Nutrition Therapy in Critically Ill Patients with Liver Disease: A Narrative Review
Abstract
:1. Introduction
2. Materials and Methods
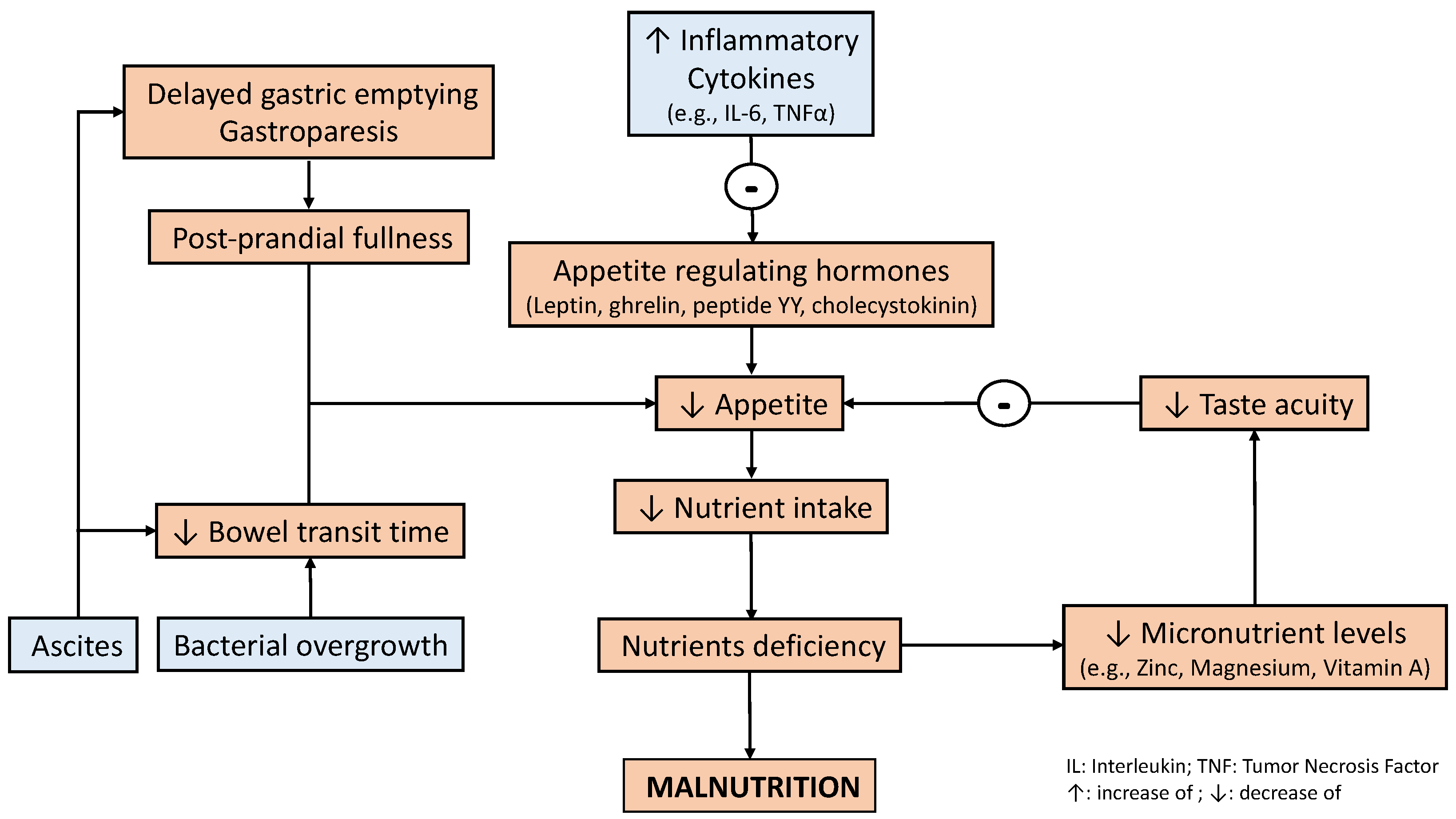
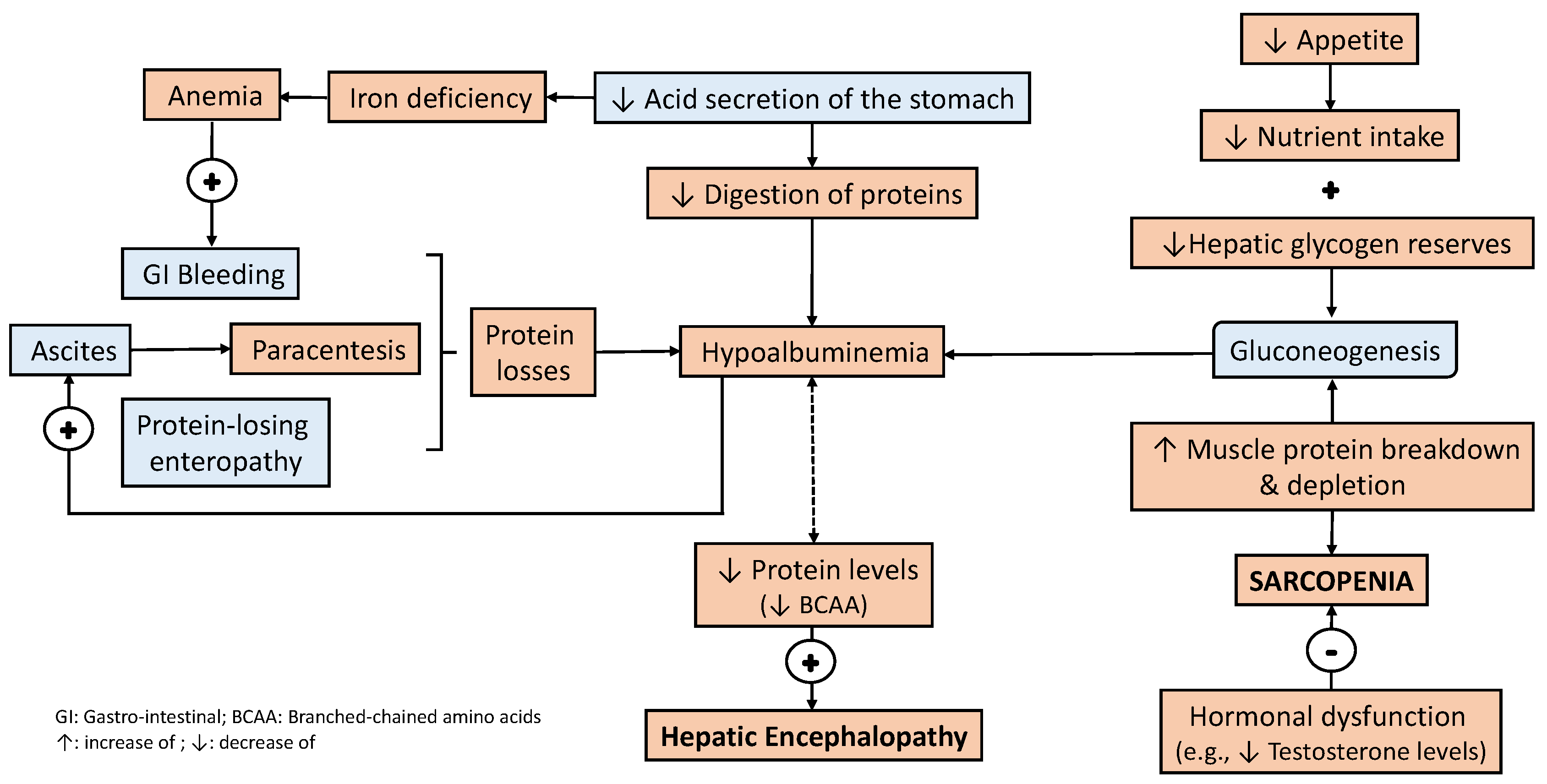
3. Bidirectional Relationship between Liver Disease and Nutritional Status
4. Nutrition Management in Patients with Liver Disease
4.1. Evaluation of Nutritional Status in Patients with Liver Disease
4.2. What Do Clinical Practice Guidelines Recommend?
4.3. A Practical Approach to Nutrition Therapy in Patients with Liver Disease
4.4. Considerations in the Perioperative Patient
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patton, H.M. Nutritional assessment of patients with chronic liver disease. Gastroenterol. Hepatol. 2012, 8, 687–690. [Google Scholar]
- Sevastianos, V.A.; Dourakis, S.P. Malnutrition and Sarcopenia in Advanced Liver Disease. J. Nutr. Food Sci. 2016, 6, 487. [Google Scholar]
- Huynh, D.K.; Selvanderan, S.P.; Harley, H.A.; Holloway, R.H.; Nguyen, N.Q. Nutritional care in hospitalized patients with chronic liver disease. World J. Gastroenterol. 2015, 21, 12835–12842. [Google Scholar] [CrossRef] [PubMed]
- Periyalwar, P.; Dasarathy, S. Malnutrition in cirrhosis: Contribution and consequences of sarcopenia on metabolic and clinical responses. Clin. Liver Dis. 2012, 16, 95–131. [Google Scholar] [CrossRef]
- Lorencio, C.; Bonet Sarís, A.; Navas Moya, E. Recommendations for specialized nutritional-metabolic treatment of the critical patient: Nonsurgical abdominal disease. Metabolism and Nutrition Working Group of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC). Med. Intensiva. 2020, 44 (Suppl. S1), 60–64. [Google Scholar] [CrossRef] [PubMed]
- Perez Ruiz de Garibay, A.; Kortgen, A.; Leonhardt, J.; Zipprich, A.; Bauer, M. Critical care hepatology: Definitions, incidence, prognosis and role of liver failure in critically ill patients. Crit. Care 2022, 26, 289. [Google Scholar] [CrossRef]
- Shergill, R.; Syed, W.; Rizvi, S.A.; Singh, I. Nutritional support in chronic liver disease and cirrhotics. World J. Hepatol. 2018, 10, 685–694. [Google Scholar] [CrossRef]
- Saunders, J.; Brian, A.; Wright, M.; Stroud, M. Malnutrition and nutrition support in patients with liver disease. Frontline Gastroenterol. 2010, 1, 105–111. [Google Scholar] [CrossRef]
- Hammad, A.; Kaido, T.; Aliyev, V.; Mandato, C.; Uemoto, S. Nutritional Therapy in liver transplantation. Nutrients 2017, 9, 1126. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, Q.Y.; Zhang, Q.; Rong, Y.M.; Lu, C.Z.; Li, H. Role of nutritional status and nutritional support in outcome of hepatitis B virus-associated acute-on-chronic liver failure. World J. Gastroenterol. 2020, 26, 4288–4301. [Google Scholar] [CrossRef]
- Prieto-Frias, C.; Conchillo, M.; Payeras, M.; Inarrairaegui, M.; Davola, D.; Fruhbeck, G.; Salvador, J.; Rodriguez, M.; Richter, J.A.; Mugueta, C.; et al. Factors related to increased resting energy expenditure in men with liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Wang, Y.Y.; Sheu, W.H. Increased serum leptin concentrations correlate with soluble tumour necrosis factor receptor levels in patients with cirrhosis. Clin. Endocrinol. 2002, 57, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzakis, E. Gastrointestinal dysfunction in liver cirrhosis. World J. Gastroenterol. 2014, 20, 14686–14695. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, R.; Gerasimidis, K.; Forrest, E. The Role of Micronutrients in the Pathogenesis of Alcohol-Related Liver Disease. Alcohol Alcohol. 2022, 57, 275–282. [Google Scholar] [CrossRef]
- Saeed, A.; Dullaart, R.P.F.; Schreuder, T.C.M.A.; Blokzijl, H.; Faber, K.N. Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2017, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Perry, B.; Wang, Y. Appetite regulation and weight control: The role of gut hormones. Nutr. Diabetes 2012, 2, e26. [Google Scholar] [CrossRef]
- Thuluvath, P.J.; Triger, D.R. Autonomic neuropathy and chronic liver disease. Q. J. Med. 1989, 72, 737–747. [Google Scholar]
- Shindo, K.; Machida, M.; Miyakawa, K.; Fukumura, M. A syndrome of cirrhosis, achlorhydria, small intestinal bacterial overgrowth, and fat malabsorption. Am. J. Gastroenterol. 1993, 88, 2084–2091. [Google Scholar]
- Lee, J.H.; Kwon, Y.J.; Park, K.; Lee, H.S.; Park, H.K.; Han, J.H.; Ahn, S.B. Metabolic Score for Insulin Resistance Is Inversely Related to Incident Advanced Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2022, 14, 3039. [Google Scholar] [CrossRef]
- Liu, K.; Yang, L.; Wang, G.; Liu, J.; Zhao, X.; Wang, Y.; Li, J.; Yang, J. Metabolic stress drives sympathetic neuropathy within the liver. Cell Metab. 2021, 33, 666–675.e4. [Google Scholar] [CrossRef]
- Marchesini, G.; Bianchi, G.; Lucidi, P.; Villanova, N.; Zoli, M.; De Feo, P. Plasma ghrelin concentrations, food intake, and anorexia in liver failure. J. Clin. Endocrinol. Metab. 2004, 89, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.F. The continuing importance of bile acids in liver and intestinal disease. Arch. Intern. Med. 1999, 159, 2647–2658. [Google Scholar] [CrossRef] [PubMed]
- Lodato, F.; Azzaroli, F.; Di Girolamo, M.; Feletti, V.; Cecinato, P.; Lisotti, A.; Festi, D.; Roda, E.; Mazzella, G. Proton pump inhibitors in cirrhosis: Tradition or evidence-based practice? World J. Gastroenterol. 2008, 14, 2980–2985. [Google Scholar] [CrossRef] [PubMed]
- Kappus, M.R. Acute Hepatic Failure and Nutrition. Nutr. Clin. Pract. 2020, 35, 30–35. [Google Scholar] [CrossRef]
- Li, B.R.; Pan, J.; Du, T.T.; Liao, Z.; Ye, B.; Zou, W.B.; Chen, H.; Ji, J.T.; Zheng, Z.H.; Wang, D.; et al. Risk Factors for Steatorrhea in Chronic Pancreatitis: A Cohort of 2153 Patients. Sci. Rep. 2016, 6, 21381. [Google Scholar] [CrossRef]
- Correnti, J.M.; Gottshall, L.; Lin, A.; Williams, B.; Oranu, A.; Beck, J.; Chen, J.; Bennett, M.J.; Carr, R.M. Ethanol and C2 ceramide activate fatty acid oxidation in human hepatoma cells. Sci. Rep. 2018, 8, 12923. [Google Scholar] [CrossRef]
- Kinny-Koster, B.; Bartels, M.; Becker, S.; Scholz, M.; Thiery, J.; Ceglarek, U.; Kaiser, T. Plasma Amino Acid Concentrations Predict Mortality in Patients with End-Stage Liver Disease. PLoS ONE 2016, 11, e0159205. [Google Scholar] [CrossRef]
- Anastácio, L.R.; Davisson Correia, M.I. Nutrition therapy: Integral part of liver transplant care. World J. Gastroenterol. 2016, 22, 1513–1522. [Google Scholar] [CrossRef]
- Petersen, K.F.; Krssak, M.; Navarro, V.; Chandramouli, V.; Hundal, R.; Schumann, W.C.; Landau, B.R.; Shulman, G.I. Contributions of net hepatic glycogenolysis and gluconeogenesis to glucose production in cirrhosis. Am. J. Physiol. 1999, 276, E529–E535. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Ferreira Martins, A.I.; Cunha, C.E.; Anastácio, L.R.; Lima, A.S.; Correia, M.I. Negative energy balance secondary to inadequate dietary intake of patients on the waiting list for liver transplantation. Nutrition 2013, 29, 1252–1258. [Google Scholar] [CrossRef]
- Ridola, L.; Gioia, S.; Faccioli, J.; Riggio, O.; Nardelli, S. Gut liver muscle brain axis: A comprehensive viewpoint on prognosis in cirrhosis. J. Hepatol. 2022, 77, 262–263. [Google Scholar] [CrossRef]
- Tantai, X.; Liu, Y.; Yeo, Y.H.; Praktiknjo, M.; Mauro, E.; Hamaguchi, Y.; Engelmann, C.; Zhang, P.; Jeong, J.Y.; van Vugt, J.L.A.; et al. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J. Hepatol. 2022, 76, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; DiMartini, A.; Feng, S.; Brown, R.; Fallon, M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 2014, 59, 1144–1165. [Google Scholar] [CrossRef]
- Preiser, J.C.; van Zanten, A.R.; Berger, M.M.; Biolo, G.; Casaer, M.P.; Doig, G.S.; Griffiths, R.D.; Heyland, D.K.; Hiesmayr, M.; Iapichino, G.; et al. Metabolic and nutritional support of critically ill patients: Consensus and controversies. Crit. Care 2015, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Bhanji, R.A.; Mazurak, V.C.; Montano-Loza, A.J. Sarcopenia in cirrhosis: From pathogenesis to interventions. J. Gastroenterol. 2019, 54, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.; Hoermann, R.; Gani, L.; Chan, I.; Cheung, A.; Gow, P.J.; Li, A.; Zajac, J.D.; Angus, P. Low testosterone levels as an independent predictor of mortality in men with chronic liver disease. Clin. Endocrinol. 2012, 77, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Nishiguchi, S.; Iijima, H. Sarcopenic Obesity in Liver Cirrhosis: Possible Mechanism and Clinical Impact. Int. J. Mol. Sci. 2021, 22, 1917. [Google Scholar] [CrossRef]
- Dasarathy, S.; Merli, M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J. Hepatol. 2016, 65, 1232–1244. [Google Scholar] [CrossRef]
- Sreedhara, R.; Avram, M.M.; Blanco, M.; Batish, R.; Avram, M.M.; Mittman, N. Prealbumin is the best nutritional predictor of survival in hemodialysis and peritoneal dialysis. Am. J. Kidney Dis. 1996, 28, 937–942. [Google Scholar] [CrossRef]
- Dağ, Z.; Köseoğlu, H.; Kekilli, M. The use of prealbumin as a predictor of malnutrition in cirrhotic patients and the effect of nutritional support in patients with low prealbumin levels. Turk. J. Med. Sci. 2020, 50, 398–404. [Google Scholar] [CrossRef]
- Hasse, J.; Strong, S.; Gorman, M.A.; Liepa, G. Subjective global assessment: Alternative nutrition-assessment technique for liver-transplant candidates. Nutrition 1993, 9, 339–343. [Google Scholar] [PubMed]
- Bakshi, N.; Singh, K. Nutrition assessment in patients undergoing liver transplant. Indian J. Crit. Care Med. 2014, 18, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Hasse, J. Liver transplantation: The benefits of nutrition therapy in the liver transplant patient. In Recent Developments in Transplantation Medicine: Liver Transplantation, 1st ed.; Klintmalm, G., Ed.; Physicians and Scientists Publishing Co.: Glenview, IL, USA, 1996; Volume 3, pp. 81–100. [Google Scholar]
- Yasutake, K.; Koga, S.; Hokko, Y.; Ikemoto, M.; Yaguchi, Y.; Sakai, H.; Murata, Y.; Ohe, K.; Kohjima, M.; Nakamuta, M.; et al. Relevance of the Mini Nutritional Assessment in cirrhotic liver disease patients. Asia Pac. J. Clin. Nutr. 2018, 27, 300–305. [Google Scholar] [PubMed]
- Wu, Y.; Zhu, Y.; Feng, Y.; Wang, R.; Yao, N.; Zhang, M.; Liu, X.; Liu, H.; Shi, L.; Zhu, L.; et al. Royal Free Hospital-Nutritional Prioritizing Tool improves the prediction of malnutrition risk outcomes in liver cirrhosis patients compared with Nutritional Risk Screening 2002. Br. J. Nutr. 2020, 124, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B. ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Nishikawa, H.; Yoh, K.; Enomoto, H.; Ishii, N.; Iwata, Y.; Takata, R.; Nishimura, T.; Aizawa, N.; Sakai, Y.; Ikeda, N.; et al. The Relationship between Controlling Nutritional (CONUT) Score and Clinical Markers among Adults with Hepatitis C Virus Related Liver Cirrhosis. Nutrients 2018, 10, 1185. [Google Scholar] [CrossRef]
- Casas Deza, D.; Betoré Glaria, M.E.; Sanz-París, A.; Lafuente Blasco, M.; Fernández Bonilla, E.M.; Bernal Monterde, V.; Arbonés Mainar, J.M.; Fuentes Olmo, J. Mini Nutritional Assessment—Short Form Is a Useful Malnutrition Screening Tool in Patients with Liver Cirrhosis, Using the Global Leadership Initiative for Malnutrition Criteria as the Gold Standard. Nutr. Clin. Pract. 2021, 36, 1003–1010. [Google Scholar] [CrossRef]
- Booi, A.N.; Menendez, J.; Norton, H.J.; Anderson, W.E.; Ellis, A.C. Validation of a screening tool to identify undernutrition in ambulatory patients with liver cirrhosis. Nutr. Clin. Pract. 2015, 30, 683–689. [Google Scholar] [CrossRef]
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: Characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J. Parenter. Enteral. Nutr. 2012, 36, 275–283. [Google Scholar] [CrossRef]
- Borhofen, S.M.; Gerner, C.; Lehmann, J.; Fimmers, R.; Gortzen, J.; Hey, B.; Geiser, F.; Strassburg, C.P.; Trebicka, J. The Royal Free Hospital-Nutritional Prioritizing Tool Is an Independent Predictor of Deterioration of Liver Function and Survival in Cirrhosis. Dig. Dis. Sci. 2016, 61, 1735–1743. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, A.; Papatheodoridis, G.V.; Alexopoulou, A.; Deutsch, M.; Vlachogiannakos, I.; Ioannidou, P.; Papageorgiou, M.V.; Papadopoulos, N.; Tsibouris, P.; Prapa, A.; et al. Evaluation of the effectiveness of eight screening tools in detecting risk of malnutrition in cirrhotic patients: The KIRRHOS study. Br. J. Nutr. 2019, 122, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Mazurak, V.C.; Tandon, P.; Montano-Loza, A.J. Nutrition and the transplant candidate. Liver Transpl. 2017, 23, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Jang, J.W. Sarcopenia in the prognosis of cirrhosis: Going beyond the MELD score. World J. Gastroenterol. 2015, 21, 7637–7647. [Google Scholar] [CrossRef]
- Cruz, R.J., Jr.; Dew, M.A.; Myaskovsky, L.; Goodpaster, B.; Fox, K.; Fontes, P.; DiMartini, A. Objective radiologic assessment of body composition in patients with end-stage liver disease: Going beyond the BMI. Transplantation 2013, 95, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Englesbe, M.J.; Patel, S.P.; He, K.; Lynch, R.J.; Schaubel, D.E.; Harbaugh, C.; Holcombe, S.A.; Wang, S.C.; Segev, D.L.; Sonnenday, C.J. Sarcopenia and mortality after liver transplantation. J. Am. Coll. Surg. 2010, 211, 271–278. [Google Scholar] [CrossRef]
- Pirlich, M.; Schütz, T.; Spachos, T.; Ertl, S.; Weiss, M.L.; Lochs, H.; Plauth, M. Bioelectrical impedance analysis is a useful bedside technique to assess malnutrition in cirrhotic patients with and without ascites. Hepatology 2000, 32, 1208–1215. [Google Scholar] [CrossRef]
- Tandon, P.; Ney, M.; Irwin, I.; Ma, M.M.; Gramlich, L.; Bain, V.G.; Esfandiari, N.; Baracos, V.; Montano-Loza, A.J.; Myers, R.P. Severe muscle depletion in patients on the liver transplant wait list: Its prevalence and independent prognostic value. Liver Transpl. 2012, 18, 1209–1216. [Google Scholar] [CrossRef]
- Meza-Junco, J.; Montano-Loza, A.J.; Baracos, V.E.; Prado, C.M.; Bain, V.G.; Beaumont, C.; Esfandiari, N.; Lieffers, J.R.; Sawyer, M.B. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J. Clin. Gastroenterol. 2013, 47, 861–870. [Google Scholar] [CrossRef]
- Krell, R.W.; Kaul, D.R.; Martin, A.R.; Englesbe, M.J.; Sonnenday, C.J.; Cai, S.; Malani, P.N. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl. 2013, 19, 1396–1402. [Google Scholar] [CrossRef]
- Montano-Loza, A.J.; Duarte-Rojo, A.; Meza-Junco, J.; Baracos, V.E.; Sawyer, M.B.; Pang, J.X.; Beaumont, C.; Esfandiari, N.; Myers, R.P. Inclusion of sarcopenia within MELD (MELD-sarcopenia) and the prediction of mortality in patients with cirrhosis. Clin. Transl. Gastroenterol. 2015, 16, e102. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Shirabe, K.; Ikegami, T.; Harimoto, N.; Yoshizumi, T.; Soejima, Y.; Uchiyama, H.; Ikeda, T.; Baba, H.; Maehara, Y. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transpl. 2014, 20, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Macías-Rodríguez, R.U.; Ruiz-Margáin, A.; Román-Calleja, B.M.; Moreno-Tavarez, E.; Weber-Sangri, L.; González-Arellano, M.F.; Fernández-Del-Rivero, G.; Ramírez-Soto, K. Exercise prescription in patients with cirrhosis: Recommendations for clinical practice. Rev. Gastroenterol. Mex. 2019, 84, 326–343. [Google Scholar] [CrossRef]
- West, J.; Gow, P.J.; Testro, A.; Chapman, B.; Sinclair, M. Exercise physiology in cirrhosis and the potential benefits of exercise interventions: A review. J. Gastroenterol. Hepatol. 2021, 36, 2687–2705. [Google Scholar] [CrossRef]
- Tandon, P.; Ismond, K.P.; Riess, K.; Duarte-Rojo, A.; Al-Judaibi, B.; Dunn, M.A.; Holman, J.; Howes, N.; Haykowsky, M.J.F.; Josbeno, D.A.; et al. Exercise in cirrhosis: Translating evidence and experience to practice. J. Hepatol. 2018, 69, 1164–1177. [Google Scholar] [CrossRef]
- García-Pagàn, J.C.; Santos, C.; Barberá, J.A.; Luca, A.; Roca, J.; Rodriguez-Roisin, R.; Bosch, J.; Rodés, J. Physical exercise increases portal pressure in patients with cirrhosis and portal hypertension. Gastroenterology 1996, 111, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Plauth, M.; Burgos Peláez, R.; Rivera Irigoin, R. ESPEN Practical Guideline: Clinical nutrition in liver disease. Nutr. Hosp. 2022, 39, 434–472. [Google Scholar] [CrossRef]
- Vasques, J.; Guerreiro, C.S.; Sousa, J.; Pinto, M.; Cortez-Pinto, H. Nutritional support in cirrhotic patients with sarcopenia. Clin. Nutr. ESPEN 2019, 33, 12–17. [Google Scholar] [CrossRef]
- Koretz, R.L. Nutritional support in liver disease—An updated systematic review. Curr. Opin. Gastroenterol. 2023, 39, 115–124. [Google Scholar] [CrossRef]
- Aslam, M.; Farooq, S.; Rizwan, B.; Asghar, A. Assessment of nutritional status of the cirrhotic patients on enteral and parenteral feeding. Nutr. Health 2022, 28, 69–76. [Google Scholar] [CrossRef]
- Besen, B.A.; Gobatto, A.L.; Melro, L.M.; Maciel, A.T.; Park, M. Fluid and electrolyte overload in critically ill patients: An overview. World J. Crit. Care. Med. 2015, 4, 116–129. [Google Scholar] [CrossRef]
- Lucchinetti, E.; Lou, P.H.; Wawrzyniak, P.; Wawrzyniak, M.; Scharl, M.; Holtzhauer, G.A.; Krämer, S.D.; Hersberger, M.; Rogler, G.; Zaugg, M. Novel Strategies to Prevent Total Parenteral Nutrition-Induced Gut and Liver Inflammation, and Adverse Metabolic Outcomes. Mol. Nutr. Food Res. 2021, 65, e1901270. [Google Scholar] [CrossRef]
- Videla, L.A.; Hernandez-Rodas, M.C.; Metherel, A.H.; Valenzuela, R. Influence of the nutritional status and oxidative stress in the desaturation and elongation of n-3 and n-6 polyunsaturated fatty acids: Impact on non-alcoholic fatty liver disease. Prostaglandins Leukot. Essent Fatty Acids 2022, 181, 102441. [Google Scholar] [CrossRef]
- Fallahzadeh, M.A.; Rahimi, R.S. Hepatic Encephalopathy and Nutrition Influences: A Narrative Review. Nutr. Clin. Pract. 2020, 35, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter. Enteral. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef]
- Plauth, M.; Cabré, E.; Riggio, O.; Assis-Camilo, M.; Pirlich, M.; Kondrup, J.; DGEM (German Society for Nutritional Medicine); Ferenci, P.; Holm, E.; Vom Dahl, S.; et al. ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin. Nutr. 2006, 25, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Kouz, J.; Vincent, C.; Leong, A.; Dorais, M.; Räkel, A. Weight gain after orthotopic liver transplantation: Is nonalcoholic fatty liver disease cirrhosis a risk factor for greater weight gain? Liver Transpl. 2014, 20, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.S.; Saigal, S.; Saraf, N.; Mohanka, R.; Rastogi, A.; Goja, S.; Menon, P.B.; Mishra, S.; Mittal, A.; Soin, A.S. Sarcopenic obesity with metabolic syndrome: A newly recognized entity following living donor liver transplantation. Clin. Transpl. 2015, 29, 211–215. [Google Scholar] [CrossRef]
- Parekh, J.; Corley, D.A.; Feng, S. Diabetes, hypertension and hyperlipidemia: Prevalence over time and impact on long-term survival after liver transplantation. Am. J. Transpl. 2012, 12, 2181–2187. [Google Scholar] [CrossRef]
- Anastácio, L.R.; Ferreira, L.G.; de Sena Ribeiro, H.; Liboredo, J.C.; Lima, A.S.; Correia, M.I.T.D. Metabolic syndrome after liver transplantation: Prevalence and predictive factors. Nutrition 2011, 27, 931–937. [Google Scholar] [CrossRef] [PubMed]
| Studies (Year) | n | Incidence of Sarcopenia | Main Results Related to the Occurrence of Sarcopenia |
|---|---|---|---|
| Merli et al. (2010) [56] | 38 | 53% | Increased LOS in the hospital and ICU and increased incidence of perioperative infections in patients with sarcopenia |
| Englesbe et al. (2010) [57] | 163 | 25% | Higher mortality after LT |
| Montano-Loza et al. (2012) [58] | 112 | 40% | Sarcopenia was independently associated with mortality |
| Tandon et al. (2012) [59] | 142 | 41% | Sarcopenia was associated with increased mortality on the LT waiting list |
| Meza-Junco et al. (2013) [60] | 116 | 30% | Sarcopenia was independently associated with mortality |
| Krell et al. (2013) [61] | 207 | 33% | Higher risk of infectious complications and mortality after LT |
| DiMartini et al. (2013) [62] | 338 | 68% | Muscle mass predicted longer LOS in ICU, total LOS, and a longer time on mechanical ventilation |
| Masuda et al. (2014) [63] | 204 | 47% | Sarcopenia was an independent prognostic factor for post-LT mortality and postoperative sepsis |
| Type of Parameter | Value Associated with the Presence of Malnutrition | |
|---|---|---|
| Anthropometric measurement | BMI | <18.5 kg/m2 and >30 kg/m2 |
| Laboratory biomarkers | Albumin | <3 g/dL2 |
| Prealbumin | <160 mg/dL2 | |
| Vitamin levels | Consider malnutrition in the presence of low levels | |
| Nutrition Scores | SGA | SGA B (mild) or C (severe) |
| LDUST | ≥2 boxes in columns B or C | |
| RFH–NPT | Scores 1 and 2–7 correspond to moderate and high risk | |
| Liver Scores | CTP | B (mild) or C (severe) |
| MELD | >15 | |
| Muscle mass | BIA | Evaluate muscle mass and the presence of sarcopenia |
| CT scan | ||
| MRI | ||
| Physical activity | HRQoL | >3 reflects a poor quality of life |
| Nutritional and metabolic assessment | |
| Should be performed regularly in all patients to detect malnutrition, especially those with acute liver failure | Evaluation should include measurement of weight, height, body mass index (BMI), arm circumference, and serum albumin/prealbumin |
| Evaluate the occurrence of hypoglycemia in the setting of acute liver failure | |
| Screening of alcohol consumption | Alcohol worsens liver disease and increases its associated complications |
| Dietary advice | |
| Patients at risk of or diagnosed with malnutrition | Individualized dietary advice from a registered dietitian/nutritionist. |
| Nutrition medical therapy and nutritional considerations | |
| Protein intake | High protein intake to avoid muscle loss and sarcopenia |
| Consider frequent monitoring of ammonia levels (i.e., 24–48 h) to modulate protein intake | |
| Energy intake | Adequate to maintain the patient’s nutritional status. In some cases, it may be necessary to increase energy intake to compensate for increased energy expenditure due to acute complications or malnutrition |
| Carbohydrates as the main source of energy | |
| Fats should be limited to avoid liver damage from dyslipidemia | |
| Consider nutritional supplements | To improve nutritional status or achieve nutritional requirements |
| Consumption of a snack at night (if possible oral route) with at least 50 g of carbohydrates | |
| Careful sodium administration | Adequate to avoid ascites/fluid retention |
| Metabolic disturbances | |
| Careful control of acid–base balance | Prevent complications such as lactic acidosis |
| Correction of electrolyte disturbances | Hyponatremia, hypokalemia, and hypomagnesemia |
| Route of administration | |
| Oral feeding | Route of choice in the absence of severe HE |
| Enteral feeding by nasogastric tube | Insufficient oral intake or not tolerated due to complications such as ascites or HE |
| Consider post-pyloric tube if high risk of bronchial aspiration | |
| Consider parenteral nutrition | Total or supplementary PN if they cannot tolerate enteral feedings or if they have compromised intestinal absorption. |
| Prevention and treatment of sarcopenia | |
| Protein supplementation combined with physiotherapy (e.g., resistance exercise training) | Avoid muscle depletion |
| L-leucine | Reverse the decrease in muscle protein balance due to hyperammonemia |
| Dietary recommendations | High protein diet (≈1.5 g/kg/day) with 30–40 kcal/kg/day |
| Consider nutritional supplements | |
| Specific recommendations | |
| Hepatic encephalopathy | Reduce protein intake to avoid worsening of hepatic encephalopathy |
| Consider supplementation with BCAAs | |
| Caution with the enteral route due to the high risk of bronchial aspiration (i.e., HE III–IV) | |
| Bleeding complications | Prevent (i.e., frequent screening of coagulation disorder) and treat coagulopathy (i.e., vitamin K administration) |
| Liver-disease-related complications (i.e., portal hypertension, hepatic encephalopathy, and ascites) | Prompt drug treatment to prevent or treat complications that ultimately may affect the nutritional status |
| Vitamin D | Correction of deficiency |
| Screening of nutrition status | |||
| |||
| General considerations for the delivery of nutrition therapy | |||
| |||
| Nutrition therapy route | |||
| EN |
| ||
| PN | Consider in the presence of absolute or relative contraindications for EN:
| ||
| Nutritional requirements | |||
| Energy | 20–25 Kcal/Kg/day | ||
| Protein | 1.2–2 g/Kg/day | ||
| Slowly increase EN rate considering clinical state and EN tolerance for 48–72 h | |||
| Strategies to optimize nutrition therapy and avoid complications | |||
| Recommended strategy | Objective | ||
| Bed position > 30–45° | Avoid regurgitation, vomiting, and aspiration | ||
| Prokinetic agents (i.e., Erythromycin and/or Metoclopramide) |
| ||
| Post-pyloric NG tube placement | |||
| Optimize drugs that interfere with gastrointestinal function (e.g., opioids) |
| ||
| Avoid fluid overload | |||
| Early mobilization and physiotherapy | Avoid progression or development of sarcopenia | ||
| Vitamin and trace element supplementation | Avoid micronutrient deficiency | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo-Blanco, M.Á.; Lopez-Delgado, J.C.; Sarria-Guerrero, J.A. Nutrition Therapy in Critically Ill Patients with Liver Disease: A Narrative Review. Livers 2023, 3, 529-544. https://doi.org/10.3390/livers3030036
Hidalgo-Blanco MÁ, Lopez-Delgado JC, Sarria-Guerrero JA. Nutrition Therapy in Critically Ill Patients with Liver Disease: A Narrative Review. Livers. 2023; 3(3):529-544. https://doi.org/10.3390/livers3030036
Chicago/Turabian StyleHidalgo-Blanco, Miguel Ángel, Juan Carlos Lopez-Delgado, and José Antonio Sarria-Guerrero. 2023. "Nutrition Therapy in Critically Ill Patients with Liver Disease: A Narrative Review" Livers 3, no. 3: 529-544. https://doi.org/10.3390/livers3030036
APA StyleHidalgo-Blanco, M. Á., Lopez-Delgado, J. C., & Sarria-Guerrero, J. A. (2023). Nutrition Therapy in Critically Ill Patients with Liver Disease: A Narrative Review. Livers, 3(3), 529-544. https://doi.org/10.3390/livers3030036







