1. Introduction
Hepatitis B virus (HBV) is a DNA virus belonging to the family
Hepadnaviridae. HBV-infected persons may express various HBV-related antigens: hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), and hepatitis B core antigen (HBcAg), as well as HBV DNA in the sera and different tissues. Based on the expression of various HBV-related antigens or antibodies, the pathological status of the patients can be ascertained. An estimated two billion people in the world are infected with HBV at some point in their life, and usually, antibody to HBcAg (anti-HBc) is detected in their blood. A group of these patients expressing anti-HBc also express antibodies to HBsAg (anti-HBs). These HBV-infected subjects with anti-HBs are usually protected from future HBV infection. However, due to superimposed pathological conditions or the use of immune-suppressive drugs, these HBV-infected patients with anti-HBs may allow HBV replication and develop HBV-related complications [
1].
The World Health Organization (WHO) estimates that HBV has chronically infected about 296 million people worldwide. These people express HBsAg and HBV DNA in the blood. Thus, on the one hand, these people are living and permanent reservoirs of HBV infection, and they may transmit the virus to healthy persons. The WHO also estimates that about 12–25% of these patients should be treated immediately, as these patients also show evidence of liver damages (patients chronic hepatitis B (CHB)). If they remain untreated, there is a strong possibility of developing complications like cirrhosis of the liver (LC) and hepatocellular carcinoma (HCC). In 2015, the WHO proposed a program of “Elimination of Hepatitis by 2030”. They found that <1% of CHB patients were under some management strategy. However, the WHO target was to start therapy for several million CHB patients by 2030 [
2].
The management of CHB patients and HBV-infected persons has been provided by international liver organizations like the American Association for the Study of Liver Diseases (AASLD) [
3], the European Association for the Study of the Liver (EASL) [
4], and the Asia-Pacific Association for the Study of the Liver (APASL) [
5]. Most of the regional, national and local liver organizations usually follow the treatment recommendations of the AASLD, EASL, and APASL, with some local adjustments. It is now evident that the use of commercially available antiviral drugs will not be able to meet the target of “Elimination of Hepatitis by 2030”, as these drugs are endowed some inherent limitations:
Drugs of interferon (IFN) groups are costly and require administration by injection. The efficacy is also limited and mostly unable to block progression to LC and HCC.
Nucleoside analogs (NAs) can be given by the oral route and are comparatively cheap, but these drugs must be given for a prolonged period and even for life. Stopping NAS may induce a flare of hepatitis, and this may be life-threatening. NAs have limited efficacy to block complications like LC and HCC.
Thus, there is a pressing need to develop new and novel drugs for chronic HBV infection. Several drugs, both with antiviral and immunomodulatory capacities, have been developed within the last 40 years. The review will provide a bird’ eye view of the scopes and limitations of the ongoing and newly developed treatment options for CHB patients. To have broad insights into these variables, proper understandings about virology, epidemiology, and the genesis of therapy for CHB should be briefly described.
2. The Life Cycle of HBV and Development of Evidence-Based Therapeutic for CHB Patients
Hepatitis B virus (HBV) is a DNA virus belonging to the family
Hepadnaviridae and is mainly a hepatotropic virus, although the HBV has been detected in tissues other than the liver and among different immunocytes. However, is it elusive if the replication of HBV takes place in these cells and organs? The HBV enters hepatocytes mainly by sodium taurocholate transporting a polypeptide (NTCP) receptor. After entry to hepatocytes, the relaxed circular DNA of the viral genome is released at the nucleus. The HBV virion is formed by a lipid-based spherical structure, and three envelope proteins (small, middle, and large) are detected. The large envelope protein containing the receptor-binding domain is involved in viral entry into the cytoplasm by receptor-mediated endocytosis, and thus, its role in the entry of the virus into hepatocytes is highly important [
6]. In the cytoplasm, the relaxed circular DNA (rcDNA) genome of HBV is formed. The entry of the virus genome into the nucleus of the hepatocyte is mediated via microtubule-mediated transport. Various host factors, most of which are not clearly known, help the rcDNA to be converted into covalently closed circular DNA (cccDNA) in the nucleus. These cccDNA represents a stable mini-chromosomes [
7]. As and when required by cellular metabolic or other demands, the cccDNA is transcribed into pre-genomic RNA (pgRNA). This is then translated to the nucleocapsid protein and serves thereafter as the template for cDNA synthesis. Finally, the nucleocapsid with partially double-stranded HBV DNA is enveloped, and the virion is secreted. A part of the nucleocapsid is recycled into the nucleus, and the rcDNA is converted to cccDNA again, and thus, the pool of cccDNA is maintained over the years [
8]. Proper understanding of the entry of HBV DNA into the cytoplasm; their translocation; and the production of rcDNA, pgRNA, and cccDNA are extremely important for developing drugs and treating HBV-infected persons.
3. Epidemiology of HBV and Fixing Target Population
About 2 billion people of the world have been infected with HBV at some point in their life. This can be assessed by evaluating various HBV-related serological markers. According to the World Health Organization (WHO) estimates, about 296 million are chronically infected with HBV. They have expressed hepatitis B surface antigen (HBsAg) for more than six months in their blood. Most of these chronic HBV-infected persons also express HBV DNA in the blood. All chronic HBV-infected persons are living, permanent reservoirs of the HBV and may transmit HBV to healthy, noninfected persons. Thus, they maintain the permanent pool of future HBV infections [
2].
4. Primary Approaches of Repurposing Drugs for CHB
As CHB is induced by HBV, handling HBV has been the main target of therapy of CHB patients during the last 40 years. However, CHB patients bear two different spectrums. The first is the persistency of the HBV in the liver and the blood, and the next is the fact that CHB is associated with liver damages of variable degrees. Initially, it was assumed that control of the virus would downregulate the extent of liver damages, and all efforts were centered towards the complete or partial stoppage of HBV replication. This opened the path for interferon and its derivatives to be used as the first-line of drugs against CHB, as IFN is well-known to stop the replication of different viruses. Prior to this, IFN has been used in different viral diseases and cancers. Thus, IFN is the first repurposed drug recommended for the treatment of CHB [
9]. IFN-α has multiple antiviral, antiproliferative, and immunomodulatory activities. IFN can work by activating different pathways of antiviral defense in infected and noninfected cells, HBV replication blocking the RNA-containing core particle formation and accelerating their decay, degrading pre-genomic RNA, and modulating the nuclear viral mini-chromosome (covalently closed circular DNA) activity by targeting its epigenetic regulation and both innate and adaptive immune responses. This is mainly manifested by activating IFN-stimulated genes [
10]. However, IFN has major limitations like cost and route of administration. Moreover, IFN was found to be only partially efficacious in CHB patients. During the last 45 years, the beneficial effects of IFN have been clear to all. After careful consideration of all these facts, it is almost clear that the role of IFN in the treatment of CHB is limited; however, opportunity still remains for the usage of IFN as a part of combination therapy [
11].
During the middle of the 1990s, another group of repurposed drugs, nucleotide(s) analogs (NAs), started to be used for treating CHB patients. As of today, seven NAs (lamivudine, telbivudine, adefovir, entecavir, tenofovir disoproxil fumarate, and tenofovir alafenamide fumarate) are used on the global level. In addition, some NAs are used on a country basis. Lamivudine is the first NA that has been repurposed for the treatment of CHB. Lamivudine blocked reverse transcriptase activity and was used to block replication of the human immune-deficiency virus (HIV). The FDA approved lamivudine for usage in HIV treatment in 1995, and in 1996, it got approval for use in HIV in Europe. In 1998, lamivudine was approved for use in CHB patients, as this replication of HBV is dependent on reverse transcriptase activity. All other NAs also work with the similar principle of inhibiting polymerase enzyme activity and thus block HBV replication and induce the downregulation of HBV DNA in the sera [
12,
13]. The limitations of ongoing antiviral therapies against CHB are shown in
Table 1.
Although there remain several limitations of nucleoside analogs, these are mainly pronounced in developing and resource-constrained countries. However, this is one side of the coin. There are many inspiring aspects of NAs. The combined uses of NAs with another NAs or immune modulators may be effective for the containment of progression of liver damage. Additionally, a new form of NAs, nucleoside prodrugs, may be of benefit for CHB patients. Until these variables are properly addressed, it is too early to provide a notion about the usage of NA in CHB. However, the development of innovative therapy for CHB remains a pressing need, as the HBV induces noncytopathic and liver damage progress preferentially in some, but not all, patients [
14].
5. Pathogenesis of CHB and Role of Repurposed Drugs for CHB
5.1. Drugs Based on Life Cycle of HBV Are Yet to Be Developed
Chronic HBV infection is not merely a viral infection. The pathogenic process of CHB is quite complex. In short, CHB is an HBV-induced immune-mediated disease. In CHB patients, the HBV remains as replicating virus with a constant production of virion particles and their antigens. These can be estimated in the blood. Additionally, the hepatocytes contain the cccDNA in the nucleus. One hepatocyte may contain 5–50 cccDNA. The half-life of cccDNA is about ten days to months, depending on the data of in vitro and in vivo studies. Using a cccDNA quantitative assay, it has been shown that treatment with adefovir, entecavir, or lamivudine for 48 weeks may reduce intrahepatic cccDNA by 0.8–1.0 log. HBV DNA and cccDNA are not cytopathic in nature, and they are unable to induce any direct damage to hepatocytes [
15].
Thus, it is natural to ask what events are responsible for inflammation (hepatitis), fibrosis (LC), and carcinogenesis (HCC) following HBV infection. In fact, there remain complex interactions between the host and the virus, and the nature of host immunity may have some dominant roles in these pathological processes. Many of these cellular and molecular mechanisms could not be fully explored due to a lack of studies in the proper model of CHB. Although HBV transgenic mice (HBV TM) have been available since 1985 for analyzing these issues, natural models of HBV TM are not representative of CHB. HBV TM harbors HBV DNA and various HBV-related antigens, but they do not show evidence of liver damages, hepatic fibrosis, and hepatocellular carcinoma. Studies in patients with CHB are also limited to the nature of immunity and its specific role during the pathogenesis, progression, and regression of CHB. Although some serological data about these factors are available in the literature, little is known regarding the pathogenic features in the liver [
16,
17]. Maini et al. showed that HBV-specific immunity, especially HBcAg-specific immunity, is protective, whereas polyclonal immunity is usually pathogenic [
18]. It is challenging to reach a consensus about the nature and properties of these immunities, because there is a paucity of information regarding these controversial issues. Nevertheless, circumstantial evidence primarily supports these conceptions, and these would provide blueprints of the development of innovative therapies for CHB.
5.2. Why NAs Are Unable to Cure CHB in a Majority of Patients
NA has an inhibitory action on the polymerase activity of HBV in CHB patients. Thus, NA can inhibit viral replication, and the usage of NA leads to a reduction of HBV DNA. Even HBV DNA negativity may be an outcome of NA usage in CHB patients. The role of NA on cccDNA is not prominent. NA cannot eradicate cccDNA from infected hepatocytes. However, the regular usage of NA may keep cccDNA within the control, and thus, the prolonged usage of NA is required. Taken together, NA is a potent antiviral agent for CHB patients. However, a drug has not been developed to take care of cccDNA, the most critical viral variable of CHB patients. Moreover, inflammation of the liver is not induced directly by HBV or cccDNA. Instead, it is an immune-mediated fact. Let us check the immune-modulatory capacity of NA. NA induces the restoration of immunity. However, the restored immunity is of an antigen-nonspecific type, and that is polyclonal [
19] in nature. Studies have revealed that antigen-nonspecific immunity may not be protective for CHB patients. Taken together, NA has prominent action on replicating HBV DNA, minimal suppressive properties on cccDNA, and endowed some capacity to restore antigen-nonspecific immunity. However, protective immunity in CHB patients requires the induction and restoration of HBV-specific immunity. Thus, NA has limited beneficial activity on CHB patients, even if NAs are used for a prolonged duration. The reality is that no antiviral drug has been developed based on the complex life cycle of HBV. Although several liver organizations are updating their recommendations of NA usage for more benefits, the fundamental limitation of NA for the treatment of CHB is eminent. In addition to these facts, NA is not patient-friendly for CHB patients of developing and resource-constrained countries. It should be used for an infinite duration. Periodic follow-up is required during the use of NA. Paradoxically, most of the CHB patients of the world live in these countries of Asia and Africa. Most of these people are unaware of their HBV infection status and the dangerous outcome of being a CHB patient. However, if the missing millions of CHB patients are exposed, one major limitation will be the ability of the health services of these countries to treat these patients [
20]. Thus, the target of “Elimination of Hepatitis by 2030” assumes that starting therapy for 65% of CHB patients by 2030 is an unrealistic goal, and this should be optimized based on reality and evidence.
5.3. Realistic Situation of CHB and Ongoing Antiviral Therapy
Although IFNs and NAs are endowed with severe limitations for treating CHB, there remains almost no drug at hand for the treatment of millions of CHB patients. NAs have been on the market for more than two decades, and several trials have been accomplished with these drugs. We know NAs more than any other antiviral drugs for CHB patients. The good side of NAs include their oral usage and low cost compared to IFNs. As the cellular and molecular mechanisms of CHB are becoming clearer day by day, the time has come to develop new and innovative drugs that can replace NAs. A short description of the evolving and innovative drugs will be provided.
Inference: The ongoing therapeutic approaches for treating CHB are directed towards the containment of HBV. It seems that CHB is regarded as merely a viral infection. Drugs have been developed to contain the virus. However, no drug has been developed based on the life cycle of HBV. Only some drugs that have been used for other pathological conditions have been repurposed for CHB. Neither IFNs nor the NAs can destroy all forms of HBV, including cccDNA. In addition, CHB is an HBV-induced, immune-mediated pathology. HBV DNA’s reduction or negativity by ongoing available antiviral drugs may be associated with the containment of liver damages, but that is not the rule. Future drug development should consider either three spectrums or one or two of these steps: (1) complete eradication of all forms of HBV, including cccDNA, the (2) downregulation of liver damages, and (3) blocking progression to hepatic fibrosis and carcinogenesis. It is not an easy task, but proper insights about cellular and molecular mechanisms underlying the pathogenesis of CHB and the natural course of HBV infection may help attain the goal.
6. Innovative Therapy (Scope and Limitation)
6.1. Therapies Targeting Viral Factors
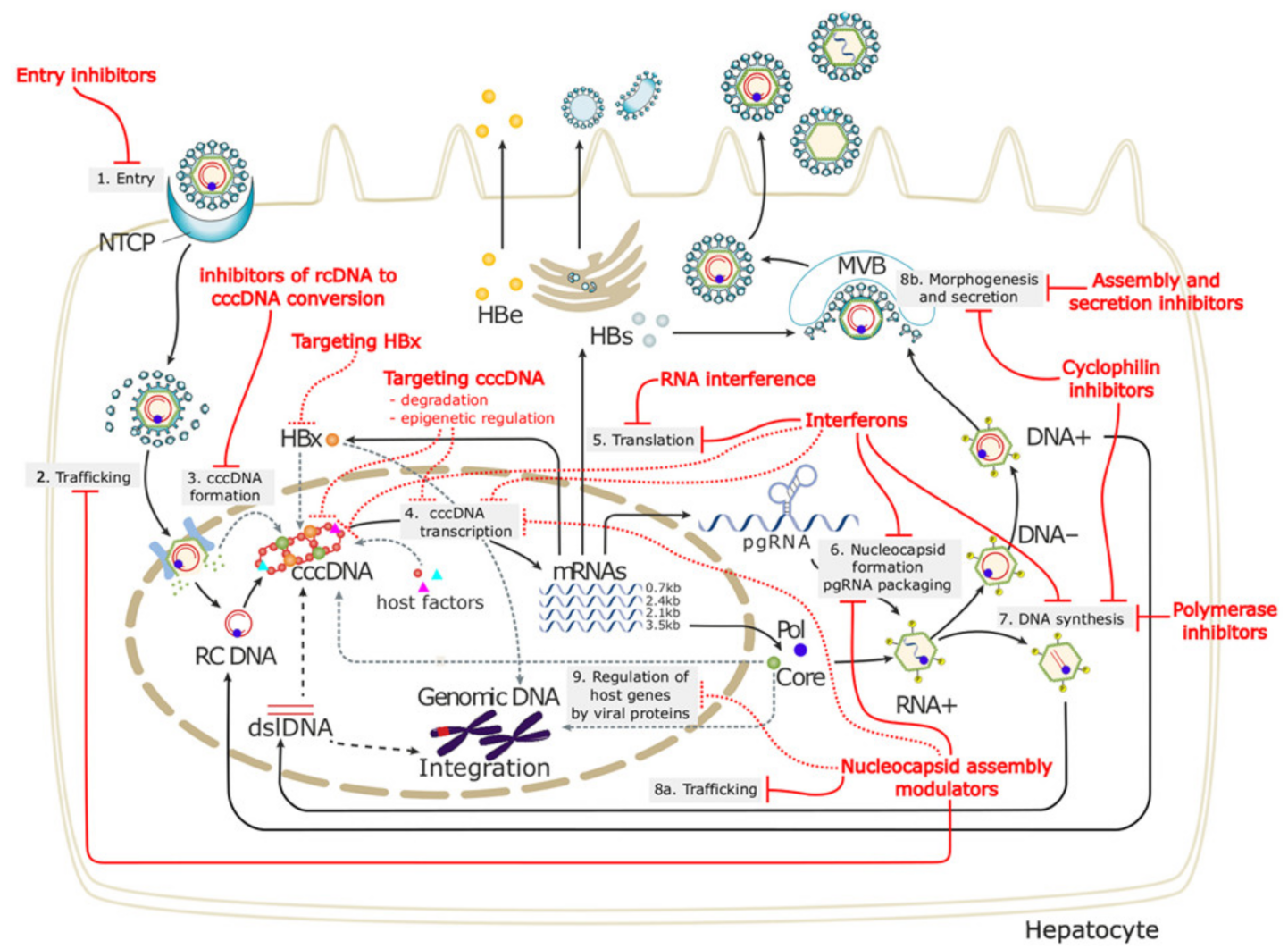
These types of therapies are summarized in
Figure 1. The points of interferences are shown. An ideal innovative therapy should have the following characteristics shown in
Table 2.
6.1.1. Entry Inhibitors
As NAs can mostly reduce replicating HBV, investigators have checked for other antiviral compounds that can be effective in different forms of HBV. One such approach is to target the entry of the virus through sodium taurocholate cotransporting polypeptide (NTCP) that acts as an entry receptor of HBV into hepatocytes. NTCP inhibitors bind to NTCP, and thus, de novo infection with the HBV can be blocked. The use of this entry inhibitor reduced the HBV DNA levels, but the eradication of HBV DNA could not be achieved. Patients enrolled in clinical trials with both HBV and HDV have shown a loss of HBsAg by the entry inhibitor and pegylated IFN. The entry inhibitor, bulevertile and other drugs, has been approved for clinical usage to block the entry of HBsAg and hepatitis delta virus into hepatocytes [
22,
23,
24,
25]. Some of the data are inspiring regarding the control of viral replication. However, it is not clear if this drug would have any role in containing liver damages or progression to LC and HCC. Clinical trials have recently started with entry inhibitors, and future data should be checked properly about their scope and limitation. When the management of CHB is dependent on controlling cccDNA, and the mechanism of immune-mediated liver damages is most relevant, what roles can entry inhibitors play by inhibiting HBV entry in vivo? However, as a complex mechanism prevailing in CHB pathogenesis, entry inhibitors may be used as a part of combination therapy in the future, with other treatment modalities accomplishing other immune-mediated jobs.
6.1.2. RNA Interference
Another mode of control of HBV replication may be accomplished by RNA interference (RNAi). RNAi can target HBV transcripts directly, which may lead to their degradation of the viral genome. Overall, this may reduce the HBV DNA levels in the sera. RNAi has been shown to block HBsAg production [
26]. The role of HBsAg in the immune regulation of CHB patients has been shown, and thus, it is assumed that, by blocking HBsAg production, RNAi may restore the host immunity. Thus, we come back to the need to restore an effective immune system that will ultimately bring the liver damage under control. It is still not clear what type of immunity would be restored by RNAi. If that is HBV-specific immunity, then the therapeutic effect may be sustained. However, if that is polyclonal in nature, more damage and inflammation may follow.
6.1.3. Capsid Assembly Modulator
The HBV core protein is essential for HBV pgRNA packaging and reverses transcription. Several compounds that are able to modulate the assembly of the core protein capsid may have potential utility in the treatment of CHB patients, especially for containing HBV replication [
27]. This drug has been used with pegylated IFN, and it revealed its potent antiviral capacity. However, like all other innovative antiviral drugs, this one also lacks any capacity to modulate liver damages.
6.1.4. Drug Targeting Inhibition of HBsAg Release
HBsAg is a vital antigen of CHB patients, as immunity to HBsAg is protective in nature. On the other hand, HBsAg is the most useful marker of HBV infection. HBsAg is produced in the cytoplasm of hepatocytes and released into the blood. However, circulating HBsAg comes mainly from noninfectious HBV subviral particles (SVPs). Thus, the production of HBsAg is not representative of the viral load. On the other hand, excess HBsAg seems to be responsible for the impaired immunity of CHB patients. This has led the investigators to develop nucleic acid polymers (REP 2139) to block HBsAg release from infective hepatocytes [
28]. Thus, by using these polymers, the levels of HBsAg will be reduced in the blood. Now, the questions are how a reduction of HBsAg would help to cure CHB patients, whereas the pathogenesis of CHB is not dependent on the HBsAg levels. One solution might explain that low levels of HBsAg would allow having adequate HBsAg-specific immunity. However, the protective nature of this immunity has yet to be assessed. Moreover, HBsAg-specific immunity that has been induced by vaccine therapy was not able to have therapeutic outcomes in CHB patients. Finally, the investigators have not checked the outcome of REP 2139 regarding immune restoration. However, the importance of HBsAg levels is confusing. Any direct role of HBsAg could not be substantiated. Several patients with very high levels of HBsAg exhibit no evidence of liver damages.
On the other hand, patients developing LC and HCC harbor shallow levels of HBsAg. The importance of HBsAg reduction bears some implications when HBsAg is reduced due to antiviral therapy. The reduction of HBsAg by antiviral therapy versus the inhibition of HBsAg by polymers is not of similar clinical significance. The reduction by antiviral therapy indicates a reduction of cccDNA or silence towards the replication cycle of HBsAg from cccDNA. This seems to be meaningful, as the HBsAg levels show a progressive decline by antiviral therapy. The major problem with the advent of innovative therapy seems to be related to the misunderstanding about the pathogenesis of CHB and attempts to develop drugs on the basis of elusive considerations.
6.1.5. Drug Targeting cccDNA
Even after containing HBV DNA and ensuring HBV DNA negativity in CHB patients, the patient is not cured, because cccDNA acts as a template for new viral particles. From this point of view, if the production of cccDNA can be controlled, an effective therapeutic option may arise for CHB patients. However, concerns will remain about managing liver damages, as it is unlikely that reducing the cccDNA would alter the course of liver damages due to the reduction of cccDNA. Numerous small molecules can block the formation, enhance the destruction, and silence the transcription of cccDNA while stimulating cell division [
29]. These include cleaving sequence-specific DNA targets and gene-editing using the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated 9 (Cas9) system. However, these maneuvers are in development, and their real implication for CHB patients is yet to be evaluated.
Inference: Innovative therapies targeting the virus represent novel approaches, but the design should be such that it can eradicate all forms of HBV, including cccDNA. One or two inspiring studies are beneficial, but preclinical studies should be followed by phase I, II, and III clinical trials. Additionally, follow-up data are required for designing new studies. The nature of an ideal innovative therapy targeting viral factors is shown in
Table 2.
6.2. Immune Therapy Targeting the Host
6.2.1. HBV Antigen-Nonspecific Polyclonal Immune Therapy
It is well-accepted that HBV infection leads to the dysfunction of immunocytes. Almost all immune system cells were found to have impaired or defective functions in animal models of chronic HBV infection or patients with CHB. However, some differences like the defect were also visible, due to the study design. This led to developing immune therapy for CHB patients. Immune therapy for CHB can be divided into two major disciplines: polyclonal immune modulators-based immune therapy and HBV antigen-based immune therapy. Initially, it was assumed that the immune responses of CHB patients are diminished because they are unable to induce the immunity to circulating HBV-related antigens, such as HBsAg. These facts are relevant, as all patients with CHB harbor moderate-to-high levels of HBsAg in the blood. However, anti-HBs are not usually detected in the sera. These findings led to the foundation of immune therapy in CHB patients. There were a wide range of polyclonal immune modulators (HBV antigen-nonspecific), such as interleukin (IL)-2, IL-12, granulocyte–macrophage colony-stimulating factor, levamisole, thymus humoral factor-gamma 2, alpha galactosylceramide, propagermanium, liver extract, thymosin-alpha 1, and others, used for CHB patients during the 1990s [
30,
31,
32,
33,
34,
35,
36,
37,
38,
39]. If the outcome of these immune therapeutic approaches is compiled, it seems that either a biologically active dose of IL-2 treatment was of no therapeutic value or recombinant IL-2 was not useful as a monotherapy for the treatment of CHB patients. The treatment of CHB by IL-12 therapy has been endowed with considerable adverse effects, or the role of a granulocyte–macrophage colony-stimulating factor did not have therapeutic implications in CHB patients. Similarly, the combination of levamisole with IFN or thymus humoral factor-gamma 2 or alpha-galactosylceramide or propagermanium or liver extracted proteins or thymosin alpha did not show any notable clinical effects in CHB patients. However, these immune modulators upregulated the immune systems of CHB patients. Thus, a mere increase in the immune status would not be sufficient for CHB patients. When the adverse outcomes of these therapeutic approaches were analyzed, it was found that the study design was of some pilot study, follow-up data were mostly unavailable, and the mechanism of action was not explored. Thus, the potential benefits of non-antigen-specific immune therapy by polyclonal immune modulators, if any, for CHB patients remain undissected until now. However, the present information does not have a favorable concept about the use of polyclonal immune modulators as treatment options for CHB.
6.2.2. Toll-like Receptor Agonists
TLR-mediated pathways may be used to suppress HBV replication and restore HBV-specific adaptive immunity. GS-9688, a TLR-8 agonist, is now in a phase II study. Although it reduced the HBV DNA, the HBsAg loss was not notable [
40]. Another study with TLR agonist plus TAF against a placebo plus TAF in patients with CHB showed that selgantolimod is safe and well-tolerated, with a decline in HBsAg. However, there cannot be a proper assessment until a phase III study with these immune therapeutic agents is completed.
6.2.3. Immune Checkpoint Inhibitors
The efficacy of targeting checkpoint inhibitors, such as programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1), has been widely used in cancer patients. Anti-PD-L1 may be a therapeutic candidate in patients with CHB, as it is expected to restore the antiviral T-cell functions. In combination with entecavir treatment and DNA vaccination, the in vivo blockade of the PD-1/PD-L1 pathway in CD8 T cells was shown to enhance the function of virus-specific T cells [
41]. A phase I pilot study evaluated anti-PD-1 (nivolumab) treatment with or without GS-4774 (therapeutic vaccine) in HBeAg-negative CHB patients. At week 24, 14% (3/22) of the patients had >0.5 log 10 reduction in the HBsAg levels [
42]. However, follow-up data are mostly unavailable about this trial.
6.3. HBV Antigen-Specific Immune Therapy for CHB
6.3.1. HBsAg-Based Immune Therapy for CHB
HBsAg-based immunotherapy was first reported by Pol et al. in 1994 [
43]. Subsequently, several clinical trials of HBsAg-based vaccines have been performed in CHB patients. As the commercially available HB vaccine has been used as a source of HBsAg, this therapy has been named “vaccine therapy” [
44,
45,
46,
47,
48,
49]. If these data are analyzed as a holistic approach, it is clear that HBsAg-based immune therapy is mostly safe and results in reduced HBV DNA levels, HBeAg negativity, and anti-HBe seroconversion in some CHB patients. However, sustained effects of the HBsAg-based vaccine on HBV DNA, ALT, fibrosis, and HCC could not be documented. Furthermore, there is a paucity of information regarding the effect’s therapeutic vaccination during the follow-up period. Most of these were pilot studies and exhibited considerable heterogeneity in the dose and composition of the vaccine and the treatment duration. Moreover, the mechanism of action of vaccine therapy has remained chiefly elusive.
6.3.2. Other Faces of HBV Antigen-Based Immune Therapy
Attempts were made to develop different types of antigen-based immune therapy for CHB patients. This led to the production of the antigen–antibody complex vaccine, DNA-based vaccine expressing HBsAg, and a combination of antiviral and HBV antigen-based vaccines. However, the final outcome was not inspiring in CHB patients, and a phase III study has not been accomplished to formulate an immune therapeutic strategy for clinical usage.
6.3.3. HBsAg/HBcAg-Based Vaccine Therapy
Recently, insights have been gained into the importance of hepatitis B core antigen (HBcAg)-based immunity in CHB patients concerning the control of HBV replication and the containment of liver damage. The study of Maini et al. revealed that patients with CHB who control HBV replication and contain progressive liver damage harbor significantly higher numbers of HBcAg-specific cytotoxic T lymphocytes in the liver than patients with CHB who are unable to control HBV replication and exhibit liver damage. Heathcote et al. used an HBcAg-based epitope vaccine to treat CHB patients in the 1990s [
50]. However, a follow-up study was not found later. As both HBsAg and HBcAg-specific immunity is essential for the control of HBV replication and the containment of liver damage in CHB patients, a therapeutic vaccine containing both HBsAg and HBcAg (HBsAg/HBcAg) (NASVAC) has been used in CHB patients. NASVAC has been designed to use as a therapeutic vaccine in a systematic manner. It was first assessed in HBV transgenic mice [
51]. Then, the safety and efficacy were tested in normal volunteers [
52]. This was followed up by a phase-I/II clinical trial in CHB patients, and the study reported that NASVAC was safe and induced HBV DNA negativity in 50% of CHB patients [
53]. Additionally, the persistent normalization of ALT was recorded in all patients in this study. A phase III clinical trial of NASVAC revealed sustained HBV DNA control, ALT normalization, and reduced fibrosis in CHB patients. The mechanism of action revealed that the activation of antigen-presenting dendritic cells and development of antigen-specific immunocytes prevailed in NASVAC recipients compared to the controls [
54]. NASVAC can be given by the nasal route, and now, a NASVAC trial is going on in Japan in NUC-experienced patients. NASVAC has been registered in some countries as an immune therapeutic drug. Long-term follow-up data of NASVAC is warranted to develop insights about the scope and limitation of NASVAC as a therapeutic vaccine.
Inference: Host-targeting therapy and immune therapies are of paramount interest, as these therapies combined with viral-targeting drugs may ultimately resolve the complex problem of CHB. However, if an immune modulator is found to be safe, clinical trials with follow-up data are necessary, even if those seem to be negative data.
7. Summary
The number of patients with chronic HBV infection is about 290 million in the world, and the majority of these patients reside in the developing and resource-constrained countries of Asia and Africa. About 30–70 million HBV-infected people are CHB, indicating that these patients are express HBV DNA and HBsAg, and they also exhibit the exacerbation and remission of hepatitis (measured by fluctuating ALT).
The 290 million chronic HBV-infected patients are living and permanent reservoirs of HBV infection and transmit the infection to healthy individuals. On the other hand, millions of CHB patients will spread HBV infection, and considerable numbers will eventually develop complications like LC and HCC. As of 2019, about 892,000 patients have died due to HBV-related complications. The study presented here concentrated on the scope and limitation of ongoing and innovative therapies for CHB patients.
IFNs and NAs may have beneficial effects for CHB patients of developed and advanced countries; where the health insurance systems support the treatment of the patients, periodic follow-up is possible, and treatment for the infinite duration is feasible. Even the safety and efficacy outcome would give some benefits to merely 10–20% of patients. Only a minor population of CHB patients of developing and resource-constrained countries can use IFNs and NAs. This is clear from the statistics of the WHO, which reveal that <1% of patients are under some treatment at the global level.
Several innovative therapies following two lines have been developed during the last 40 years. One of them has targeted the viral factor, and these therapeutic modalities may have limited scope, as already available NAs are potent inhibitors of HBV replication but cannot stand the test of time as a therapeutic option for CHB patients (
Table 3). The other innovative therapy has targeted the host factors; however, most studies are a pilot study in nature or limited-scale clinical trials. There should be phase I/II/II clinical trials to explore the mechanisms of action to validate their usage as therapeutic modalities. CHB is a complex pathological entity. The development of drugs for CHB will be more complex, and scientific evidence should be followed to develop innovative therapy (
Table 4).
Author Contributions
Conceptualization: S.M.F.A.; Formal analysis: S.M.F.A., M.A.M., and Y.H.; Writing—original draft: S.M.F.A. and O.Y.; and Writing—review and editing: S.M.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported in part by a grant-in-aid from the Japan Agency for Medical Research and Development (AMED) to Sheikh Mohammad Fazle Akbar (grant number 20fk0310103h1905).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- MacLachlan, J.H.; Cowie, B.C. Hepatitis B virus epidemiology. Cold Spring Harb. Perspect. Med. 2015, 5, a021410. [Google Scholar] [CrossRef] [Green Version]
- WHO. Global Hepatitis Report 2017; World Health Organization: Geneva, Switzerland, 2017; Available online: https://apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf (accessed on 15 October 2021).
- Terrault, N.A.; Bzowej, N.H.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Murad, M.; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016, 63, 261–283. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarin, S.K.; Kumar, M.; Lau, G.K.; Abbas, Z.; Chan, H.L.Y.; Chen, D.D.; Chen, H.L.; Chen, P.J.; Chien, R.N.; Dockmeci, A.K.; et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol. Int. 2016, 10, 1–98. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, M.; Sureau, C. Infectivity determinants of the hepatitis B virus pre-S domain are confined to the N-terminal 75 amino acid residues. J. Virol. 2007, 81, 5841–5849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, B.; Hildt, E. Intracellular Trafficking of HBV Particles. Cells 2020, 9, 2023. [Google Scholar] [CrossRef] [PubMed]
- Kann, M.; Schmitz, A.; Rabe, B. Intracellular transport of hepatitis B virus. World J. Gastroenterol. 2007, 13, 39. [Google Scholar] [CrossRef]
- Bourlière, M.; Rabiega, P.; Ganne-Carrie, N.; Serfaty, L.; Marcellin, P.; Barthe, Y.; Thabut, D.; Guyader, D.; Hezode, C.; Picon, M.; et al. Effect on HBs antigen clearance of addition of pegylated interferon alfa-2a to nucleos(t)ide analogue therapy versus nucleos(t)ide analogue therapy alone in patients with HBe antigen-negative chronic hepatitis B and sustained undetectable plasma hepatitis B virus DNA: A randomised, controlled, open-label trial. Lancet Gastroenterol. Hepatol. 2017, 2, 177–188. [Google Scholar] [PubMed]
- Katze, M.G.; He, Y.; Gale, M., Jr. Viruses and interferon a fight for supremacy. Nat. Rev. Immunol. 2002, 2, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Viganò, M.; Grossi, G.; Loglio, A.; Lampertico, P. Treatment of hepatitis B: Is there still a role for interferon? Liver Int. 2018, 38 (Suppl. 1), 79–83. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Liang, Y.; Zhang, M.; Liu, F.; Zhang, L.; Yang, B.; Wang, L. Nucleoside/nucleotide analog consolidation therapy in hepatitis B e-antigen-postive chronic hepatitis B patients: Three years should be preferred. Hepatol. Res. 2021, 51, 633–640. [Google Scholar] [CrossRef]
- Charlton, M.R.; Alam, A.; Shukla, A.; Dashtseren, B.; Lesmana, C.R.A.; Duger, D.; Payawal, D.A.; Do Cuong, D.; Jargalsaikhan, G.; Cua, I.H.Y.; et al. An expert review on the use of tenofovir alafenamide for the treatment of chronic hepatitis B virus infection in Asia. J. Gastroenterol. 2020, 55, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, Y.; Wen, X.; Ma, H. Current prodrug strategies for improving oralabsorption of nucleoside analogues. Asian J. Pharm. Sci. 2014, 9, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Allweiss, L.; Dandri, M. The Role of cccDNA in HBV Maintenance. Viruses 2017, 9, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidotti, L.G.; Chisari, F.V. Immunobiology and pathogenesis of viral hepatitis. Annu. Rev. Pathol. 2006, 1, 23–61. [Google Scholar] [CrossRef] [Green Version]
- Eddleston, A.L.; Mondelli, M. Immunopathological mechanisms of liver cell injury in chronic hepatitis B virus infection. J. Hepatol. 1986, 3 (Suppl. 2), S17–S23. [Google Scholar] [CrossRef]
- Maini, M.K.; Pallett, L.J. Defective T-cell immunity in hepatitis B virus infection: Why therapeutic vaccination needs a helping hand. Lancet Gastroenterol. Hepatol. 2018, 3, 192–202. [Google Scholar] [CrossRef]
- Akbar, S.M.; Al-Mahtab, M.; Khan, M.S. Non-antigen-specific and antigen-specific immune therapies for chronic hepatitis B: Evidences from laboratory benches and patient’s bedsides. Expert Opin. Biol. Ther. 2013, 13, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.M.; Hiasa, Y.; Mishiro, S.; Onji, M. Treatment of hepatitis B virus-infected patients: Utility of therapeutic recommendations in developing countries. Expert Opin. Pharmacother. 2009, 10, 1605–1614. [Google Scholar] [CrossRef]
- Lok, A.S.-F. Hepatitis B treatment: What we know now and what remains to be researched. Hepatol. Commun. 2018, 3, 8–19. [Google Scholar]
- Urban, S.; Bartenschlager, R.; Kubitz, R.; Zoulim, F. Strategies to inhibit entry of HBV and HDV into hepatocytes. Gastroenterology 2014, 147, 48–64. [Google Scholar] [CrossRef]
- Urban, S.; Neumann-Haefelin, C.; Lampertico, P. Hepatitis D virus in 2021: Virology, immunology and new treatment approaches for a difficult-to-treat disease. Gut 2021, 70, 1782–1794. [Google Scholar] [CrossRef]
- Ligat, G.; Verrier, E.R.; Nassal, M.; Baumert, T.F. Hepatitis B virus-host interactions and novel targets for viral cure. Curr. Opin. Virol. 2021, 49, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Wedemeyer, H.; Schoneweis, K.; Bogomolov, P.O.; Voronkova, N.; Chulanov, V.; Stepanova, T.; Bremer, B.; Allweiss, L.; Dandri, M.; Burhenne, J.; et al. Final results of a multicenter, open-label phase 2 clinical trial (MYR203) to assess safety and efficacy of myrcludex B in cwith PEG-interferon Alpha 2a in patients with chronic HBV/HDV co-infection. J. Hepatol. 2019, 70, 81. [Google Scholar] [CrossRef]
- Nayagam, J.S.; Cargill, Z.C.; Agarwal, K. The Role of RNA Interference in Functional Cure Strategies for Chronic Hepatitis B. Curr. Hepatol. Rep. 2020, 19, 362–369. [Google Scholar] [CrossRef]
- Li, C.; Wu, M.; Zhang, H.; Mai, J.; Yang, L.; Ding, Y.; Niu, J.; Mao, J.; Wu, W.; Zhang, D.; et al. Safety, Tolerability, and Pharmacokinetics of the novel Hepatitis B Virus Capsid Assembly Modulator GST-HG141 in Healthy Chinese Subjects: A First-in-Human Single- and Multiple-Dose Escalation Trial. Antimicrob. Agents Chemother. 2021, 65, e0122021. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, M.; Pântea, V.; Placinta, G.; Moscalu, I.; Cebotarescu, V.; Cojuhari, L.; Jimbei, P.; Iarovoi, L.; Smesnoi, V.; Musteata, T.; et al. Safety and Efficacy of 48Weeks REP 2139 or REP 2165, Tenofovir Disoproxil, and Pegylated Interferon Alfa-2a in Patients With Chronic HBV Infection Naïve to Nucleos(t)ide Therapy. Gastroenterology 2020, 158, 2180–2194. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, R.; Chen, S.; Guo, D.; Chen, Y. Inhibition of hepatitis B virus by the CRISPR/Cas9 system via targeting the conserved regions of the viral genome. J. Gen. Virol. 2015, 96, 2252–2261. [Google Scholar] [CrossRef]
- Tilg, H.; Vogel, W.; Tratkiewicz, J.; Aulizky, W.E.; Herold, M.; Gruber, M.; Geissler, D.; Umalauft, F.; Judmaier, G.; Schwuelra, U. Pilot study of natural human interleukin-2 in patients with chronic hepatitis B. Immunomodulatory and antiviral effects. J. Hepatol. 1993, 19, 259–267. [Google Scholar] [CrossRef]
- Artillo, S.; Pastore, G.; Alberti, A.; Milella, M.; Santantonio, T.; Fattovitch, G.; Guistina, G.; Ryff, J.C.; Chaneac, M.; Bartolome, J.; et al. Double-blind, randomized controlled trial of interleukin-2 treatment of chronic hepatitis B. J. Med. Virol. 1998, 54, 167–172. [Google Scholar] [CrossRef]
- Carreño, V.; Zeuzem, S.; Hopf, U.; Marcellin, P.; Cooksley, W.G.; Fevery, J.; Diuago, M.; Reddy, R.; Peters, M.; Rittweger, K.; et al. A phase I/II study of recombinant human interleukin-12 in patients with chronic hepatitis B. J. Hepatol. 2000, 32, 317–324. [Google Scholar] [CrossRef]
- Martín, J.; Quiroga, J.A.; Bosch, O.; Carreño, V. Changes in cytokine production during therapy with granulocyte-macrophage colony-stimulating factor in patients with chronic hepatitis B. Hepatology 1994, 20, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moreno, M.; García, R.; Rua, M.J.; Serrano, B.; Moraleda, G.; Feijoo, E.; Bartolome, J.; Ortiz, F.; Castillo, I.; Carreno, V. Levamisole and interferon in children with chronic hepatitis B. Hepatology 1993, 18, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Farhat, B.A.; Marinos, G.; Daniels, H.M.; Naoumov, N.V.; Williams, R. Evaluation of efficacy and safety of thymus humoral factor-gamma 2 in the management of chronic hepatitis B. J. Hepatol. 1995, 23, 21–27. [Google Scholar] [CrossRef]
- Woltman, A.M.; Ter Borg, M.J.; Binda, R.S.; Spoengers, D.; Von Blumberg, B.M.; Scheper, R.J.; Hayashi, K.; Nishi, N.; Boonstra, A.; van der Molen, R.; et al. Alpha-galactosylceramide in chronic hepatitis B infection: Results from a randomized placebo-controlled Phase I/II trial. Antivir. Ther. 2009, 14, 809–818. [Google Scholar] [CrossRef] [Green Version]
- Hirayama, C.; Suzuki, H.; Ito, M.; Okumura, M.; Oda, T. Propagermanium: A nonspecific immune modulator for chronic hepatitis B. J. Gastroenterol. 2003, 38, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Israeli, E.; Safadi, R.; Melhem, A.; Pappo, O.; Shibolet, O.; Klein, A.; Hemed, N.; Thalenfeld, B.; Engelhardt, D.; Rabbani, E.; et al. Induction of oral immune regulation towards liver-extracted proteins for treatment of chronic HBV and HCV hepatitis: Results of a phase I clinical trial. Liver Int. 2004, 24, 295–307. [Google Scholar] [CrossRef]
- Iino, S.; Toyota, J.; Kumada, H.; Kiyosawa, K.; Kakumu, S.; Sata, M.; Suzuki, H.; Martins, E.B. The efficacy and safety of thymosin alpha-1 in Japanese patients with chronic hepatitis B; results from a randomized clinical trial. J. Viral Hepat. 2005, 12, 300–306. [Google Scholar] [CrossRef]
- Ma, Z.; Cao, Q.; Xiong, Y.; Zhang, E.; Lu, M. Interaction between Hepatitis B Virus and Toll-Like Receptors: Current Status and Potential Therapeutic Use for Chronic Hepatitis B. Vaccines 2018, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Janssen, H.L.; Brunetto, M.R.; Kim, Y.J.; Ferrari, C.; Massetto, B.; Nguyen, A.H.; Joshi, A.; Woo, J.; Lau, A.H.; Gaggar, A.; et al. Safety, efficacy and pharmacodynamics of vesatolimod (GS-9620) in virally suppressed patients with chronic hepatitis B. J. Hepatol. 2018, 68, 431–440. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, E.; Ma, Z.; Wu, W.; Kosinska, A.; Zhang, X.; Möller, I.; Seiz, P.; Glebe, D.; Wang, B.; et al. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS Pathog. 2014, 10, e1003856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pol, S.; Driss, F.; Michel, M.L.; Nalpas, B.; Berthelot, P.; Brechot, C. Specific vaccine therapy in chronic hepatitis B infection. Lancet 1994, 344, 342. [Google Scholar] [CrossRef]
- Senturk, H.; Tabak, F.; Akdogan, M.; Erdem, L.; Mert, A.; Ozaras, R.; Sander, E.; Ozbay, G.; Badur, S. Therapeutic vaccination in chronic hepatitis B. J. Gastroenterol. Hepatol. 2002, 17, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Zhang, X.X.; Yao, X.; Jiang, J.-H.; Xie, Y.-H.; Yuan, Z.-H.; Wen, Y.-M. Serum HBeAg sero-conversion correlated with decrease of HBsAg and HBV DNA in chronic hepatitis B patients treated with a therapeutic vaccine. Vaccine 2010, 28, 8169–8174. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.M.; Wu, X.H.; Hu, D.C.; Zhang, Q.P.; Guo, S.Q. Hepatitis B vaccine and anti-HBs complex as approach for vaccine therapy. Lancet 1995, 345, 1575–1576. [Google Scholar] [CrossRef]
- Xu, D.Z.; Zhao, K.; Guo, L.M.; Li, A.-L.; Xie, Q.; Ren, H.; Zhang, J.-M.; Xu, M.; Wang, H.-F.; Huang, W.-X.; et al. A randomized controlled phase IIb trial of antigen-antibody immunogenic complex therapeutic vaccine in chronic hepatitis B patients. PLoS ONE 2008, 3, e2565. [Google Scholar] [CrossRef]
- Dahmen, A.; Herzog-Hauff, S.; Böcher, W.O.; Galle, P.R.; Lohr, H.F. Clinical and immunological efficacy of intradermal vaccine plus lamivudine with or without interleukin-2 in patients with chronic hepatitis B. J. Med. Virol. 2002, 66, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Horiike, N.; Akbar, S.M.F.; Michitaka, K.; Joukou, K.; Yamamoto, K.; Kojima, N.; Hiasa, Y.; Abe, M.; Onji, M. In vivo immunization by vaccine therapy following virus suppression by lamivudine: A novel approach for treating patients with chronic hepatitis B. J. Clin. Virol. 2005, 32, 156–161. [Google Scholar] [CrossRef]
- Heathcote, J.; McHutchchinson, J.; Lee, S.; Tong, M.; Benner, K.; Minuk, G.; Wright, T.; Fikes, J.; Livingston, B.; Sette, A.; et al. A pilot study of the CY-1899 T-cell vaccine in subjects chronically infected with hepatitis B virus. The CY1899 T Cell Vaccine Study Group. Hepatology 1999, 30, 531–536. [Google Scholar] [CrossRef]
- Akbar, S.M.; Chen, S.; Al-Mahtab, M.; Abe, M.; Hiasa, Y.; Onji, M. Strong and multi-antigen specific immunity by hepatitis B core antigen (HBcAg)-based vaccines in a murine model of chronic hepatitis B: HBcAg is a candidate for a therapeutic vaccine against hepatitis B virus. Antivir. Res. 2012, 96, 59–64. [Google Scholar] [CrossRef]
- Betancourt, A.A.; Delgado, C.A.; Estévez, Z.C.; Martinez, J.C.; Rios, G.V.; Aureoles-Rosello, S.R.M.; Guzman, M.A.; Baile, N.F.; Reyes, P.A.D.; Ruano, L.O. Phase I clinical trial in healthy adults of a nasal vaccine candidate containing recombinant hepatitis B surface and core antigens. Int. J. Infect. Dis. 2007, 11, 394–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Mahtab, M.; Akbar, S.M.; Aguilar, J.C.; Uddin, M.H.; Khan, M.S.; Rahman, S. Therapeutic potential of a combined hepatitis B virus surface and core antigen vaccine in patients with chronic hepatitis B. Hepatol. Int. 2013, 7, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahtab, M.; Akbar, S.M.F.; Aguilar, J.C.; Guillen, G.; Penton, E.; Tuero, A.; Yoshida, O.; Hiasa, Y.; Onji, M. Treatment of chronic hepatitis B naïve patients with a therapeutic vaccine containing HBs and HBc antigens (a randomized, open and treatment-controlled phase III clinical trial). PLoS ONE 2018, 13, e0201236. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).