1. Background
The liver possesses the unique property of being able to regenerate organ-specific cells to maintain homeostatic function [
1,
2,
3,
4]. Unlike true regenerative mechanisms, liver regeneration functions by increasing the size of remaining liver structures to meet biological needs rather than obtaining the original gross anatomical structure [
2]. Conversely, enlarged liver mass is corrected by apoptosis [
3]. While other solid organs adjust to tissue loss without reaching original homeostatic levels, the regenerative and apoptotic properties of the liver allow it to reach 100% of what is required for homeostasis [
1]. This regenerative capacity has been demonstrated in both human and animal studies, highlighting the liver’s distinct mechanisms [
1,
2,
3,
4].
The mechanisms of liver regeneration have been thoroughly studied in the past. Liver regeneration is a complex process which involves various cell types and intercellular interactions mediated by various growth factors [
5,
6,
7,
8,
9]. While in vitro human studies, created by culturing isolated hepatocytes, are beneficial by minimizing the effect of confounding variables, the complex process of liver regeneration requires consideration of these variables in in vivo animal models.
In the past, various strategies were explored to encourage the regeneration of the first stage liver prior to performing extended liver resection in the second stage. It was found in human studies that the regeneration of the first stage liver is promoted by causing controlled damage to the future resected liver lobe (FRL). This restoration of volume and function of the first stage liver improves the tolerance to the subsequent major hepatectomy performed as a second stage operation. Portal vein occlusion techniques, including portal vein embolization (PVE) and portal vein ligation (PVL), were initially established to improve resectability. However, these techniques failed to induce sufficient hypertrophy or prevent tumor growth progression [
10].
Thus, the two-stage hepatectomy, a procedure that consists of two sequential liver resections to clear all tumors located in the FLR (the left lobe) followed by PVE of the right lobe combined with portal vein occlusion, was introduced. Performing a small additional resection (20%PHx) did not fully prevent atrophy of the ligated liver lobe. However, by performing a large additional resection (70%PHx), there was an induction of proliferation, a down-regulation of apoptosis, and an up-regulation of autophagy in the ligated lobe. This combined procedure induced more hepatocyte proliferation and a faster course of liver hypertrophy in the FLR than after PVL alone, as simultaneous PVL and PHx was found to be a well-tolerated procedure even when reducing the size of the healthy FLR to only 10% of the original liver mass. Additional PHx was found to not only promote regeneration of the FLR but also reduce the tumor load in the first step, thereby preventing the increase of the tumor burden during the waiting period [
10].
However, despite improved outcomes following major hepatectomy, major vascular invasion has led to subsequent higher risks for increased intraoperative blood loss, which was also identified as an independent predictor of early postoperative mortality [
11]. Thus, the Pringle maneuver (PM) was introduced as a common and evidence-based method of hepatic inflow control, as it was found to decrease intraoperative blood loss, the amount of blood products transfused, and operation time. However, an associative risk with the PM procedure is an ischemia-reperfusion injury of the liver, which negatively affects hepatocyte metabolism and increases the rate of posthepatectomy liver failure (PHLF). Furthermore, there have been limited studies on the effects of the PM on intra-and postoperative outcomes in extended hepatectomy which has a higher risk of intraoperative bleeding than minor hepatectomies [
12].
Therefore, the objective of this study is to evaluate a novel bile duct-saving portal ligation technique for performing a 90% subtotal hepatectomy in rats, focusing on minimizing intraoperative blood loss and promoting liver regeneration during the immediate postoperative period, without the need for preoperative liver hypertrophy induction techniques. By combining previous techniques, this approach aims to minimize blood loss and enhance liver regeneration. This could potentially make it a more efficient and safer alternative to multi-stage procedures for substantial liver resection in HCC cases. Additionally, our technique has potential benefits for cases requiring repeated liver resections involving substantial tissue excision or patients with compromised liver function. In such cases, preservation of biliary function, minimizing blood loss, and reducing the risk of liver ischemia-reperfusion injury are critical for improving patient outcomes. In living donor liver transplantations, performing subtotal hepatectomies while ensuring sufficient regeneration and decreasing postoperative complications could contribute to optimizing donor hepatic function and expanding the pool of eligible donors. Ultimately, our technique may improve patient outcomes by reducing complications traditionally associated with hepatectomy procedures, such as increased bile leaks and higher morbidity, increasing patient safety.
2. Methods
2.1. Preoperative Preparation
One hour prior to operation, the rat is administered a dose of Buprenorphine HCl-ER analgesic at 1.0 mg/kg injected subcutaneously and an inhaled isoflurane anesthetic, inducted at 4% and maintained at 2%. Immediately before operation, the stomach area of the rat is shaved and wiped with chlorhexidine and 70% ethanol twice.
2.2. Obtaining Intraperitoneal Access and Mobilizing the Liver
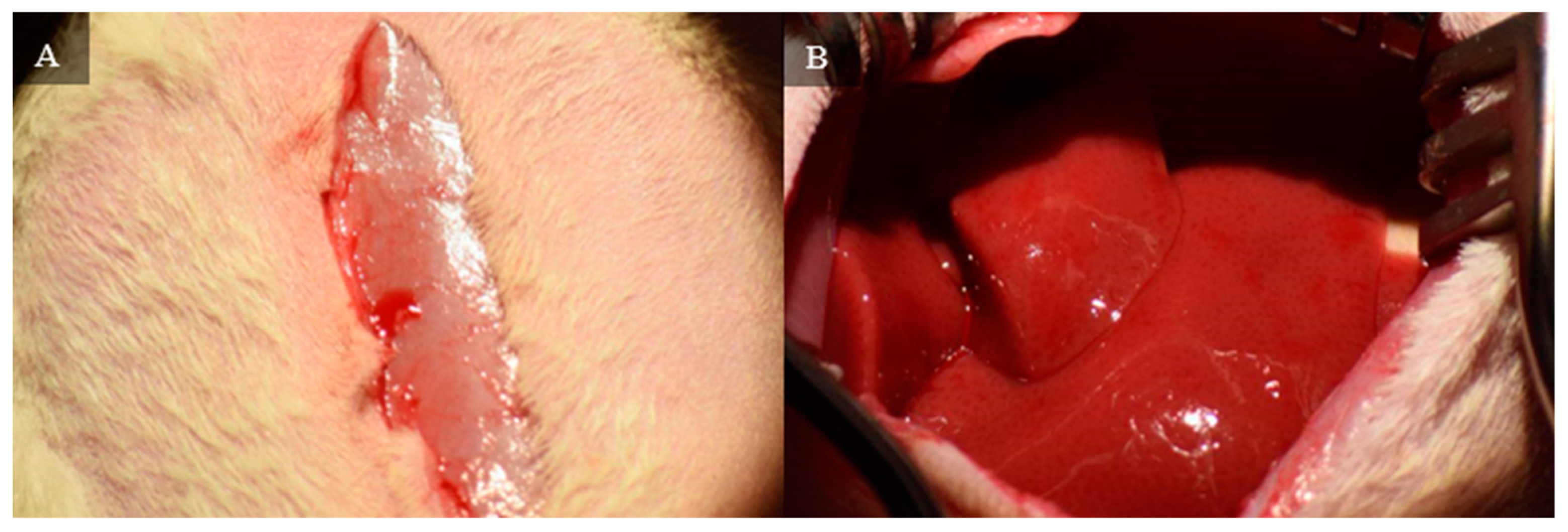
A midline incision is made on the skin to expose the muscle layer. The incision is about 3 cm long and starts at the xiphoid process. A small incision is then made to gain access to the peritoneal cavity. Taking care to ensure that the animal’s internal organs are not at risk of being cut, a midline incision is made on the muscle layer to gain complete access to the peritoneal cavity. The cavity is held open with a retractor.
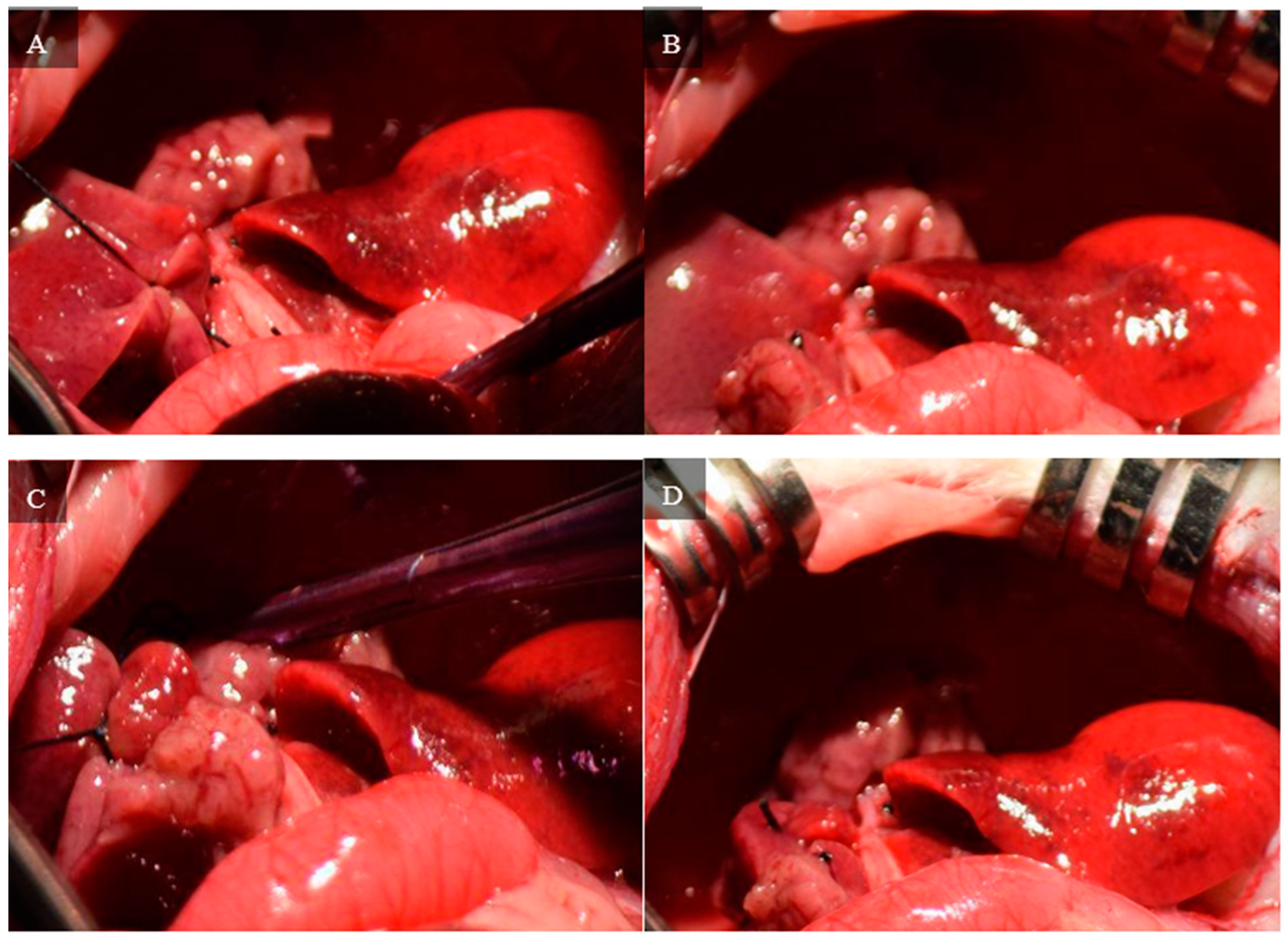
Figure 1 demonstrates the opening of the skin and muscle layer separately to expose the liver.
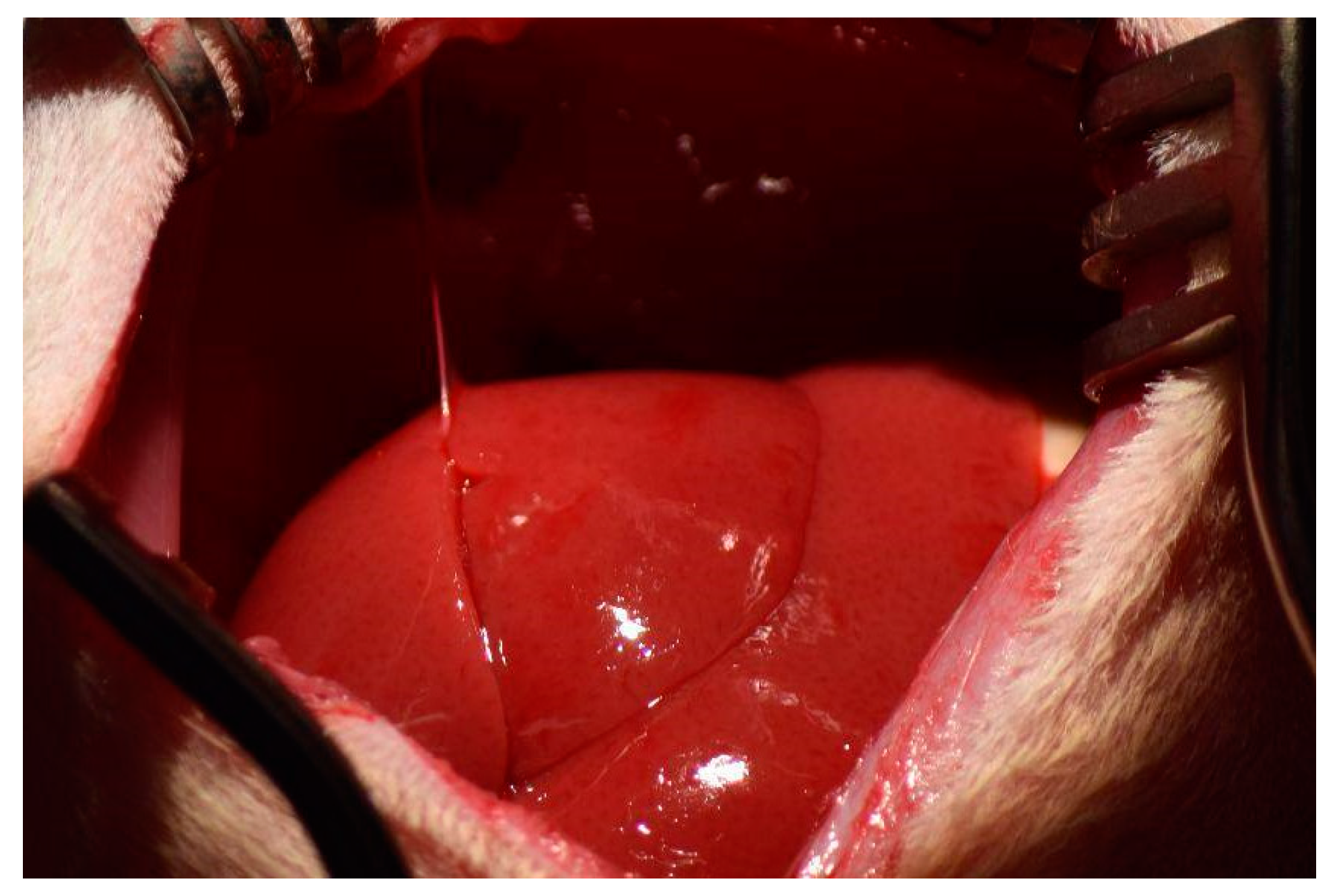
Suspending ligaments were cut to mobilize the liver and allow for resection of liver lobes. The ligaments include: the falciform ligament attaching the median lobe to the diaphragm (
Figure 2), the left triangular ligament connecting the left lateral lobe to the diaphragm, the interlobar ligament connecting the left lateral lobe to the superior caudate lobe, and the right triangular ligament connecting the superior right lateral lobe to the diaphragm.
Figure 3 depicts the resection of these four ligaments.
2.3. Bile Duct-Saving Portal Ligation via Hilum Dissection
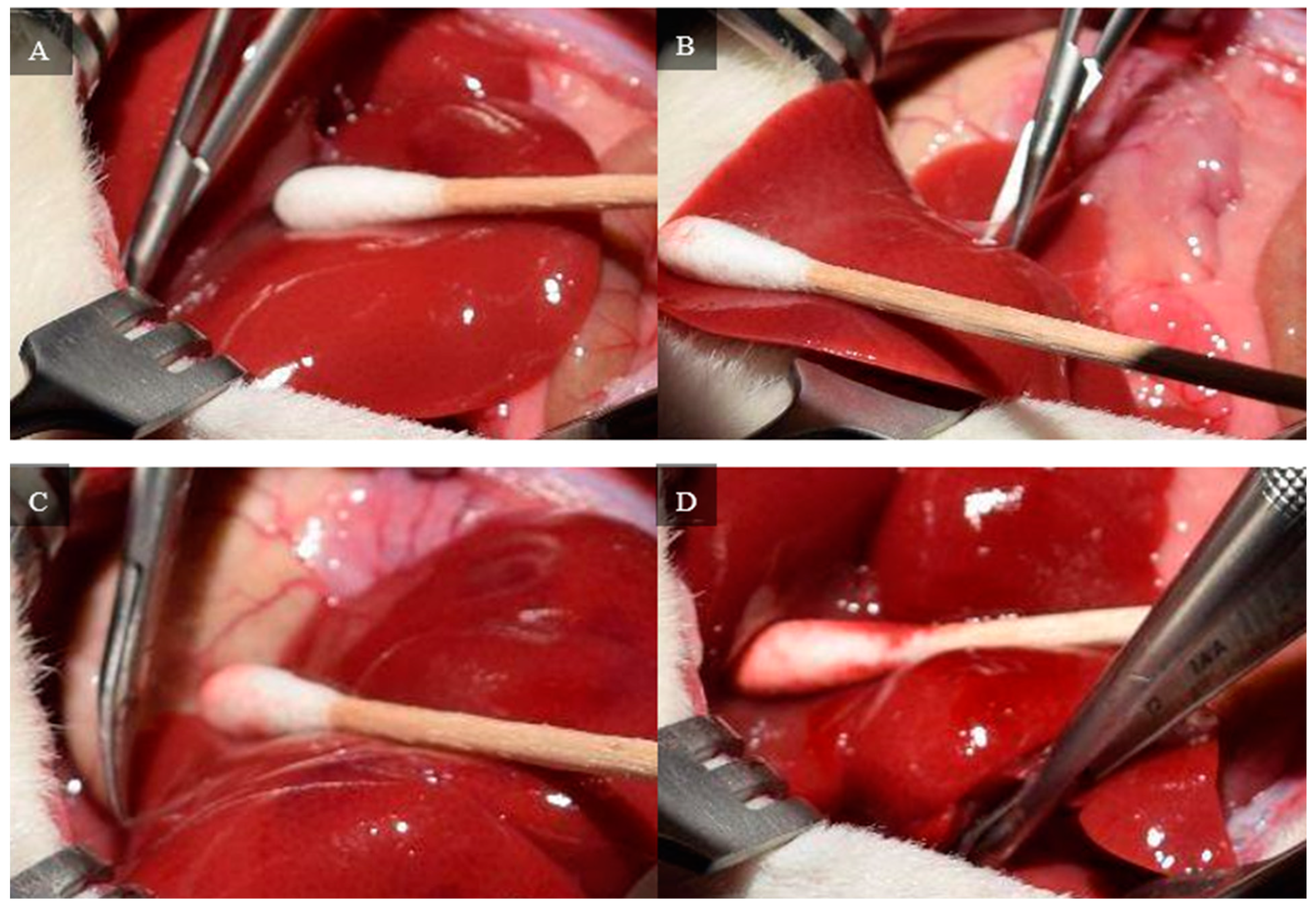
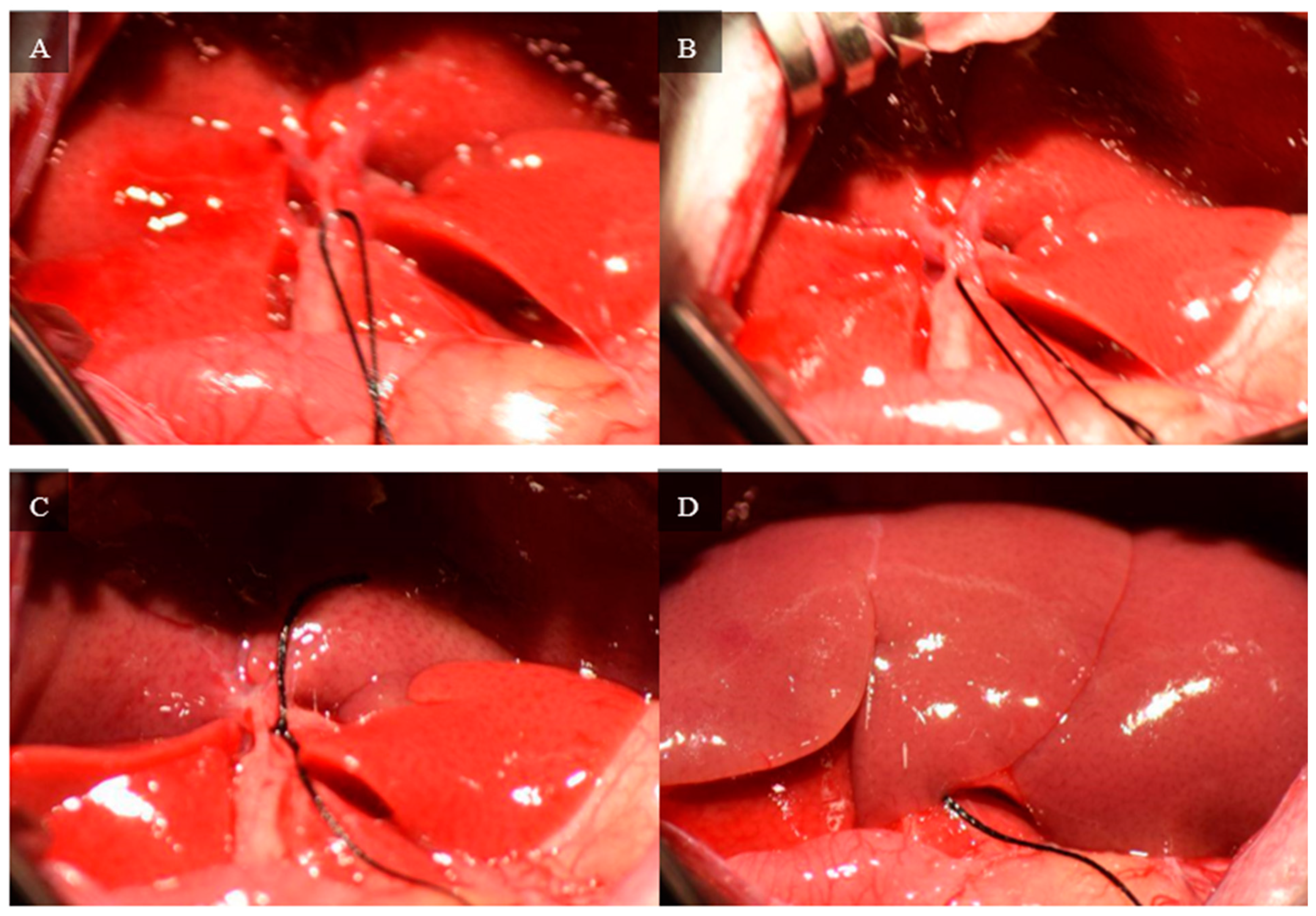
The medial and left lateral lobes are lifted to expose the hilum (
Figure 4). Using a Leica Wilde M651 surgical microscope (Leica Microsystems, Bensheim, Germany) at 20 × power magnification, visual access is focused on the portion of the hilum that supplies the median and left lateral lobes. The membrane between the bile duct and hepatic artery is punctured using a curved jaw micro needle holder, and 4-0 silk thread is pulled through the separation. The membrane between the portal vein and inferior vena cava is then punctured using a curved jaw micro needle holder, and one end of the 4-0 silk thread is pulled through the separation. As a result, the thread holds the hepatic artery and portal vein that supplies the median and left lateral lobes. The hepatic artery and portal vein are ligated, which stops all inflow to those lobes. Visual confirmation of successful ligation can be obtained through a color change in the lobes from bright pink to brown.
Figure 5 depicts each stop of this hilum dissection and ligation.
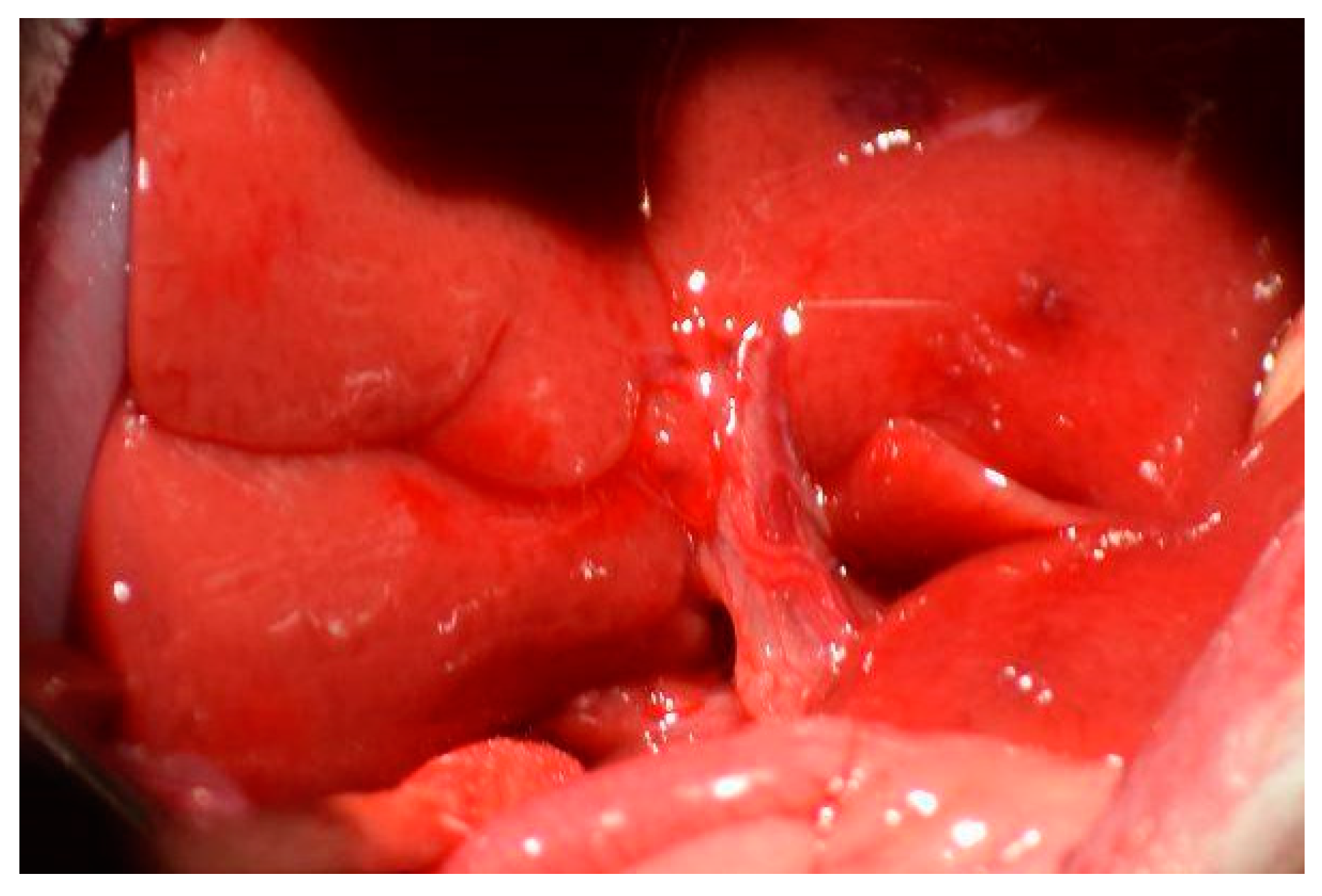
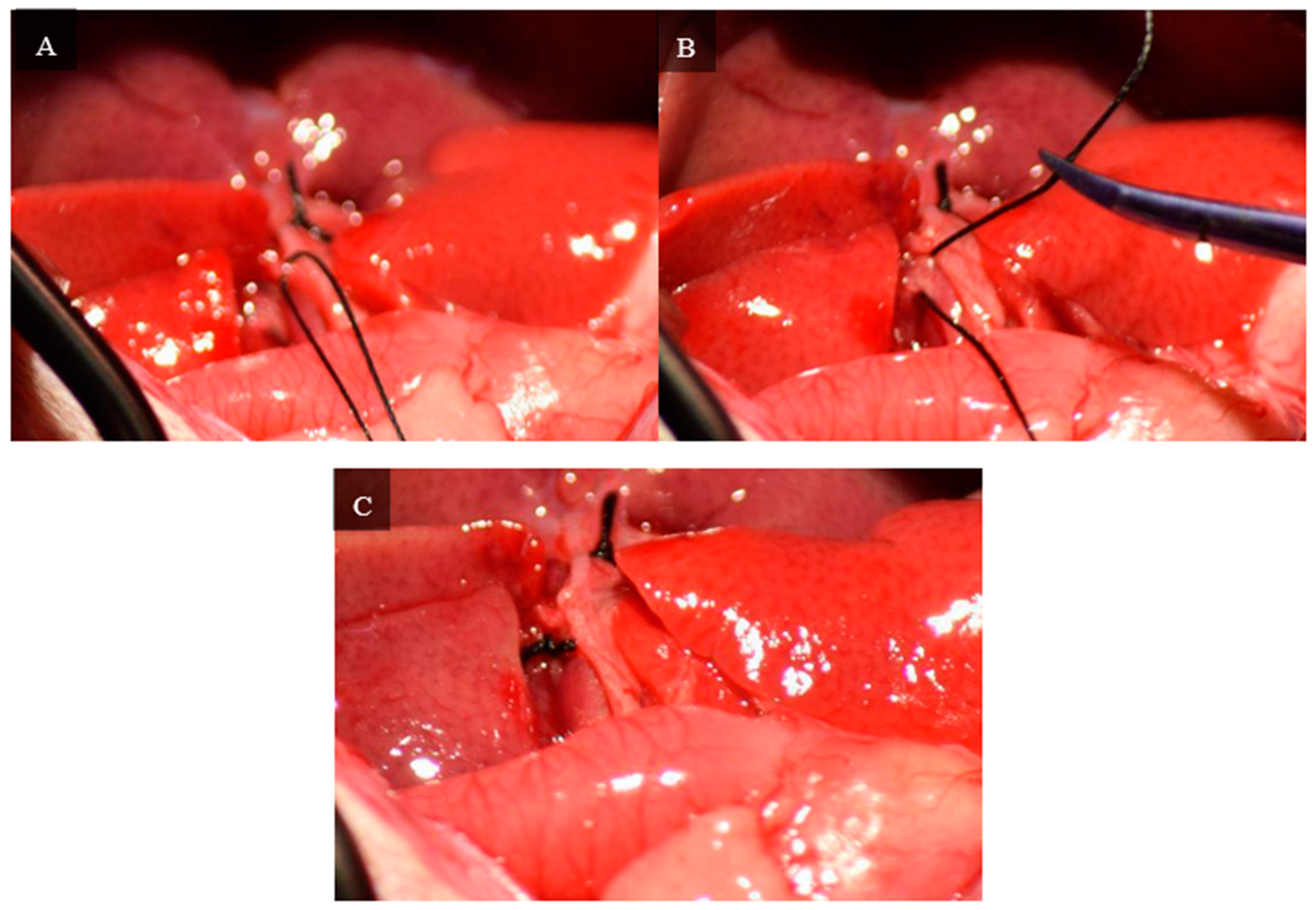
A second dissection of the hilum is required to control the inflow to the right superior and right inferior lobes. The branch of the hilum that supplies the right lateral lobe is isolated. Similar to the initial dissection, the membrane separating the bile duct and hepatic artery is penetrated with curved jaw micro needle holders, and 4-0 silk thread is passed through. One end of the thread is placed through the membrane between the portal vein and inferior vena cava, so the thread only holds the portal vein and hepatic artery. The portal vein and hepatic artery are ligated with 4-0 silk thread, and visual confirmation of successful ligation can be seen by a color change in the right superior and right inferior lobes.
Figure 6 depicts the steps of this portal ligation.
2.4. Ligation and Resection of Liver Lobes
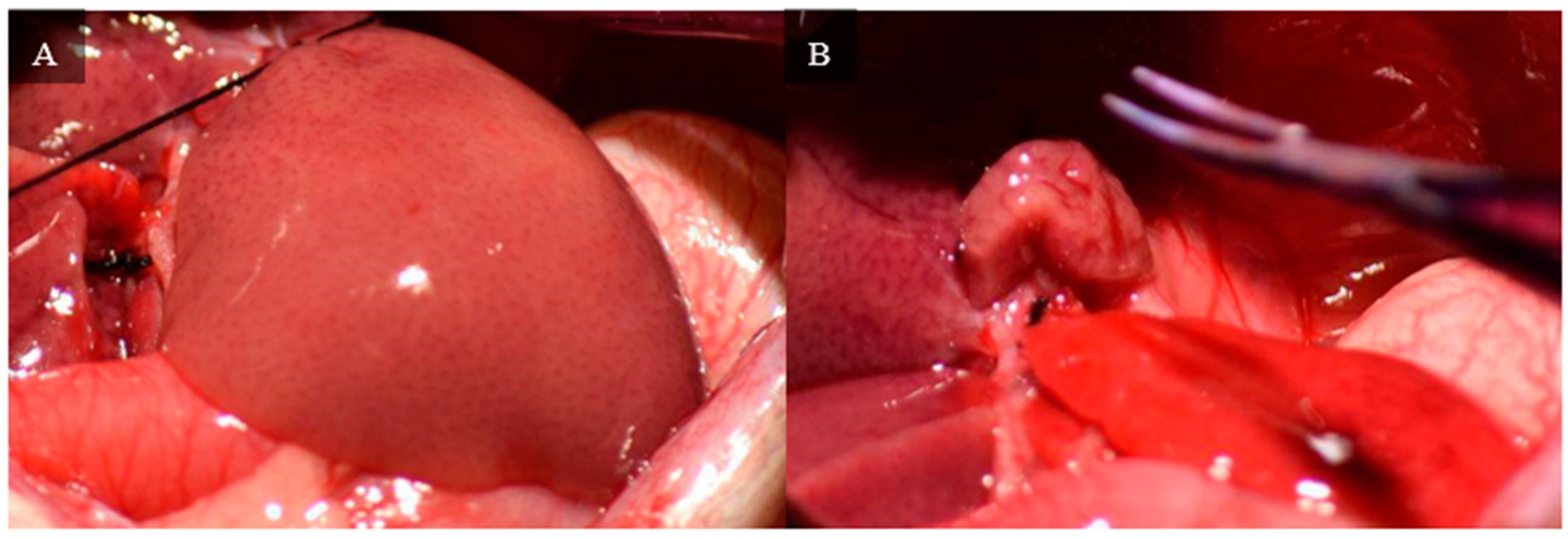
A 4-0 silk thread is placed around the neck of the lateral lobe using microdissection forceps and a curved jaw micro needle holder. The thread is tied and the left lateral lobe is cut using curved jaw micro dissecting scissors distal to the tie, leaving a small stump of the left lateral lobe.
Figure 7 depicts ligation of the left lateral lobe and the remaining stump.
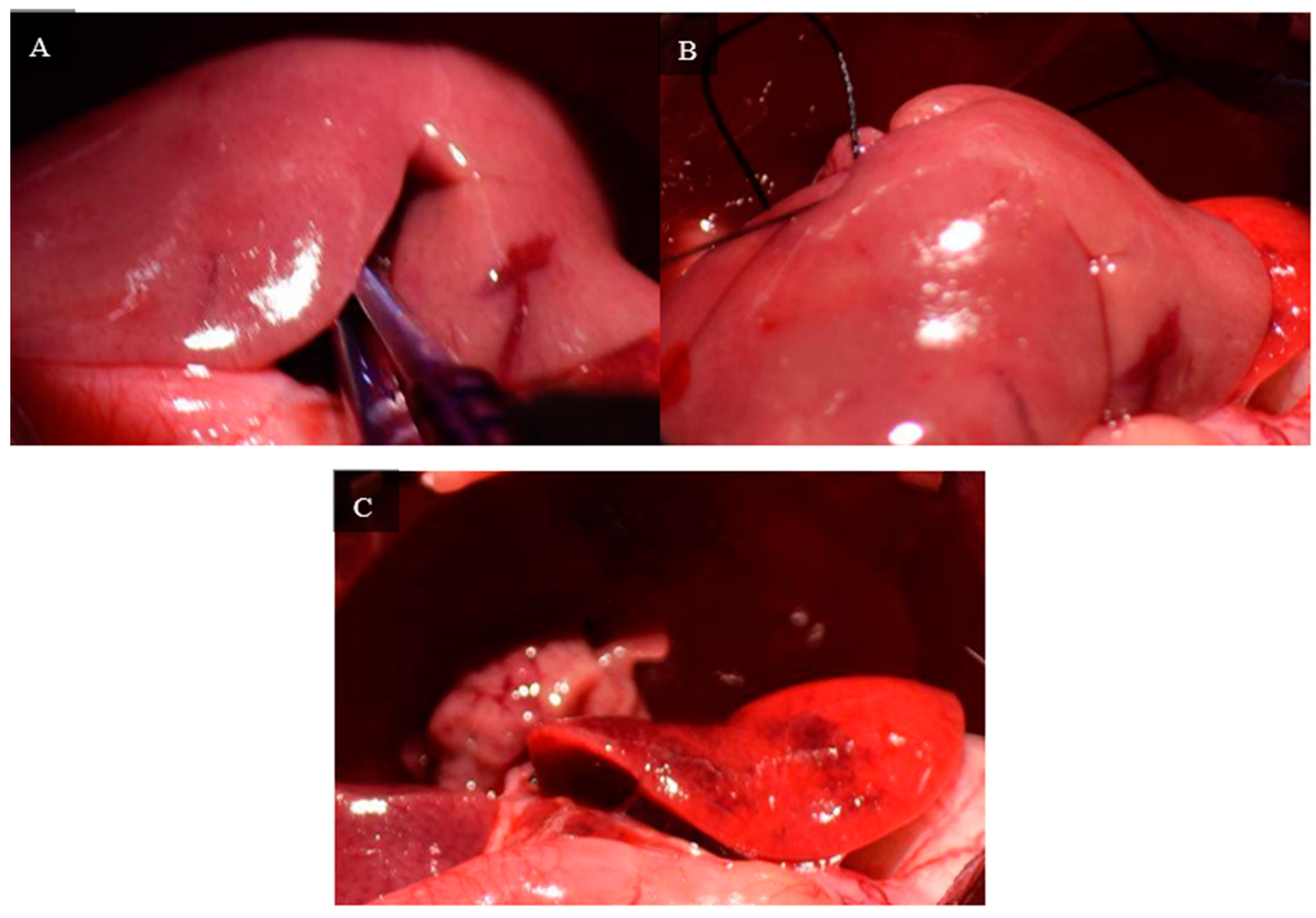
A curved jaw micro needle holder is passed between the right and left portions of the median lobe starting from the dorsal side of the lobe about 5 mm above the neck of the median lobe. Using a curved jaw micro needle holder, 2 4-0 silk threads are passed between the right and left portions of the median lobe, and each portion is ligated separately. The median lobe is cut using curved jaw micro dissecting scissors, leaving a small stump of the median lobe.
Figure 8 depicts ligation and the remaining stump of the median lobe after resection.
The right inferior lobe is resected by placing a 4-0 silk tie around the neck using microdissecting forceps and a curved jaw micro needle holder. The lobe is ligated and cut using curved jaw microdissecting scissors, leaving a small stump. The right superior lobe is resected using the same method.
Figure 9 depicts the ligation and remaining stumps of the right inferior and superior lobes.
2.5. Closing and Post-Operational Care
Prior to closing, the rat is given 5 mL of 10% glucose solution intraperitoneally. The muscle layer is closed with a simple continuous suture using 4-0 vicryl. The skin layer is closed with a running subcuticular suture using 5-0 monocryl to avoid the animal ripping out its sutures during the post-operative period.
The rat is placed in a cage with ClearH2O DietGel Recovery(ClearH2O, Westbrook, ME, USA), a gel-based formula for hydration and nutrients sourced from the company ClearH2O, and an ad libitum 20% dextrose solution. The DietGel Recovery and dextrose solution are replaced every other day during the post-operative period until the rat is sacrificed. A total of 72 h after the operation, if the rat is showing any symptoms of pain, it is administered a second dose of Buprenorphine HCl-ER at 0.5 mg/kg. The rats’ weight and behavior are closely monitored during the week-long period of survival. Rats which experience significant losses in weight or show signs of fatigue are provided 5–10 mL of normal saline or lactate ringer solution as needed.
2.6. Experimental Design
Five Sprague–Dawley male rats, sourced from Charles River laboratories, were used, all being between 300 g–400 g at the time of the operation. The mass of resected liver tissue was weighed and total liver mass was estimated using Equation (1) where mass of the resected liver (M
R) is divided by 0.9 to estimate the total pre-surgical liver mass (M
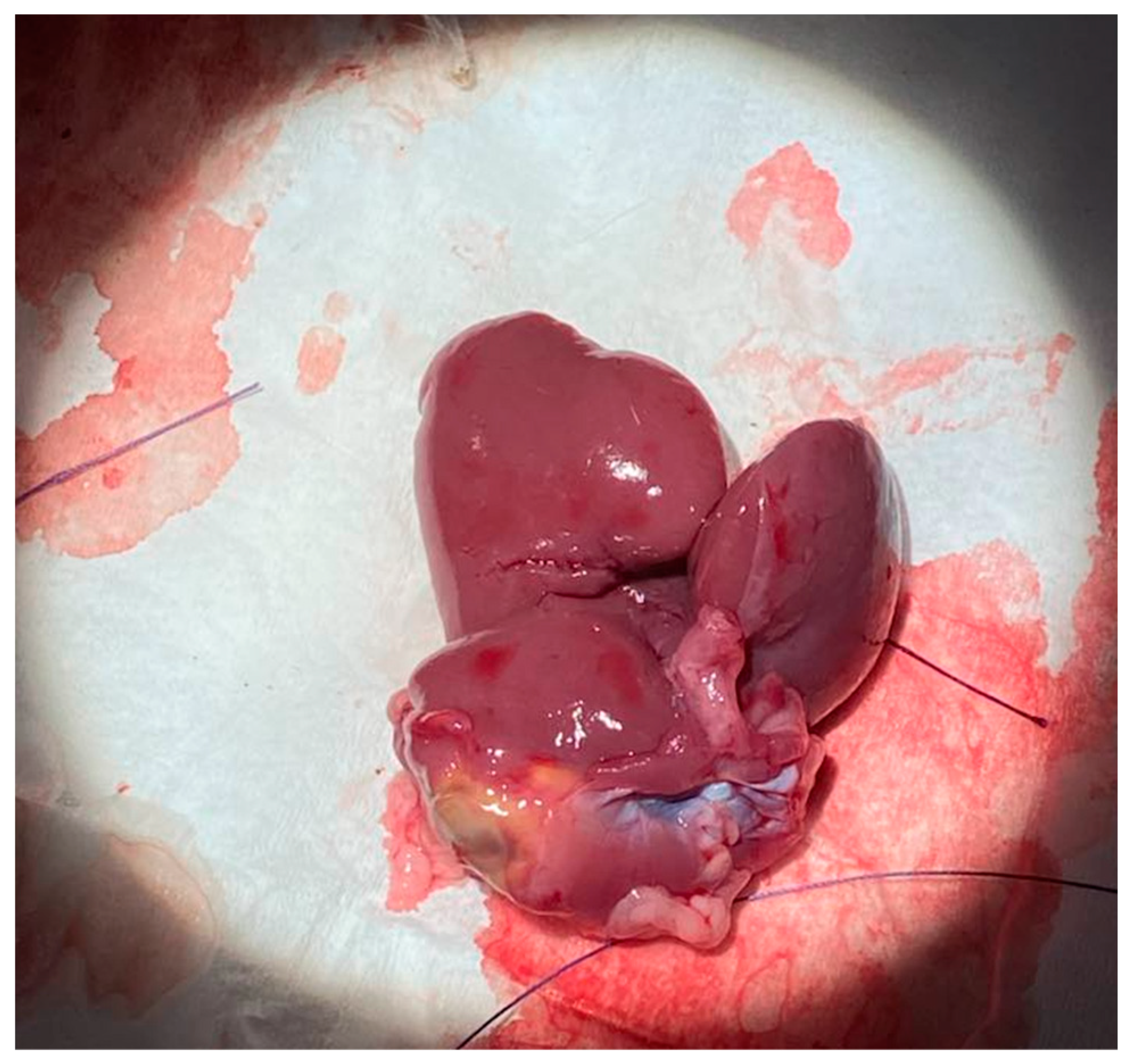
T). All five rats were monitored daily for the seven-day post-surgical period to adjust post-op care as necessary. Additionally, the mass of each rat was weighed for the first three days of the post-operative period. Following the seven-day period, all rats were euthanized and sacrificed for blood and liver samples. The total remaining liver was harvested and weighed.
Figure 10 depicts the explanted liver after sacrifice on postoperative day 7.
4. Discussion
This study demonstrated a novel surgical technique to perform subtotal hepatectomy procedures on rats, with minimal complications and acceptable early survival outcomes. The bile duct-saving portal ligation technique involves a microsurgery technique to dissect the hilum twice. The first hilum dissection inhibits the blood supply to the entire median and left lateral lobes. The second hilum dissection inhibits blood supply to superior and inferior right lateral lobes. Following the ligation and resection of the lobes, only the superior and inferior caudate lobes remain, which account for 10% of the original liver volume. This novel technique was performed on five rats to confirm safety and efficacy. All five rats survived and demonstrated significant liver regeneration within the 7-day post-operative period.
Current models of liver resection in rats involve the immediate ligation and resection of the liver lobes at the neck of each lobe [
13]. A more refined technique involves the use of a Pringle’s maneuver to temporarily stop the inflow to specific lobes [
14]. Using both of these techniques, the stubs of the resected lobes still receive blood supply, which leads to postoperative blood loss. Additionally, in the former, continuous inflow to the lobes leads to significant perioperative blood loss. When mass ligation is placed at the base of the lobes, resection further carries the risk of constriction of the vena cava. Utilizing the novel technique described in this study eliminates this concern by permanently stopping blood flow into the lobes prior to resection and distal resection of liver lobes from the site of ligation. The benefit of this is twofold, first, that perioperative blood loss is minimized, and second, that blood flow to the remaining stubs is stopped with prevention of vena cava constriction. Ultimately this contributes to less blood loss, improved survival rates, and the ability to perform more subtotal hepatectomies.
Complete hilum ligation is not a feasible surgical alternative, as blockage of the bile duct can lead to biloma. Therefore, dissection of the hilum to preserve the bile duct is essential for higher acuity resections such as those performed in this study. By preserving the bile duct and selectively ligating the portal vein and hepatic artery, this technique minimizes the risks of postoperative complications associated with bile duct obstruction and ensures greater safety and efficacy of the procedure [
15]. Ligation of the bile duct can also induce liver fibrosis and acute obstructive jaundice with progression to cirrhosis with portal fibrosis. Bile duct ligation induces an increase in the expression of pro-inflammatory cytokines and a subsequent depletion of Küpffer cells, further increasing development of liver fibrosis and potential liver failure [
16].
However, through ligation of both the portal vein and hepatic artery, there is a redistribution of arterial and portal circulation, creating an optimal environment for liver remnant hypertrophy. In prior studies, the rate of hepatic regeneration increases when both the portal vein and hepatic artery are ligated compared to only the portal vein [
17]. Additionally, ligation of both vessels enhances the release of inflammatory cytokines associated with superior hepatic regeneration. Subsequently, there is a rapid rise in portal venous pressure and arterial hyperperfusion in the remaining non-ligated liver lobes, playing a crucial role in stimulating liver growth via compensatory hypertrophy and highlighting the inherent regenerative capacity of the liver. Acute portal hypertension serves as a key initiator in post-resection liver regeneration and is driven by a rapid reduction in the volume of the intrahepatic portal bed from liver excision [
18].
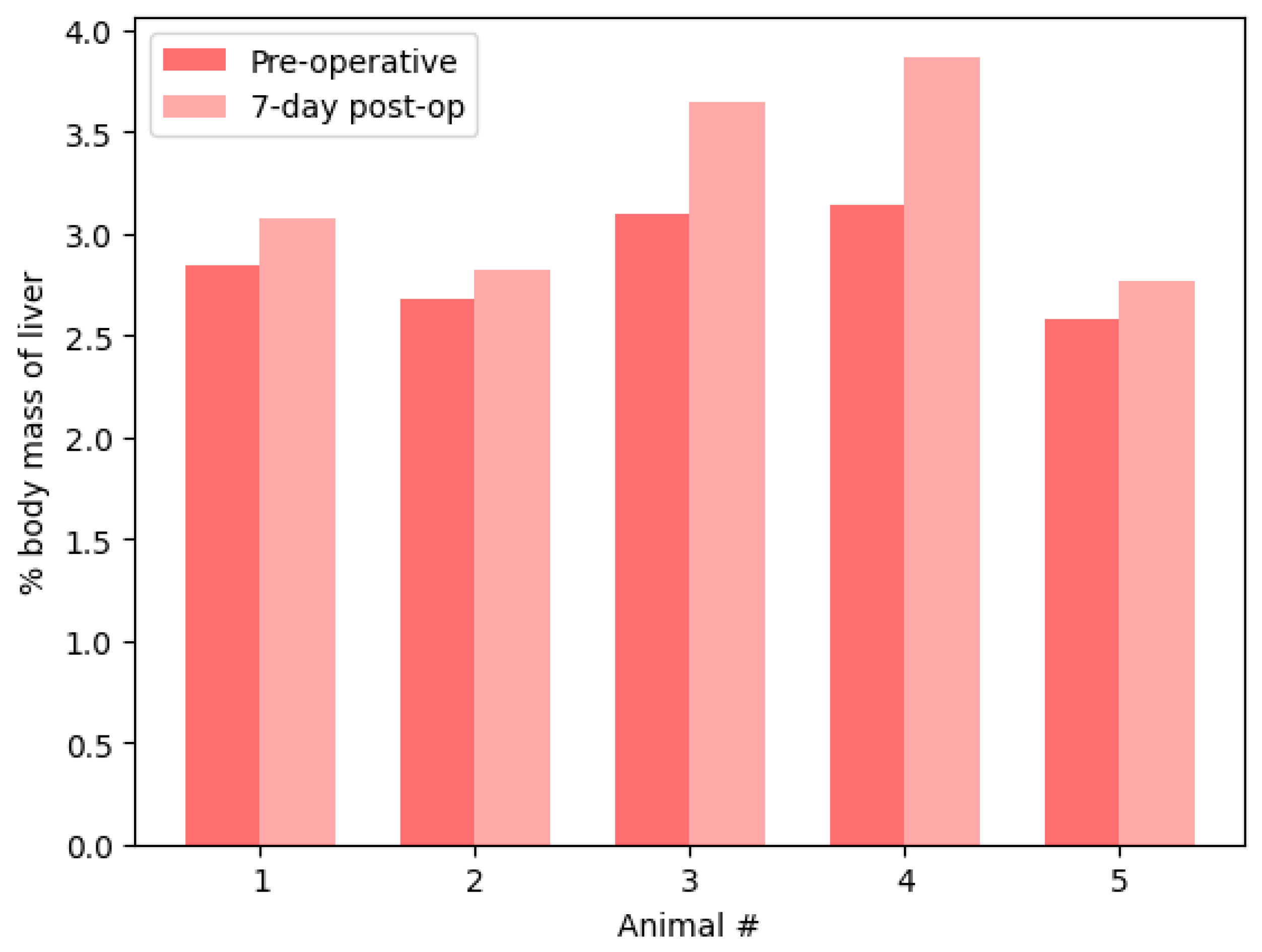
This study demonstrated that complete liver regeneration can occur in 90% hepatectomy models of rats after a 7-day postoperative period. The average change in liver mass was an increase of 3.1%, with the changes in liver mass ranging from −4.2% to +8.1%. Furthermore, despite the estimated decrease in liver mass that three animals experienced, every animal demonstrated an increase in liver mass relative to their total body mass. One week after 90% hepatectomy, the liver recovered more than 95% of its original mass on average. Therefore, this study demonstrates the effectiveness of a novel bile duct-saving portal ligation technique for subtotal hepatectomy survival procedures on rats. Through dissecting the hilum and selective ligation of the portal vein and hepatic artery, this approach provides more precise control over blood flow to target liver lobes, minimizing perioperative blood loss and postoperative complications. The successful outcomes, 100% survival, and significant liver regeneration within the 7-day postoperative period demonstrate the use of this technique as a potential tool for hepatic surgery.
4.1. Limitations
Limitations of the novel bile duct-saving portal ligation technique discussed within this paper include its challenging surgical nature within clinical settings, requiring significant practice and having a steep learning curve. Performing the delicate dissections of the hilum and the ligations of the portal vein and hepatic artery without disruption of the bile duct demands precise microsurgical skill, limiting its widespread adoption without proper training and experience. Furthermore, variability in the anatomy of the hepatic vasculature among individual rats can pose challenges during surgery and impact overall success rates of the procedure. Specifically, the differences in branching patterns of the portal vein and hepatic artery that can occur among subjects require intraoperative adjustments, which may contribute to inconsistent outcomes [
19]. Additionally, the rats in this animal model were healthy and lacked any underlying liver diseases, such as cirrhosis, commonly seen in clinical patients. The absence of such pathological conditions may limit the model’s applicability when translating its findings to human cases, where disease can alter both hepatic vasculature and procedural outcomes.
4.2. Future Directions
Inclusion of earlier sacrifice dates to measure the rate of liver growth during the postoperative period is one avenue of exploration for future studies. With a larger sample size, sacrificing rats at various time points following surgery can provide insight into the molecular kinetics of liver regeneration for the better understanding of the early phases of hepatic tissue regeneration. The effectiveness of administering growth factors through the inferior vena cava is another potential avenue for evaluating the stimulation of hepatocyte proliferation and angiogenesis within the process of liver regeneration. Through delivery of growth factors, such as hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF), future studies can evaluate methods to accelerate liver regeneration and shorten recovery times, highlighting therapeutic potential within clinical settings where patients are undergoing acute partial hepatectomies.