The Effects of Aromatherapy on Sleep Quality in Menopausal Women: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Results
2.1. Search Results
2.2. Study Characteristics
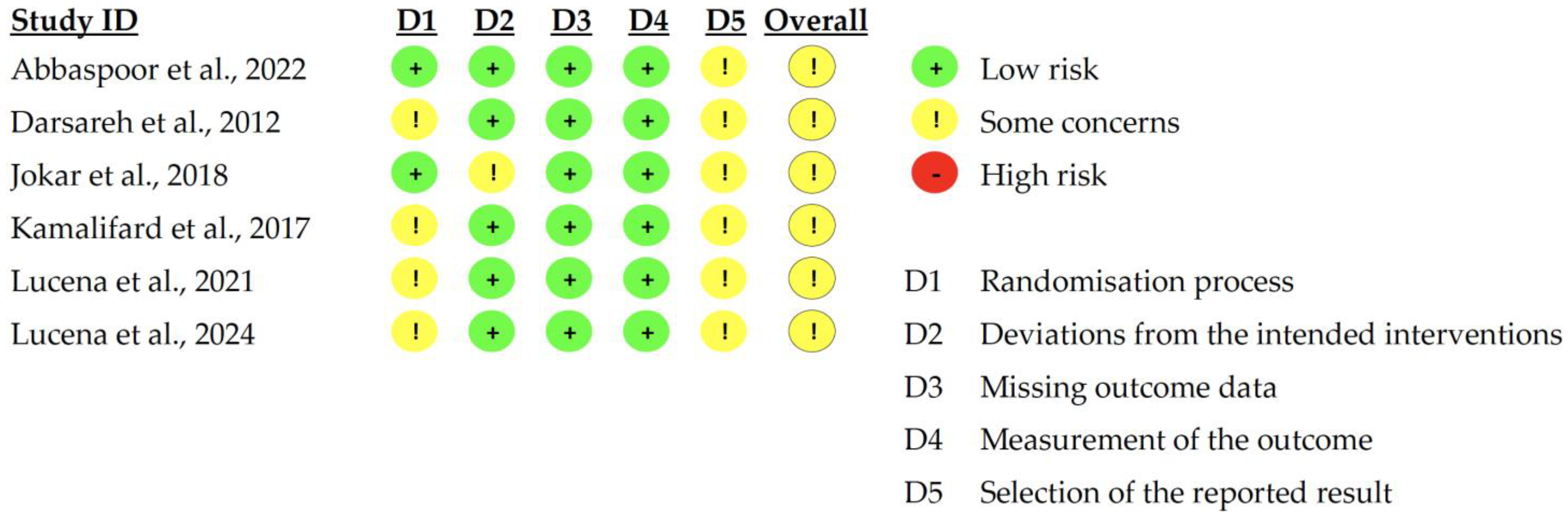
2.3. Quality Assessment of Included Studies
2.4. Meta-Analysis
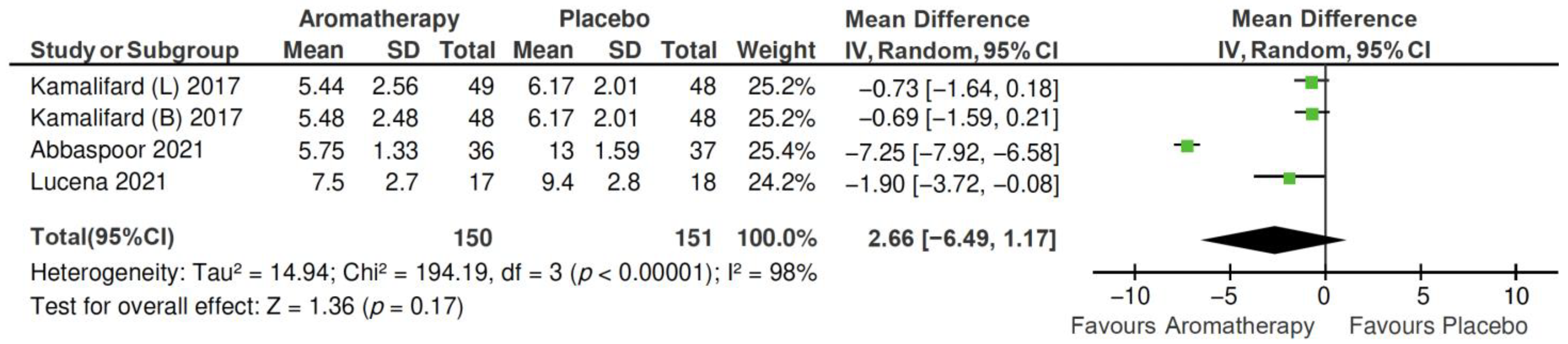
2.4.1. Total PSQI Score
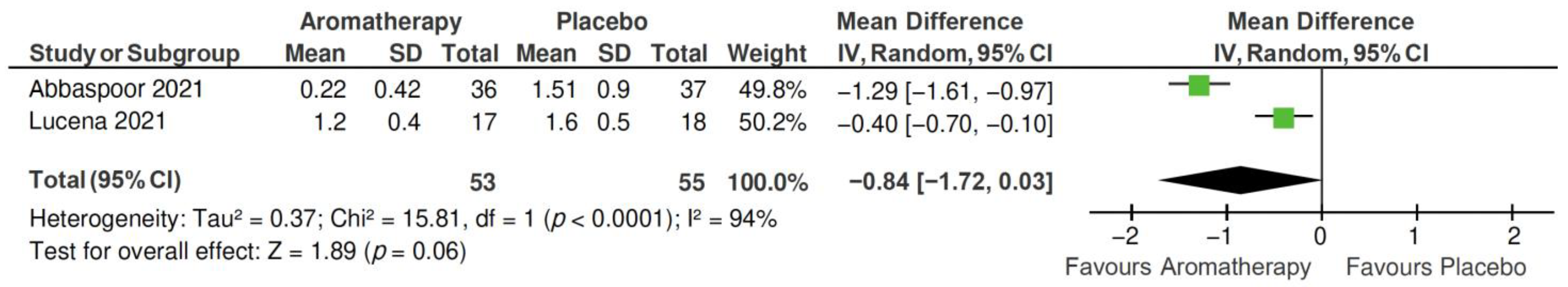
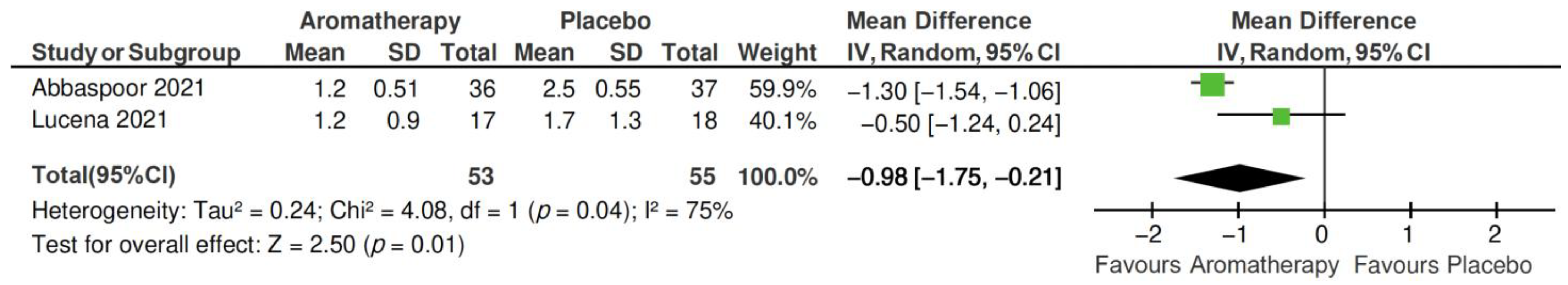
2.4.2. PSQI Subscales
- 1.
- Subjective sleep quality subscale
- 2.
- Sleep latency subscale
- 3.
- Sleep duration subscale
- 4.
- Sleep efficiency subscale
- 5.
- Sleep disturbances subscale
- 6.
- Daytime drowsiness subscale
2.5. Publication Bias
3. Discussion
4. Materials and Methods
4.1. Protocol and Registration
4.2. Search Strategy
4.3. Eligibility Criteria and Study Selection
- 1.
- Population/participants: Postmenopausal women (defined as having no menstrual periods for at least 12 consecutive months) without restrictions on nationality or comorbidities;
- 2.
- Intervention: Aromatherapy using essential oils, without restrictions on type of oil, method of administration, dosage, frequency, or duration;
- 3.
- Comparison/control group: Participants receiving no treatment, placebo, or routine care not involving aromatherapy;
- 4.
- Outcomes: Sleep quality assessed using validated subjective or objective measurements.
4.4. Data Extraction
4.5. Quality Assessment
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ESS | Epworth Sleepiness Scale |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PSQI | Pittsburgh Sleep Quality Index |
| REVMAN | Review Manager |
| ROB 2 | Risk of Bias 2 Tool |
| RCTs | Randomized controlled trials |
| MRS | Menopausal Rating Scale |
| HRT | Hormone replacement therapy |
| WASO | Wake after sleep onset |
| SOL | Sleep onset latency |
| TST | Total sleep time |
| SE | Sleep efficiency |
| ISI | Insomnia Severity Index |
| KMI | Kupperman Menopausal Index |
| MENQOL-1 | Menopause-specific Quality of Life Questionnaire |
| AHI | Apnea Hypopnea Index |
| MD | Mean difference |
References
- Gatenby, C.; Simpson, P. Menopause: Physiology, definitions, and symptoms. Best Pract. Res. Clin. Endocrinol. Metab. 2024, 38, 101855. [Google Scholar] [CrossRef] [PubMed]
- Talaulikar, V. Menopause transition: Physiology and symptoms. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, 81, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Hachul, H.; Hachul de Campos, B.; Lucena, L.; Tufik, S. Sleep During Menopause. Sleep Med. Clin. 2023, 18, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N.; Epperson, C.N.; Mathews, S.B. Menopausal Symptoms and Their Management. Endocrinol. Metab. Clin. N. Am. 2015, 44, 497–515. [Google Scholar] [CrossRef]
- Shkurenko, Y.V.; Ibatov, A.D.; Kapyrina, T.D. Insomnia in the menopause. S.S. Korsakov J. Neurol. Psychiatry 2023, 123, 26–30. [Google Scholar] [CrossRef]
- Tandon, V.R.; Sharma, S.; Mahajan, A.; Mahajan, A.; Tandon, A. Menopause and Sleep Disorders. J. Midlife Health 2022, 13, 26–33. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Hatvate, N.T.; Naren, P.; Khan, S.; Chavda, V.P.; Balar, P.C.; Gandhi, J.; Khatri, D.K. Essential oils for clinical aromatherapy: A comprehensive review. J. Ethnopharmacol. 2024, 330, 118180. [Google Scholar] [CrossRef]
- Yuksel, N.; Evaniuk, D.; Huang, L.; Malhotra, U.; Blake, J.; Wolfman, W.; Fortier, M. Guideline No. 422a: Menopause: Vasomotor Symptoms, Prescription Therapeutic Agents, Complementary and Alternative Medicine, Nutrition, and Lifestyle. J. Obstet. Gynaecol. Can. 2021, 43, 1188–1204.e1181. [Google Scholar] [CrossRef]
- Pan, M.; Zhou, J.; Pan, X.; Wang, J.; Qi, Q.; Wang, L. Drugs for the treatment of postmenopausal symptoms: Hormonal and non-hormonal therapy. Life Sci. 2023, 312, 121255. [Google Scholar] [CrossRef]
- Flores, V.A.; Pal, L.; Manson, J.E. Hormone Therapy in Menopause: Concepts, Controversies, and Approach to Treatment. Endocr. Rev. 2021, 42, 720–752. [Google Scholar] [CrossRef]
- Johnson, A.; Roberts, L.; Elkins, G. Complementary and Alternative Medicine for Menopause. J. Evid. Based Integr. Med. 2019, 24, 2515690x19829380. [Google Scholar] [CrossRef]
- Adams, L.L.; Gatchel, R.J.; Gentry, C. Complementary and alternative medicine: Applications and implications for cognitive functioning in elderly populations. Altern. Ther. Health Med. 2001, 7, 52–61. [Google Scholar]
- Caballero-Gallardo, K.; Quintero-Rincón, P.; Olivero-Verbel, J. Aromatherapy and Essential Oils: Holistic Strategies in Complementary and Alternative Medicine for Integral Wellbeing. Plants 2025, 14, 400. [Google Scholar] [CrossRef]
- Dos Reis Lucena, L.; Dos Santos-Junior, J.G.; Tufik, S.; Hachul, H. Lavender essential oil on postmenopausal women with insomnia: Double-blind randomized trial. Complement. Ther. Med. 2021, 59, 102726. [Google Scholar] [CrossRef]
- Lialy, H.E.; Mohamed, M.A.; AbdAllatif, L.A.; Khalid, M.; Elhelbawy, A. Effects of different physiotherapy modalities on insomnia and depression in perimenopausal, menopausal, and post-menopausal women: A systematic review. BMC Womens Health 2023, 23, 363. [Google Scholar] [CrossRef]
- Lucena, L.; Santos-Junior, J.G.; Tufik, S.; Hachul, H. Effect of a lavender essential oil and sleep hygiene protocol on insomnia in postmenopausal women: A pilot randomized clinical trial. Explore 2024, 20, 116–125. [Google Scholar] [CrossRef]
- Candy, B.; Armstrong, M.; Flemming, K.; Kupeli, N.; Stone, P.; Vickerstaff, V.; Wilkinson, S. The effectiveness of aromatherapy, massage and reflexology in people with palliative care needs: A systematic review. Palliat. Med. 2020, 34, 179–194. [Google Scholar] [CrossRef]
- Cheong, M.J.; Kim, S.; Kim, J.S.; Lee, H.; Lyu, Y.S.; Lee, Y.R.; Jeon, B.; Kang, H.W. A systematic literature review and meta-analysis of the clinical effects of aroma inhalation therapy on sleep problems. Medicine 2021, 100, e24652. [Google Scholar] [CrossRef]
- Cooke, B.; Ernst, E. Aromatherapy: A systematic review. Br. J. Gen. Pract. 2000, 50, 493–496. [Google Scholar]
- Farrar, A.J.; Farrar, F.C. Clinical Aromatherapy. Nurs. Clin. N. Am. 2020, 55, 489–504. [Google Scholar] [CrossRef]
- Gong, M.; Dong, H.; Tang, Y.; Huang, W.; Lu, F. Effects of aromatherapy on anxiety: A meta-analysis of randomized controlled trials. J. Affect. Disord. 2020, 274, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Nikjou, R.; Kazemzadeh, R.; Asadzadeh, F.; Fathi, R.; Mostafazadeh, F. The Effect of Lavender Aromatherapy on the Symptoms of Menopause. J. Natl. Med. Assoc. 2018, 110, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Mahdavian, M.; Mirzaii Najmabadi, K.; Hosseinzadeh, H.; Mirzaeian, S.; Badiee Aval, S.; Esmaeeli, H. Effect of the Mixed Herbal Medicines Extract (Fennel, Chamomile, and Saffron) on Menopause Syndrome: A Randomized Controlled Clinical Trial. J. Caring Sci. 2019, 8, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh, R.; Nikjou, R.; Rostamnegad, M.; Norouzi, H. Effect of lavender aromatherapy on menopause hot flushing: A crossover randomized clinical trial. J. Chin. Med. Assoc. 2016, 79, 489–492. [Google Scholar] [CrossRef]
- Smith-Francis, M.J. Complementary and Alternative Medicine for Menopause. Nurs. Clin. N. Am. 2024, 59, 551–562. [Google Scholar] [CrossRef]
- Cui, J.; Li, M.; Wei, Y.; Li, H.; He, X.; Yang, Q.; Li, Z.; Duan, J.; Wu, Z.; Chen, Q.; et al. Inhalation Aromatherapy via Brain-Targeted Nasal Delivery: Natural Volatiles or Essential Oils on Mood Disorders. Front. Pharmacol. 2022, 13, 860043. [Google Scholar] [CrossRef]
- Deepa, Y.; Vijay, A.; Nivethitha, L.; Nandhakumar, G.; Sathiya, S.; Mooventhan, A. Effects of chamomile oil inhalation on sleep quality in young adults with insomnia: A randomized controlled trial. Int. J. Psychiatry Med. 2024, 912174241301279. [Google Scholar] [CrossRef]
- Dimitriou, T.D.; Verykouki, E.; Papatriantafyllou, J.; Konsta, A.; Kazis, D.; Tsolaki, M. Non-Pharmacological interventions for the anxiety in patients with dementia. A cross-over randomised controlled trial. Behav. Brain Res. 2020, 390, 112617. [Google Scholar] [CrossRef]
- Hedigan, F.; Sheridan, H.; Sasse, A. Benefit of inhalation aromatherapy as a complementary treatment for stress and anxiety in a clinical setting—A systematic review. Complement. Ther. Clin. Pract. 2023, 52, 101750. [Google Scholar] [CrossRef]
- Song, H.; Yang, A.; Wang, Y.; Xu, R.; Hu, W. Potential roles of inhalation aromatherapy on stress-induced depression by inhibiting inflammation in the peripheral olfactory system. Neurochem. Int. 2025, 186, 105967. [Google Scholar] [CrossRef]
- Całkosiński, A.; Chruścicka, I.; Burzyńska, P.; Malinowski, K. Physical activity and aromatherapy as positive factors influencing the quality of sleep. Pol. Merkur Lek. 2021, 49, 461–463. [Google Scholar]
- Can Çiçek, S.; Demir, Ş.; Yılmaz, D.; Açıkgöz, A.; Yıldız, S.; Yis, Ö.M. The Effect of Aromatherapy on Blood Pressure and Stress Responses by Inhalation and Foot Massage in Patients with Essential Hypertension: Randomized Clinical Trial. Holist. Nurs. Pract. 2022, 36, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Bavarsad, N.H.; Bagheri, S.; Kourosh-Arami, M.; Komaki, A. Aromatherapy for the brain: Lavender’s healing effect on epilepsy, depression, anxiety, migraine, and Alzheimer’s disease: A review article. Heliyon 2023, 9, e18492. [Google Scholar] [CrossRef] [PubMed]
- Abbaspoor, Z.; Siahposh, A.; Javadifar, N.; Faal Siahkal, S.; Mohaghegh, Z.; Sharifipour, F. The Effect of Citrus Aurantium Aroma on the Sleep Quality in Postmenopausal Women: A Randomized Controlled Trial. Int. J. Community Based Nurs. Midwifery 2022, 10, 86–95. [Google Scholar] [CrossRef]
- Darsareh, F.; Taavoni, S.; Joolaee, S.; Haghani, H. Effect of aromatherapy massage on menopausal symptoms: A randomized placebo-controlled clinical trial. Menopause 2012, 19, 995–999. [Google Scholar] [CrossRef]
- Jokar, M.; Zahraseifi, F.B.; Khalili, M.; Bakhtiari, S. The effects of lavender aromatherapy on menopausal symptoms: A single-blind randomized placebo-controlled clinical trial. Int. J. Pharm. Res. 2018, 10, 182–188. [Google Scholar]
- Kamalifard, M.; Azizeh, F.-K.; Mahsa, N.; Yunes, R.; and Herizchi, S. Comparison of the effect of lavender and bitter orange on sleep quality in postmenopausal women: A triple-blind, randomized, controlled clinical trial. Women Health 2018, 58, 851–865. [Google Scholar] [CrossRef]
- Herson, M.; Kulkarni, J. Hormonal Agents for the Treatment of Depression Associated with the Menopause. Drugs Aging 2022, 39, 607–618. [Google Scholar] [CrossRef]
- Proserpio, P.; Marra, S.; Campana, C.; Agostoni, E.C.; Palagini, L.; Nobili, L.; Nappi, R.E. Insomnia and menopause: A narrative review on mechanisms and treatments. Climacteric 2020, 23, 539–549. [Google Scholar] [CrossRef]
- Hur, M.H.; Yang, Y.S.; Lee, M.S. Aromatherapy massage affects menopausal symptoms in korean climacteric women: A pilot-controlled clinical trial. Evid. Based Complement. Alternat. Med. 2008, 5, 325–328. [Google Scholar] [CrossRef]
- Cavanagh, H.M.; Wilkinson, J.M. Biological activities of lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.L.; Li, Y.; Wang, Q.; Niu, F.J.; Li, K.W.; Wang, Y.Y.; Wang, J.; Zhou, C.Z.; Gao, L.N. Chamomile: A Review of Its Traditional Uses, Chemical Constituents, Pharmacological Activities and Quality Control Studies. Molecules 2022, 28, 133. [Google Scholar] [CrossRef] [PubMed]
- Hieu, T.H.; Dibas, M.; Surya Dila, K.A.; Sherif, N.A.; Hashmi, M.U.; Mahmoud, M.; Trang, N.T.T.; Abdullah, L.; Nghia, T.L.B.; Hirayama, K.; et al. Therapeutic efficacy and safety of chamomile for state anxiety, generalized anxiety disorder, insomnia, and sleep quality: A systematic review and meta-analysis of randomized trials and quasi-randomized trials. Phytother. Res. 2019, 33, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Koulivand, P.H.; Khaleghi Ghadiri, M.; Gorji, A. Lavender and the nervous system. Evid. Based Complement. Alternat. Med. 2013, 2013, 681304. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Shankar, E.; Gupta, S. Chamomile: A herbal medicine of the past with bright future. Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [CrossRef]
- Faturi, C.B.; Leite, J.R.; Alves, P.B.; Canton, A.C.; Teixeira-Silva, F. Anxiolytic-like effect of sweet orange aroma in Wistar rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 605–609. [Google Scholar] [CrossRef]
- Lee, Y.L.; Wu, Y.; Tsang, H.W.; Leung, A.Y.; Cheung, W.M. A systematic review on the anxiolytic effects of aromatherapy in people with anxiety symptoms. J. Altern. Complement. Med. 2011, 17, 101–108. [Google Scholar] [CrossRef]
- Her, J.; Cho, M.K. Effect of aromatherapy on sleep quality of adults and elderly people: A systematic literature review and meta-analysis. Complement. Ther. Med. 2021, 60, 102739. [Google Scholar] [CrossRef]
- Takahashi, T.A.; Johnson, K.M. Menopause. Med. Clin. N. Am. 2015, 99, 521–534. [Google Scholar] [CrossRef]


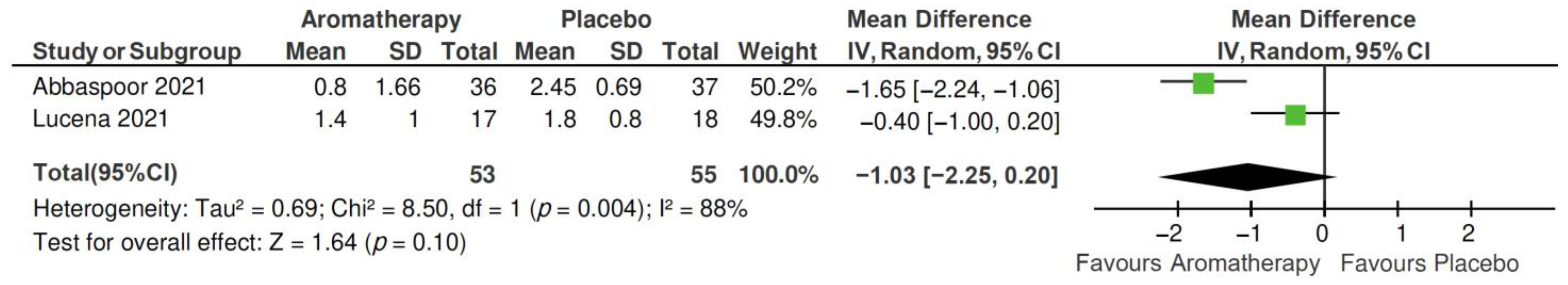

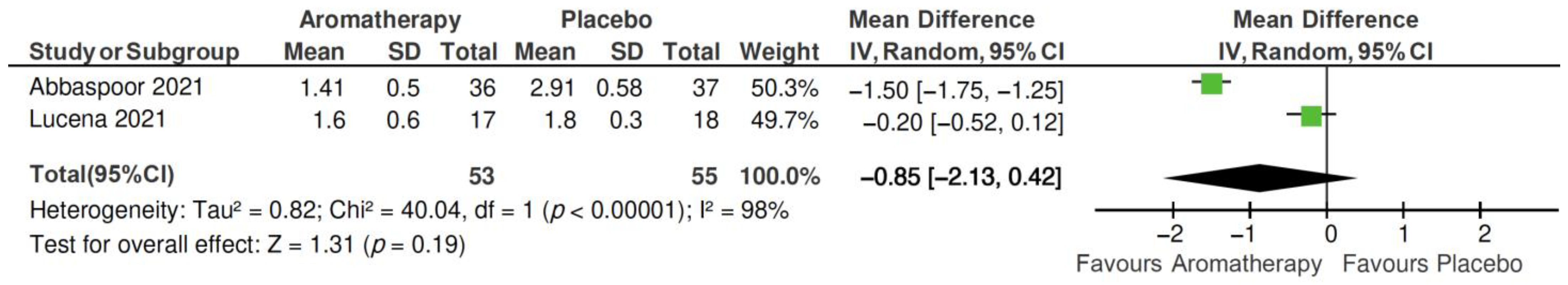
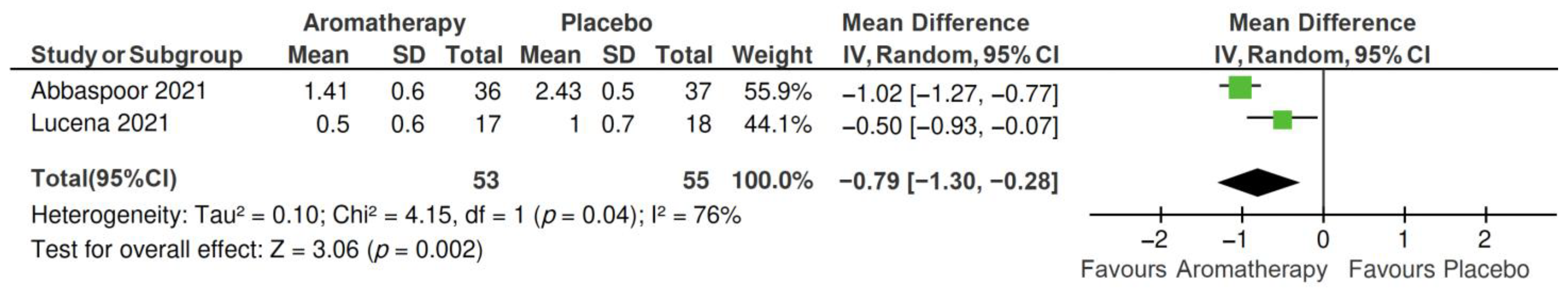
| Author, Year | Main Characteristics of Participants | Sample Size | Sleep Outcome Measurement | Outcomes |
|---|---|---|---|---|
| Abbaspoor et al., 2022 [34] |
| Total = 80
| PSQI, PSQI subscale | Aromatherapy vs. control (post-intervention comparison)
|
| Darsareh et al., 2012 [35] |
| Total = 90
| MRS with sleeping problems assessment | Aromatherapy vs. control (post-intervention comparison)
|
| Jokar et al., 2018 [36] |
| Total = 70
| Evaluated through menopausal symptoms, including insomnia | Aromatherapy vs. control (post-intervention comparison)
|
| Kamalifard et al., 2017 [37] |
| Total = 156
| PSQI | Aromatherapy vs. control (post-intervention comparison)
|
| Lucena et al., 2021 [14] |
| Total = 35
| PSQI, PSQI subscale, ISI, Polysomnography, SOL, REM latency, TST, SE, WASO, Sleep Stages, AHI | Aromatherapy vs. control (post-intervention comparison)
|
| Lucena et al., 2024 [16] |
| Total = 35
| Sleep diary: SOL, TST, SE, ESS | Aromatherapy vs. control (post-intervention comparison)
|
| Author (Year) | Aromatherapy | |||
|---|---|---|---|---|
| Type | Route | Regimen | Duration | |
| Abbaspoor et al., 2022 [34] | Citrus aurantium | Inhalation | Twice daily | 4 weeks |
| Darsareh et al., 2012 [35] | Mixed (rose, rosemary, lavender, and rose geranium) | Massage, 30 min | Twice weekly | 4 weeks |
| Jokar et al., 2018 [36] | Lavender, two drops on collar | Inhalation | Daily, before bedtime | 4 weeks |
| Kamalifard et al., 2017 [37] | Lavender, bitter orange 500 mg | Oral | Twice daily | 8 weeks |
| Lucena et al., 2021 [14] | Lavandular angustifolia | Inhalation | Daily, before bedtime | 29 days |
| Lucena et al., 2024 [16] | Lavandular angustifolia | Inhalation | Daily, before bedtime | 29 days |
| Essential Oil | Key Bioactive Components | Proposed Mechanism | References |
|---|---|---|---|
| Lavender (Lavandula angustifolia) | Linalool, Linalyl acetate | Enhances GABAergic transmission; reduces sympathetic activity | Koulivand et al., 2013 [44] |
| Chamomile (Matricaria chamomilla) | Apigenin | Binds to benzodiazepine receptors; mild sedative effect | Srivastava et al., 2010 [45] |
| Bitter orange (Citrus aurantium) | Limonene, Linalool | Modulates serotonin receptors; anxiolytic effect | Faturi et al., 2010 [46] |
| Mixed oils | Various (e.g., lavender + sweet marjoram) | Combined calming and anxiolytic actions | Lee et al., 2011 [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tangkeeratichai, C.; Segsarnviriya, C.; Kawinchotpaisan, K.; Sugkraroek, P.; Maiprasert, M. The Effects of Aromatherapy on Sleep Quality in Menopausal Women: A Systematic Review and Meta-Analysis. Women 2025, 5, 23. https://doi.org/10.3390/women5030023
Tangkeeratichai C, Segsarnviriya C, Kawinchotpaisan K, Sugkraroek P, Maiprasert M. The Effects of Aromatherapy on Sleep Quality in Menopausal Women: A Systematic Review and Meta-Analysis. Women. 2025; 5(3):23. https://doi.org/10.3390/women5030023
Chicago/Turabian StyleTangkeeratichai, Choltira, Charnsiri Segsarnviriya, Kittibhum Kawinchotpaisan, Pansak Sugkraroek, and Mart Maiprasert. 2025. "The Effects of Aromatherapy on Sleep Quality in Menopausal Women: A Systematic Review and Meta-Analysis" Women 5, no. 3: 23. https://doi.org/10.3390/women5030023
APA StyleTangkeeratichai, C., Segsarnviriya, C., Kawinchotpaisan, K., Sugkraroek, P., & Maiprasert, M. (2025). The Effects of Aromatherapy on Sleep Quality in Menopausal Women: A Systematic Review and Meta-Analysis. Women, 5(3), 23. https://doi.org/10.3390/women5030023






