Potential Explanatory Models of the Female Preponderance in Very Late Onset Schizophrenia
Abstract
1. Introduction
2. Results and Discussion
2.1. Epidemiology and Risk Factors Relevant to Sex Differences and Age at Onset of Schizophrenia
2.2. Symptomatology and Pathophysiology of Onset Categories
2.3. Sex Differences in Other Late-Life-Onset Disorders with Potential Psychosis
2.3.1. Alzheimer’s Dementia
2.3.2. Depression
2.3.3. Parkinson’s Disease
2.3.4. Delusional Disorder
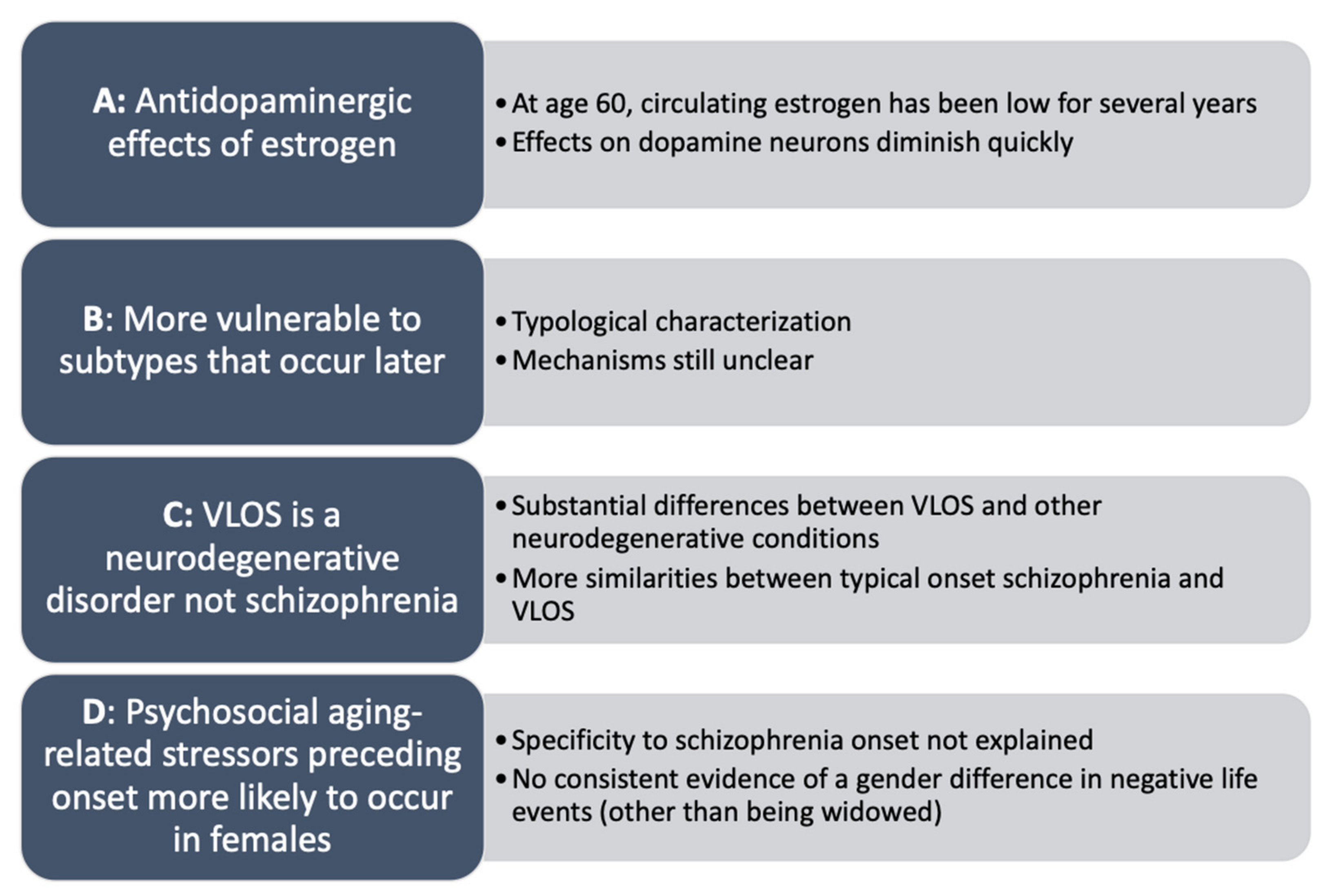
2.4. Explanatory Models of Sex Differences in Very Late Onset Schizophrenia
- (a)
- During reproductive age, females are “protected” from schizophrenia onset due to antidopaminergic effects of estrogen, wherein estrogen regulates dopaminergic transmission, “sparing” females from early onset of schizophrenia [74]. Studies have found that estradiol may increase the threshold for psychosis through regulation of dopaminergic transmission and degradation, particularly in the mesolimbic and mesocortical pathways, and in reducing oxidative stress [31]. Notably, females with early onset schizophrenia are more likely to be hypoestrogenic (i.e., lower circulating levels of estrogen), evidenced by menstrual irregularities [75,76,77]. Low estrogen is suspected to precede onset of schizophrenia due to later menarche, infertility, and lighter menstrual periods prior to symptom emergence [78]. However, effects of estrogen on dopamine alone cannot explain the observed increase in diagnoses of females with VLOS, as onset is several years after menopause has occurred (typically age 50; [79]) and circulating estrogen levels have been low and approximately equal to age-matched males for several years [27].
- (b)
- VLOS may reflect a subtype (or subtypes) of schizophrenia that is (are) more likely to onset later in life, to which females are more vulnerable [35]. Stringent diagnostic criteria for schizophrenia, relative to other schizophrenia-spectrum disorders, may exclude more females, as they tend to present more ‘atypical’ features, including marked affective symptoms in some cases. Diagnostic criteria for schizophrenia that emphasize negative symptoms and psychosocial decline may exclude more females than males across the lifespan, especially in later life [16,35]. Differential vulnerability to certain presentations or subtypes (e.g., VLOS) based on sex may explain the female preponderance as well as variations in symptom and presentation between onset categories. However, this explanation serves as more of a typological characterization, while mechanisms of development, presentation, and the cause of the sex distribution remain unanswered.
- (c)
- VLOS is reflective of a type of neurodegenerative disorder to which females are differentially prone. The neurodegenerative hypothesis may explain the predominance of positive symptoms and the absence of negative symptoms, as neurodegenerative conditions such as AD are often accompanied by hallucinations and paranoid delusions [56]. Disorders such as AD similarly have a female preponderance as well as etiological mechanisms that implicate neuroinflammation. However, as discussed above, research does not support progressive neurodegeneration in most cases of VLOS, suggesting that it is not primarily a neurodegenerative disease. There are substantial similarities in neurocognitive profiles between VLOS and other onset categories of schizophrenia as well as substantial differences relative to other neurodegenerative conditions. Furthermore, in VLOS there is a lack of neurobiological evidence of atrophy and tauopathy [44], hallmarks of neurodegeneration [80]. In fact, VLOS is a risk factor for the development of dementia and other neurodegenerative diseases [20,44,53].
- (d)
- Psychosocial aging-related factors may be more likely to occur in females (e.g., bereavement, job loss, etc.) in part due to their generally longer lifespan [7], and these may interact with neurological aging-related risk factors to contribute to onset of schizophrenia in late life [40]. However, specificity of psychosocial risk factors to onset of schizophrenia is unclear, as these can precipitate several late-life conditions, including AD [81] and geriatric depression [82]. Furthermore, the sex distribution does not appear to be entirely explained by this model, as there is no consistent evidence of a gender difference in negative life events related to aging, other than being married and subsequently widowed, which is significantly more prevalent in older females [61]. Comparatively, aging-related neurological changes may explain the very late life onset and sex distribution. For example, tardive dyskinesia is significantly more likely to occur in females over 70 years old, and symptoms are generally more severe than in males, supporting aging-related and sex-divergent dopaminergic dysfunction. Similarly, females experience significant age-associated decreases in striatal dopamine transporter that is not paralleled in males [83]; this may contribute to the dysregulation of dopaminergic signaling that is present in schizophrenia [74,84]. However, other studies have not supported the etiological role of the dopamine transporter in etiology of schizophrenia, limiting this model’s ability to fully explain VLOS [85], and the mechanisms of sex-specific dopaminergic effects are still unclear.
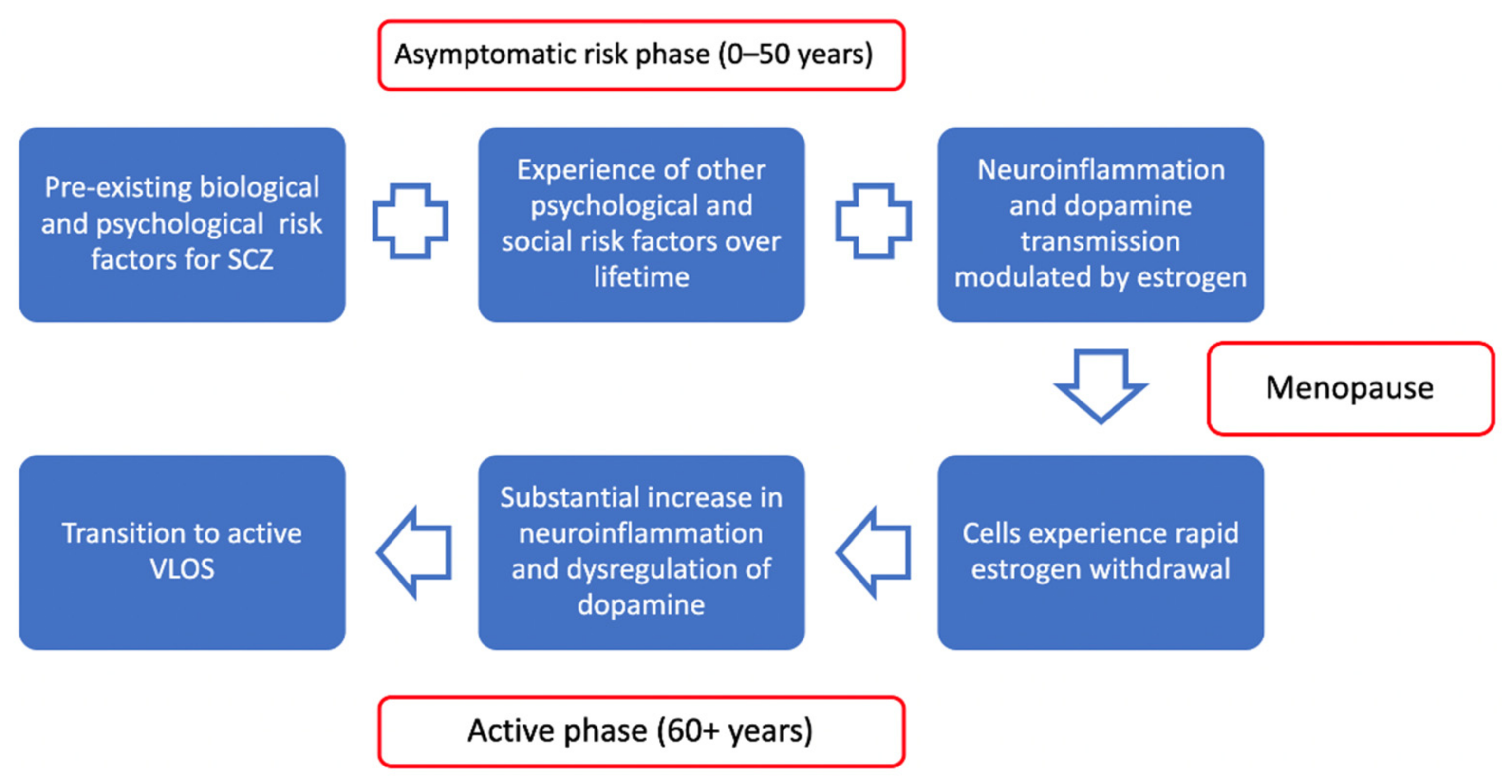
2.5. Towards a Unified Theoretical Approach
2.5.1. Neuroinflammation in Schizophrenia
2.5.2. Relevance of Estrogen to Very Late Onset Schizophrenia
2.5.3. Psychosocial Aging-Related Factors, Neuroinflammation, and Telomeres in Schizophrenia
2.5.4. Summary of Model
3. Limitations
4. Materials and Methods
4.1. Search Strategy and Inclusion Process
4.2. Synthesizing Approach
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harrison, P.J. Neuropathology of schizophrenia. Psychiatry 2008, 7, 421–424. [Google Scholar] [CrossRef]
- Diagnostic and Statistical Manual of Mental Disorders 5; American Psychiatric Association: Washington, DC, USA, 2013.
- Janoutová, J.; Janackova, P.; Sery, O.; Zeman, T.; Ambroz, P.; Kovalová, M.; Vařechová, K.; Hosák, L.; Jiřík, V.; Janout, V. Epidemiology and risk factors of schizophrenia. Neuroendocrinol. Lett. 2016, 37, 1–8. [Google Scholar] [PubMed]
- Häfner, H.; an der Heiden, W. Epidemiology of Schizophrenia. Can. J. Psychiatry 1997, 42, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.; Rabins, P.V.; Seeman, M.V.; Jeste, D.V. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: An international consensus. The International Late-Onset Schizophrenia Group. Am. J. Psychiatry 2000, 157, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Castle, D.J.; Wessely, S.; Howard, R.; Murray, R.M. Schizophrenia with onset at the extremes of adult life. Int. J. Geriatr. Psychiatry 1997, 12, 712–717. [Google Scholar] [CrossRef]
- Lemaître, J.-F.; Ronget, V.; Tidière, M.; Allainé, D.; Berger, V.; Cohas, A.; Colchero, F.; Conde, D.A.; Garratt, M.; Liker, A.; et al. Sex differences in adult lifespan and aging rates of mortality across wild mammals. Proc. Natl. Acad. Sci. USA 2020, 117, 8546–8553. [Google Scholar] [CrossRef] [PubMed]
- Kay, D.W.K. Late Paraphrenia and Its Bearing on the Aetiology of Schizophrenia. Acta Psychiatr. Scand. 1963, 39, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Herbert, M.E.; Jacobson, S. Late Paraphrenia. Br. J. Psych. 1967, 113, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Bland, R.C. Demographic aspects of functional psychoses in Canada. Acta Psychiatr. Scand. 1977, 55, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Blessed, G.; Wilson, I.D. The Contemporary Natural History of Mental Disorder in Old Age. Br. J. Psych. 1982, 141, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Grahame, P.S. Schizophrenia in Old Age (Late Paraphrenia). Br. J. Psych. 1984, 145, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, P.; Munk-Jørgensen, P. Paranoid psychosis in the elderly. Acta Psychiatr. Scand. 1985, 72, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Holden, N.L. Late Paraphrenia or the Paraphrenias? A Descriptive Study with a 10-year Follow-up. Br. J. Psychiatry 1987, 150, 635–639. [Google Scholar] [CrossRef]
- Naguib, M.; Levy, R. Late paraphrenia: Neuropsychological impairment and structural brain abnormalities on computed tomography. Int. J. Geriatr. Psychiatry 1987, 2, 83–90. [Google Scholar] [CrossRef]
- Castle, D.J.; Wessely, S.; Murray, R.M. Sex and schizophrenia: Effects of diagnostic stringency, and associations with and premorbid variables. Br. J. Psychiatry 1993, 162, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Mazeh, D.; Zemishlani, C.; Aizenberg, D.; Barak, Y. Patients With Very-Late-Onset Schizophrenia-Like Psychosis: A Follow-Up Study. Am. J. Geriatr. Psychiatry 2005, 13, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.; Blackwood, N.; Corcoran, R.; Rowse, G.; Kinderman, P.; Bentall, R.; Howard, R. Misunderstanding the Intentions of Others: An Exploratory Study of the Cognitive Etiology of Persecutory Delusions in Very Late-Onset Schizophrenia-Like Psychosis. Am. J. Geriatr. Psychiatry 2006, 14, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.S.; Kotsopoulos, E.J.; Yamin, S. Phenotypic cognitive impairment in late-onset delusional disorder. Int. Psychogeriatr. 2014, 26, 965–975. [Google Scholar] [CrossRef]
- Hanssen, M.; van der Werf, M.; Verkaaik, M.; Arts, B.; Myin-Germeys, I.; van Os, J.; Verhey, F.; Köhler, S. Comparative Study of Clinical and Neuropsychological Characteristics Between Early-, Late and Very-Late-Onset Schizophrenia-Spectrum Disorders. Am. J. Geriatr. Psychiatry 2015, 23, 852–862. [Google Scholar] [CrossRef]
- Monji, A.; Mizoguchi, Y. Neuroinflammation in Late-Onset Schizophrenia: Viewing from the Standpoint of the Microglia Hypothesis. Neuropsychobiology 2022, 81, 98–103. [Google Scholar] [CrossRef]
- Villa, A.; Vegeto, E.; Poletti, A.; Maggi, A. Estrogens, Neuroinflammation, and Neurodegeneration. Endocr. Rev. 2016, 37, 372–402. [Google Scholar] [CrossRef] [PubMed]
- Laskaris, L.E.; Di Biase, M.A.; Everall, I.; Chana, G.; Christopoulos, A.; Skafidas, E.; Cropley, V.L.; Pantelis, C. Microglial activation and progressive brain changes in schizophrenia. Br. J. Pharmacol. 2016, 173, 666–680. [Google Scholar] [CrossRef]
- Ribeiro, B.M.; do Carmo, M.R.; Freire, R.S.; Rocha, N.F.; Borella, V.C.; de Menezes, A.T.; Monte, A.S.; Gomes, P.X.; de Sousa, F.C.; Vale, M.L.; et al. Evidences for a progressive microglial activation and increase in iNOS expression in rats submitted to a neurodevelopmental model of schizophrenia: Reversal by clozapine. Schizophr. Res. 2013, 151, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Segura, C.; Becker, J.B. Why we should consider sex (and study sex differences) in addiction research. Addict. Biol. 2016, 21, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Falkenburg, J.; Tracy, D.K. Sex and schizophrenia: A review of gender differences. Psychosis 2014, 6, 61–69. [Google Scholar] [CrossRef]
- Iqbal, J.; Zaidi, M. Understanding estrogen action during menopause. Endocrinology 2009, 150, 3443–3445. [Google Scholar] [CrossRef]
- Kulkarni, J. Perimenopausal depression–an under-recognised entity. Aust. Prescr. 2018, 41, 183. [Google Scholar] [CrossRef] [PubMed]
- Hafner, H. From Onset and Prodromal Stage to a Life-Long Course of Schizophrenia and Its Symptom Dimensions: How Sex, Age, and Other Risk Factors Influence Incidence and Course of Illness. Psychiatry J. 2019, 2019, 9804836. [Google Scholar] [CrossRef] [PubMed]
- Sampathkumar, N.K.; Bravo, J.I.; Chen, Y.; Danthi, P.S.; Donahue, E.K.; Lai, R.W.; Lu, R.; Randall, L.T.; Vinson, N.; Benayoun, B.A. Widespread sex dimorphism in aging and age-related diseases. Hum. Genet. 2020, 139, 333–356. [Google Scholar] [CrossRef]
- Brand, B.A.; de Boer, J.N.; Sommer, I.E.C. Estrogens in schizophrenia: Progress, current challenges and opportunities. Curr. Opin. Psychiatry 2021, 34, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Meesters, P.D.; de Haan, L.; Comijs, H.C.; Stek, M.L.; Smeets-Janssen, M.M.J.; Weeda, M.R.; Eikelenboom, P.; Smit, J.H.; Beekman, A.T.F. Schizophrenia Spectrum Disorders in Later Life: Prevalence and Distribution of Age at Onset and Sex in a Dutch Catchment Area. Am. J. Geriatr. Psychiatry 2012, 20, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Stafford, J.; Howard, R.; Dalman, C.; Kirkbride, J.B. The Incidence of Nonaffective, Nonorganic Psychotic Disorders in Older People: A Population-based Cohort Study of 3 Million People in Sweden. Schizophr. Bull. 2019, 45, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Frederikse, M.; Lu, A.; Aylward, E.; Barta, P.; Sharma, T.; Pearlson, G. Sex differences in inferior parietal lobule volume in schizophrenia. Am. J. Psychiatry 2000, 157, 422–427. [Google Scholar] [CrossRef]
- Castle, D.J.; Murray, R.M. The neurodevelopmental basis of sex differences in schizophrenia. Psychol. Med. 1991, 21, 565–575. [Google Scholar] [CrossRef]
- Stusiński, J.M. Lew-Starowicz. Gender identity in schizophrenia. Psychiatr. Pol. 2018, 52, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Neill, E.; Tan, E.J.; Toh, W.L.; Selvendra, A.; Morgan, V.A.; Rossell, S.L.; Castle, D.J. Examining which factors influence age of onset in males and females with schizophrenia. Schizophr. Res. 2020, 223, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Selvendra, A.; Stewart, A.; Castle, D. Risk factors in early and late onset schizophrenia. Compr. Psychiatry 2018, 80, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Selvendra, A.; Toh, W.L.; Neill, E.; Tan, E.J.; Rossell, S.L.; Morgan, V.A.; Castle, D.J. Age of onset by sex in schizophrenia: Proximal and distal characteristics. J. Psychiatr. Res. 2022, 151, 454–460. [Google Scholar] [CrossRef]
- Ayano, G. Schizophrenia: A concise overview of etiology, epidemiology diagnosis and management: Review of literatures. J. Schizophr. Res. 2016, 3, 2–7. [Google Scholar]
- Jeste, D.V.; Gladsjo, J.A.; Lindamer, L.A.; Lacro, J.P. Medical Comorbidity in Schizophrenia. Schizophr. Bull. 1996, 22, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Barak, Y.; Aizenberg, D.O.V.; Mirecki, I.; Mazeh, D.; Achiron, A. Very Late-Onset Schizophrenia-Like Psychosis: Clinical and Imaging Characteristics in Comparison with Elderly Patients with Schizophrenia. J. Nerv. Ment. Dis. 2002, 190, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Suen, Y.N.; Wong, S.M.Y.; Hui, C.L.M.; Chan, S.K.W.; Lee, E.H.M.; Chang, W.C.; Chen, E.Y.H. Late-onset psychosis and very-late-onset-schizophrenia-like-psychosis: An updated systematic review. Int. Rev. Psychiatry 2019, 31, 523–542. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, L.; Morrens, M.; Luyten, P.; Van de Ven, L.; Vandenbulcke, M. The neuropsychology and neurobiology of late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: A critical review. Neurosci. Biobehav. Rev. 2017, 83, 604–621. [Google Scholar] [CrossRef] [PubMed]
- Wynn Owen, P.A.; Castle, D.J. Late-Onset Schizophrenia. Drugs Aging 1999, 15, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.P.; Howard, R.J.; Levy, R.; David, A.S. Psychotic States Arising in Late Life (Late Paraphrenia): Psychopathology and Nosology. Br. J. Psych. 1995, 166, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Vahia, I.V.; Palmer, B.W.; Depp, C.; Fellows, I.; Golshan, S.; Kraemer, H.C.; Jeste, D.V. Is late-onset schizophrenia a subtype of schizophrenia? Acta Psychiatr. Scand. 2010, 122, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.; Brodaty, H.; Rose, N.; Cathcart, S. Schizophrenia with onset after age 50 years. 2: Neurological, neuropsychological and MRI investigation. Br. J. Psychiatry 1999, 175, 416–421. [Google Scholar] [CrossRef]
- Brichant-Petitjean, C.; Legauffre, C.; Ramoz, N.; Ades, J.; Gorwood, P.; Dubertret, C. Memory deficits in late-onset schizophrenia. Schizophr Res. 2013, 151, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Smeets-Janssen, M.M.; Meesters, P.D.; Comijs, H.C.; Eikelenboom, P.; Smit, J.H.; de Haan, L.; Beekman, A.T.; Stek, M.L. Theory of Mind differences in older patients with early-onset and late-onset paranoid schizophrenia. Int. J. Geriatr. Psychiatry. 2013, 28, 141. [Google Scholar] [CrossRef] [PubMed]
- Rajji, T.K.; Ismail, Z.; Mulsant, B.H. Age at onset and cognition in schizophrenia: Meta-analysis. Br. J. Psychiatry 2009, 195, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.M.; O’Callaghan, E.; Castle, D.J.; Lewis, S.W. A neurodevelopmental approach to the classification of schizophrenia. Schizophr. Bull. 1992, 18, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Kørner, A.; Lopez, A.G.; Lauritzen, L.; Andersen, P.K.; Kessing, L.V. Acute and transient psychosis in old age and the subsequent risk of dementia: A nationwide register-based study. Geriatr. Gerontol. Int. 2009, 9, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Ropacki, S.A.; Jeste, D.V. Epidemiology of and risk factors for psychosis of Alzheimer’s disease: A review of 55 studies published from 1990 to 2003. Am. J. Psychiatry 2005, 162, 2022–2030. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Filon, J.R.; Intorcia, A.J.; Sue, L.I.; Vazquez Arreola, E.; Wilson, J.; Davis, K.J.; Sabbagh, M.N.; Belden, C.M.; Caselli, R.J.; Adler, C.H.; et al. Gender Differences in Alzheimer Disease: Brain Atrophy, Histopathology Burden, and Cognition. J. Neuropathol. Exp. Neurol. 2016, 75, 748–754. [Google Scholar] [CrossRef]
- Eikelboom, W.S.; Pan, M.; Ossenkoppele, R.; Coesmans, M.; Gatchel, J.R.; Ismail, Z.; Lanctôt, K.L.; Fischer, C.E.; Mortby, M.E.; Van Den Berg, E.; et al. Sex differences in neuropsychiatric symptoms in Alzheimer’s disease dementia: A meta-analysis. Alzheimer’s Res. Ther. 2022, 14, 48. [Google Scholar] [CrossRef]
- Cholerton, B.; Gleason, C.E.; Baker, L.D.; Asthana, S. Estrogen and Alzheimer’s disease: The story so far. Drugs Aging 2002, 19, 405–427. [Google Scholar] [CrossRef] [PubMed]
- Ratnakumar, A.; Zimmerman, S.E.; Jordan, B.A.; Mar, J.C. Estrogen activates Alzheimer’s disease genes. Alzheimer’s Dement. 2019, 5, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Girgus, J.S.; Yang, K.; Ferri, C.V. The Gender Difference in Depression: Are Elderly Women at Greater Risk for Depression Than Elderly Men? Geriatrics 2017, 2, 35. [Google Scholar] [CrossRef]
- Brodaty, H.; Luscombe, G.; Parker, G.; Wilhelm, K.; Hickie, I.; Austin, M.P.; Mitchell, P. Increased rate of psychosis and psychomotor change in depression with age. Psychol. Med. 1997, 27, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Gournellis, R.; Oulis, P.; Rizos, E.; Chourdaki, E.; Gouzaris, A.; Lykouras, L. Clinical correlates of age of onset in psychotic depression. Arch. Gerontol. Geriatr. 2011, 52, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Schoevers, R.A.; Geerlings, M.I.; Beekman, A.T.F.; Penninx, B.W.J.H.; Deeg, D.J.H.; Jonker, C.; Tilburg, W.V. Association of depression and gender with mortality in old age. Br. J. Psychiatry 2000, 177, 336–342. [Google Scholar] [CrossRef]
- Alexopoulos, G.S.; Morimoto, S.S. The inflammation hypothesis in geriatric depression. Int. J. Geriatr. Psychiatry 2011, 26, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Emamzadeh, F.N.; Surguchov, A. Parkinson’s Disease: Biomarkers, Treatment, and Risk Factors. Front. Neurosci. 2018, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, H.H.; Lapane, K.L.; Ott, B.R.; Friedman, J.H. Gender differences in the frequency and treatment of behavior problems in Parkinson’s disease. Mov. Disord. 2000, 15, 490–496. [Google Scholar] [CrossRef]
- Haaxma, C.A.; Bloem, B.R.; Borm, G.F.; Oyen, W.J.; Leenders, K.L.; Eshuis, S.; Booij, J.; Dluzen, D.E.; Horstink, M.W. Gender differences in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2007, 78, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Frentzel, D.; Judanin, G.; Borozdina, O.; Klucken, J.; Winkler, J.; Schlachetzki, J.C.M. Increase of Reproductive Life Span Delays Age of Onset of Parkinson’s Disease. Front. Neurol. 2017, 8, 397. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, B.; Dhamija, K.; Guru, P.; Sharma, S.S. Parkinson’s disease in women: Mechanisms underlying sex differences. Eur. J. Pharmacol. 2021, 895, 173862. [Google Scholar] [CrossRef] [PubMed]
- Tansey, M.G.; Goldberg, M.S. Neuroinflammation in Parkinson’s disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 2010, 37, 510–518. [Google Scholar] [CrossRef]
- Peralta, V.; Cuesta, M.J. An empirical study of five sets of diagnostic criteria for delusional disorder. Schizophr. Res. 2019, 209, 164–170. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, A.; Seeman, M.V.; Izquierdo, E.; Natividad, M.; Guàrdia, A.; Román, E.; Monreal, J.A. Delusional Disorder in Old Age: A Hypothesis-Driven Review of Recent Work Focusing on Epidemiology, Clinical Aspects, and Outcomes. Int. J. Environ. Res. Public Health 2022, 19, 7911. [Google Scholar] [CrossRef] [PubMed]
- Brisch, R.; Saniotis, A.; Wolf, R.; Bielau, H.; Bernstein, H.-G.; Steiner, J.; Bogerts, B.; Braun, K.; Jankowski, Z.; Kumaratilake, J.; et al. The Role of Dopamine in Schizophrenia from a Neurobiological and Evolutionary Perspective: Old Fashioned, but Still in Vogue. Front. Psychiatry 2014, 5, 47. [Google Scholar] [PubMed]
- Bergemann, N.; Mundt, C.; Parzer, P.; Jannakos, I.; Nagl, I.; Salbach, B.; Klinga, K. Runnebaum, B. Resch, F. Plasma concentrations of estradiol in women suffering from schizophrenia treated with conventional versus atypical antipsychotics. Schizophr. Res. 2005, 73, 357–366. [Google Scholar] [CrossRef]
- Huber, T.J.; Rollnik, J.; Wilhelms, J.; von zur Mühlen, A.; Emrich, H.M.; Schneider, U. Estradiol levels in psychotic disorders. Psychoneuroendocrinology 2001, 26, 27–35. [Google Scholar] [CrossRef]
- Riecher-Rössler, A.; Häfner, H.; Stumbaum, M.; Maurer, K.; Schmidt, R. Can Estradiol Modulate Schizophrenic Symptomatology? Schizophr. Bull. 1994, 20, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Gogos, A.; Sbisa, A.M.; Sun, J.; Gibbons, A.; Udawela, M.; Dean, B. A Role for Estrogen in Schizophrenia: Clinical and Preclinical Findings. Int. J. Endocrinol. 2015, 2015, 615356. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, S.M. The normal menopause transition: An overview. Maturitas 1996, 23, 137–145. [Google Scholar] [CrossRef]
- Stancu, I.C.; Lodder, C.; Botella Lucena, P.; Vanherle, S.; Gutiérrez De Ravé, M.; Terwel, D.; Bottelbergs, A.; Dewachter, I. The NLRP3 inflammasome modulates tau pathology and neurodegeneration in a tauopathy model. Glia 2022, 70, 1117–1132. [Google Scholar] [CrossRef]
- Burke, S.L.; Cadet, T.; Alcide, A.; O’Driscoll, J.; Maramaldi, P. Psychosocial risk factors and Alzheimer’s disease: The associative effect of depression, sleep disturbance, and anxiety. Aging Ment. Health 2018, 22, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Sachs-Ericsson, N.; Corsentino, E.; Moxley, J.; Hames, J.L.; Rushing, N.C.; Sawyer, K.; Joiner, T.; Selby, E.A.; Zarit, S.; Gotlib, I.H.; et al. A longitudinal study of differences in late- and early-onset geriatric depression: Depressive symptoms and psychosocial, cognitive, and neurological functioning. Aging Ment. Health 2013, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Nam, H.Y.; Lee, M.J.; Pak, K.; Kim, K.; Kim, I.J. Effect of sex on aging-related decline of dopamine transporter in healthy subjects. Ann. Nucl. Med. 2021, 35, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, H.; Pavey, G.; Dean, B. Altered levels of dopamine transporter in the frontal pole and dorsal striatum in schizophrenia. NPJ Schizophr. 2019, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Lavalaye, J.; Linszen, D.H.; Booij, J.; Dingemans, P.M.A.J.; Reneman, L.; Habraken, J.B.A.; Gersons, B.P.R.; van Royen, E.A. Dopamine transporter density in young patients with schizophrenia assessed with (123)FP-CIT SPECT. Schizophr. Res. 2001, 47, 59–67. [Google Scholar] [CrossRef]
- Khandaker, G.M.; Dantzer, R.; Jones, P.B. Immunopsychiatry: Important facts. Psychol. Med. 2017, 47, 2229–2237. [Google Scholar] [CrossRef]
- Mondelli, V.; Vernon, A.C.; Turkheimer, F.; Dazzan, P.; Pariante, C.M. Brain microglia in psychiatric disorders. Lancet Psychiatry 2017, 4, 563–572. [Google Scholar] [CrossRef]
- Pape, K.; Tamouza, R.; Leboyer, M.; Zipp, F. Immunoneuropsychiatry—Novel perspectives on brain disorders. Nat. Rev. Neurol. 2019, 15, 317–328. [Google Scholar] [CrossRef]
- Czepielewski, L.S.; Massuda, R.; Panizzutti, B.; da Rosa, E.D.; de Lucena, D.; Macêdo, D.; Grun, L.K.; Barbé-Tuana, F.M.; Gama, C.S. Telomere length in subjects with schizophrenia, their unaffected siblings and healthy controls: Evidence of accelerated aging. Schizophr. Res. 2016, 174, 39–42. [Google Scholar] [CrossRef]
- Flanary, B.E.; Sammons, N.W.; Nguyen, C.; Walker, D.; Streit, W.J. Evidence That Aging And Amyloid Promote Microglial Cell Senescence. Rejuvenation Res. 2007, 10, 61–74. [Google Scholar] [CrossRef]
- Scheffold, A.; Holtman, I.R.; Dieni, S.; Brouwer, N.; Katz, S.-F.; Jebaraj, B.M.C.; Kahle, P.J.; Hengerer, B.; Lechel, A.; Stilgenbauer, S.; et al. Telomere shortening leads to an acceleration of synucleinopathy and impaired microglia response in a genetic mouse model. Acta Neuropathol. Commun. 2016, 4, 87. [Google Scholar] [CrossRef]
- Wium-Andersen, M.K.; Ørsted, D.D.; Nordestgaard, B.G. Elevated C-reactive protein associated with late- and very-late-onset schizophrenia in the general population: A prospective study. Schizophr. Bull. 2014, 40, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.M.; Manly, J.J.; Schupf, N.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Association of C-reactive protein with cognitive impairment. Arch. Neurol. 2010, 67, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Takano, A.; Arakawa, R.; Ito, H.; Tateno, A.; Takahashi, H.; Matsumoto, R.; Okubo, Y.; Suhara, T. Peripheral benzodiazepine receptors in patients with chronic schizophrenia: A PET study with [11C]DAA1106. Int. J. Neuropsychopharmacol. 2010, 13, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Meijide, A.; Rodriguez-Perez, A.I.; Diaz-Ruiz, C.; Guerra, M.J.; Labandeira-Garcia, J.L. Dopamine modulates astroglial and microglial activity via glial renin-angiotensin system in cultures. Brain Behav. Immun. 2017, 62, 277–290. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Liu, L.; Wang, X.; Ding, C.; Tian, Z.; Zhou, R. Dopamine Controls Systemic Inflammation through Inhibition of NLRP3 Inflammasome. Cell 2015, 160, 62–73. [Google Scholar] [CrossRef]
- Huck, J.H.; Freyer, D.; Böttcher, C.; Mladinov, M.; Muselmann-Genschow, C.; Thielke, M.; Gladow, N.; Bloomquist, D.; Mergenthaler, P.; Priller, J. De Novo Expression of Dopamine D2 Receptors on Microglia after Stroke. J. Cereb. Blood Flow Metab. 2015, 35, 1804–1811. [Google Scholar] [CrossRef]
- Pacheco, R. Targeting dopamine receptor D3 signalling in inflammation. Oncotarget 2017, 8, 7224–7225. [Google Scholar] [CrossRef]
- Hanamsagar, R.; Bilbo, S.D. Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development. J. Steroid Biochem. Mol. Biol. 2016, 160, 127–133. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, Z.; Guo, Y.; Sultana, M.S.; Wu, K.; Lang, X.; Lv, Q.; Huang, X.; Yi, Z.; Li, Z. Sex difference in the interrelationship between TNF-α and oxidative stress status in first-episode drug-naïve schizophrenia. J. Neuroinflammation 2021, 18, 202. [Google Scholar] [CrossRef]
- Akyol, Ö.; Herken, H.; Uz, E.; Fadıllıoǧlu, E.; Ünal, S.; Söǧüt, S.; Özyurt, H.; Savaş, H.A. The indices of endogenous oxidative and antioxidative processes in plasma from schizophrenic patients: The possible role of oxidant/antioxidant imbalance. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2002, 26, 995–1005. [Google Scholar] [CrossRef]
- Mahadik, S.P.; Mukherjee, S.; Scheffer, R.; Correnti, E.E.; Mahadik, J.S. Elevated Plasma Lipid Peroxides at the Onset of Nonaffective Psychosis. Biol. Psychiatry 1998, 43, 674–679. [Google Scholar] [CrossRef]
- Sohrabji, F.; Williams, M. Stroke neuroprotection: Oestrogen and insulin-like growth factor-1 interactions and the role of microglia. J. Neuroendocrinol. 2013, 25, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Vegeto, E.; Belcredito, S.; Ghisletti, S.; Meda, C.; Etteri, S.; Maggi, A. The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology 2006, 147, 2263–2272. [Google Scholar] [CrossRef][Green Version]
- Lei, D.L.; Long, J.M.; Hengemihle, J.; O’Neill, J.; Manaye, K.F.; Ingram, D.K.; Mouton, P.R. Effects of estrogen and raloxifene on neuroglia number and morphology in the hippocampus of aged female mice. Neuroscience 2003, 121, 659–666. [Google Scholar] [CrossRef]
- Yanguas-Casás, N. Physiological sex differences in microglia and their relevance in neurological disorders. Neuroimmunol. Neuroinflammation 2020, 7, 13–22. [Google Scholar] [CrossRef]
- Milan-Mattos, J.C.; Anibal, F.F.; Perseguini, N.M.; Minatel, V.; Rehder-Santos, P.; Castro, C.A.; Vasilceac, F.A.; Mattiello, S.M.; Faccioli, L.H.; Catai, A.M. Effects of natural aging and gender on pro-inflammatory markers. Braz. J. Med. Biol. Res. 2019, 52, e8392. [Google Scholar] [CrossRef]
- Calcia, M.A.; Bonsall, D.R.; Bloomfield, P.S.; Selvaraj, S.; Barichello, T.; Howes, O.D. Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology 2016, 233, 1637–1650. [Google Scholar] [CrossRef] [PubMed]
- Gracia-García, P.; de-la-Cámara, C.; Santabárbara, J.; Lopez-Anton, R.; Quintanilla, M.A.; Ventura, T.; Marcos, G.; Campayo, A.; Saz, P.; Lyketsos, C.; et al. Depression and Incident Alzheimer Disease: The Impact of Disease Severity. Am. J. Geriatr. Psychiatry 2015, 23, 119–129. [Google Scholar] [CrossRef]
- Wilson, R.S.; Barnes, L.L.; Bennett, D.A.; Li, Y.; Bienias, J.L.; de Leon, C.F.M.; Evans, D.A. Proneness to psychological distress and risk of Alzheimer disease in a biracial community. Neurology 2005, 64, 380. [Google Scholar] [CrossRef]
- Piirainen, S.; Youssef, A.; Song, C.; Kalueff, A.V.; Landreth, G.E.; Malm, T.; Tian, L. Psychosocial stress on neuroinflammation and cognitive dysfunctions in Alzheimer’s disease: The emerging role for microglia? Neurosci. Biobehav. Rev. 2017, 77, 148–164. [Google Scholar] [CrossRef]
- Russo, P.; Prinzi, G.; Proietti, S.; Lamonaca, P.; Frustaci, A.; Boccia, S.; Amore, R.; Lorenzi, M.; Onder, G.; Marzetti, E.; et al. Shorter telomere length in schizophrenia: Evidence from a real-world population and meta-analysis of most recent literature. Schizophr. Res. 2018, 202, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Aas, M.; Elvsåshagen, T.; Westlye, L.T.; Kaufmann, T.; Athanasiu, L.; Djurovic, S.; Melle, I.; Van Der Meer, D.; Martin-Ruiz, C.; Steen, N.E.; et al. Telomere length is associated with childhood trauma in patients with severe mental disorders. Transl. Psychiatry 2019, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Balzan, R.P.; Dhillon, V.S.; Liu, D.; Hahn, L.; Fenech, M.F.; Galletly, C. Shorter telomere length in people with schizophrenia who live alone? Schizophr. Res. 2018, 199, 422–423. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Mulsant, B.H.; Voineskos, A.N.; Rajji, T.K. Brain-Derived Neurotrophic Factor Expression in Individuals With Schizophrenia and Healthy Aging: Testing the Accelerated Aging Hypothesis of Schizophrenia. Curr. Psychiatry Rep. 2017, 19, 36. [Google Scholar] [CrossRef]
- Lindqvist, D.; Epel, E.S.; Mellon, S.H.; Penninx, B.W.; Révész, D.; Verhoeven, J.E.; Lin, J.; Mahan, L.; Hough, C.M.; Rosser, R.; et al. Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging. Neurosci. Biobehav. Rev. 2015, 55, 333–364. [Google Scholar] [CrossRef]
- Lapham, K.; Kvale, M.N.; Lin, J.; Connell, S.; Croen, L.A.; Dispensa, B.P.; Fang, L.; Hesselson, S.; Hoffmann, T.J.; Iribarren, C.; et al. Automated Assay of Telomere Length Measurement and Informatics for 100,000 Subjects in the Genetic Epidemiology Research on Adult Health and Aging (GERA) Cohort. Genetics 2015, 200, 1061–1072. [Google Scholar] [CrossRef]
- Lee, D.-C.; Im, J.-A.; Kim, J.-H.; Lee, H.-R.; Shim, J.-Y. Effect of long-term hormone therapy on telomere length in postmenopausal women. Yonsei Med. J. 2005, 46, 471–479. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Christman, S.; Bermudez, C.; Hao, L.; Landman, B.A.; Boyd, B.; Albert, K.; Woodward, N.; Shokouhi, S.; Vega, J.; Andrews, P.; et al. Accelerated brain aging predicts impaired cognitive performance and greater disability in geriatric but not midlife adult depression. Transl. Psychiatry 2020, 10, 317. [Google Scholar] [CrossRef]
- Au, A.; Feher, A.; McPhee, L.; Jessa, A.; Oh, S.; Einstein, G. Estrogens, inflammation and cognition. Front. Neuroendocrinol. 2016, 40, 87–100. [Google Scholar] [CrossRef]
- Gupta, R.; Assalman, I.; Bottlender, R. Menopause and schizophrenia. Menopause Int. 2012, 18, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Brzezinski, A.; Brzezinski-Sinai, N.A.; Seeman, M.V. Treating schizophrenia during menopause. Menopause 2017, 24, 582–588. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, A.; Guàrdia, A.; Monreal, J.A. Peri- and Post-Menopausal Women with Schizophrenia and Related Disorders Are a Population with Specific Needs: A Narrative Review of Current Theories. J. Pers. Med. 2021, 11, 849. [Google Scholar] [CrossRef]
- Bianchi, V.E. The Anti-Inflammatory Effects of Testosterone. J. Endocr. Soc. 2019, 3, 91–107. [Google Scholar] [CrossRef]
| Reference | Country/Setting | Diagnostic Criteria | Age of Onset | Number of Cases | F:M Ratio |
|---|---|---|---|---|---|
| Kay, 1963 [8] | County of Northumberland, UK | Late Paraphrenia | >65 | N = 57 | 5.3:1 |
| Herbert & Jacobson, 1967 [9] | UK | Late Paraphrenia (systematized delusions and/or hallucinations) | >65 | N = 47 | 22.5:1 |
| Bland, 1977 [10] | Canada | ICD-8 Schizophrenia (first admission) | ≥60 | N = 192 | 2:1 |
| Blessed & Wilson, 1982 [11] | Newcastle upon Tyne, UK | Late Paraphrenia1 | ≥65 | N = 28 | 6:1 |
| Grahame, 1984 [12] | UK | Late Paraphrenia | ≥60 | N = 25 | 3.2:1 |
| Jørgensen & Munk- Jørgensen, 1985 [13] | Aarhus, Denmark | ICD-8 Schizophrenia and related disorders | ≥60 | N = 106 | 2.2:1 |
| Holden, 1987 [14] | UK | Late paraphrenia (‘functional’) | >60 | N = 34 | 7:1 |
| Late paraphrenia (‘organic’) | N = 13 | 3:1 | |||
| Naguib & Levy, 1987 [15] | UK | Paraphrenia | ≥60 | N = 43 | 6.2:1 |
| Castle et al., 1993 [16] | UK | ICD-9 Schizophrenia and related disorders | >60 | N = 513 | 4.4:1 |
| Mazeh et al., 2005 [17] | Israel | DSM-IV Schizophrenia | ≥70 | N = 21 | 2.5:1 |
| Moore et al., 2006 [18] | South London and North West England | Very-late-onset Schizophrenia-like Psychosis (Howard et al., 2000) | >60 | N = 29 | 1.9:1 |
| Harris et al., 2014 [19] | Monash, Australia | DSM-IV-TR Delusional Disorder | >65 | N = 19 | 3.75:1 |
| Hanssen et al., 2015 [20] | The Netherlands | DSM-IV diagnosis of non-affective psychotic disorder | ≥60 | N = 28 | 7:1 |
| Risk Factor | Early Onset | Very Late Onset Schizophrenia | Reference |
|---|---|---|---|
| Sex | Male | Female | Castle et al., 1993 [16] |
| Pregnancy and birth-related complications | More prevalent | Less prevalent | Janoutová et al., 2016 [3] |
| Traumatic life experiences | More prevalent | Less prevalent | Janoutová et al., 2016 [3] |
| Genetic risk factors | More prevalent | Less prevalent | Janoutová et al., 2016 [3] |
| Ventricle size | Larger | Not observed | Castle & Murray, 1991 [35] |
| Poor premorbid social adjustment | More prevalent | Less prevalent | Neill et al., 2020 [36] |
| Family history of psychiatric illness | More prevalent | Less prevalent | Neill et al., 2020 [36] |
| Family history of schizophrenia | More prevalent | Less prevalent | Ayano, 2016 [39] |
| Loss of close relative | More prevalent | Less prevalent | Neill et al., 2020 [36] |
| Alcohol or drug abuse | More prevalent | Less prevalent | Selvendra et al., 2022 [38] |
| Immigration status | Non-migrant status | Migrant status | Neill et al., 2020 [36] |
| Physical and childhood sexual abuse | More prevalent | Less prevalent | Selvendra et al., 2022 [38] |
| Features | Earlier Onset | Later Onset | Reference |
|---|---|---|---|
| Positive Symptoms | Less Prevalent | More Prevalent | Hanssen et al., 2015 [20] |
| Partition Delusions | Less Prevalent | More Prevalent | Van Assche et al., 2017 [44] |
| Paranoid Delusions | Less Prevalent | More Prevalent | Van Assche et al., 2017 [44] |
| Persecutory Delusions | Less Prevalent | More Prevalent | Castle et al., 1997 [6] |
| Hallucinations | More Prevalent | Less Prevalent | Castle et al., 1997 [6] |
| Psychotic Episodes | More Prevalent | Less Prevalent | Hanssen et al., 2015 [20] |
| Negative Symptoms | More Prevalent | Less Prevalent | Howard et al., 2000 [5] |
| Restricted affect | More Prevalent | Less Prevalent | Castle et al., 1997 [6] |
| Formal thought disorder | More Prevalent | Less Prevalent | Almeida et al., 1995 [46] |
| Inappropriate affect | More Prevalent | Less Prevalent | Castle et al., 1997 [6] |
| Lifetime diagnosis of Major Depression | More Prevalent | Less Prevalent | Hanssen et al., 2015 [20] |
| Cognitive deficits | Significant deficits | Some deficits | Van Assche, et al., 2017 [44] |
| Intelligence | Significant deficits | Some deficits | Vahia et al., 2010 [47]; Sachdev et al., 1999 [48] |
| Processing Speed | Significant deficits | Some deficits | Vahia et al., 2010 [47]; Sachdev et al., 1999 [48] |
| Executive Functioning | Significant deficits | Significant deficits | Brichant-Petitjean et al., 2013 [49]; Hanssen et al., 2015 [20] |
| Attention | Significant deficits | Significant deficits | Brichant-Petitjean et al., 2013 [49]; Hanssen et al., 2015 [20] |
| Verbal learning and memory ** | Some deficits | Some deficits | Hanssen et al., 2015 [20] |
| Social cognitive functioning | Primarily impaired | Primarily intact | Moore et al., 2006 [18]; Smeets-Janssen et al., 2013 [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnstone, S.; Dela Cruz, G.A.; Girard, T.A.; Rajji, T.K.; Castle, D.J. Potential Explanatory Models of the Female Preponderance in Very Late Onset Schizophrenia. Women 2022, 2, 353-370. https://doi.org/10.3390/women2040033
Johnstone S, Dela Cruz GA, Girard TA, Rajji TK, Castle DJ. Potential Explanatory Models of the Female Preponderance in Very Late Onset Schizophrenia. Women. 2022; 2(4):353-370. https://doi.org/10.3390/women2040033
Chicago/Turabian StyleJohnstone, Samantha, Gil Angela Dela Cruz, Todd A. Girard, Tarek K. Rajji, and David J. Castle. 2022. "Potential Explanatory Models of the Female Preponderance in Very Late Onset Schizophrenia" Women 2, no. 4: 353-370. https://doi.org/10.3390/women2040033
APA StyleJohnstone, S., Dela Cruz, G. A., Girard, T. A., Rajji, T. K., & Castle, D. J. (2022). Potential Explanatory Models of the Female Preponderance in Very Late Onset Schizophrenia. Women, 2(4), 353-370. https://doi.org/10.3390/women2040033






