Phase Characterization of (Mn, S) Inclusions and Mo Precipitates in Reactor Pressure Vessel Steel from Greifswald Nuclear Power Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Materials
2.2. Methods
2.2.1. Optical Emission Spectroscopy (OES)
2.2.2. Radioanalytical Methods
2.2.3. Neutron Fluence Calculation
2.2.4. Scanning Electron Microscopy/Electron Dispersive X-Ray Spectroscopy (SEM/EDS)
2.2.5. X-Ray Photoelectron Spectroscopy (XPS)
2.2.6. Raman Spectroscopy
3. Results
3.1. Elemental Analysis of the RPV Steel Samples (OES)
3.2. Radioanalytical Characterization
3.3. Microstructural Characterization and Elemental Analysis (SEM/EDS)
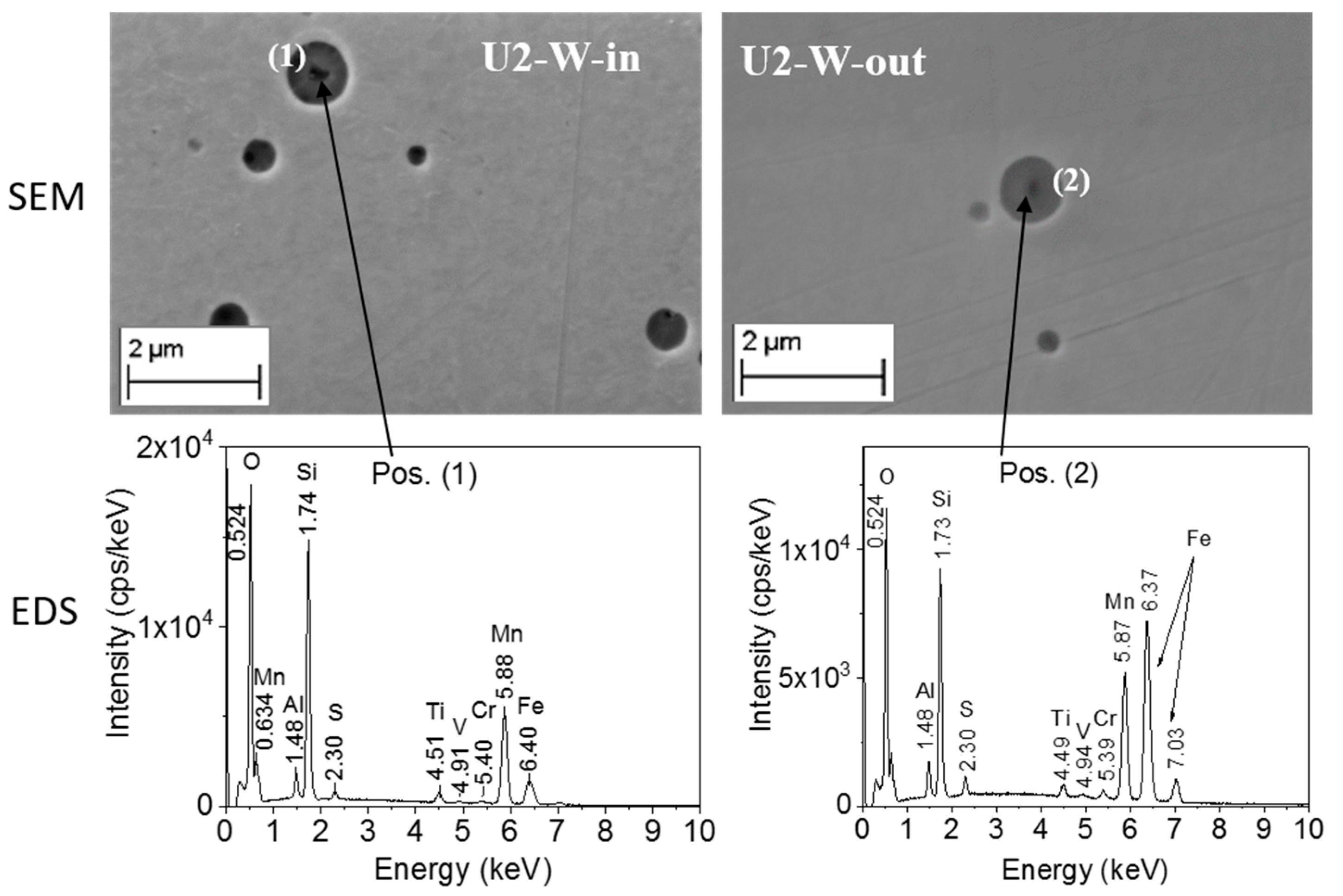
3.3.1. Characterization of the RPV First-Generation (U1 and U2) Samples
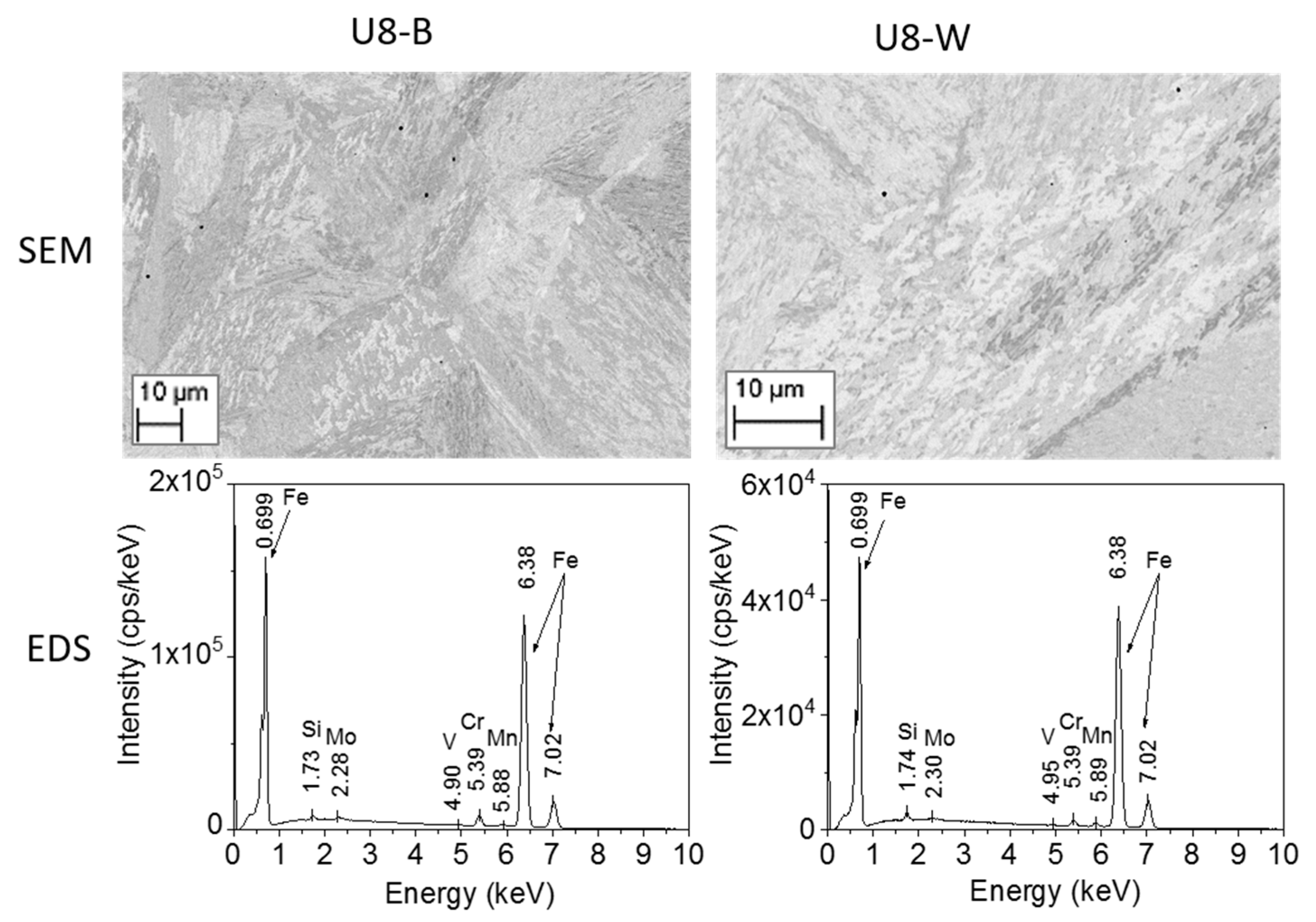
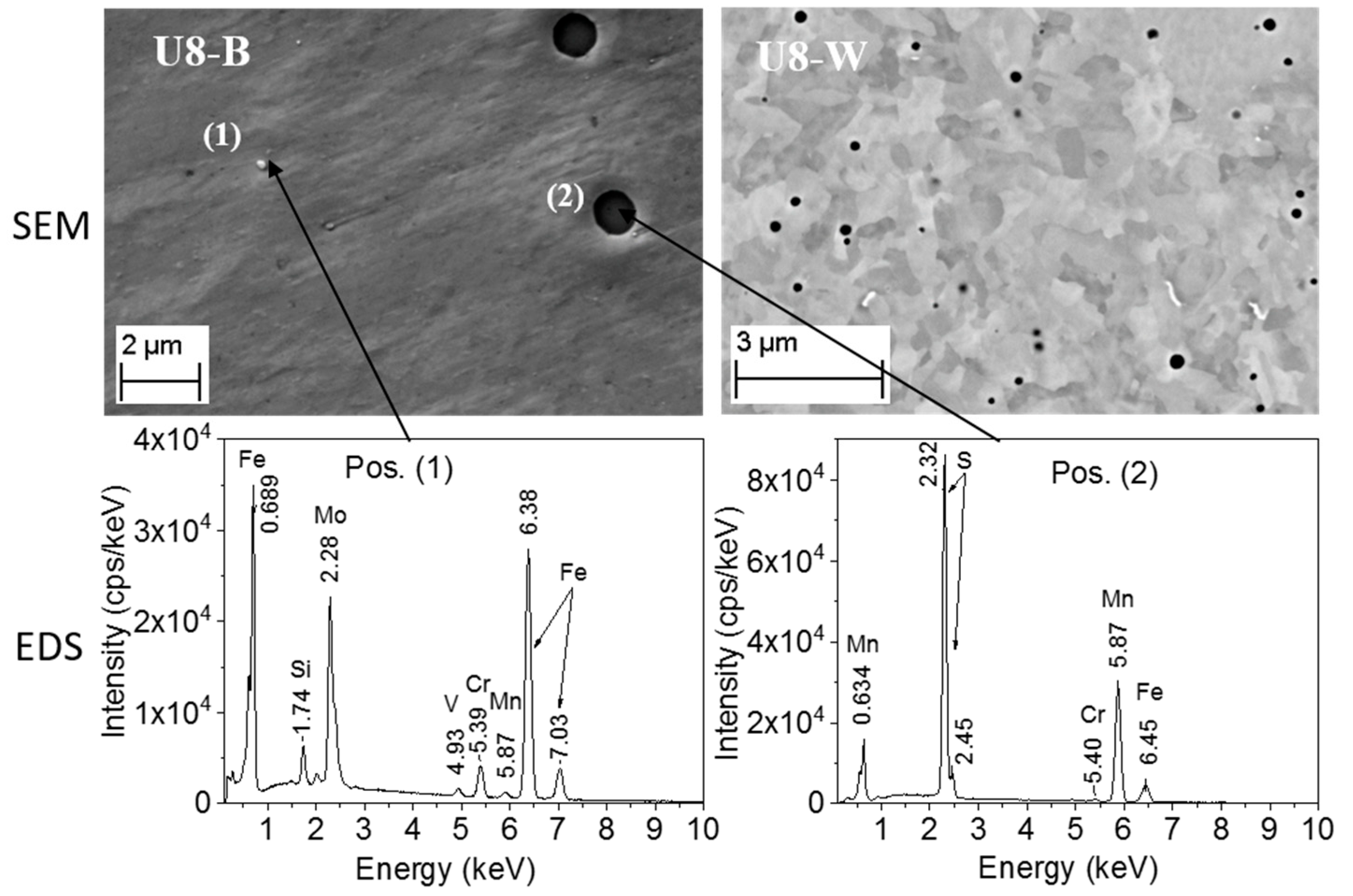
3.3.2. Characterization of the RPV Second-Generation U8 Samples
3.4. Spectroscopic Characterization of Steel Inclusions
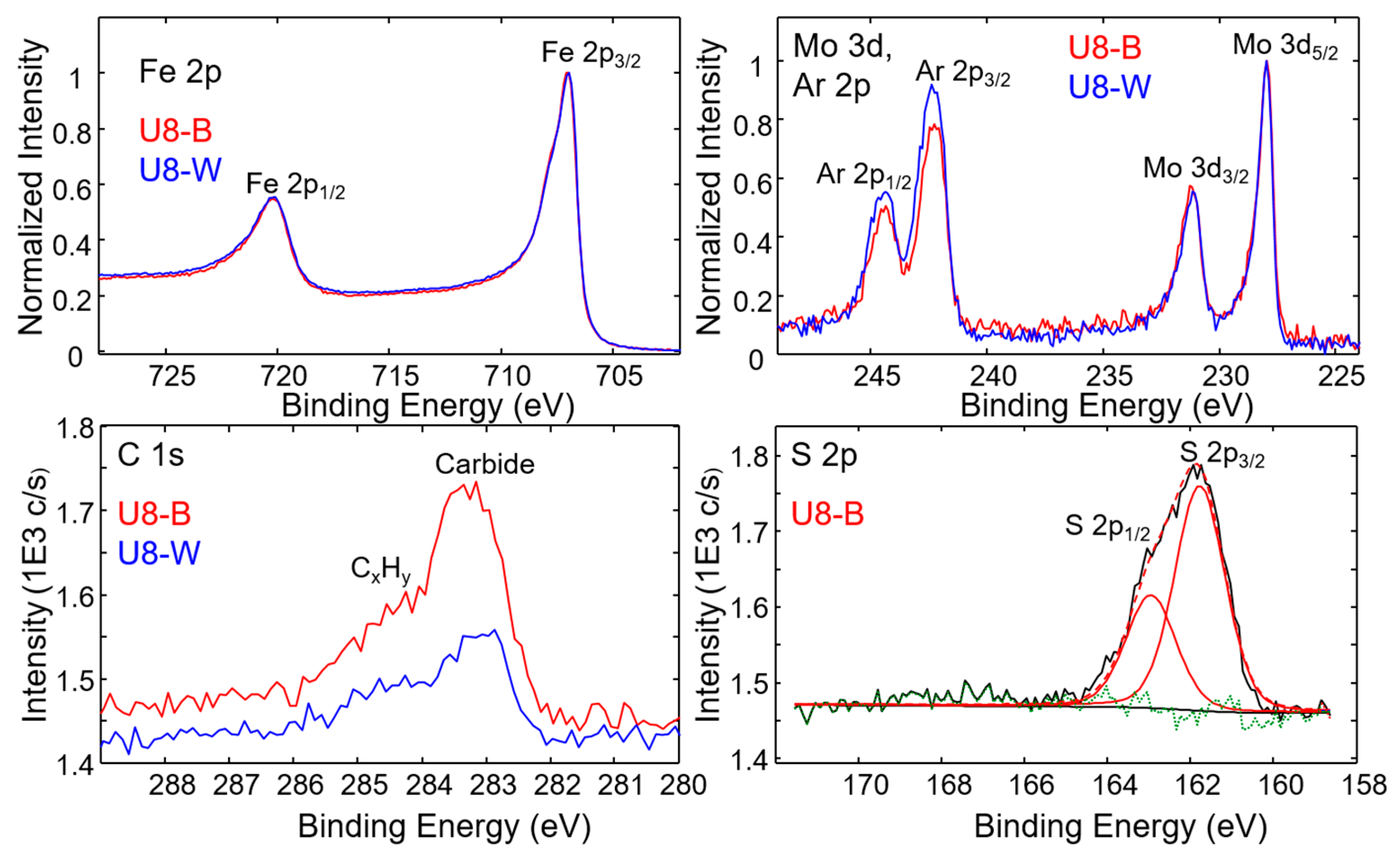
3.4.1. X-Ray Photoelectron Spectroscopy (XPS)
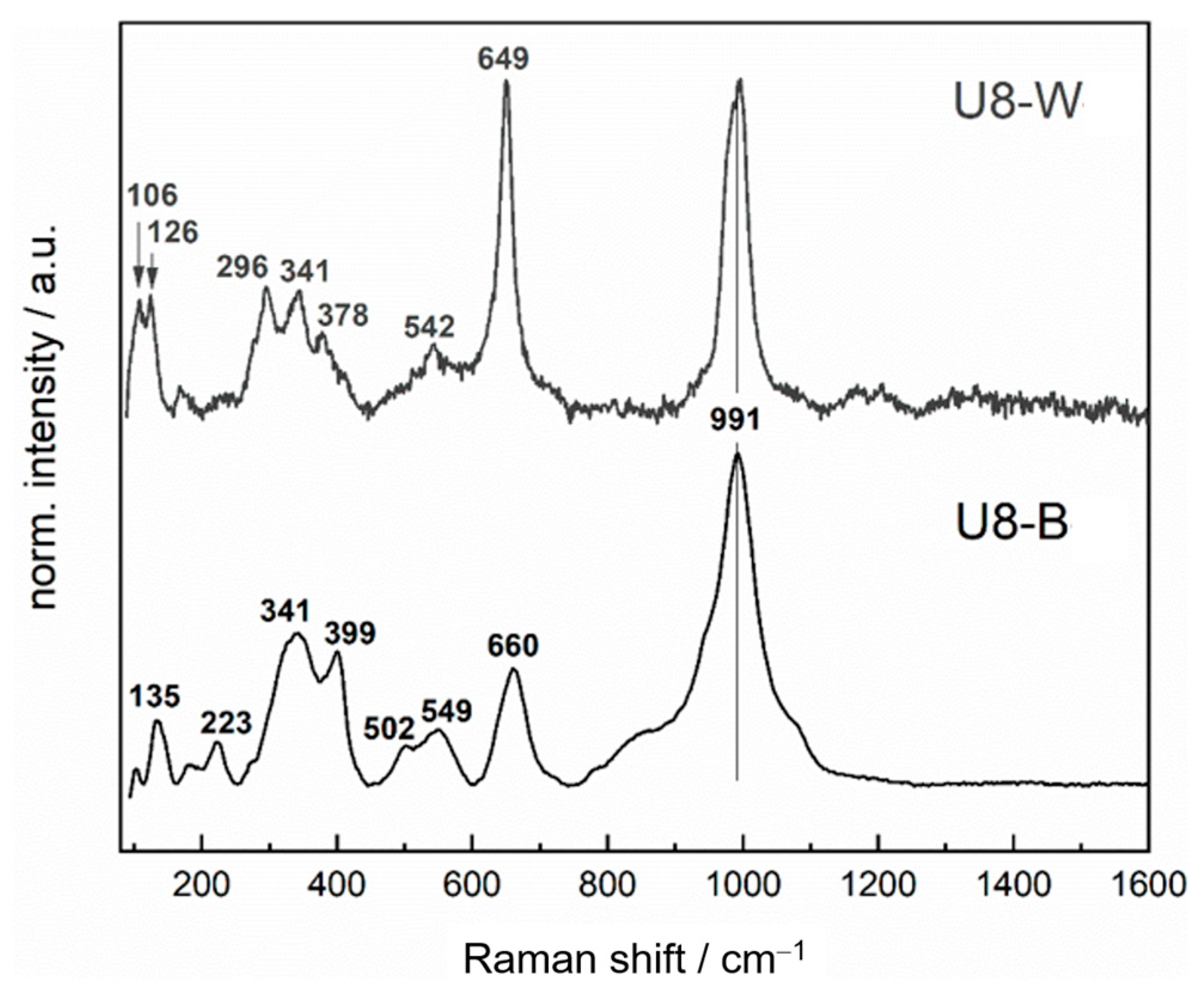
3.4.2. Raman Spectroscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hou, X.L.; Roos, P. Critical comparison of radiometric and mass spectrometric methods for the determination of radionuclides in environmental, biological and nuclear waste samples. Anal. Chim. Acta 2008, 608, 105–139. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Q.; Fang, S.; Li, H. Research on the induced radioactivity of HTR-PM’s reactor pressure vessel: A comparative study between FLUKA, KORIGEN and QAD-CGA. Ann. Nucl. Energy 2018, 114, 129–135. [Google Scholar] [CrossRef]
- Robertson, D.E.; Thomas, C.W.; Wynhoff, N.L.; Hetzer, D.C. Characterization of long-lived activation products in spent fuel assembly hardware and reactor pressure-vessel steel. Nucl. Eng. Des. 1990, 118, 463–486. [Google Scholar] [CrossRef]
- Das, A. Fracture complexity of pressure vessel steels. Philos. Mag. 2017, 97, 3084–3141. [Google Scholar] [CrossRef]
- Odette, G.R.; Lucas, G.E. Recent progress in understanding reactor pressure vessel steel embrittlement. Radiat. Eff. Defects Solids 1998, 144, 189–231. [Google Scholar] [CrossRef]
- Emerson, J.N.; Marrero-Jackson, E.H.; Nemets, G.A.; Okuniewski, M.A.; Wharry, J.P. Nuclear Reactor Pressure Vessel Welds: A Critical and Historical Review of Microstructures, Mechanical Properties, Irradiation Effects, and Future Opportunities. Mater. Des. 2024, 244, 34. [Google Scholar] [CrossRef]
- Zhou, L.J.; Dai, J.; Li, Y.; Dai, X.; Xie, C.S.; Li, L.Z.; Chen, L.S. Research Progress of Steels for Nuclear Reactor Pressure Vessels. Materials 2022, 15, 8761. [Google Scholar] [CrossRef]
- Kim, J.; Choi, K.J.; Bahn, C.B.; Kim, J.H. In situ Raman spectroscopic analysis of surface oxide films on Ni-base alloy/low alloy steel dissimilar metal weld interfaces in high-temperature water. J. Nucl. Mater. 2014, 449, 181–187. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.H.; Choi, K.J.; Bahn, C.B.; Hwang, I.S.; Kim, J.H. In-situ investigation of thermal aging effect on oxide formation in Ni-base alloy/low alloy steel dissimilar metal weld interfaces. Corros. Sci. 2014, 86, 295–303. [Google Scholar] [CrossRef]
- Maslar, J.E.; Hurst, W.S.; Bowers, W.J.; Hendricks, J.H.; Aquino, M.I. In situ Raman spectroscopic investigation of aqueous iron corrosion at elevated temperatures and pressures. J. Electrochem. Soc. 2000, 147, 2532–2542. [Google Scholar] [CrossRef]
- Wu, X.Q.; Katada, Y. Role of inclusions and carbide bands in corrosion fatigue of pressure vessel steel in high-temperature water. Corrosion 2004, 60, 1045–1057. [Google Scholar] [CrossRef]
- Xing, R.S.; Yu, D.J.; Xie, G.F.; Yang, Z.H.; Wang, X.X.; Chen, X. Effect of thermal aging on mechanical properties of a bainitic forging steel for reactor pressure vessel. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2018, 720, 169–175. [Google Scholar] [CrossRef]
- Hytonen, N.; Que, Z.Q.; Arffman, P.; Lydman, J.; Nevasmaa, P.; Ehrnsten, U.; Efsing, P. Effect of weld microstructure on brittle fracture initiation in the thermally-aged boiling water reactor pressure vessel head weld metal. Int. J. Miner. Metall. Mater. 2021, 28, 867–876. [Google Scholar] [CrossRef]
- Que, Z.Q.; Lindroos, M.; Lydman, J.; Hytonen, N.; Lindqvist, S.; Efsing, P.; Nevasmaa, P.; Arffman, P. Brittle fracture initiation in decommissioned boiling water reactor pressure vessel head weld. J. Nucl. Mater. 2022, 569, 15. [Google Scholar] [CrossRef]
- Ulbricht, A.; Altstadt, E.; Bergner, F.; Viehrig, H.W.; Keiderling, U. Small-angle neutron scattering investigation of as-irradiated, annealed and reirradiated reactor pressure vessel weld material of decommissioned reactor. J. Nucl. Mater. 2011, 416, 111–116. [Google Scholar] [CrossRef]
- Viehrig, H.W.; Altstadt, E.; Houska, M.; Valo, M. Fracture mechanics characterisation of the beltline welding seam of the decommissioned WWER-440 reactor pressure vessel of nuclear power plant Greifswald Unit 4. Int. J. Press. Vessel. Pip. 2012, 89, 129–136. [Google Scholar] [CrossRef]
- Lindgren, K.; Boasen, M.; Que, Z.Q.; Stiller, K.; Efsing, P.; Thuvander, M. Post-irradiation annealing of high flux irradiated and surveillance material reactor pressure vessel weld metal. J. Nucl. Mater. 2022, 562, 10. [Google Scholar] [CrossRef]
- Lindgren, K.; Boasen, M.; Stiller, K.; Efsing, P.; Thuvander, M. Evolution of precipitation in reactor pressure vessel steel welds under neutron irradiation. J. Nucl. Mater. 2017, 488, 222–230. [Google Scholar] [CrossRef]
- Lindgren, K.; Stiller, K.; Efsing, P.; Thuvander, M. On the Analysis of Clustering in an Irradiated Low Alloy Reactor Pressure Vessel Steel Weld. Microsc. Microanal. 2017, 23, 376–384. [Google Scholar] [CrossRef]
- Saroun, J.; Kocik, J.; Garcia-Matres, E.; Muransky, O.; Strunz, P. Characterisation of radiation-induced precipitates in reactor pressure vessel steels. Z. Fur Krist. 2006, S23, 393–398. [Google Scholar]
- Ulbricht, A.; Hernandez-Mayoral, M.; Onorbe, E.; Etienne, A.; Radiguet, B.; Hirschmann, E.; Wagner, A.; Hein, H.; Bergner, F. Effect of Neutron Flux on an Irradiation-Induced Microstructure and Hardening of Reactor Pressure Vessel Steels. Metals 2022, 12, 369. [Google Scholar] [CrossRef]
- Viehrig, H.W.; Altstadt, E.; Houska, M. Radiation response of the overlay cladding from the decommissioned WWER-440 Greifswald Unit 4 reactor pressure vessel. Nucl. Eng. Des. 2015, 286, 227–236. [Google Scholar] [CrossRef]
- Wagner, A.; Bergner, F.; Chaouadi, R.; Hein, H.; Hernandez-Mayoral, M.; Serrano, M.; Ulbricht, A.; Altstadt, E. Effect of neutron flux on the characteristics of irradiation-induced nanofeatures and hardening in pressure vessel steels. Acta Mater. 2016, 104, 131–142. [Google Scholar] [CrossRef]
- Kapoor, G.; Chekhonin, P.; Kaden, C.; Vogel, K.; Bergner, F. Microstructure-informed prediction and measurement of nanoindentation hardness of an Fe-9Cr alloy irradiated with Fe-ions of 1 and 5 MeV energy. Nucl. Mater. Energy 2022, 30, 9. [Google Scholar] [CrossRef]
- Kumar, N.; Li, C.; Leonard, K.J.; Bei, H.; Zinkle, S.J. Microstructural stability and mechanical behavior of FeNiMnCr high entropy alloy under ion irradiation. Acta Mater. 2016, 113, 230–244. [Google Scholar] [CrossRef]
- Wagner, A.; Ulbricht, A.; Bergner, F.; Altstadt, E. Influence of the copper impurity level on the irradiation response of reactor pressure vessel steels investigated by SANS. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. At. 2012, 280, 98–102. [Google Scholar] [CrossRef]
- Ghoneim, M.M.; Hammad, F.H. Pressure vessel steels: Influence of chemical composition on irradiation sensitivity. Int. J. Press. Vessel. Pip. 1997, 74, 189–198. [Google Scholar] [CrossRef]
- Rindelhardt, U.; Viehrig, H.W.; Konheiser, J.; Schuhknecht, J.; Noack, K.; Gleisberg, B. RPV material investigations of the former VVER-440 Greifswald NPP. Nucl. Eng. Des. 2009, 239, 1581–1590. [Google Scholar] [CrossRef]
- Viehrig, H.W.; Houska, M.; Altstadt, E. Radiation and annealing response of WWER 440 beltline welding seams. J. Nucl. Mater. 2015, 456, 334–343. [Google Scholar] [CrossRef]
- Werner, C.J. MCNP® User’s Manual; Code Version 6.2. 2017, LA-UR-17-29981; Los Alamos National Laboratory: Los Alamos, NM, USA, 2017. [Google Scholar]
- Rindelhardt, U.; Viehrig, H.W.; Konheiser, J.; Schuhknecht, J. Weld Material Investigations of a WWER-440 Reactor Pressure Vessel: Results from the First Trepan Taken from the Former Greifswald NPP. J. Eng. Gas Turbines Power-Trans. Asme. 2009, 131, 7. [Google Scholar] [CrossRef]
- Poenitz, E.; Konheiser, J. Investigation of RPV samples of the Greifswald NPP with focus on retrospective dosimetry. EPJ Web Conf. ISRD-17 2024, 308, 03009. [Google Scholar] [CrossRef]
- Seah, M.P.; Gilmore, L.S.; Beamson, G. XPS: Binding energy calibration of electron spectrometers 5—Re-evaluation of the reference energies. Surf. Interface Anal. 1998, 26, 642–649. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy; Chastain, J., King, R.C., Jr., Eds.; ULVAC-PHI, Inc.: Chigasaki, Japan, 1992. [Google Scholar]
- Sun, J.L.; Tang, H.J.; Wang, C.L.; Han, Z.; Li, S.S. Effects of Alloying Elements and Microstructure on Stainless Steel Corrosion: A Review. Steel Res. Int. 2022, 93, 19. [Google Scholar] [CrossRef]
- Wang, Y.; Karasev, A.; Jonsson, P.G. Characterization of Nonmetallic Inclusions in Different Ferroalloys used in the Steelmaking Processes. Steel Res. Int. 2021, 92, 12. [Google Scholar] [CrossRef]
- Wang, Y.; Karasev, A.; Park, J.H.; Jonsson, P.G. Non-metallic Inclusions in Different Ferroalloys and Their Effect on the Steel Quality: A Review. Metall. Mater. Trans. B-Process Metall. Mater. Process. Sci. 2021, 52, 2892–2925. [Google Scholar] [CrossRef]
- Kepka, M.; Kletecka, Z.; Barackova, L. Chemische Inhomogenität und Güte großer Schmiedeblöcke. Stahl Und Eisen 1982, 102, 127–130. [Google Scholar]
- GRS. Sicherheitsbeurteilung des Kernkraftwerks Greifswald, Block 1—4; Gesellschaft für Reaktorsicherheit (GRS) mbH: Koln, Germany, 1990. [Google Scholar]
- Viehrig, H.W.; Schuhknecht, J. Fracture mechanics characterisation of the WWER-440 reactor pressure vessel beltline welding seam. Int. J. Press. Vessel. Pip. 2009, 86, 239–245. [Google Scholar] [CrossRef]
- Rindelhardt, U.; Viehrig, H.W.; Konheiser, J.; Schuhknecht, J. Asme. In Weld Material Investigations of a WWER-440 Reactor Pressure Vessel: Results Form the First Trepan Taken from the Former Greifswald NPP, Proceedings of the 16th International Conference on Nuclear Engineering, Orlando, FL, USA, 11–15 May 2008; The American Society of Mechanical Engineers: New York, NY, USA, 2008. [Google Scholar]
- Leskinen, A.; Salminen-Paatero, S.; Gautier, C.; Räty, A.; Tanhua-Tyrkkö, M.; Fichet, P.; Kekki, T.; Zhang, W.Z.; Bubendorff, J.; Laporte, E.; et al. Intercomparison exercise on difficult to measure radionuclides in activated steel: Statistical analysis of radioanalytical results and activation calculations. J. Radioanal. Nucl. Chem. 2020, 324, 1303–1316. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Bainite in Steels, 3rd ed.; CRC Press: London, UK, 2015. [Google Scholar]
- Ghoneim, M.M.; Nasreldin, A.M.; Elsayed, A.A.; Pachur, D.; Hammad, F.H. Instrumented impact properties of some advanced nuclear reactor pressure vessel steels. J. Mater. Eng. Perform. 1996, 5, 328–334. [Google Scholar] [CrossRef]
- NIST. NIST X-Ray Photoelectron Spectroscopy Database, Version 4.1, 2012; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012.
- Maslar, J.E.; Hurst, W.S.; Bowers, W.J.; Hendricks, J.H. In situ Raman spectroscopic investigation of stainless steel hydrothermal corrosion. Corrosion 2002, 58, 739–747. [Google Scholar] [CrossRef]
- Li, S.X.; Hihara, L.H. Identification of inclusions in carbon steels using micro-Raman spectroscopy. J. Raman Spectrosc. 2017, 48, 137–142. [Google Scholar] [CrossRef]
- Alanazi, A.M.; McNaughter, P.D.; Alam, F.; Vitorica-yrezabal, I.J.; Whitehead, G.F.S.; Tuna, F.; O’Brien, P.; Collison, D.; Lewis, D.J. Structural Investigations of alpha-MnS Nanocrystals and Thin Films Synthesized from Manganese(II) Xanthates by Hot Injection, Solvent-Less Thermolysis, and Doctor Blade Routes. ACS Omega 2021, 6, 27716–27725. [Google Scholar] [CrossRef]
- Bourdoiseau, J.A.; Jeannin, M.; Remazeilles, C.; Sabota, R.; Refait, P. The transformation of mackinawite into greigite studied by Raman spectroscopy. J. Raman Spectrosc. 2011, 42, 496–504. [Google Scholar] [CrossRef]
- Dywel, P.; Szczesny, R.; Domanowski, P.; Skowronski, L. Structural and Micromechanical Properties of Nd:YAG Laser Marking Stainless Steel (AISI 304 and AISI 316). Materials 2020, 13, 2168. [Google Scholar] [CrossRef] [PubMed]
- He, X.B.; Wang, L.J.; Chou, K.C. Basicity Contributions to Interfacial Structure and Oxygen Potential of CaO-MnO-SiO2 slag. Metall. Mater. Trans. B-Process Metall. Mater. Process. Sci. 2022, 53, 1583–1592. [Google Scholar] [CrossRef]
- Huidobro, J.; Aramendia, J.; Garcia-Florentino, C.; Ruiz-Galende, P.; Torre-Fdez, I.; Castro, K.; Arana, G.; Madariaga, J.M. Mineralogy of the RBT 04262 Martian meteorite as determined by micro-Raman and micro-X-ray fluorescence spectroscopies. J. Raman Spectrosc. 2022, 53, 450–462. [Google Scholar] [CrossRef]
- Takahashi, N.; Tsujimori, T.; Kamada, S.; Nakamura, M. In-situ Raman spectroscopic analysis of dissolved silica structures in Na2CO3 and NaOH solutions at high pressure and temperature. Contrib. Mineral. Petrol. 2022, 177, 15. [Google Scholar] [CrossRef]
- Girish, M.; Dhandayuthapani, T.; Sivakumar, R.; Sanjeeviraja, C. MnS thin films prepared by a simple and novel nebulizer technique: Report on the structural, optical, and dispersion energy parameters. J. Mater. Sci. Mater. Electron. 2015, 26, 3670–3684. [Google Scholar] [CrossRef]
- Maslar, J.E.; Hurst, W.S.; Bowers, W.J.; Hendricks, J.H.; Aquino, M.I.; Levin, I. In situ Raman spectroscopic investigation of chromium surfaces under hydrothermal conditions. Appl. Surf. Sci. 2001, 180, 102–118. [Google Scholar] [CrossRef]
- Molchan, I.S.; Thompson, G.E.; Lindsay, R.; Skeldon, P.; Likodimos, V.; Romanos, G.E.; Falaras, P.; Adamova, G.; Iliev, B.; Schubert, T.J.S. Corrosion behaviour of mild steel in 1-alkyl-3-methylimidazolium tricyanomethanide ionic liquids for CO2 capture applications. RSC Adv. 2014, 4, 5300–5311. [Google Scholar] [CrossRef]
- Rafique, M.Y.; Pan, L.Q.; Javed, Q.U.; Iqbal, M.Z.; Qiu, H.M.; Farooq, M.H.; Guo, Z.G.; Tanveer, M. Growth of monodisperse nanospheres of MnFe2O4 with enhanced magnetic and optical properties. Chin. Phys. B 2013, 22, 7. [Google Scholar] [CrossRef]
- Ramki, S.; Pandi, K.; Chen, S.M.; Ye, Y.T.; Chen, T.W.; Hao, Q.L. Hydrothermal Synthesis of Manganese Sulfide Decorated Graphene Oxide for Effective Electrochemical Sensing of Dopamine. Int. J. Electrochem. Sci. 2019, 14, 1069–1081. [Google Scholar] [CrossRef]
- Avril, C.; Malavergne, V.; Caracas, R.; Zanda, B.; Reynard, B.; Charon, E.; Bobocioiu, E.; Brunet, F.; Borensztajn, S.; Pont, S.; et al. Raman spectroscopic properties and Raman identification of CaS-MgS-MnS-FeS-Cr2FeS4 sulfides in meteorites and reduced sulfur-rich systems. Meteorit. Planet. Sci. 2013, 48, 1415–1426. [Google Scholar] [CrossRef]
- Rémazeilles, C.; Saheb, M.; Neff, D.; Guilminot, E.; Tran, K.; Bourdoiseau, J.A.; Sabot, R.; Jeannin, M.; Matthiesen, H.; Dillmann, P.; et al. Microbiologically influenced corrosion of archaeological artefacts: Characterisation of iron(II) sulfides by Raman spectroscopy. J. Raman Spectrosc. 2010, 41, 1425–1433. [Google Scholar] [CrossRef]
- Snovski, R.; Grinblat, J.; Sougrati, M.T.; Jumas, J.C.; Margel, S. Synthesis and characterization of iron, iron oxide and iron carbide nanostructures. J. Magn. Magn. Mater. 2014, 349, 35–44. [Google Scholar] [CrossRef]
- Upadhyay, S.; Pandey, O.P. One-pot synthesis of pure phase molybdenum carbide (Mo2C and MoC) nanoparticles for hydrogen evolution reaction. Int. J. Hydrogen Energ. 2020, 45, 27114–27128. [Google Scholar] [CrossRef]
- Grover, V.; Shukla, R.; Kumari, R.; Mandal, B.P.; Kulriya, P.K.; Srivastava, S.K.; Ghosh, S.; Tyagi, A.K.; Avasthi, D.K. Effect of grain size and microstructure on radiation stability of CeO2: An extensive study. Phys. Chem. Chem. Phys. 2014, 16, 27065–27073. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, S.X.; Zhang, C.H.; Yang, Y.T.; Lv, K.Y. Raman Spectra and Microstructure of Zinc Oxide irradiated with Swift Heavy Ion. Crystals 2019, 9, 395. [Google Scholar] [CrossRef]
- Karcher, S.; Mohun, R.; Olds, T.; Weber, M.; Kriegsman, K.; Zhao, X.D.; Guo, X.F.; Corkhill, C.; Field, D.; McCloy, J. Benefits of using multiple Raman laser wavelengths for characterizing defects in a UO2 matrix. J. Raman Spectrosc. 2022, 53, 988–1002. [Google Scholar] [CrossRef]
- Bing, B.; Han, X.; Jia, L.; He, X.; Zhang, C.; Yang, W. Influence analysis of alloy elements on irradiation embrittlement of RPV steel based on deep neural network. Int. J. Adv. Nucl. React. Des. Technol. 2023, 5, 44–51. [Google Scholar] [CrossRef]
- Druyts, F.; Caes, S.; Thomas, P. Carbon-14 release and speciation from carbon steel in highly alkaline conditions. Radiocarbon 2018, 60, 1683–1690. [Google Scholar] [CrossRef]
- Cvetkovic, B.Z.; Salazar, G.; Kunz, D.; Tits, J.; Szidat, S.; Wieland, E. Quantification of dissolved organic 14C-containing compounds by accelerator mass spectrometry in a corrosion experiment with irradiated steel. Radiocarbon 2018, 60, 1711–1727. [Google Scholar] [CrossRef]
- De Visser-Tynová, E.; Swanton, S.W.; Williams, S.J.; Stijkel, M.P.; Walker, A.J.; Otlet, R.L. 14C release from irradiated stainless steel. Radiocarbon 2018, 60, 1671–1681. [Google Scholar] [CrossRef]
- Guillemot, T.; Salazar, G.; Rauber, M.; Kunz, D.; Szidat, S.; Wieland, E. Carbon-14 release and speciation during corrosion of irradiated steel under radioactive waste disposal conditions. Sci. Total Environ. 2022, 817, 9. [Google Scholar] [CrossRef]
- Mibus, J.; Diomidis, N.; Wieland, E.; Swanton, S.W. Release and speciation of 14C during the corrosion of activated steel in deep geological repositories for the disposal of radioactive waste. Radiocarbon 2018, 60, 1657–1670. [Google Scholar] [CrossRef]
- Rodríguez, M.; Gascón, J.L.; Magro, E.; Piña, G.; Lara, E.; Sevilla, L. 14C release from steels under aerobic conditions. Radiocarbon 2018, 60, 1729–1741. [Google Scholar] [CrossRef]
- Wieland, E.; Cvetkovic, B.Z.; Kunz, D.; Salazar, G.; Szidat, S. Carbon-14 Speciation During Anoxic Corrosion of Activated Steel in a Repository Environment. Atw-Int. J. Nucl. Power 2018, 63, 34–37. [Google Scholar]
- Lin, C.C. A review of corrosion product transport and radiation field buildup in boiling water reactors. Prog. Nucl. Energy 2009, 51, 207–224. [Google Scholar] [CrossRef]


| Sample Code | Sample Description | Sample Position | ||
|---|---|---|---|---|
| Axial from Reactor Core Bottom (cm) | Azimuthal (°) | Horizontal from Inner Wall (mm) | ||
| U1-B-in | Base metal | 275 | 300 | 39 ± 1 |
| U1-B-out | 71 ± 1 | |||
| U2-W-in | Weld metal | −161 | 300 | 39 ± 1 |
| U2-W-out | 71 ± 1 | |||
| U8-B | Base metal | |||
| U8-W | Weld metal | |||
| C | Si | Mn | Cr | Ni | V | Mo | Cu | Co | Al | Ti | S | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nominal values for RPV crude steel (CrMoV) [38] | ||||||||||||
| Min | 0.13 | 0.17 | 0.30 | 2.50 | 0.00 | 0.25 | 0.60 | 0.00 | 0.00 | n.i. | n.i. | 0.00 |
| Max | 0.18 | 0.37 | 0.60 | 3.00 | 0.40 | 0.35 | 0.80 | 0.15 | 0.020 | n.i. | n.i. | 0.025 |
| U8-B | ||||||||||||
| p.w. | 0.15 | 0.26 | 0.47 | 2.87 | 0.074 | 0.29 | 0.72 | 0.05 | 0.009 | 0.011 | 0.0002 | 0.012 |
| SD* | 0.006 | 0.003 | 0.003 | 0.01 | 0.0006 | 0.001 | 0.008 | 0.0002 | 0.0006 | 0.0001 | 0.0001 | 0.0009 |
| U8-W | ||||||||||||
| p.w. | 0.05 | 0.55 | 1.08 | 1.4 | 0.053 | 0.17 | 0.53 | 0.083 | 0.005 | 0.013 | 0.003 | 0.012 |
| SD* | 0.002 | 0.009 | 0.002 | 0.0003 | 0.0001 | 0.001 | 0.006 | 0.005 | 0.0004 | 0.0001 | 0.0001 | 0.0005 |
| U1-B | ||||||||||||
| [39] | 0.16 | 0.28 | 0.45 | 2.73 | 0.16 | 0.27 | 0.67 | 0.15 | n.i. | n.i. | n.i. | 0.015 |
| p.w. | 0.141 | 0.29 | 0.425 | 3.06 | 0.18 | 0.265 | 0.68 | 0.173 | 0.015 | 0.001 | 0.0004 | 0.01 |
| SD* | 0.007 | 0.008 | 0.004 | 0.014 | 0.003 | 0.001 | 0.01 | 0.002 | 0.0004 | 0.0004 | 0.0001 | 0.001 |
| U2-W | ||||||||||||
| [39] | 0.08 | 0.17 | 0.63 | 1.50 | 0.19 | 0.17 | 0.46 | 0.18 | n.i. | n.i. | 0.06 | 0.013 |
| p.w. | 0.06 | 0.38 | 0.95 | 1.62 | 0.17 | 0.14 | 0.45 | 0.16 | 0.0165 | 0.001 | 0.004 | 0.01 |
| SD* | 0.01 | 0.01 | 0.006 | 0.04 | 0.001 | 0.003 | 0.006 | 0.002 | 0.001 | 0.004 | 0.0006 | 0.001 |
| Sample | Neutron Fluence ϕ (> 0.5 MeV) (n/cm2) | 60Co (Bq/g) | 14C (Bq/g) |
|---|---|---|---|
| U1-B-in | (1.1 ± 0.2) × 1018 | 273 ± 50 | 3.0 ± 2.1 |
| U1-B-out | (0.9 ± 0.2) × 1018 | 208 ± 17 | 1.5 ± 0.6 |
| U2-W-in | (1.6 ± 0.3) × 1015 | 4.3 ± 1.3 | 0.31 ± 0.03 |
| U2-W-out | (2.4 ± 0.5) × 1015 | 7.4 ± 1.5 | 0.21 ± 0.07 |
| Raman Shift (cm−1) U8-B | Raman Shift (cm−1) U8-W | Assignment | Reference |
|---|---|---|---|
| 991 | 991 | (Mn-)SiO3 | ~1000 cm−1 [53] ~1000 cm−1 [51] |
| 660 | Fe3O4 γ-Fe2O3 NiCr2O4 | 665 cm−1 [10] 657 cm−1 [10] 665 cm−1 [8] | |
| 649 | γ-Fe2O3 MnFe2O4 | 657 cm−1 [10] 643 cm−1 [47] | |
| 549 | Cr2O3 | 550 cm−1 [8] | |
| 502 | γ-Fe2O3 α-Fe2O3 NiCr2O4 | 507 cm−1 [10] 494 cm−1 [10] 508 cm−1 [8] | |
| 399 | α-Fe2O3 | 406 cm−1 [10] 400 cm−1 [50] | |
| 341 | 341 | γ-Fe2O3 | 339 cm−1 [10] |
| 296 | α-Fe2O3 FeS | 290 cm−1 [10] 280 cm−1 [50] 296 cm−1 [49] | |
| 223 | α-Fe2O3 | 224 cm−1 [10] 220 cm−1 [50] |
| Raman Shift (cm−1) U1-B | Assignment | Reference |
|---|---|---|
| 520–680 | Fe3O4 γ-Fe2O3 NiCr2O4 MnFe2O4 Cr2O3 MnS | 665 cm−1 [10] 657 cm−1 [10] 665 cm−1 [8] 643 cm−1 [47] 550 cm−1 [8] 635 cm−1 [48] |
| 483, 223 | S | 474 cm−1, 221 cm−1 [52] |
| 282 | (Mn, Fe)S FeS | 276 cm−1 [47] 282 cm−1 [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yassin, G.; Pönitz, E.; Huittinen, N.M.; Schild, D.; Konheiser, J.; Müller, K.; Barkleit, A. Phase Characterization of (Mn, S) Inclusions and Mo Precipitates in Reactor Pressure Vessel Steel from Greifswald Nuclear Power Plant. J. Nucl. Eng. 2025, 6, 12. https://doi.org/10.3390/jne6020012
Yassin G, Pönitz E, Huittinen NM, Schild D, Konheiser J, Müller K, Barkleit A. Phase Characterization of (Mn, S) Inclusions and Mo Precipitates in Reactor Pressure Vessel Steel from Greifswald Nuclear Power Plant. Journal of Nuclear Engineering. 2025; 6(2):12. https://doi.org/10.3390/jne6020012
Chicago/Turabian StyleYassin, Ghada, Erik Pönitz, Nina Maria Huittinen, Dieter Schild, Jörg Konheiser, Katharina Müller, and Astrid Barkleit. 2025. "Phase Characterization of (Mn, S) Inclusions and Mo Precipitates in Reactor Pressure Vessel Steel from Greifswald Nuclear Power Plant" Journal of Nuclear Engineering 6, no. 2: 12. https://doi.org/10.3390/jne6020012
APA StyleYassin, G., Pönitz, E., Huittinen, N. M., Schild, D., Konheiser, J., Müller, K., & Barkleit, A. (2025). Phase Characterization of (Mn, S) Inclusions and Mo Precipitates in Reactor Pressure Vessel Steel from Greifswald Nuclear Power Plant. Journal of Nuclear Engineering, 6(2), 12. https://doi.org/10.3390/jne6020012