Breath Analysis in Children with Ketogenic Glycogen Storage Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Exhaled Breath Collection and Analysis
2.2. Statistical Analysis
3. Results
3.1. Participant Characteristics
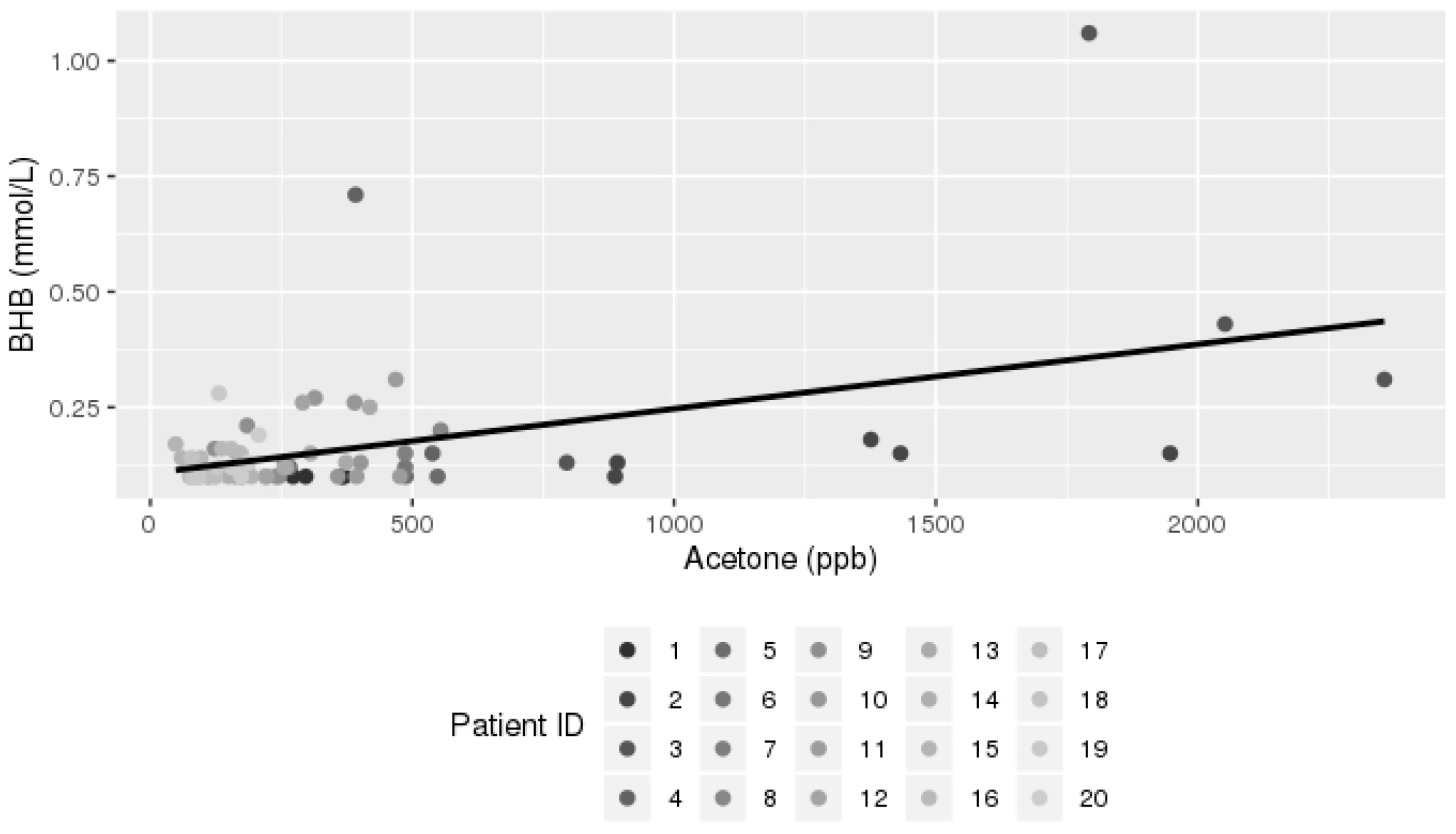
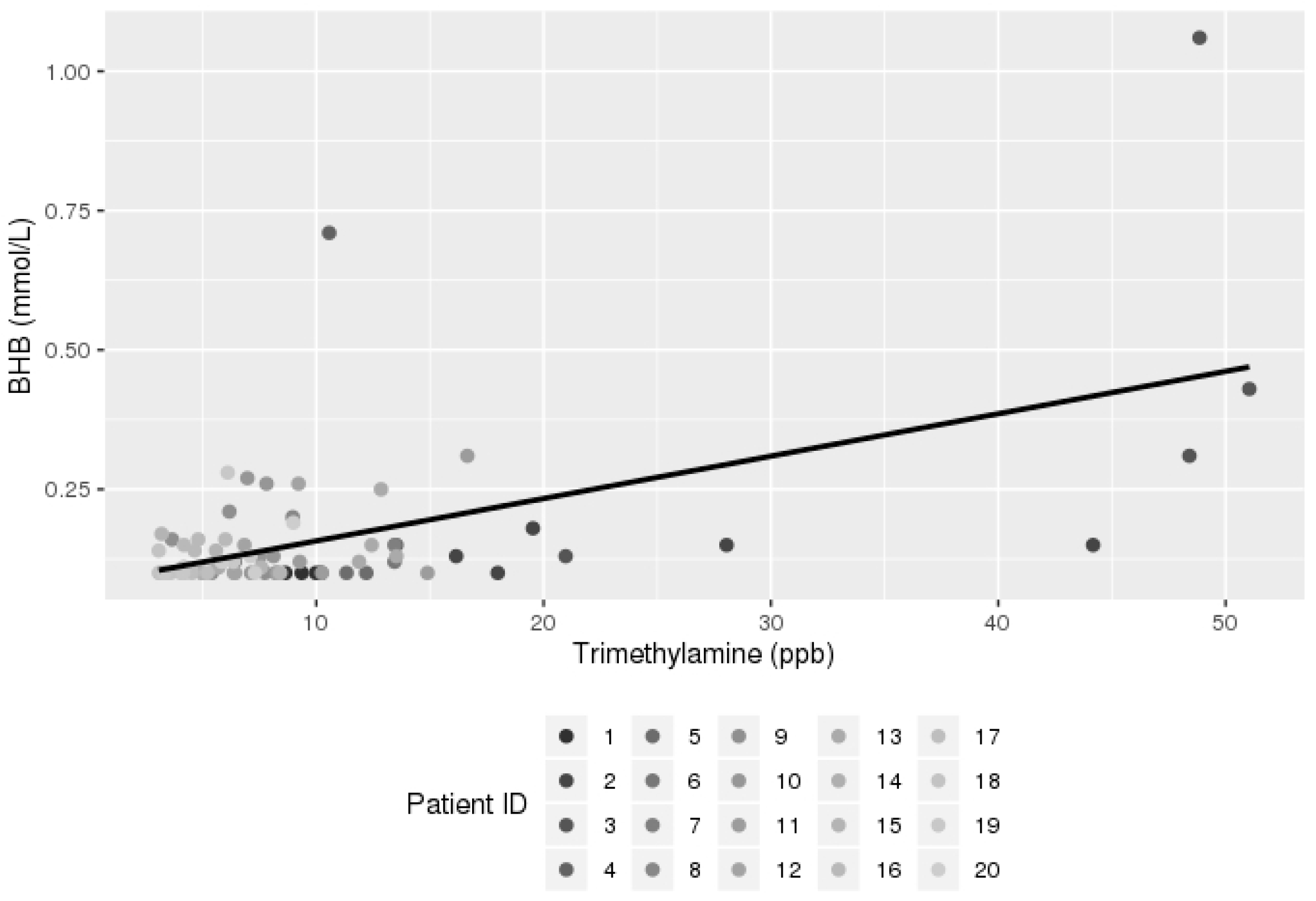
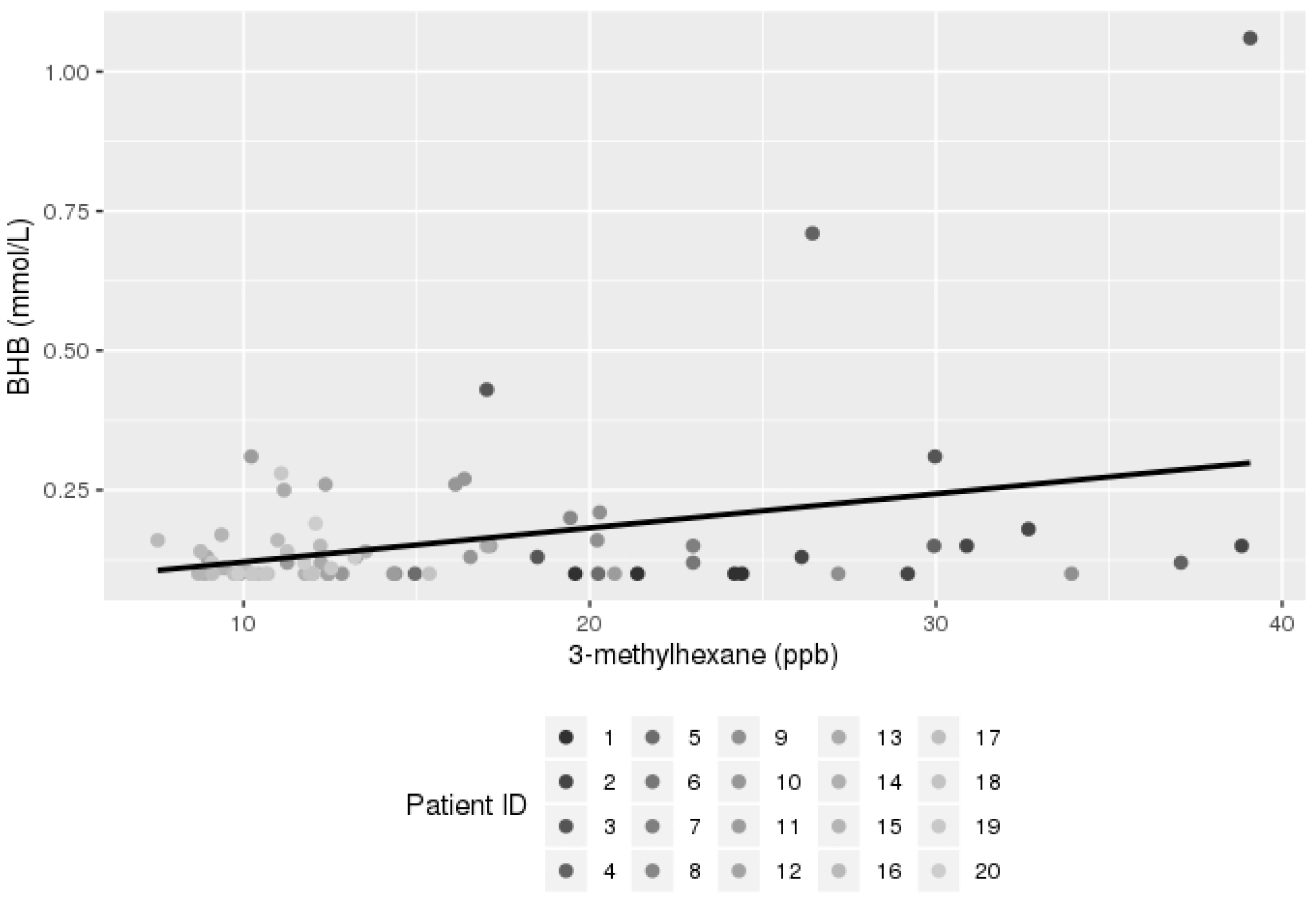
3.2. Breath Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hendriksz, C.J.; Gissen, P. Glycogen storage disease. Paediatr. Child Health 2015, 25, 139–144. [Google Scholar] [CrossRef]
- Ozen, H. Glycogen storage diseases: New perspectives. World J. Gastroenterol. 2007, 13, 2541–2553. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.A.; Weinstein, D.A. Glycogen storage diseases: Diagnosis, treatment and outcome. Transl. Sci. Rare Dis. 2016, 1, 45–72. [Google Scholar] [CrossRef]
- Tsilianidis, L.A.; Fiske, L.M.; Siegel, S.; Lumpkin, C.; Hoyt, K.; Wasserstein, M.; Weinstein, D.A. Aggressive therapy improves cirrhosis in glycogen storage disease type, I.X. Mol. Genet. Metab. 2013, 109, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Kuru, B.; Sever, M.; Aksay, E.; Dogan, T.; Yalcin, N.; Eren, E.S.; Ustuner, F. Comparing finger-stick β-hydroxybutyrate with dipstick urine tests in the detection of ketone bodies. Turkiye Acil Tip Derg. 2014, 14, 47–52. [Google Scholar] [CrossRef]
- Shirasu, M.; Touhara, K. The scent of disease: Volatile organic compounds of the human body related to disease and disorder. J. Biochem. 2011, 150, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Paschke, K.M.; Mashir, A.; Dweik, R.A. Clinical applications of breath testing. F1000 Med. Rep. 2010, 2, 56. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, K.K.; Gupta, S. Biochemistry, Ketogenesis; StatPearls: Treasure Island, FL, USA, 2018; p. 493179. [Google Scholar]
- Ercal, B.; Crawford, P.A. 37—Ketone Body Metabolism in the Neonate, 5th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Pasquel, F.J.; Umpierrez, G.E. Hyperglycemic Crises, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Ruzsányi, V.; Kalapos, M.P. Breath acetone as a potential marker in clinical practice. J. Breath Res. 2017, 11, 024002. [Google Scholar] [CrossRef] [PubMed]
- Musa-Veloso, K.; Likhodii, S.S.; Rarama, E.; Benoit, S.; Liu, Y.M.C.; Chartrand, D.; Curtis, R.; Carmant, L.; Lortie, A.; Comeau, F.J.E.; et al. Breath acetone predicts plasma ketone bodies in children with epilepsy on a ketogenic diet. Nutrition 2006, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Gao, Z.; Liu, Y.; Cheng, Y.; Yu, M.; Zhao, L.; Duan, Y.; Liu, Y. Breath ketone testing: A new biomarker for diagnosis and therapeutic monitoring of diabetic ketosis. BioMed Res. Int. 2014, 2014, 869186. [Google Scholar] [CrossRef] [PubMed]
- Tanda, N.; Hinokio, Y.; Washio, J.; Takahashi, N. Analysis of ketone bodies in exhaled breath and blood of ten healthy Japanese at OGTT using a portable gas chromatograph. J. Breath Res. 2014, 8, 046008. [Google Scholar] [CrossRef] [PubMed]
- Fraser, T.R.; Gilmour, A.; Prentice, E. Tension is a more certain form of treatment on both measurements in diabetes conclusion tion with a secondary disorder of coagulation in the the fibrinoid lesion and red-blood-cell fragmentation patients overnight fasting levels of both breath acetone P-hydroxybutyrate sampled during prolonged fasting (10–36 days). Forty-Five 1969, 1282, 1282–1286. [Google Scholar]
- Wang, C.; Mbi, A.; Shepherd, M. A Study on Breath Acetone in Diabetic Patients Using a Cavity Ringdown Breath Analyzer: Exploring Correlations of Breath Acetone with Blood Glucose and Glycohemoglobin A1C. IEEE Sens. J. 2010, 10, 54–63. [Google Scholar] [CrossRef]
- Brown, J.M.; Hazen, S.L. The Gut Microbial Endocrine Organ: Bacterially Derived Signals Driving Cardiometabolic Diseases. Annu. Rev. Med. 2015, 66, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.M.; Wang, Z.; Lee, R.; Meng, Y.; Che, N.; Charugundla, S.; Qi, H.; Wu, J.; Pan, C.; Brown, J.M.; et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J. Lipid Res. 2015, 56, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Kohlmüller, D.; Kochen, W. Is n-pentane really an index of lipid peroxidation in humans and animals? A methodological reevaluation. Anal. Biochem. 1993, 210, 268–276. [Google Scholar] [CrossRef]
- Amann, A.; de Lacy Costello, B.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Kannan, K.; Lim, G. Ketosis (acetoacetate) can generate oxygen radicals and cause increased lipid peroxidation and growth inhibition in human endothelial cells. Free Radic. Biol. Med. 1998, 25, 1083–1088. [Google Scholar] [CrossRef]
- Eng, K.; Alkhouri, N.; Cikach, F.; Patel, N.; Yan, C.; Grove, D.; Lopez, R.; Rome, E.; Dweik, R.A. Analysis of breath volatile organic compounds in children with chronic liver disease compared to healthy controls. J. Breath Res. 2015, 9, 26002. [Google Scholar] [CrossRef] [PubMed]
- You, K.W.; Ge, Y.S.; Qian, Y.X.; Liu, W.; Feng, B.; Zhang, Y.N.; Ning, Z.W.; Hu, B.; Zhao, S.T. Volatile organic compounds concentrations and sources inside new air-conditioned bus. Huan Jing Ke Xue = Huanjing Kexue 2008, 29, 1436–1440. Available online: http://www.ncbi.nlm.nih.gov/pubmed/18624220 (accessed on 21 June 2018). [PubMed]

| Molecule | Precursor | Product Mass |
|---|---|---|
| Acetone | H3O+ | 59 |
| NO+ | 88 | |
| Pentane | O2+ | 42 |
| 72 | ||
| 3-methylhexane | NO+ | 99 |
| O2+ | 71 | |
| Carbon Disulfide | O2+ | 76 |
| Trimethylamine | H3O+ | 58 |
| 60 | ||
| NO+ | 59 | |
| O2+ | 58 | |
| 59 |
| Total (N = 20) Mean (Range) | |
|---|---|
| Age (year) | 8.9 (5.7–15.7) |
| BMI (kg/m2) | 20 (14.7–31.7) |
| BMI percentile | 72.4 (20.4–99.8) |
| WBC (k/µL) | 5.5 (3.3–8.5) |
| Hemoglobin (g/dL) | 12.7 (9.9–14.0) |
| MCV (fL) | 81.3 (69.9–88.6) |
| Platelets (k/µL) | 249.2 (176.0–398.0) |
| AST (U/L) | 54.4 (14–487) |
| ALT(U/L) | 46.1 (10.0–409.0) |
| ALK. Phosphatase (U/L) | 224.3 (110.0–407.0) |
| Total Bilirubin (mg/dL) | 0.3 (0.2–0.7) |
| Albumin (g/dL) | 4.2 (3.6–4.9) |
| Total Cholesterol (mg/dL) | 143.6 (105.0–216.0) |
| LDL (mg/dL) | 78.9 (38.0–157.0) |
| HDL (mg/dL) | 49.9 (21.0–74.0) |
| TG (mg/dL) | 76.4 (27.0–222.0) |
| CK (U/L) | 269.3(41.0–3040.0) |
| ESR (mm/h) | 7.6 (2.0–26.0) |
| Ferritin (ng/mL) | 35.1 (6.8–122.1) |
| TIBC (µg/dL) | 348.6 (251.0–514.0) |
| Prealbumin (mg/dL) | 17.6 (10.0–27.0) |
| Breath Element | Univariate Mixed Model Estimate | Standard Error | p Value |
|---|---|---|---|
| Acetone | 0.000141 | 0.000032 | <0.0001 |
| Trimethylamine (TMA) | 0.007635 | 0.001357 | <0.0001 |
| Pentane | 0.003248 | 0.000777 | 0.0001 |
| 3-methylhexane | 0.006502 | 0.002203 | 0.0047 |
| Carbon disulfide | 0.03799 | 0.01894 | 0.0499 |
| GSD Type | Eponym | Enzyme Involved |
|---|---|---|
| 0 | Glycogen synthetase | |
| III | Cori | Debranchig enzyme (Amylo-1,6 glucosidase) |
| VI | Hers | Liver phosphorylase |
| IX | Phosphorylase kinase |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabbany, M.N.; Conjeevaram Selvakumar, P.K.; Han, X.; Wang, X.; Grove, D.; Tonelli, A.R.; Dweik, R.A.; Minarich, L.; Radhakrishnan, K.; Alkhouri, N. Breath Analysis in Children with Ketogenic Glycogen Storage Diseases. Livers 2022, 2, 336-343. https://doi.org/10.3390/livers2040025
Kabbany MN, Conjeevaram Selvakumar PK, Han X, Wang X, Grove D, Tonelli AR, Dweik RA, Minarich L, Radhakrishnan K, Alkhouri N. Breath Analysis in Children with Ketogenic Glycogen Storage Diseases. Livers. 2022; 2(4):336-343. https://doi.org/10.3390/livers2040025
Chicago/Turabian StyleKabbany, Mohammad Nasser, Praveen Kumar Conjeevaram Selvakumar, Xiaozhen Han, Xiaofeng Wang, David Grove, Adriano R. Tonelli, Raed A. Dweik, Laurie Minarich, Kadakkal Radhakrishnan, and Naim Alkhouri. 2022. "Breath Analysis in Children with Ketogenic Glycogen Storage Diseases" Livers 2, no. 4: 336-343. https://doi.org/10.3390/livers2040025
APA StyleKabbany, M. N., Conjeevaram Selvakumar, P. K., Han, X., Wang, X., Grove, D., Tonelli, A. R., Dweik, R. A., Minarich, L., Radhakrishnan, K., & Alkhouri, N. (2022). Breath Analysis in Children with Ketogenic Glycogen Storage Diseases. Livers, 2(4), 336-343. https://doi.org/10.3390/livers2040025







