Abstract
Primary sclerosing cholangitis (PSC) is a chronic inflammatory disease, which is associated with an increased risk of cholangiocarcinoma (CCA). Novel markers, to complement or replace CA19-9, are urgently needed for the screening of PSC-associated biliary neoplasia. Previous studies have suggested that serum trypsinogen-2 and human chorionic gonadotropin β-subunit (hCGβ) may serve as such markers. Using highly specific in-house immunoassays, we studied trypsin(ogen)-2 and -3, SPINK1 and hCGβ in bile samples of 214 patients, referred for endoscopic retrograde cholangiography. We found that biliary trypsinogen-2 was decreased (p = 0.027) and hCGβ was elevated (p < 0.001) in PSC patients who were diagnosed 1.6 years (median, range 0.1–8.8 years) later with CCA or in whom biliary dysplasia was observed at least twice in brush cytology (n = 11) as compared to PSC patients without CCA or repeated dysplasia (n = 171). The other studied markers did not show significant differences between these groups. Our results warrant further evaluation of hCGβ as a predictive marker for PSC-associated biliary neoplasia.
Keywords:
cholangitis; sclerosing; cholangiocarcinoma; hCG-beta; biochemical markers; bile; trypsinogen; SPINK1 1. Introduction
Primary sclerosing cholangitis (PSC) is a chronic inflammatory disease leading to strictures of bile ducts causing cholestasis and biliary cirrhosis [1]. PSC is associated with a markedly increased risk of cholangiocarcinoma (CCA), the lifetime risk being up to 20% and the annual incidence being 0.6–1.5% [1,2]. CCA is preceded by biliary dysplasia, which is a highly significant predictor of later development of CCA [3]. CCA is the most common reason for death among patients with PSC [4]. Treatment options for CCA are limited, especially when diagnosed at later stages [2]. Thus, an early diagnosis of both PSC and CCA is important but challenging, as 27–50% of CCA cases are diagnosed simultaneously at the time of PSC or within the first year after diagnosis of PSC [4,5]. Presently, serum carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA) are routinely used for the screening of CCA, but neither of them is a sensitive or specific marker for biliary neoplasia [1].
Previously, serum trypsinogen-1 and -2 and serine protease inhibitor Kazal-type 1 (SPINK1, also known as tumor-associated trypsin inhibitor (TATI) and pancreatic secretory trypsin inhibitor (PSTI)) have been found to be elevated in CCA patients [6,7]. Importantly, serum trypsinogen-2 concentrations were found to differentiate between PSC and PSC-associated CCA better than trypsinogen-1, SPINK1, CEA and CA19-9 [7]. In that study, human chorionic gonadotropin (hCG) β-subunit (hCGβ) also showed better accuracy than CA19-9 for the detection of CCA.
In humans, three different genes (PRSS1, PRSS2 and PRSS3) encode trypsin precursors, namely, trypsinogens-1, -2 and -3, which are either autoactivated or activated by other proteases, such as enteropeptidase, to trypsin-1, -2 and -3, respectively [8]. While trypsins and SPINK1 are highly expressed in the pancreas, they are also expressed in many other tissues. In cancer, the expression is often increased, which agrees with their proposed roles in the promotion of tumor growth and metastatic dissemination [8,9,10]. Thus, serum trypsin(ogen)s and SPINK1 are useful markers for various pathological conditions, including pancreatitis and several cancers [8,9,10,11]. Although high levels of trypsinogen-1 and -2 and SPINK1 have been reported in the bile of patients with non-neoplastic and malignant biliary tract diseases and pancreatic cancer [12], it is not known whether their biliary concentrations are changed in biliary neoplasia.
Glycoprotein hormone hCG, which is a heterodimer of hCGβ and α-subunit of hCG, is a well-known pregnancy hormone, which acts through the LH/hCG receptor (LHCGR) in the corpus luteum. However, many nontrophoblastic tumors, such as those in various gastrointestinal tract tissues, produce free hCGβ, but very rarely intact hCG [10]. For hCGβ, several, likely LHCGR-independent, cancer progression-relevant functions have been described, including the promotion of cell migration and invasion, which are associated with epithelial-to-mesenchymal transition-like changes in the cells [13]. This is in line with studies showing that serum levels of hCGβ are strongly associated with adverse prognosis in many nontrophoblastic cancers [10].
Here, we aimed to identify novel potential markers for the prediction of biliary neoplasia in PSC patients. We analyzed bile samples, which should reflect the biliary disease state more accurately than serum. In addition to trypsin(ogen)-2, SPINK1 and hCGβ, we analyzed trypsin(ogen)-3 concentration, not previously addressed in PSC or CCA patients.
2. Patients and Methods
2.1. Patients
The study included 214 consecutive patients from Helsinki University Hospital PSC registry, referred for endoscopic retrograde cholangiography (ERC), and from whom bile samples were available. The study period was 2010–2012, with follow-up to 2020 for hard end points, such as CCA, liver transplantation or death. Patient demographics are shown in Table 1 and in Results Section 3.1. The indications for ERC were (1) documentation of PSC diagnosis due to elevated or fluctuating plasma alkaline phosphatase levels in conjunction with inflammatory bowel disease (IBD), magnetic resonance cholangiography findings or liver biopsy suggestive of PSC and (2) surveillance of disease progression and/or biliary dysplasia [14]. While the PSC diagnoses were mainly based on ERC findings, liver biopsy was performed in 82% (150) of PSC patients. According to present guidelines, liver biopsy is recommended only for the diagnosis of PSC with AIH-like features (PSC/AIH overlap syndrome) and in suspicion of so called ‘small duct PSC’. The absence of PSC was confirmed histologically in 22 (66%) of control patients in addition to ERC.

Table 1.
Clinical characteristics and laboratory findings.
During ERC, a bile sample was aspirated from bile ducts with balloon occlusion technique and then stored at −80 °C. Brush cytology from intra- and extra-hepatic bile ducts was routinely collected from all patients, regardless of the presence of strictures, for Papanicolaou staining and grading for inflammation and dysplasia. ERC findings were scored according to the Helsinki score [14], modified from the Amsterdam score. Blood, serum and plasma samples were also collected at the time of ERC and were analyzed in an accredited hospital laboratory (Huslab, Helsinki, Finland) as part of clinical routine for blood hemoglobin and thrombocytes, plasma alanine transaminase, aspartate transaminase, plasma alkaline phosphatase, albumin and total cholesterol, and serum total bilirubin and CA19-9.
All the patients included in the PSC registry gave written informed consent. The study was performed following the principles of good clinical practice and in accordance with the ethical guidelines of the 1975 Declaration of Helsinki (sixth revision, 2008). The study protocol was approved by Helsinki University Hospital Ethical Committee IV, HUS/1566/2020.
2.2. Immunofluorometric Assays
The concentrations of trypsin(ogen)-2 and -3, SPINK1 and hCGβ in bile samples were determined as previously described using in-house immunofluorometric assays [11,15,16,17,18]. The assays for trypsin(ogen)-2 and -3 detect both active trypsins and pro-forms, trypsinogens. The limits of detection for trypsin(ogen)-2 and -3 and SPINK1 assays are 0.24 µg/L, 0.4 µg/L and 0.13 µg/L, respectively, and, for all assays, intra- and inter-assay CVs are <13% [11,15,16]. The cross-reaction of trypsinogen-1 in the trypsin(ogen)-2 assay is <0.1%. The trypsin(ogen)-3 assay shows <0.1% cross-reactivity with trypsinogen-1 and -2. The detection limit of hCGβ assay is 0.27 pmol/L and the intra-assay CVs are <15% [18]. The results below the limits of detection were assigned a value half of the detection limits.
2.3. Data Analyses
Mann–Whitney U test was used to evaluate differences in marker concentrations between different groups and Spearman’s rho to assess correlations between the markers (IBM SPSS Statistics and SigmaPlot (Systat Software Inc., San Jose, CA, USA)). Two-sided/tailed p-values are reported, and p < 0.05 was considered significant. Unless otherwise stated, the marker concentrations and values of other continuous variables are reported as median and, in parenthesis, interquartile range (IQR). Receiver operating characteristic (ROC) curves, differences between AUCs (paired analysis) and marker levels at which the sensitivities were equal to the specificities (considered as optimal cut-off values) were deduced using SigmaPlot. Positive and negative predictive values (PPV and NPV, respectively), i.e., the proportions of true positive and true negative results, were calculated using the formula PPV (NPV) = 100% * number of true positives (negatives)/number of all positives (negatives).
3. Results
3.1. Patients
Of the 214 patients included in the study, 85% (n = 182) had confirmed PSC, whereas 32 patients with normal ERC served as controls (Table 1). The median PSC duration at the time of sample collection was 4 years (IQR 6.75 years). In total, 73% of the PSC patients had concomitant IBD, while of the non-PSC controls, only 25% presented with IBD. Of the patients with IBD, 72.3% had ulcerative colitis, 23.4% Crohn’s disease and 4.3% IBD unclassified. Median IBD duration at the time of bile sample collection was 11.5 years (IQR 14 years). During the follow-up of 9.1 years (median, IQR 0.9 years), CCA was diagnosed in 5 of the PSC patients (median 1.6 years, range 0.1–8.8 years, after the sample collection) and hepatocellular carcinoma (5.8 years, 0.0–9.1 years) in 5, and 22 underwent liver transplantation (3.7 years, range 0.33–9.1 years). Of the patients who underwent liver transplantation, five were transplanted for suspicion of malignancy. In explant, dysplasia was found in three of them. None of the patients transplanted for end-stage liver disease (n = 12) had dysplasia in the explant, but one had three small HCC lesions, all less than 20 mm. In patients transplanted for symptoms (n = 5), no dysplasia was found either in the explant, and four of them had cirrhosis. Of all PSC patients, nine patients died (three of them had CCA and one hepatocellular carcinoma) (4.0 years, 0.94–6.1 years). In eight patients, biliary dysplasia was observed at least twice (up to four times) in brush cytology, two of these were diagnosed with CCA and four underwent liver transplantation for suspicion of CCA. Patients who were diagnosed with CCA or with biliary dysplasia at least twice during the follow-up were combined into a group that developed biliary neoplasia during the follow-up (n = 11).
3.2. Detection of Markers in Bile
Trypsin(ogen)-2, SPINK1 and hCGβ were often detectable in bile in that 100%, 94% and 94%, respectively, of the samples (n = 214) contained concentrations above the limits of detection. Trypsinogen-3 was measurable only in 52% of the samples. Trypsin(ogen)-2 and -3 levels were significantly correlated (ρ = 0.46, p < 10−11). SPINK1 correlated negatively with trypsin(ogen)-2 (ρ = −0.41, p < 10−9) and positively with hGCβ (ρ = 0.19, p = 0.005). Serum CA19-9 did not correlate with biliary trypsin(ogen)s, SPINK1 or hCGβ (for all p > 0.17). None of these markers were significantly different in PSC patients as compared to controls without PSC (Table 1), and only trypsin(ogen)-2 was higher in PSC patients with IBD (259 (947) µg/L, median (IQR), p = 0.006) as compared to those without IBD (133 (347) µg/L).
3.3. Biliary hCGβ Levels Are Higher in Patients with Biliary Neoplasia
Patients who, during the follow-up of 9.1 years (median, IQR 0.9 years), were diagnosed with CCA (after 1.6 years (median, range 0.1–8.8 years) follow-up) or in whom biliary dysplasia was detected at least twice in brush cytology were combined to represent the biliary neoplasia group (n = 11). In this group, as compared to the rest of the PSC patients, biliary hCGβ was increased (from 1.41 (2.48) in controls to 5.51 (11.7) pmol/L, median (IQR), p < 0.001), while biliary trypsin(ogen)-2 levels were decreased (from 248 (851) in controls to 55.6 (133) µg/L, median (IQR), p = 0.027) (Table 1). No change in serum CA19-9 was observed (p = 0.31). While no correlation between biliary hCGβ and serum CA19-9 was observed in PSC patients (ρ = 0.10, p = 0.22, n = 161), biliary hCGβ correlated with plasma ALT and ALP and serum bilirubin (for all ρ > 0.16, p < 0.05, n = 177–179).
3.4. Biliary hCGβ Has Better Diagnostic Accuracy Than Serum CA19-9 for Detecting Biliary Neoplasia
ROC curves were drawn using data from PSC patients from whom results were available for all studied novel markers and CA19-9 (n = 161) (Figure 1). As deduced from the AUCs, biliary hCGβ showed a better diagnostic accuracy than serum CA19-9 for the prediction of neoplasia (n = 10, p < 0.05). AUC was 0.82 for hCGβ (optimal cut-off value 2.7 pmol/L at sensitivity and specificity level of 72%), 0.69 for trypsin(ogen)-2 (cut-off 128 µg/L at 66%), 0.60 for serum CA19-9 (cut-off 6.0 kU/L at 50%) and 0.082 for the ratio of hCGβ to trypsin(ogen)-2 (cut-off 0.035 at 77%). Positive and negative predictive values (PPV and NPV, respectively) were determined using these cut-off levels. The ratio between hCGβ and trypsin(ogen)-2 showed a PPV of 20.6% (NPV 94.5%), while that of hCGβ was 14.3% (NPV 97.3), trypsin(ogen)-2 was 10.3% (NPV 96.1%) and CA19-9 was 6.6% (NPV 94.1%). The PPV of the combination of hCGβ and CA19-9 (i.e., both > cut-off levels) was 18.2% and NPV was 95.7%.
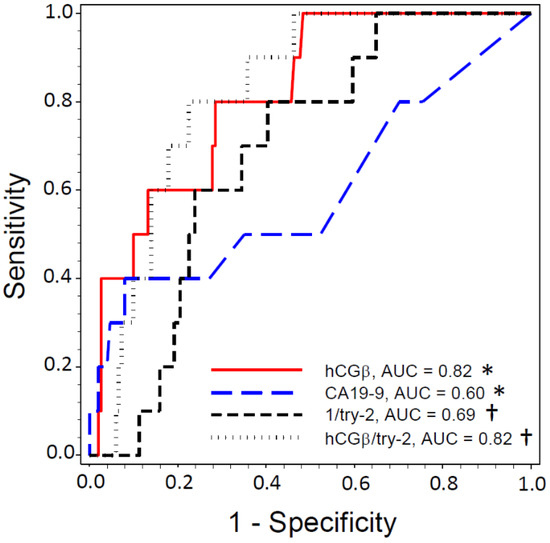
Figure 1.
ROC curve analysis for identification of PSC patients developing biliary neoplasia. Ability of serum CA19-9 and biliary hCGβ, trypsin(ogen)-2 (try-2) and the ratio between hCGβ and try-2 (hCGβ/try-2) to identify PCS patients who developed neoplasia (i.e., in whom CCA was diagnosed during the follow-up or in whom biliary dysplasia documented twice or more often (n = 10)). Only PSC patients from whom the levels of all markers were available are included (n = 161). As trypsin(ogen)-2 levels were lower in patients developing biliary neoplasia, the reciprocal was used for ROC analyses. *, †, p < 0.05 in comparison of AUCs (paired analysis).
4. Discussion
There is a great need for novel biomarkers in PSC to predict the development of biliary neoplasia. Trypsin(ogen)-2 and hCGβ have previously been found to be elevated in the serum of patients with CCA, irrespective of the concomitant PSC, and they distinguish CCA better than the most widely used, but poorly performing, markers, such as CA19-9 and CEA [7]. Here, we evaluated whether hCGβ, trypsin(ogen)-2 or -3 or trypsin inhibitor SPINK1 are elevated in bile already before the development of biliary neoplasia. The main finding was the increased biliary hCGβ levels in PSC patients who progressed to CCA during the follow-up period of about a decade or in whom biliary dysplasia was observed at least twice, indicating an increased risk for the later development of CCA [3]. Contrary to hCGβ, biliary trypsin(ogen)-2 levels were decreased in these patients, whereas no change in serum CA19-9 was observed.
Previously, elevated serum levels of hCGβ have been found to be associated with adverse prognosis in CCA, as well as in several other non-trophoblastic cancers [7,10]. Since an increased protein expression of hCGβ has also been observed in tumor tissue, including extrahepatic CCA [19], it is likely that the associations with poor prognosis reflect the increased expression of hCGβ in malignant tissue. While it remains to be studied, it is plausible that hCGβ has a cancer-promoting role in CCA similar to that in other cancers [13], which would explain the increase in hCGβ levels observed before the diagnosis of CCA.
Since the main purpose of the present study was to identify novel markers for PSC patients at risk for the development of CCA, we analyzed bile samples, which were assumed to reflect the disease state and the risk for CCA more accurately than serum markers. This also enabled us to focus on the markers produced in the liver, circumventing the relative high background observed in serum due to the high expression of trypsin(ogen)s and SPINK1 in the pancreas, especially in patients with pancreatitis or pancreatic cancer [6,9,10]. A drawback is that bile is difficult to collect and needs an ERC. Thus, it is not practical to use for routine screening. When aspirating the samples from bile ducts, we used the balloon occlusion technique, which minimized the risk of contamination with pancreatic fluid.
The present study also has some other limitations, mostly due to the small number of patients with biliary neoplasia, reflecting the disease prevalence. Thus, we were not able to fully evaluate the clinical performance of the biliary hCGβ for the prediction or early detection of biliary neoplasia and, especially, CCA. Furthermore, the commonly used serum markers, CEA and CA19-9, were not measured from biliary samples, and, at present, their performance in bile, compared to that of hCGβ, is unknown.
In addition to biomarkers and brush cytology, methods such as peroral cholangioscopy (POCS) with targeted biopsies have been suggested for the diagnosis of PSC and provide information about PSC-associated biliary neoplasia [20,21]. However, brush cytology with the possibility of reaching bile ducts, including intrahepatic ones, has more widely been found to have a better diagnostic accuracy than POCS for indeterminate biliary strictures [22]. Furthermore, biomarkers that are functionally involved or associated with the development of neoplasia may be able to detect or predict the development of neoplasia at a much earlier state than visualization-based approaches. However, all this warrants further studies.
Overall, in our study cohort, biliary hCGβ was increased in patients with biliary dysplasia or CCA, but not in IBD or PSC patients without biliary neoplasia. In contrast, biliary trypsin(ogen)-2 was elevated in PSC patients with IBD but lower in those with biliary dysplasia. Based on ROC curves, the diagnostic accuracy of biliary hCGβ exceeded that of serum CA19-9. Biliary hCGβ and serum CA19-9 concentrations did not show any correlation, suggesting that they are independent of each other. Thus, the monitoring of hCGβ may be justified in PSC patients, and our results warrant larger clinical studies.
Author Contributions
Conceptualization, H.K., U.-H.S. and M.F.; patient sample collection and laboratory analyses, H.K., S.B., J.A., K.J., R.K., A.L., K.H., U.-H.S., M.F. ; primary data analysis and interpretation, H.K., U.-H.S. and M.F.; writing—original draft preparation, H.K. and M.F.; writing—review and editing, H.K., S.B., J.A., K.J., R.K., A.L., K.H., U.-H.S., M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sigrid Jusélius Foundation and State funding for university-level health research.
Institutional Review Board Statement
The study was performed following the principles of good clinical practice and according to the guidelines of the 1975 Declaration of Helsinki (sixth revision, 2008). The study protocol was approved by Helsinki University Hospital Ethical Committee IV, HUS/1566/2020.
Informed Consent Statement
All the patients included in the PSC registry gave written informed consent.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
We would like to thank Annikki Löfhjelm, Maarit Leinimaa, Taina Grönholm and Kristiina Nokelainen for excellent technical assistance. Study nurses Virpi Pelkonen and Pirkko Tuukkala are acknowledged for patient recruitment and data recording in PSC registry.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Dyson, J.K.; Beuers, U.; Jones, D.E.J.; Lohse, A.W.; Hudson, M. Primary sclerosing cholangitis. Lancet 2018, 391, 2547–2559. [Google Scholar] [CrossRef]
- Fung, B.M.; Tabibian, J.H. Cholangiocarcinoma in patients with primary sclerosing cholangitis. Curr. Opin. Gastroenterol. 2020, 36, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.T.; Talwalkar, J.A.; Rosen, C.B.; Smyrk, T.C.; Abraham, S.C. Precancerous bile duct pathology in end-stage primary sclerosing cholangitis, with and without cholangiocarcinoma. Am. J. Surg. Pathol. 2010, 34, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, K.; Weersma, R.K.; van Erpecum, K.J.; Rauws, E.A.; Spanier, B.W.M.; Poen, A.C.; van Nieuwkerk, K.M.; Drenth, J.P.; Witteman, B.J.; Tuynman, H.A.; et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 2013, 58, 2045–2055. [Google Scholar] [CrossRef]
- Weismüller, T.J.; Trivedi, P.J.; Bergquist, A.; Imam, M.; Lenzen, H.; Ponsioen, C.Y.; Holm, K.; Gotthardt, D.; Färkkilä, M.A.; Marschall, H.U.; et al. Patient Age, Sex, and Inflammatory Bowel Disease Phenotype Associate with Course of Primary Sclerosing Cholangitis. Gastroenterology 2017, 152, 1975–1984.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedström, J.; Haglund, C.; Haapiainen, R.; Stenman, U.-H. Serum trypsinogen-2 and trypsin-2-alpha(1)-antitrypsin complex in malignant and benign digestive-tract diseases. Preferential elevation in patients with cholangiocarcinomas. Int. J. Cancer 1996, 66, 326–331. [Google Scholar] [CrossRef]
- Lempinen, M.; Isoniemi, H.; Mäkisalo, H.; Nordin, A.; Halme, L.; Arola, J.; Höckerstedt, K.; Stenman, U.-H. Enhanced detection of cholangiocarcinoma with serum trypsinogen-2 in patients with severe bile duct strictures. J. Hepatol. 2007, 47, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Paju, A.; Stenman, U.H. Biochemistry and clinical role of trypsinogens and pancreatic secretory trypsin inhibitor. Crit. Rev. Clin. Lab. Sci. 2006, 43, 103–142. [Google Scholar] [CrossRef] [PubMed]
- Räsänen, K.; Itkonen, O.; Koistinen, H.; Stenman, U.-H. Emerging Roles of SPINK1 in Cancer. Clin. Chem. 2016, 62, 449–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenman, U.H. Biomarker development, from bench to bedside. Crit. Rev. Clin. Lab. Sci. 2016, 53, 69–86. [Google Scholar] [CrossRef]
- Oiva, J.; Itkonen, O.; Koistinen, R.; Hotakainen, K.; Zhang, W.-M.; Kemppainen, E.; Puolakkainen, P.; Kylänpää, L.; Stenman, U.-H.; Koistinen, H. Specific Immunoassay Reveals Increased Serum Trypsinogen 3 in Acute Pancreatitis. Clin. Chem. 2011, 57, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Hedström, J.; Haglund, C.; Leinonen, J.; Nordling, S.; Stenman, U.-H.; Hedström, J. Trypsinogen-1, -2 and tumour-associated trypsin-inhibitor in bile and biliary tract tissues from patients with biliary tract diseases and pancreatic carcinomas. Scand. J. Clin. Lab. Investig. 2001, 61, 111–118. [Google Scholar] [PubMed]
- Koistinen, H.; Stenman, U.-H. LH/hCG-receptor independent activities of hCG and hCGβ. In 100 Years of Human Chorionic Gonadotropin: Reviews and New Perspectives, 1st ed.; Cole, L., Butler, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 261–268. [Google Scholar]
- Boyd, S.; Tenca, A.; Jokelainen, K.; Mustonen, H.; Krogerus, L.; Arola, J.; Färkkilä, M.A. Screening primary sclerosing cholangitis and biliary dysplasia with endoscopic retrograde cholangiography and brush cytology: Risk factors for biliary neoplasia. Endoscopy 2016, 48, 432–439. [Google Scholar] [CrossRef]
- Itkonen, O.; Kylänpää, L.; Zhang, W.-M.; Stenman, U.-H. Reference intervals for and validation of recalibrated immunoassays for trypsinogen-1 and trypsinogen-2. Clin. Chem. 2012, 58, 1494–1496. [Google Scholar] [CrossRef] [PubMed]
- Janeiro, E.; Guimarães, J.; Stenman, U.-H.; Catarino, M.; Itkonen, O. Validation and comparison of tumor-associated trypsin inhibitor (TATI) immunoassays. Clin. Chim. Acta 2012, 413, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Lempiäinen, A.; Stenman, U.-H.; Blomqvist, C.; Hotakainen, K. Free beta-subunit of human chorionic gonadotropin in serum is a diagnostically sensitive marker of seminomatous testicular cancer. Clin. Chem. 2008, 54, 1840–1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfthan, H.; Haglund, C.; Dabek, J.; Stenman, U.-H. Concentrations of human chorionic gonadotropin, its β-subunit and the core fragment of the β-subunit in serum and urine of men and nonpregnant women. Clin. Chem. 1992, 38, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Louhimo, J.; Nordling, S.; Alfthan, H.; von Boguslawski, K.; Stenman, U.-H.; Haglund, C. Specific staining of human chorionic gonadotropin beta in benign and malignant gastrointestinal tissues with monoclonal antibodies. Histopathology 2001, 38, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Ushio, M.; Takahashi, S.; Yamagata, W.; Takasaki, Y.; Suzuki, A.; Okawa, Y.; Ochiai, K.; Tomishima, K.; Ishii, S.; et al. Role of Peroral Cholangioscopy in the Diagnosis of Primary Sclerosing Cholangitis. Diagnostics 2020, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Arnelo, U.; von Seth, E.; Bergquist, A. Prospective Evaluation of the Clinical Utility of Single-Operator Peroral Cholangioscopy in Patients with Primary Sclerosing Cholangitis. Endoscopy 2015, 47, 696–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Vries, A.B.; van der Heide, F.; Ter Steege, R.W.F.; Koornstra, J.J.; Buddingh, K.T.; Gouw, A.S.H.; Weersma, R.K. Limited Diagnostic Accuracy and Clinical Impact of Single-Operator Peroral Cholangioscopy for Indeterminate Biliary Strictures. Endoscopy 2020, 52, 107–114. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).