg | 124 (20) * | 138 (29) * | 125 (17) * | 71 (10) |
| BMI, kg/m2 | 41.8 (5.1) * | 44.6 (8.4) * | 42.4 (5.4) * | 24.4 (2.2) |
| Waist-hip ratio | 0.88 (0.11) | 0.95 (0.13) | 0.98 (0.08) *,† | 0.83 (0.10) |
| Systolic blood pressure, mmHg | 126 (12) | 130 (14) | 131 (16) | 117 (11) |
| Diastolic blood pressure, mmHg | 81 (8) | 82 (9) | 82 (11) | 77 (9) |
| Heart rate (BPM) | 72 (12) | 76 (13) | 76 (14) | 69 (7) |
| ALT, U/L | 28 (10) | 34 (15) | 39 (15) *,† | 21 (9) |
| AST, U/L | 24 (6) | 25 (9) | 27 (7) | 21 (4) |
| Fasting plasma glucose, mmol/L | 5.9 (0.7) | 6.8 (1.5) | 7.0 (2.2) | 5.5 (0.4) |
| C-peptide pmol/L | 1162 (343) * | 1217 (257) * | 1649 (523) *,†,§ | 791 (204) |
| Fasting insulin pmol/L | 118.8 (47.3) | 122.78 (44.6) | 208.6 (88.9) *,†,§ | 63.3 (30.4) |
| HbA1c | 35 (3) | 41 (8) *,† | 37 (4) * | 32 (3) |
| HOMA-IR | 4.4 (1.8) | 5.2 (1.7) | 9.1 (4.2) *,†,§ | 2.2 (1.1) |
| LDL cholesterol, mmol/L | 2.79 (0.98) | 2.25 (0.56) | 2.02 (0.61) | 2.59 (0.53) |
| HDL cholesterol, mmol/L | 1.22 (0.27) | 1.20 (0.41) | 1.09 (0.15) | 1.39 (0.23) |
| VLDL cholesterol, mmol/L | 0.58 (0.24) | 0.69 (0.43) | 0.75 (0.33) | 0.50 (0.21) |
| Triglycerides, mmol/L | 1.29 (0.52) | 1.53 (0.95) | 1.66 (0.74) | 1.13 (0.50) |
| HsCRP (mg/L) | 5.2 (4.1) | 9.2 (10.7) * | 5.1 (3.7) | 1.5 (1.3) |
| Protein Name | Gene Name | CON Log2 Intensity | LS− Log2 Intensity | LS+ Log2 Intensity | MASH Log2 Intensity | Location in Cell | Tissue Specificity | Main Function(s) |
|---|---|---|---|---|---|---|---|---|
| Upregulated liver proteins in SWO/MASLD groups | ||||||||
| Metabolism | ||||||||
| Isocitrate dehydrogenase 2 | IDH2 | 22.57 | 22.81 | 22.97 * | 23.03 * | M, C, PX | Low | TCA |
| Glycerol-3-phosphate dehydrogenase 1 | GPD1 | 22.12 | 22.71 * | 22.86 * | 22.84 * | C | Low | CaM, LiM, LiB |
| OBG like ATPase 1 | OLA1 | 18.86 | 19.20 * | 19.33 * | 19.35 * | C | Low | CP, ATPH, GTPH |
| ATP citrate lyase | ACLY | 18.40 | 18.06 | 18.76 † | 18.72 † | C | Low | ACoA, LiM, LiB |
| Mevalonate kinase | MVK | 17.99 | 18.11 | 18.79 | 18.92 *,† | C, PX | Low | ChoB, ChoM, LiM, LiB, SterB, SterM |
| 15-hydroxyprostaglandin dehydrogenase | HPGD | 18.07 | 18.77 | 19.37 *,† | 19.32 * | C, N | Placenta, liver, GI tract | FAM, LiM, PGM |
| Perilipin 1 | PLIN1 | 14.24 | 14.99 | 16.09 *,† | 16.25 *,† | Adipose tissue | LiMM, LiBi | |
| Perilipin 2 | PLIN2 | 15.09 | 15.08 | 16.87 † | 17.09 † | Adipose tissue | LiMM, LiBi | |
| Transport and carriers | ||||||||
| Apolipoprotein 3 | APOL3 | 15.03 | 16.21 * | 16.51 * | 16.64 * | C | Low | LiT |
| Solute carrier family 25 member 42 | SLC25A42 | 15.03 | 16.31 * | 16.51 * | 16.57 * | M | Liver | MTC, MTT, ADPT, ATPT, AMPT, ACoAT |
| Archain 1 | ARCN1 | 18.23 | 18.26 | 18.38 *,† | 18.31 | V, ER | Low | ERGT, PT |
| Protein transport protein Sec24A | SEC24A | 16.65 | 16.81 | 17.00 *,† | 17.01 *,† | V | Low | ERGT, PT |
| Cytoskeleton, ECM and signal transduction | ||||||||
| Collagen type XVIII alpha 1 chain | COL18A1 | 18.62 | 19.11 * | 19.23 * | 19.40 * | ECM | Liver | EMO |
| Radixin | RDX | 20.70 | 20.93 * | 20.98 * | 21.04 * | PM | Adrenal gland | CO, CA, ST |
| Moesin | MSN | 20.38 | 20.96 * | 20.93 * | 20.76 * | PM | Low | CO, CA, ST |
| Protein kinase cAMP-dependent type I regulatory subunit alpha | PRKAR1A | 18.37 | 18.61 * | 18.64 * | 18.67 * | C | Low | cAMP, ST |
| Cell, quality, cell cycle, and apoptosis | ||||||||
| Tumor protein p53 inducible protein 3 | TP53I3 | 13.96 | 15.13 * | 15.84 * | 16.03 *,† | C | Intestine | Apop(+), SR |
| Retinoblastoma binding protein 9 | RBBP9 | 18.01 | 18.17 | 18.31 * | 18.28 * | N | Low | SH, TS, CCR |
| Downregulated liver proteins in SWO/MASLD groups | ||||||||
| Metabolism | ||||||||
| Glycerate kinase | GLYCKTK | 19.80 | 19.36 * | 19.09 * | 19.15 * | C, G | Liver | FC, SD |
| Glyoxylate hydroxypyrovate dehydrogenase | GRHPR | 24.04 | 23.82 | 23.59 *,† | 23.69 * | C, N | Liver | GlyoxM, PyroM |
| GTP cyclohydrolase I feedback regulator | GCHFR | 20.09 | 19.70 * | 19.51 * | 19.55 * | C, N | Liver | RP |
| Methionine adenosyltransferase 1A | MAT1A | 21.67 | 21.57 | 21.38 | 21.34 *,† | C | Liver | 1CM, MT, MetC |
| Adenosylhomocysteinase | ACHY | 22.53 | 22.22 * | 22.08 * | 22.13 * | C | Low | 1CM, MT |
| Hydroxysteroid 17-beta dehydrogenase 2 | HSD17B2 | 19.79 | 19.32 | 19.00 * | 18.94 * | ER | Liver, intestine, placenta | LiB, LiM, SteB |
| Hydroxysteroid 17-beta dehydrogenase 11 | HSD17B11 | 19.51 | 19.33 | 19.98 | 18.82 * | ER, LD | Immune cells, intestine | LiB, LiM, SterB, AndC, EstB |
| Cytochrome P450 family 3 subfamily A member 4 | CYP3A4 | 21.85 | 21.71 | 21.01 *,† | 21.19 | C, ER | Liver | FAM, CholM, LiM, LiB, SterB, SterM, DrugM |
| Mitochondrial | ||||||||
| Sideroflexin 2 | SFXN2 | 17.88 | 17.36 | 17.14 *,† | 17.65 | M | low | MTTT, AAT |
| Cytochrome b-c1 complex (CIII) | CYC1 | 19.74 | 19.35 * | 19.35 * | 19.23 * | M | low | ET, ATPS, RCP |
| Intracellular transport and carriers | ||||||||
| VAMP associated protein A | VAPA | 19.30 | 19.25 | 19.14 | 19.06 *,† | ER, N, PM | Low | ERGT, PT, MF |
| Cell quality, cell cycle, apoptosis | ||||||||
| Death associated protein | DAP | 17.97 | 17.65 | 17.44 | 17.24 *,† | V, M, N | Pancreas | Autop(−), Apop(−), NFkaBTF(−) |
| Heat shock protein family A (Hsp70) member 1B | HSPA1B | 20.93 | 20.97 | 20.65 † | 20.78 | V, C, N | Vagina | CHA, PF, SR |
| Cyclin-dependent kinase inhibitor | CDKN1B | 14.78 | 14.83 | 14.40 | 13.49† | N, V | Low | CCP |
| Protein Name | Gene Name | CON Log2 Intensity | LS− Log2 Intensity | Ls+ Log2 Intensity | Mash Log2 Intensity | Location in Cell | Tissue Specificity | Main Function(s) |
|---|---|---|---|---|---|---|---|---|
| Upregulated VAT proteins in MASLD | ||||||||
| Metabolism | ||||||||
| L-lactate dehydrogenase B chain | LDHB | 16.53 | 17.96 * | 18.51 * | 18.40 * | C | Heart, kidney | CM, PyrvM, Ferm |
| Inorganic pyrophosphatase | PPA1 | 13.47 | 15.30 * | 15.28 * | 15.68 * | C | Low | PhosM |
| Lipid metabolism | ||||||||
| Sulfotransferase 1A1 | SULT1A1 | 13.36 | 14.96 * | 14.50 | 15.36 * | C | Liver | CateM, LiM, SterM, XM |
| Fatty aldehyde dehydrogenase | ALDH3A2 | 12.41 | 14.19 * | 14.09 * | 14.37 * | PXM, ERM | Low | FAM, LiM, SB |
| Aldo-keto reductase family 1 member C1 | AKR1C1 | 16.83 | 18.12 * | 18.28 * | 18.47 * | C | Liver | PM, SterM |
| 3-oxo-5-beta-steroid 4-dehydrogenase | AKR1D1 | 14.86 | 16.97 * | 17.16 * | 17.85 * | C | Liver | AndM, BAB, ChoC, SterM |
| Aldo-keto reductase family 1 member B | AKR1B1 | 13.24 | 14.44 | 14.42 * | 14.45 * | C | Adrenal gland | CM(PP), SteM, PGM, SIG |
| Aldo-keto reductase family 1 member C3 | AKR1C3 | 15.07 | 16.06 * | 16.51 * | 16.53 * | C | Liver | M, SteM, ProglM, AndM, CellD(+), Apop(+) ROSM(+) |
| Liver carboxylesterase 1 | CES1 | 17.31 | 18.45 * | 18.95 * | 18.94 * | ER | Liver | FAM, XM, ChoB |
| Epoxide hydrolase 1 | EPHX1 | 15.91 | 17.91 * | 17.95 * | 17.78 * | ER | Liver, adrenal gland | AHC, DtX |
| Alkylglycerone phosphate synthase | AGPS | 7.80 | 10.68 * | 10.68 * | 10.16 * | PX | Low | LiB, LiM |
| Mitochondrial | ||||||||
| Mitochondrial carnitine/acylcarnitine carrier protein | SLC25A20 | 11.57 | 13.17 | 13.02 * | 13.36 * | M | Low | CarnS |
| Sulfide:quinone oxidoreductase, mitochondrial | SQOR | 13.72 | 12.83 | 12.89 | 13.85 †,§ | M | Low | H2SM |
| Inflammation | ||||||||
| Complement C1q subcomponent subunit C | C1QC | 15.19 | 16.37 * | 16.61 * | 16.26 * | XC (extra cellular) | Lymphoid tissue (monocytes) | ComP, ImR(+) |
| Complement C1r | C1QR | 12.93 | 14.41 * | 14.10 * | 14.00 * | XC | Lymphoid tissue (monocytes) | ComP, ImR(+) |
| Alpha-1-microglobulin | AMBP | 14.86 | 16.41 * | 16.27 * | 16.59 * | G, S, XC | Liver | HVI, ImR(−) |
| Intracellular transport and carriers | ||||||||
| Member RAS oncogene family | RAB5C | 15.49 | 16.45 * | 16.36 * | 16.50 * | EN | Low | PT |
| VPS35 endosomal protein sorting factor like | VPS35 | 14.76 | 15.43 * | 15.43* | 15.69 * | EN, PM | Low | GPMT, PT |
| Coatomer subunit beta’ | COPB2 | 13.56 | 14.27 * | 14.41 * | 14.37 * | C | Low | ERGT, PT |
| ADP-ribosylation factor 3 | ARF3 | 15.05 | 16.11 * | 16.11 * | 16.13 * | ER | Brain | ERGT, PT |
| MAL proteolipid protein 2 | MAL2 | 11.19 | 14.12 * | 14.23 * | 14.42 * | PM | Esophagus | TC (PIGR) |
| Cytoskeleton and ECM | ||||||||
| Keratin, type II cytoskeletal 1 | KRT1 | 14.85 | 16.45 * | 16.06 * | 16.35 * | PM | Skin | CO, ST, ComA |
| Vimentin | VIM | 21.16 | 22.49 * | 22.47 * | 22.49 * | PM, CS, N | Low | CO, HVI |
| Collagen alpha-2 (IV) chain | COL4A2 | 18.08 | 19.55 * | 19.90 * | 19.92 * | ECM | Placenta | EMO |
| Tubulin beta-2A chain | TUBB2A | 14.90 | 15.41 | 15.80 * | 15.87 * | CS | Brain | CO, MCC |
| Lipoma-preferred partner | LPP | |||||||
| Signal transduction and regulation, apoptosis | ||||||||
| Tyrosine 3-monooxygenase | YWHAZ | 18.42 | 19.21 * | 19.28 * | 19.44 * | C, N | low | ST, Apop(−), Angio(+) |
| Phospholysine phosphohistidine inorganic pyrophosphate phosphatase | LHPP | 12.19 | 13.88 * | 13.90 * | 13.71 * | C | Brain | PDP |
| Annexin A5 | ANXA5 | 17.59 | 18.94 * | 19.15 * | 19.13 * | NM | Low | ST, BC(−), Apop(−) |
| Pleckstrin homology-like domain family B member 1 | PHLDB1 | 12.23 | 13.88 * | 13.95 * | 13.86 * | N, MiS | Brain | Reg |
| Dual specificity mitogen-activated protein kinase kinase 1 | MAP2K1 | 11.92 | 13.10 * | 13.29 * | 13.49 * | C, PM | Low | MAPKSC(+), PPARGSC(+) |
| Receptor-type tyrosine-protein phosphatase S | PTPRS | 9.16 | 12.15 * | 11.74 * | 10.86 | C, PM | Low | ST, MAPK(−), ImR(−) |
| Cell cycle, cell quality, and apoptosis | ||||||||
| Atlastin-3 | ATL3 | 12.63 | 14.00 * | 14.24 * | 14.21 * | ER | Low | ERQ |
| Reticulon-4 | RTN4 | 13.33 | 13.90 | 14.17 * | 14.28 * | ER | Low | ERS, Angio(+), Infl(+) |
| Crystallin alpha B | CRYAB | 18.29 | 20.31 * | 20.76 * | 20.62 * | C, PM | Muscle | CHA |
| Heat shock protein beta-6 | HSPB6 | 16.24 | 18.22 * | 18.23 * | 18.35 * | C, G | Muscle | CHA, SR, Angio(+) |
| Heterogeneous nuclear ribonucleoproteins A2/B1 | HNRNPA2B1 | 16.40 | 17.68 * | 17.63 * | 17.69 * | N | Low | mRNAp, mRNAs, mRNAt, HVI, ImR(+) |
| Parkinsonism associated deglycase | PARK7 | 15.51 | 17.17 * | 16.96 * | 17.11 * | C, N | Low | CHA, PRep, PDeglyc, OxSS, MtHom |
| Follistatin-related protein 1 | FSTL1 | 10.56 | 11.50 * | 11.67 * | 11.98 * | C, V | Low | CP, CD |
| Coactosin-like protein | COTL1 | 14.19 | 15.28 * | 15.32 * | 15.25 * | C | Blood, lymphoid tissue | CHA, LeuS |
| Miscellaneous | ||||||||
| Myoglobin | MB | 9.99 | 12.72 * | 12.05 | 13.07 * | C | Muscle | OxT, OxR |
| Cystatin-B | CSTB | 16.15 | 17.57 * | 17.47 * | 17.63 * | C, N | Esophagus, tongue | Pro(−) |
| Unknown function | ||||||||
| FUN14 domain-containing protein 2 | FUNDC2 | 12.99 | 14.22 * | 14.31 * | 14.23 * | N, M | Low | MTautop |
| Downregulated VAT proteins in MASLD | ||||||||
| Mitochondrial metabolism | ||||||||
| 2-oxoisovalerate dehydrogenase subunit alpha, mitochondrial | BCKDHA | 13.00 | 12.43 | 11.32 * | 11.04 * | M | Low | BCAAC |
| Pyruvate dehydrogenase protein X component, mitochondrial | PDHX | 13.88 | 12.32 * | 12.31 * | 11.24 * | M | Low | AcoAB, PyrvM |
| ATP synthase subunit alpha, mitochondrial | ATP5A1 | 18.58 | 17.85 * | 17.89 * | 17:54 * | M | Low | ATPsyn |
| GTP:AMP phosphotransferase AK3, mitochondrial | AK3 | 16.21 | 15.25 * | 15.08 * | 15.51 * | M | Low | NPI |
| Sulfide:quinone oxidoreductase, mitochondrial | SQOR | 13.72 | 12.83 | 12.89 | 13.85 †,§ | M | Low | H2SM |
| Lipid metabolism | ||||||||
| Very-long-chain enoyl-CoA reductase | TECR | 15.21 | 14.34 * | 14.37 * | 14.33 * | ER | Low | FAB, FAE, FAM, LiB, LiM, SteM, SM |
| Enoyl-CoA delta isomerase 1, mitochondrial | ECI1 | 15.00 | 14.49 | 14.13* | 13.98 *,† | M | Muscle | FAOX, FAM, LiM |
| Cytochrome b5 | CYP5A | 17.46 | 16.51 * | 16.47 * | 16.00 * | C, V | Liver | ET |
| Signal transduction | ||||||||
| Calcium/calmodulin-dependent protein kinase II delta | CAMK2 | 14.66 | 14.04 * | 13.70 * | 13.92 * | C, PM | Low | CaB, ST |
| Intracellular transport | ||||||||
| AP-1 complex subunit mu-1 | AP1M1 | 15.54 | 13.93 * | 13.93 * | 14.36 | C, G | Low | PT, HVI |
| Cytoskeleton, ECM and signaling | ||||||||
| Tight junction protein 2 | TJP2 | 12.89 | 11.71 * | 11.22 * | 11.05 * | PM, CJ | Low | CA, TJ |
| Cell cycle, cell quality, and apoptosis | ||||||||
| SH3 domain-containing kinase-binding protein 1 | SH3KBP1 | 14.08 | 12.72 * | 12.65 * | 13.08 | CS | Low | ST, CO, CA |
| Serine/threonine-protein phosphatase 2A activator | PTPA | 13.94 | 13.01 * | 13.85 † | 13.01 | C, N | Low | PF, DNArep, CCP |
| Antioxidant defense | ||||||||
| Probable hydrolase PNKD | PNKD | 13.28 | 12.41 * | 12.26 * | 12.22 * | C, M | Low | GlutB(+) |
| Miscellaneous | ||||||||
| Sperm-associated antigen-17 | SPAG17 | 20.56 | 18.68 * | 18.70 * | 18.88 * | C, CS | Testis, epididymis, brain | CilB, CilF |
| Family with sequence similarity member A2 | FAM114A2 | 11.54 | 10.26 * | 11.30 † | 11.09 | V | low | PuNB |
| Hemoglobin subunit alpha | HBA1 | 25.86 | 24.55 * | 24.36 * | 25.02 | C | Bone marrow | OxT |
| Protein Name | Gene Name | q-Value | Pearson’s r | Location in Cell | Tissue Specificity | Main Function(s) |
|---|---|---|---|---|---|---|
| Hemoglobin subunit gamma-1 | HBG1 | 0.0000 | 0.717 | C | Placenta | FetalHG, Oxb, OxT |
| Immunoglobulin heavy constant alpha 2 | IGHA2 | 0.0000 | 0.630 | PM, secreted | Low | ImR |
| Angiotensinogen | AGT | 0.0000 | 0.624 | Secreted | Liver | RAAS, BPReg |
| Ribosyldihydronicotinamide dehydrogenase [quinone] | NQO2 | 0.0000 | 0.603 | C | Low | DeTox, OxStress protector |
| Glutathione S-transferase theta-1 | GSTT1 | 0.0001 | 0.595 | C | Breast | GluthB, GluthM |
| NAD(P)HX epimerase | NAXE | 0.0001 | 0.586 | C, N | Low | NAD(P)HXrep |
| Immunoglobulin heavy constant mu | IGHM | 0.0002 | 0.557 | PM, secreted | Low | ImR |
| Complement C4-A | C4A | 0.0005 | 0.554 | Secreted | Liver | CP, ImR(+) |
| Copine-1 | CPNE1 | 0.0006 | 0.535 | N, NM | Low | TNFaSig, TF |
| Enoyl-CoA hydratase domain-containing protein 3, mitochondrial | ECHDC3 | 0.0006 | 0.532 | M | Liver, muscle | FAM, LiM |
| Complement C4-B | C4B | 0.0010 | 0.521 | Secreted | Liver | CP, ImR(+) |
| Afamin | AFM | 0.0025 | 0.5 | ECM, Se | Liver | PT, FAB |
| N-acetylneuraminate lyase | NPL | 0.0452 | 0.492 | VE, PM | Blood | CM |
| Epsin-1 | EPN1 | 0.0041 | 0.491 | PM, C | Low | Endocytosis |
| Acyl-coenzyme A thioesterase 1 | ACOT1 | 0.0064 | 0.476 | M | Liver | FAM, AcoAM |
| Phosphoglucomutase-1 | PGM1 | 0.0068 | 0.473 | C | Muscle | CM, GluM |
| Glycogenin-2 | GYG2 | 0.0290 | 0.44 | C, N | Adipose tissue, brain, breast | GlycB |
| Tyrosine-protein kinase CSK | CSK | 0.0313 | 0.433 | C, V | Lymphoid tissue | Reg, Imm |
| Heat shock protein HSP 90-alpha | HSP90AA1 | 0.0360 | 0.428 | C | Vagina | Cha, HVI, SR |
| Interferon-induced protein with tetratricopeptide repeats 1 | IFIT1 | 0.0367 | 0.426 | C | Low | HVI, Imm |
| Histidine-rich glycoprotein | HRG | 0.0455 | 0.415 | Secreted | Liver | Angio(+), BC, Chem |
| Tetratricopeptide repeat protein 38 | TTC38 | 0.0498 | 0.411 | C, secreted | Liver, intestine | Unknown |
| 60S ribosomal protein L38 | RPL38 | 0.0453 | −0.416 | ER, C | Low | RibP, translation |
| RAB14 member RAS oncogene family | RAB14 | 0.0450 | −0.419 | Er, C | Low | RibP, translation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedersen, J.S.; Niu, L.; Wewer Albrechtsen, N.J.; Kristiansen, V.B.; Poulsen, I.M.; Serizawa, R.R.; Hansen, T.; Gluud, L.L.; Madsbad, S.; Bendtsen, F. Comparative Analysis of the Human Proteome Profile in Visceral Adipose and Liver Tissue in Individuals with Obesity with and Without MASLD and MASH. Livers 2025, 5, 16. https://doi.org/10.3390/livers5020016
Pedersen JS, Niu L, Wewer Albrechtsen NJ, Kristiansen VB, Poulsen IM, Serizawa RR, Hansen T, Gluud LL, Madsbad S, Bendtsen F. Comparative Analysis of the Human Proteome Profile in Visceral Adipose and Liver Tissue in Individuals with Obesity with and Without MASLD and MASH. Livers. 2025; 5(2):16. https://doi.org/10.3390/livers5020016
Chicago/Turabian StylePedersen, Julie S., Lili Niu, Nicolai J. Wewer Albrechtsen, Viggo B. Kristiansen, Inge Marie Poulsen, Reza R. Serizawa, Torben Hansen, Lise Lotte Gluud, Sten Madsbad, and Flemming Bendtsen. 2025. "Comparative Analysis of the Human Proteome Profile in Visceral Adipose and Liver Tissue in Individuals with Obesity with and Without MASLD and MASH" Livers 5, no. 2: 16. https://doi.org/10.3390/livers5020016
APA StylePedersen, J. S., Niu, L., Wewer Albrechtsen, N. J., Kristiansen, V. B., Poulsen, I. M., Serizawa, R. R., Hansen, T., Gluud, L. L., Madsbad, S., & Bendtsen, F. (2025). Comparative Analysis of the Human Proteome Profile in Visceral Adipose and Liver Tissue in Individuals with Obesity with and Without MASLD and MASH. Livers, 5(2), 16. https://doi.org/10.3390/livers5020016






