Is There a Place for Somatostatin Analogues for the Systemic Treatment of Hepatocellular Carcinoma in the Immunotherapy Era?
Abstract
1. Introduction
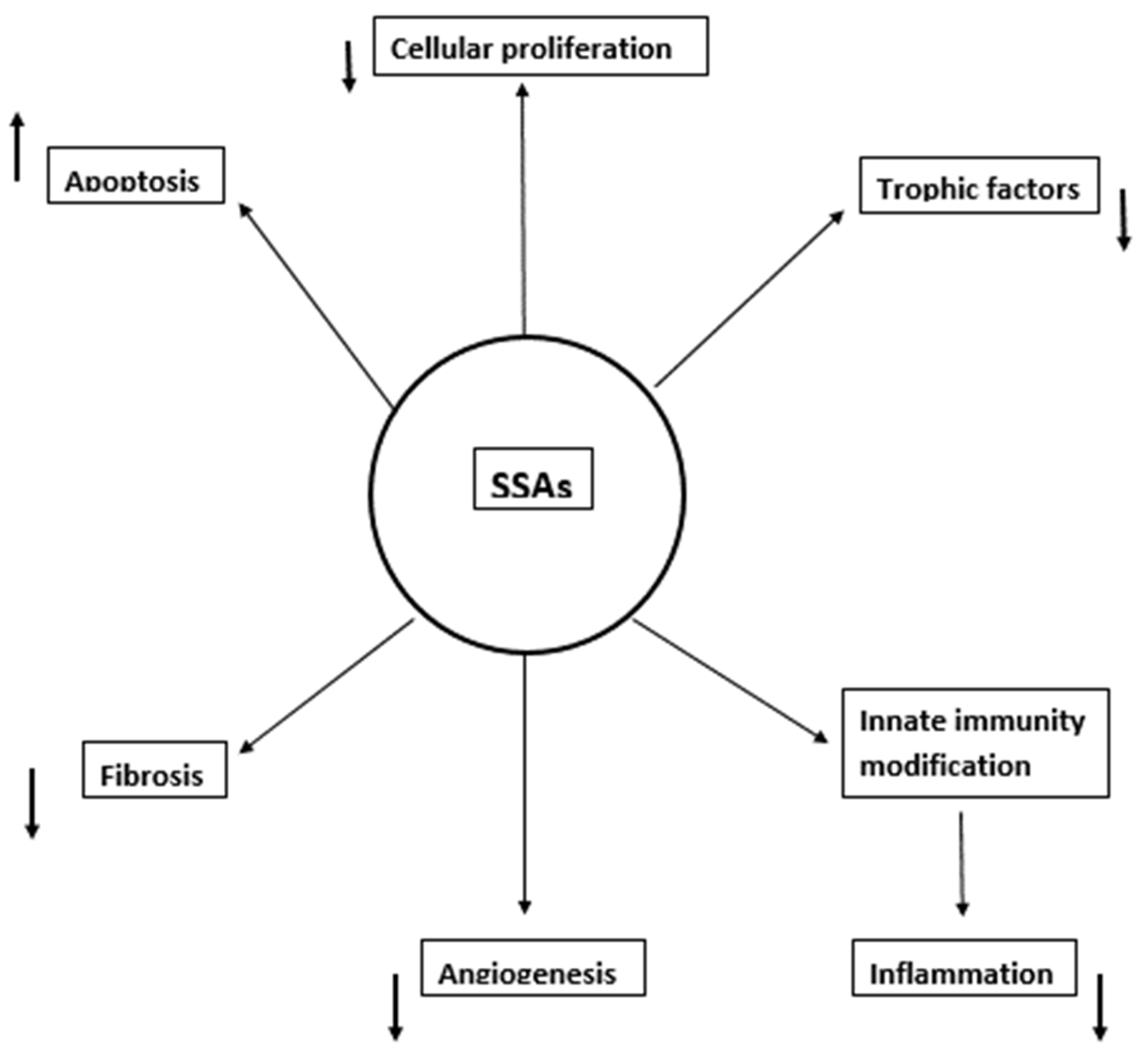
2. Mechanisms of SSAs’ Activity in HCC
3. Somatostatin Analogues as a Systemic Treatment of HCC
3.1. Positive Studies (Table 1)
| Authors | Type of Study | No. of Patients | SST Receptors Assessment | Intervention | Control | Outcomes |
|---|---|---|---|---|---|---|
| Kouroumalis E et al., 1998 [41] | RCT | 58 patients with advanced HCC | Histology in all cases | Octreotide 250 μg s.c. b.i.d in 28 patients | No treatment in 30 patients |
|
| Samonakis DN et al., 2002 [42] | Comparative observational study | 59 patients with inoperable HCC | N/A | Long-acting somatostatin analogues in 32 patients | No treatment in 27 patients |
|
| Raderer M et al., 2000 [44] | Uncontrolled study | 21 patients with inoperable HCC | Scintigraphy in 15 patients | Lanreotide 30 mg i.m. every 14 days | None |
|
| Patsanas T et al., 2004 [45] | Comparative observational study | 30 patients with unresectable HCC (group A: cirrhosis group B: non-cirrhosis) | N/A | Octreotide 500 μg s.c. b.i.d | None |
|
| Dimitroulopoulos D et al., 2007 [46] | RCT | 127 cirrhotic patients, stages A–B, due to chronic viral infections with advanced HCC, 61 patients with positive SSTR were randomized | Scintigraphy in all cases | 31 patients with SSTR positive were treated with octreotide 0.5 mg s.c. every 8 h for 6 wk., at the end of wk. 4–8 octreotide LAR 20 mg i.m. and at the end of wk. 12 and every 4 wks. octreotide LAR 30 mg i.m. | Oral placebo in 30 patients |
|
| Pan DY et al., 2003 [48] | RCT | 39 patients with inoperable HCC | N/A | 24 patients were treated with TAM + OCT | 15 patients were treated with regular chemotherapy |
|
| Gill ML et al., 2005 [49] | Comparative observational study | 42 patients with inoperable HCC | N/A | 22 patients were treated with long-acting octreotide 100mcg s.c. t.i.d for 2 wks. followed by 20mg i.m. monthly | 20 patients with no treatment due to socioeconomic issues |
|
| Shah U et al., 2009 [50] | Observational study | 22 patients with advanced inoperable HCC and CLIP score of 3 or higher | N/A | 22 patients were treated with long-acting octreotide 30 mg i.m. (or 20 mg) monthly | None | Treatment with long-acting octreotide showed:
|
| Siveke JT et al., 2003 [51] | Case report | 1 patient with advanced HCC | Scintigraphy | Octreotide 250 μg b.i.d followed by long-acting octreotide 10mg monthly | None |
|
| Borbath I et al., 2012 [52] | Case report | 1 patient with metastatic HCC | Histology and scintigraphy | Lanreotide 30 mg twice monthly | None |
|
| Li S et al., 2012 [53] | Observational study | 76 patients with inoperable HCC | Histology, 2 groups based on high or low expression of SSTR-2 and 5 in tumor tissue | Octreotide | None |
|
| Liu Y et al., 2013 [54] | Comparative observational study | 99 patients with HCC and cirrhosis who underwent curative liver resection | Histology | At one day post-surgery, all the patients were administered 20 mg octreotide LAR i.m. monthly for 12 mos. | None |
|
| Plentz RR et al., 2005 [55] | Observational study | 41 patients with advanced HCC and cirrhosis | N/A | Octreotide 250 μg t.i.d followed by long-acting octreotide 30 mg i.m. monthly | None |
|
| Schöniger-Hekele M et al., 2009 [56] | Retrospective observational study | 95 patients with HCC (BCLC stage A or B) | N/A | Octreotide | TACE, multimodal therapy, palliative care |
|
| Jia W et al., 2012 [57] | RCT | 147 patients with HCC receiving 2–4 TACE treatments | N/A | 84 patients were treated with heparin + octreotide for 12 mos. | No treatment in 63 patients |
|
| Tong H et al., 2017 [58] | RCT | 71 patients with inoperable HCC | N/A | 36 patients were treated with TACE + C + L | 35 patients were treated with TACE | During a 3-year follow-up period:
|
| Montella L et al., 2008 [59] | Observational study | 35 patients with advanced HCC and Child A or B cirrhosis | N/A | 35 patients treated with RFA + octreotide | None |
|
| Feun LG et al., 2018 [60] | Observational study | 20 patients with advanced or metastatic HCC (BCLC stage C or D) | N/A | 20 patients were treated with pasireotide LAR 60 mg i.m. monthly | None |
|
3.2. Negative Studies (Table 2)
| Authors | Type of Study | No.of Patients | SST Receptors Assessment | Intervention | Control | Outcomes |
|---|---|---|---|---|---|---|
| Rabe C et al., 2002 [61] | Retrospective multicenter cohort study | 63 patients with unresectable HCC and cirrhosis Child A–C) | N/A | Initially: 43 cases received long-acting octreotide 20–30 mg/mo. 20 cases received octreotide 50 t.i.d-300 ug t.i.d. s.c. Later: 11 patients of the s.c. group were converted to long-acting octreotide | None | At 3 mos.:
At 6 mons:
|
| Yuen MF et al., 2002 [62] | RCT | 70 patients with advanced HCC | N/A | 35 patients received a 2-week course of 250 μg short-acting octreotide twice daily followed by Sandostatin LAR 30 mg injection once every 4 wks. for 6 doses | 35 patients received placebo |
|
| Slijkhuis WA et al., 2005 [65] | Prospective cohort study | 30 patients with advanced HCC | N/A | Long-acting octreotide 30 mg i.m. every 4 to 6 wks. | None |
|
| Cebon J et al., 2006 [67] | Observational study | 63 patients with advanced HCC | Scintigraphy | Long-acting octreotide 20 mg i.m. monthly | None |
|
| Sanoff HK et al., 2015 [68] | Multicenter observational study | 24 patients with advanced HCC (46% metastatic disease) | N/A | Pasireotide LAR 60 mg i.m. monthly + everolimus 7.5 mg per os daily | None |
|
| Verset G et al., 2007 [69] | RCT | 109 patients with advanced HCC | N/A | 56 patients were treated with octreotide LAR + TAM | 53 patients were treated with TAM | No difference between the groups concerning:
|
| Becker G et al., 2007 [70] | RCT | 120 patients with advanced HCC | N/A | 60 patients were treated with long-acting octreotide 30 mg i.m. monthly | 59 patients were treated with placebo |
|
| Barbare JC et al., 2009 [71] | RCT | 272 patients with untreatable or relapsed HCC | N/A | 135 patients were treated with long-acting octreotide 30 mg i.m. monthly for 2 years | 137 patients were treated with placebo |
|
4. Is There a Place for SSAs in the Treatment of HCC Today?
5. Future Research
6. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomatarm, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer. J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ryerson, A.B.; Eheman, C.R.; Altekruse, S.F.; Ward, J.W.; Jemal, A.; Sherman, R.L.; Henley, S.J.; Holtzman, D.; Lake, A.; Noone, A.M.; et al. Annual report to the nation on the status of cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer 2016, 122, 1312–1337. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Chan, S.L.; Galle, P.R.; Rimassa, L.; Sangro, B. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J. Hepatol. 2021, 75, 960–974. [Google Scholar] [CrossRef]
- Petruzziello, A. Epidemiology of hepatitis B virus (HBV) and hepatitis C virus (HCV) related hepatocellular carcinoma. Open Virol. J. 2018, 12, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, R. Deciphering Tumor Heterogeneity in Hepatocellular Carcinoma (HCC)-Multi-Omic and Singulomic Approaches. Semin. Liver Dis. 2021, 41, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, R.C.; Pestana, R.C.; Miyamura, B.V.; Kaseb, A.O. Hepatocellular Carcinoma Immunotherapy. Annu. Rev. Med. 2022, 73, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, P. Somatostatin analogues: Multiple roles in cellular proliferation, neoplasia, and angiogenesis. Pharmacol. Ther. 2004, 102, 61–85. [Google Scholar] [CrossRef] [PubMed]
- Hasskarl, J.; Kaufmann, M.; Schmid, H.A. Somatostatin receptors in non-neuroendocrine malignancies: The potential role of somatostatin analogs in solid tumors. Future Oncol. 2011, 7, 895–913. [Google Scholar] [CrossRef]
- Kouroumalis, E.; Samonakis, D.; Notas, G. Somatostatin in hepatocellular carcinoma: Experimental and therapeutic implications. Hepatoma Res. 2018, 4, 34. [Google Scholar] [CrossRef][Green Version]
- Lahlou, H.; Saint-Laurent, N.; Estève, J.-P.; Eychene, A.; Pradayrol, L.; Pyronnet, S.; Susini, C. sst2 Somatostatin receptor inhibits cell proliferation through Ras-, Rap1-, and B-Raf-dependent ERK2 activation. J. Biol. Chem. 2003, 278, 39356–39371. [Google Scholar] [CrossRef]
- Grant, M.; Alturaihi, H.; Jaquet, P.; Collier, B.; Ujendra, U. Cell growth inhibition and functioning of human somatostatin receptor type 2 are modulated by receptor heterodimerization. Mol. Endocrinol. 2008, 22, 2278–2292. [Google Scholar] [CrossRef]
- War, S.A.; Kumar, U. Co-expression of human somatostatin receptor-2 (SSTR2) and SSTR3 modulates antiproliferative signaling and apoptosis. J. Mol. Signal. 2012, 7, 5. [Google Scholar] [CrossRef]
- Hua, Y.-P.; Yin, X.-Y.; Peng, B.-G.; Li, S.-Q.; Lai, J.-M.; Liang, H.-Z.; Liang, L.-J. Mechanisms and influence of octreotide-induced regulation of somatostatin receptor 2 on hepatocellular carcinoma. Chemotherapy 2009, 55, 312–320. [Google Scholar] [CrossRef]
- Tsagarakis, N.J.; Drygiannakis, I.; Batistakis, A.G.; Kolios, G.; Kouroumalis, E.A. Octreotide induces caspase activation and apoptosis in human hepatoma HepG2 cells. World J. Gastroenterol. 2011, 17, 313–321. [Google Scholar] [CrossRef]
- Kouroumalis, E.; Voumvouraki, A.; Augoustaki, A.; Samonakis, D.N. Autophagy in liver diseases. World J. Hepatol. 2021, 13, 6–65. [Google Scholar] [CrossRef]
- Yang, S.; Yang, L.; Li, X.; Li, B.; Li, Y.; Zhang, X.; Ma, Y.; Peng, X.; Jin, H.; Li, H. New insights into autophagy in hepatocellular carcinoma: Mechanisms and therapeutic strategies. Am. J. Cancer Res. 2019, 9, 1329–1353. [Google Scholar]
- Zou, S.; Sun, H.; Candiotti, K.A.; Peng, Y.; Zhang, Q.; Xiao, W.; Zhao, S.; Wu, L.; Yang, J. Octreotide protects against hepatic ischemia/reperfusion injury via HO-1- mediated autophagy. Acta Biochim. Biophys. Sin. Shanghai 2018, 50, 316–318. [Google Scholar] [CrossRef]
- Pivonello, C.; de Martino, M.C.; Negri, M.; Cuomo, G.; Cariati, F.; Izzo, F.; Colao, A.; Pivonello, R. The GH-IGF-SST system in hepatocellular carcinoma: Biological and molecular pathogenetic mechanisms and therapeutic targets. Infect. Agent Cancer 2014, 9, 27. [Google Scholar] [CrossRef]
- Barbieri, F.; Bajetto, A.; Pattarozzi, A.; Gatti, M.; Würth, R.; Thellung, S.; Corsaro, A.; Villa, V.; Nizzari, M.; Florio, T. Peptide receptor targeting in cancer: The somatostatin paradigm. Int. J. Pept. 2013, 2013, 926295. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.-S.; Zhai, Z.-M.; Xue, Q.; Jia, W.-D.; Xu, G.-L.; Xu, R.-N.; Sun, H.-C.; Wang, L.; Yu, J.-H. Octreotide acts as an antitumor angiogenesis compound and suppresses tumor growth in nude mice bearing human hepatocellular carcinoma xenografts. J. Cancer Res. Clin. Oncol. 2003, 129, 327–334. [Google Scholar] [CrossRef]
- de la Torre, N.G.; Wass, J.A.; Turner, H.E. Antiangiogenic effects of somatostatin analogues. Clin. Endocrinol. 2002, 57, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.H.; Wen, S.L.; Feng, S.; Yang, W.J.; Lu, Y.Y.; Tong, H.; Liu, R.; Tang, S.H.; Huang, Z.Y.; Tang, Y.M.; et al. Celecoxib and octreotide synergistically ameliorate portal hypertension via inhibition of angiogenesis in cirrhotic rats. Angiogenesis 2016, 19, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Dalm, V.A.S.H.; Hofland, L.J.; Lamberts, S.W. Future clinical prospects in somatostatin/cortistatin/somatostatin receptor field. Mol. Cell Endocrinol. 2008, 286, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Valatas, V.; Kolios, G.; Manoussou, P.; Xidakis, C.; Notas, G.; Ljumovic, D.; Kouroumalis, E. Secretion of inflammatory mediators by isolated rat Kupffer cells: The effect of octreotide. Reg. Pept. 2004, 120, 215–225. [Google Scholar] [CrossRef]
- Wan, S.; Zhao, E.; Kryczek, I.; Vatan, L.; Sadovskaya, A.; Ludema, G.; Simeone, D.M.; Zou, W.; Welling, T.H. Tumor associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology 2014, 147, 1393–1404. [Google Scholar] [CrossRef]
- Xidakis, C.; Ljumovic, D.; Manousou, P.; Notas, G.; Valatas, V.; Kolios, G.; Kouroumalis, E. Production of pro- and anti-fibrotic agents by rat Kupffer cells: The effect of octreotide. Dig. Dis. Sci. 2005, 50, 935–941. [Google Scholar] [CrossRef]
- Xidakis, C.; Kolios, G.; Valatas, V.; Notas, G.; Mouzas, I.; Kouroumalis, E. Effect of octreotide on apoptosis-related proteins in rat Kupffer cells: A possible anti-tumour mechanism. Anticancer Res. 2004, 24, 833–841. [Google Scholar]
- Zhang, C.; An, R.; Bao, Y.-W.; Meng, X.-M.; Wang, T.-Q.; Sun, H.-N.; Pan, F.-M.; Zhang, C. Inhibitory effects of octreotide on the progression of hepatic fibrosis via the regulation of Bcl-2/Bax and PI3K/AKT signaling pathways. Int. Immunopharmacol. 2019, 73, 515–526. [Google Scholar] [CrossRef]
- Baeck, C.; Wei, X.; Bartneck, M.; Fech, V.; Heymann, F.; Gassler, N.; Hittatiya, K.; Eulberg, D.; Luedde, T.; Trautwein, C.; et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) accelerates liver fibrosis regression by suppressing Ly-6C+ macrophage infiltration. Hepatology 2014, 59, 1060–1072. [Google Scholar] [CrossRef]
- Valatas, V.; Kolios, G.; Manousou, P.; Notas, G.; Xidakis, C.; Diamantis, I.; Kouroumalis, E. Octreotide regulates CC but not CXC LPS-induced chemokine secretion in rat Kupffer cells. Br. J. Pharmacol. 2004, 141, 477–487. [Google Scholar] [CrossRef]
- Neaud, V.; Faouzi, S.; Guirouilh, J.; Le Bail, B.; Balabaud, C.; Bioulac-Sage, P.; Rosenbaum, J. Human hepatic myofibroblasts increase invasiveness of hepatocellular carcinoma cells: Evidence for a role of hepatocyte growth factor. Hepatology 1997, 26, 1458–1466. [Google Scholar] [CrossRef]
- Reynaert, H.; Rombouts, K.; Jia, Y.; Urbain, D.; Chatterjee, N.; Uyama, N.; Geerts, A. Somatostatin at nanomolar concentration reduces collagen I and III synthesis by, but not proliferation of activated rat hepatic stellate cells. Br. J. Pharmacol. 2005, 146, 77–88. [Google Scholar] [CrossRef]
- Klironomos, S.; Notas, G.; Sfakianaki, O.; Kiagiadaki, F.; Xidakis, C.; Kouroumalis, E. Octreotide modulates the effects on fibrosis of TNF-α, TGF-β and PDGF in activated rat hepatic stellate cells. Regul Pept. 2014, 188, 5–12. [Google Scholar] [CrossRef]
- Fujita, T.; Narumiya, S. Roles of hepatic stellate cells in liver inflammation: A new perspective. Inflamm. Regen. 2016, 36, 1. [Google Scholar] [CrossRef]
- Lang, A.; Sakhnini, E.; Fidder, H.H.; Maor, Y.; Bar-Meir, S.; Chowers, Y. Somatostatin inhibits pro-inflammatory cytokine secretion from rat hepatic stellate cells. Liver Int. 2005, 25, 808–816. [Google Scholar] [CrossRef]
- Aziz, N.M.; Ragy, M.M.; Ahmed, S.M. Somatostatin analogue, Octreotide, improves restraint stress-induced liver injury by ameliorating oxidative stress, inflammatory response, and activation of hepatic stellate cells. Cell Stress Chaperones. 2018, 23, 1237–1245. [Google Scholar] [CrossRef]
- Schindel, D.T.; Grosfeld, J.L. Hepatic resection enhances growth of residual intrahepatic and subcutaneous hepatoma, which is inhibited by octreotide. J. Pediatr. Surg. 1997, 32, 995–997. [Google Scholar] [CrossRef]
- Jia, W.-D.; Xu, G.-L.; Wang, W.; Wang, Z.-H.; Li, J.-S.; Ma, J.-L.; Ren, W.-H.; Ge, Y.-S.; Yu, J.-H.; Liu, W.-B. A somatostatin analogue, octreotide, inhibits the occurrence of second primary tumors and lung metastasis after resection of hepatocellular carcinoma in mice. Tohoku J. Exp. Med. 2009, 218, 155–160. [Google Scholar] [CrossRef][Green Version]
- Xie, Y.; Chen, S.; Wang, C.-H.; Tang, C.-W. SOM230 combined with celecoxib prolongs survival in nude mice with HepG-2 xenografts. Cancer Biol. Ther. 2011, 12, 86–92. [Google Scholar] [CrossRef][Green Version]
- Borbath, I.; Leclercq, I.A.; Sempoux, C.; Abarca-Quinones, J.; Desaeger, C.; Horsmans, Y. Efficacy of lanreotide in preventing the occurrence of chemically induced hepatocellular carcinoma in rats. Chem. Biol. Interact. 2010, 183, 238–248. [Google Scholar] [CrossRef]
- Kouroumalis, E.; Skordilis, P.; Thermos, K.; Vasilaki, A.; Moschandrea, J.; Manousos, O.N. Treatment of hepatocellular carcinoma with octreotide: A randomised controlled study. Gut 1998, 42, 442–447. [Google Scholar] [CrossRef][Green Version]
- Samonakis, D.N.; Moschandreas, J.; Arnaoutis, T.; Skordilis, P.; Leontidis, C.; Vafiades, I.; Kouroumalis, E. Treatment of hepatocellular carcinoma with long acting somatostatin analogues. Oncol. Rep. 2002, 9, 903–907. [Google Scholar] [CrossRef]
- Samonakis, D.N.; Kouroumalis, E.A. Systemic treatment for hepatocellular carcinoma: Still unmet expectations. World J. Hepatol. 2017, 9, 80–90. [Google Scholar] [CrossRef]
- Raderer, M.; Hejna, M.H.; Müller, C.; Kornek, G.V.; Kurtaran, A.; Virgolini, I.; Fiebieger, W.; Hamilton, G.; Scheithauer, W. Treatment of hepatocellular cancer with the long- acting somatostatin analog lanreotide in vitro and in vivo. Int. J. Oncol. 2000, 16, 1197–1201. [Google Scholar] [CrossRef]
- Patsanas, T.; Kapetanos, D.; Ilias, A.; Gessiou, C.; Tzarou, V.; Kokozidis, G.; Kitis, G. Octreotide in the treatment of inoperable hepatocellular carcinoma. Ann. Gastroenterol. 2004, 17, 69–74. [Google Scholar]
- Dimitroulopoulos, D.; Xinopoulos, D.; Tsamakidis, K.; Zisimopoulos, A.; Andriotis, E.; Panagiotakos, D.; Fotopoulou, A.; Chrysohoou, C.; Bazinis, A.; Daskalopoulou, D.; et al. Long acting octreotide in the treatment of advanced hepatocellular cancer and overexpression of somatostatin receptors: Randomized placebo-controlled trial. World J. Gastroenterol. 2007, 13, 3164–3170. [Google Scholar] [CrossRef]
- Pivonello, R.; Sarno, A.D.; Vitale, G.; Ferraioli, G.; Guerra, E.; de Stefano, G. Somatostatin analogs in the treatment of hepatocellular carcinoma: Correlation between sst2 receptor expression and patients’ survival. Endocr. Rev. 2006, 34, 917–924. [Google Scholar]
- Pan, D.-Y.; Qiao, J.-G.; Chen, J.-W.; Huo, Y.-C.; Zhou, Y.-K.; Shi, H.-A. Tamoxifen combined with octreotide or regular chemotherapeutic agents in treatment of primary liver cancer: A randomized controlled trial. Hepatobiliary Pancreat. Dis. Int. 2003, 2, 211–215. [Google Scholar]
- Gill, M.L.; Atiq, M.; Sattar, S.; Khokhar, N. Treatment outcomes with long- acting octreotide in inoperable hepatocellular carcinoma: A local experience and review of literature. J. Pak. Med. Assoc. 2005, 55, 135–138. [Google Scholar]
- Shah, U.; O’Neil, B.; Allen, J.; Goldberg, R.M.; Bernard, S.; Moore, D.; Venook, A.P.; Morse, M.M. A phase II study of long-acting octreotide in patients with advanced hepatocellular carcinoma and CLIP score of 3 or higher. Gastrointest Cancer Res. 2009, 3, 45–48. [Google Scholar] [PubMed]
- Siveke, J.T.; Folwaczny, C.; Herberhold, C. Complete regression of advanced HCC with long- acting octreotide. Gut 2003, 52, 1531. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Borbath, I.; Lhommel, R.; Guiot, Y.; Coche, E.; Sempoux, C. Lanreotide treatment of metastatic hepatocellular carcinoma resulting in partial regression and more than 3 years of progression-free survival. Acta Gastroenterol. Belg. 2012, 75, 270–273. [Google Scholar] [PubMed]
- Li, S.; Liu, Y.; Shen, Z. Characterization of somatostatin receptor 2 and 5 expression in operable hepatocellular carcinomas. Hepatogastroenterology 2012, 59, 2054–2058. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, L.; Mu, Y. Somatostatin receptor subtypes 2 and 5 are associated with better survival in operable hepatitis B-related hepatocellular carcinoma following octreotide long-acting release treatment. Oncol. Lett. 2013, 6, 821–828. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Plentz, R.R.; Tillmann, H.L.; Kubicka, S.; Bleck, J.S.; Gebel, M.; Manns, M.P.; Rudolph, K.L. Hepatocellular carcinoma and octreotide: Treatment results in prospectively assigned patients with advanced tumor and cirrhosis stage. J. Gastroenterol. Hepatol. 2005, 20, 1422–1428. [Google Scholar] [CrossRef]
- Schöniger-Hekele, M.; Kettenbach, J.; Peck-Radosavljevic, M.; Müller, C. Octreotide treatment of patients with hepatocellular carcinoma–a retrospective single centre -controlled study. J. Exp. Clin. Cancer Res. 2009, 28, 142. [Google Scholar] [CrossRef]
- Jia, W.; Feng, K.; Fan, P.; Fan, G.; Yang, S.; Zhang, T.; Wei, Q.; Qian, L. Post-TACE combination therapy of heparin and octreotide results in decreased tumor metastasis in extrahepatic tumorigenesis. Cell Biochem Biophys. 2012, 62, 35–40. [Google Scholar] [CrossRef]
- Tong, H.; Wei, B.; Chen, S.; Xie, Y.-M.; Zhang, M.-G.; Zhang, L.-H.; Huang, Z.-Y.; Tang, C.-W. Adjuvant celecoxib and lanreotide following transarterial chemoembolisation for unresectable hepatocellular carcinoma: A randomized pilot study. Oncotarget 2017, 8, 48303–48312. [Google Scholar] [CrossRef]
- Montella, L.; Addeo, R.; Caraglia, M.; Faiola, V.; Guarrasi, R.; Vincenzi, B.; Palmeri, A.; Capasso, E.; Nocera, V.; Tarantino, L.; et al. Vascular endothelial growth factor monitoring in advanced hepatocellular carcinoma patients treated with radiofrequency ablation plus octreotide: A single center experience. Oncol. Rep. 2008, 20, 385–390. [Google Scholar] [CrossRef][Green Version]
- Feun, L.G.; Wangpaichitr, M.; Li, Y.-Y.; Kwon, D.; Richman, S.P.; Hosein, P.J.; Savaraj, N. Phase II trial of SOM230 (pasireotide LAR) in patients with unresectable hepatocellular carcinoma. J. Hepatocell. Carcinoma. 2018, 5, 9–15. [Google Scholar] [CrossRef]
- Rabe, C.; Pilz, T.; Allgaier, H.P.; Halm, U.; Straßer, C.; Wettstein, M.; Sauerbruch, T.; Caselmann, W.H. Clinical outcome of a cohort of 63 patients with hepatocellular carcinoma treated with octreotide. Z Gastroenterol. 2002, 40, 395–400. [Google Scholar] [CrossRef]
- Yuen, M.F.; Poon, R.T.; Lai, C.L.; Fan, S.T.; Lo, C.M.; Wong, K.W.; Wong, W.M.; Wong, B.C. A randomized placebo-controlled study of long-acting octreotide for treatment of advanced hepatocellular carcinoma. Hepatology 2002, 36, 687–691. [Google Scholar] [CrossRef]
- Kouroumalis, E.; Samonakis, D.; Skordilis, P. Octreotide treatment of hepatocellular carcinoma. Hepatology 2003, 37, 477. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Slijkhuis, W.A.; Stadheim, L.; Hassoun, Z.M.; Nzeako, U.C.; Kremers, W.K.; Talwalkar, J.A.; Gores, G.J. Octreotide therapy for advanced hepatocellular carcinoma. J. Clin. Gastroenterol. 2005, 39, 333–338. [Google Scholar] [CrossRef]
- Samonakis, D.N.; Christodoulakis, N.S.; Kouroumalis, E.A. Octreotide for unresectable hepatocellular carcinoma: Beyond the first sight. J. Clin. Gastroenterol. 2006, 40, 86–87. [Google Scholar] [CrossRef]
- Cebon, J.; Findlay, M.; Hargreaves, C.; Stockler, M.; Thompson, P.; Boyer, M.; Roberts, S.; Poon, A.; Scott, A.M.; Kalf, F.V.; et al. Somatostatin receptor expression, tumour response, and quality of life in patients with advanced hepatocellular carcinoma treated with long-acting octreotide. Br. J. Cancer 2006, 95, 853–861. [Google Scholar] [CrossRef]
- Sanoff, H.K.; Kim, R.; Ivanova, A.; Alistar, A.; McRee, A.J.; O’Neil, B.H. Everolimus and pasireotide for advanced and metastatic hepatocellular carcinoma. Investig. New Drugs 2015, 33, 505–509. [Google Scholar] [CrossRef]
- Verset, G.; Verslype, C.; Reynaert, H.; Borbath, I.; Langlet, P.; Vandebroek, A.; Peeters, M.; Houbiers, G.; Francque, S.; Arvanitakis, M.; et al. Efficacy of the combination of long-acting release octreotide and tamoxifen in patients with advanced hepatocellular carcinoma: A randomised multicentre phase III study. Br. J. Cancer 2007, 97, 582–588. [Google Scholar] [CrossRef]
- Becker, G.; Allgaier, H.-P.; Olschewski, M.; Zähringer, A.; Blum, H.E.; HECTOR Study Group. Long-acting octreotide versus placebo for treatment of advanced HCC: A randomized controlled double-blind study. Hepatology 2007, 45, 9–15. [Google Scholar] [CrossRef]
- Barbare, J.-C.; Bouché, O.; Bonnetain, F.; Dahan, L.; Lombard-Bohas, C.; Faroux, R.; Raoul, J.-L.; Cattan, S.; Lemoine, A.; Blanc, J.-F.; et al. Treatment of advanced hepatocellular carcinoma with long-acting octreotide: A phase III multicentre, randomised, double blind placebo-controlled study. Eur. J. Cancer 2009, 45, 1788–1797. [Google Scholar] [CrossRef]
- Ji, X.-Q.; Ruan, X.-J.; Chen, H.; Chen, G.; Li, S.-Y.; Yu, B. Somatostatin analogues in advanced hepatocellular carcinoma: An updated systematic review and meta-analysis of randomized controlled trials. Med. Sci. Monit. 2011, 17, RA169–RA176. [Google Scholar] [CrossRef][Green Version]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Karageorgos, S.A.; Stratakou, S.; Koulentaki, M.; Voumvouraki, A.; Mantaka, A.; Samonakis, D.; Notas, G.; Kouroumalis, E.A. Long-term change in incidence and risk factors of cirrhosis and hepatocellular carcinoma in Crete, Greece: A 25-year study. Ann. Gastroenterol. 2017, 30, 357–363. [Google Scholar] [CrossRef]
- Kaemmerer, D.; Schindler, R.; Mußbach, F.; Dahmen, U.; Altendorf-Hofmann, A.; Dirsch, O.; Sänger, J.; Schulz, S.; Lupp, A. Somatostatin and CXCR4 chemokine receptor expression in hepatocellular and cholangiocellular carcinomas: Tumor capillaries as promising targets. BMC Cancer 2017, 17, 896. [Google Scholar] [CrossRef]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef]
- Foerster, F.; Gairing, S.J.; Müller, L.; Galle, P.R. NAFLD-driven HCC: Safety and efficacy of current and emerging treatment options. J. Hepatol. 2022, 76, 446–457. [Google Scholar] [CrossRef]
- Bruix, J. Endpoints in clinical trials for liver cancer and their value in evidence-based clinical decision making: An unresolved Gordian knot. J. Hepatol. 2021, 74, 1483–1488. [Google Scholar] [CrossRef]
- Trehanpati, N.; Vyas, A.K. Immune Regulation by T Regulatory Cells in Hepatitis B Virus-Related Inflammation and Cancer. Scand. J. Immunol. 2017, 85, 175–181. [Google Scholar] [CrossRef]
- Shrestha, R.; Prithviraj, P.; Anaka, M.; Bridle, K.R.; Crawford, D.H.G.; Dhungel, B.; Steel, J.; Jayachandran, A. Monitoring immune checkpoint regulators as predictive biomarkers in hepatocellular carcinoma. Front. Oncol. 2018, 8, 269. [Google Scholar] [CrossRef]
- Langhans, B.; Nischalke, H.D.; Krämer, B.; Dold, L.; Lutz, P.; Mohr, R.; Vogt, A.; Toma, M.; Eis-Hübinger, A.M.; Nattermann, J.; et al. Role of regulatory T cells and checkpoint inhibition in hepatocellular carcinoma. Cancer Immunol. Immunother. 2019, 68, 2055–2066. [Google Scholar] [CrossRef]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef] [PubMed]
- Koulouris, A.; Tsagkaris, C.; Spyrou, V.; Pappa, E.; Troullinou, A.; Nikolaou, M. Hepatocellular Carcinoma: An Overview of the Changing Landscape of Treatment Options. J. Hepatocell. Carcinoma 2021, 8, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kudo, M.; Venook, A.P.; Ye, S.-L.; Bronowicki, J.-P.; Chen, X.-P.; Dagher, L.; Furuse, J.; Geschwind, J.-F.H.; de Guevara, L.L.; et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J. Hepatol. 2016, 65, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Targeted and immune therapies for hepatocellular carcinoma: Predictions for 2019 and beyond. World J. Gastroenterol. 2019, 25, 789–807. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Saborowski, A. Current strategies for the treatment of intermediate and advanced hepatocellular carcinoma. Cancer Treat Rev. 2020, 82, 101946. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.H.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. KEYNOTE-224 Investigators. Updated efficacy and safety of KEYNOTE-224, a phase II study of pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. Eur. J. Cancer 2022, 167, 1–12. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab as Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240, A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label phase 3 trial. Lancet Oncol 2022, 23, 77–90. [Google Scholar] [CrossRef]
- Bangaru, S.; Marrero, J.A.; Singal, A.G. Review article: New therapeutic interventions for advanced hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2020, 51, 78–89. [Google Scholar] [CrossRef]
- Dyhl-Polk, A.; Mikkelsen, M.K.; Ladekarl, M.; Nielsen, D.L. Clinical Trials of Immune Checkpoint Inhibitors in Hepatocellular Carcinoma. J. Clin. Med. 2021, 10, 2662. [Google Scholar] [CrossRef]
- Jain, A.; Chitturi, S.; Peters, G.; Yip, D. Atezolizumab and bevacizumab as first line therapy in advanced hepatocellular carcinoma: Practical considerations in routine clinical practice. World J. Hepatol. 2021, 13, 1132–1142. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150, Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef]
- de Castro, T.; Jochheim, L.S.; Bathon, M.; Welland, S.; Scheiner, B.; Shmanko, K.; Roessler, D.; Ben Khaled, N.; Jeschke, M.; Ludwig, J.M.; et al. Atezolizumab and bevacizumab in patients with advanced hepatocellular carcinoma with impaired liver function and prior systemic therapy: A real-world experience. Ther. Adv. Med. Oncol. 2022, 14, 17588359221080298. [Google Scholar] [CrossRef]
- Singh, A.; Beechinor, R.; Huynh, J.; Li, D.; Dayyani, F.; Valerin, J.; Hendifar, A.; Gong, J.; Cho, M. Immunotherapy Updates in Advanced Hepatocellular Carcinoma. Cancers 2021, 13, 2164. [Google Scholar] [CrossRef] [PubMed]
- van Doorn, D.J.; Takkenberg, R.B.; Klümpen, H.-J. Immune Checkpoint Inhibitors in Hepatocellular Carcinoma: An Overview. Pharmaceuticals 2020, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Saborowski, A. Medical therapy of HCC. J. Hepatol. 2022, 76, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Pinyol, R.; Kelley, R.K.; El-Khoueiry, A.; Reeves, H.L.; Wang, X.W.; Gores, G.J.; Villanueva, A. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat. Cancer 2022, 3, 386–401. [Google Scholar] [CrossRef]
- Sanceau, J.; Gougelet, A. Epigenetic mechanisms of liver tumor resistance to immunotherapy. World J. Hepatol. 2021, 13, 979–1002. [Google Scholar] [CrossRef]
- Rai, V.; Mukherjee, S. Targets of immunotherapy for hepatocellular carcinoma: An update. World J. Hepatol. 2022, 14, 140–157. [Google Scholar] [CrossRef]
- Finn, R.S.; Ikeda, M.; Zhu, A.X.; Sung, M.W.; Baron, A.D.; Kudo, M.; Okusaka, T.; Kobayashi, M.; Kumada, H.; Kaneko, S.; et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients with Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2020, 38, 2960–2970. [Google Scholar] [CrossRef]
- Machairas, N.; Tsilimigras, D.I.; Pawlik, T.M. Current Landscape of Immune Checkpoint Inhibitor Therapy for Hepatocellular Carcinoma. Cancers 2022, 14, 2018. [Google Scholar] [CrossRef]
- Su, D.; Wu, B.; Shi, L. Cost-effectiveness of Atezolizumab Plus Bevacizumab vs Sorafenib as First-Line Treatment of Unresectable Hepatocellular Carcinoma. JAMA Netw Open 2021, 4, e210037. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.H.; Lim, Y.S.; Yeon, J.E.; Song, T.J.; Yu, S.J.; Gwak, G.Y.; Kim, K.M.; Kim, Y.J.; Lee, J.W.; et al. Adjuvant immunotherapy with autologous cytokine- induced killer cells for hepatocellular carcinoma. Gastroenterology 2015, 148, 1383–1391e6. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.; Kim, Y.; Shuang, Z.-Y.; Zhang, Y.-J.; Lao, X.-M.; Li, Y.-Q.; Chen, M.-S.; Pawlik, T.M.; Xia, J.-C.; et al. A randomized controlled trial on patients with or without adjuvant autologous cytokine-induced killer cells after curative resection for hepatocellular carcinoma. Oncoimmunology 2015, 5, e1083671. [Google Scholar] [CrossRef]
- Jiang, Y.; Han, Q.; Zhao, H.; Zhang, J. The Mechanisms of HBV-Induced Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, 8, 435–450. [Google Scholar] [CrossRef]
- Audureau, E.; Carrat, F.; Layese, R.; Cagnot, C.; Asselah, T.; Guyader, D.; Larrey, D.; De Lédinghen, V.; Ouzan, D.; Zoulim, F.; et al. Personalized surveillance for hepatocellular carcinoma in cirrhosis—using machine learning adapted to HCV status. J. Hepatol. 2020, 73, 1434–1445. [Google Scholar] [CrossRef]
- Kurebayashi, Y.; Matsuda, K.; Ueno, A.; Tsujikawa, H.; Yamazaki, K.; Masugi, Y.; Kwa, W.T.; Effendi, K.; Hasegawa, Y.; Yagi, H.; et al. Immunovascular classification of HCC reflects reciprocal interaction between immune and angiogenic tumor microenvironments. Hepatology 2022, 75, 1139–1153. [Google Scholar] [CrossRef]
- Reynaert, H.; Colle, I. Treatment of Advanced Hepatocellular Carcinoma with Somatostatin Analogues: A Review of the Literature. Int. J. Mol. Sci. 2019, 20, 4811. [Google Scholar] [CrossRef]
- Heinrich, S.; Craig, A.J.; Ma, L.; Heinrich, B.; Greten, T.F.; Wang, X.W. Understanding tumour cell heterogeneity and its implication for immunotherapy in liver cancer using single-cell analysis. J. Hepatol. 2021, 74, 700–715. [Google Scholar] [CrossRef]
- Scheiner, B.; Pomej, K.; Kirstein, M.M.; Hucke, F.; Finkelmeier, F.; Waidmann, O.; Himmelsbach, V.; Schulze, K.; von Felden, J.; Fründt, T.W.; et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy—Development and validation of the CRAFITY score. J. Hepatol. 2022, 76, 353–363. [Google Scholar] [CrossRef]
- Cabibbo, G.; Singal, A.G. The quest for precision oncology with immune checkpoint inhibitors for hepatocellular carcinoma. J. Hepatol. 2022, 76, 262–264. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouroumalis, E.; Tsomidis, I.; Voumvouraki, A. Is There a Place for Somatostatin Analogues for the Systemic Treatment of Hepatocellular Carcinoma in the Immunotherapy Era? Livers 2022, 2, 315-335. https://doi.org/10.3390/livers2040024
Kouroumalis E, Tsomidis I, Voumvouraki A. Is There a Place for Somatostatin Analogues for the Systemic Treatment of Hepatocellular Carcinoma in the Immunotherapy Era? Livers. 2022; 2(4):315-335. https://doi.org/10.3390/livers2040024
Chicago/Turabian StyleKouroumalis, Elias, Ioannis Tsomidis, and Argryro Voumvouraki. 2022. "Is There a Place for Somatostatin Analogues for the Systemic Treatment of Hepatocellular Carcinoma in the Immunotherapy Era?" Livers 2, no. 4: 315-335. https://doi.org/10.3390/livers2040024
APA StyleKouroumalis, E., Tsomidis, I., & Voumvouraki, A. (2022). Is There a Place for Somatostatin Analogues for the Systemic Treatment of Hepatocellular Carcinoma in the Immunotherapy Era? Livers, 2(4), 315-335. https://doi.org/10.3390/livers2040024






