The Association between Intimate Partner Violence, Depression and Influenza-like Illness Experienced by Pregnant Women in Australia
Abstract
:1. Introduction
2. Materials and Methods
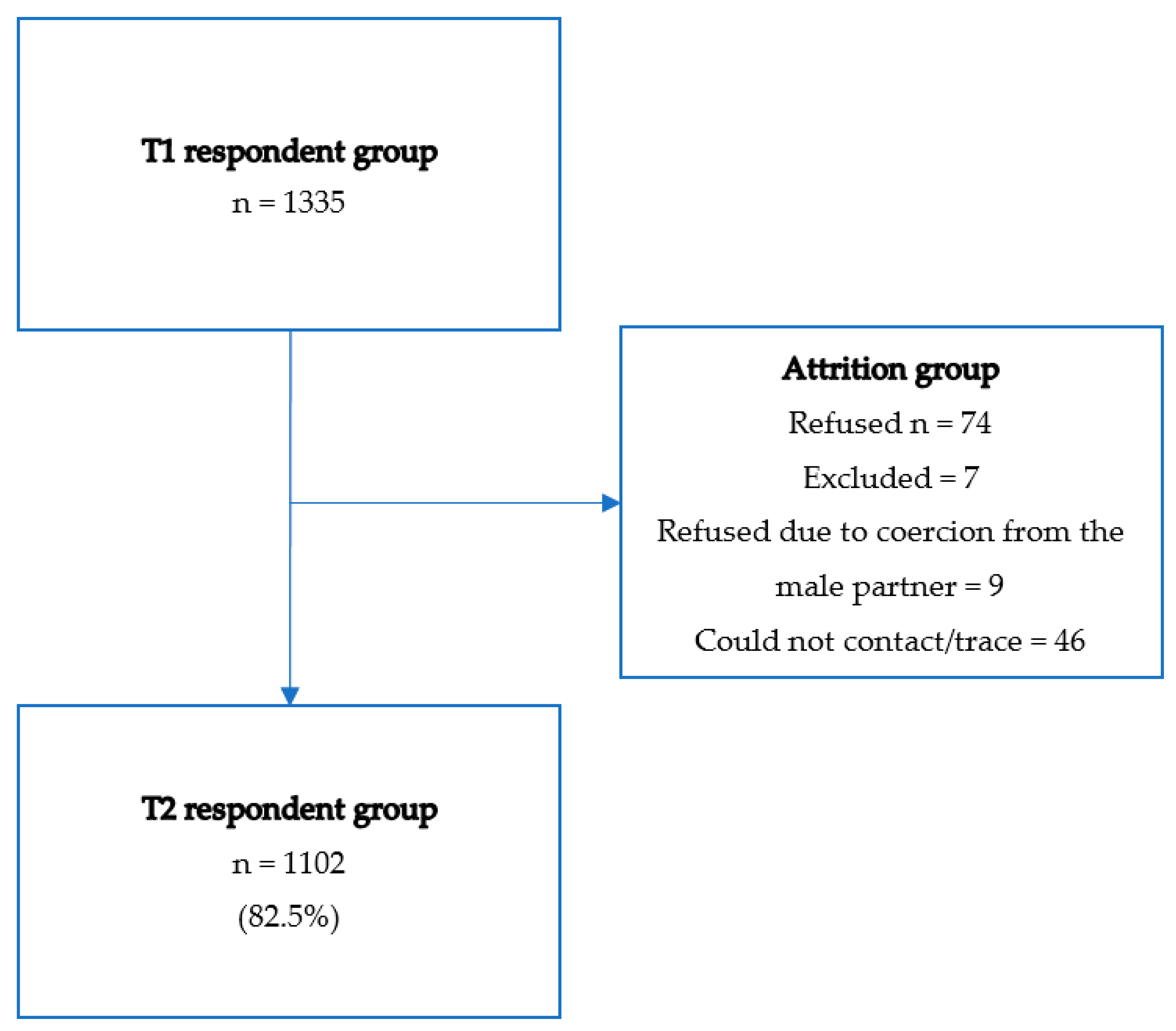
2.1. Procedure
2.2. Ethics and Research Personnel
2.3. Survey Measures
2.3.1. Cultural Accuracy
2.3.2. Sociodemographic Characteristics
2.3.3. Influenza-Like Illness
2.4. Mental Health Measures
2.5. Traumatic Events
2.6. Intimate Partner Violence
2.7. Statistical Analysis
2.8. Path Analysis
3. Results
3.1. Participant Characteristics
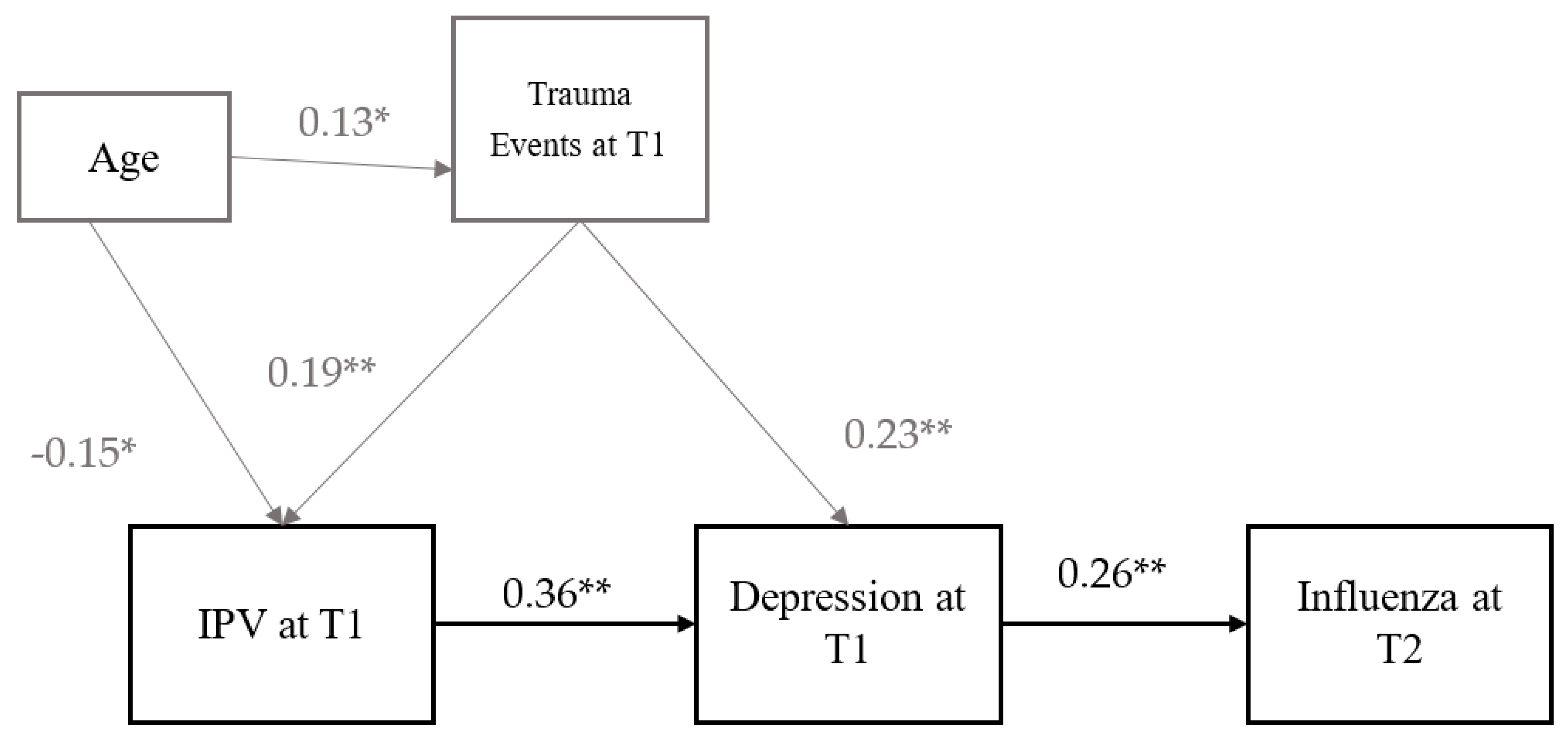
3.2. Path Analysis
4. Discussion
Future Directions for Practice and Policy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Miller, E.; McCaw, B. Intimate Partner Violence. N. Engl. J. Med. 2019, 380, 850–857. [Google Scholar] [CrossRef] [Green Version]
- Rees, S.; Silove, D.; Chey, T.; Ivancic, L.; Steel, Z.; Creamer, M.; Teesson, M.; Bryant, R.; McFarlane, R.C.; Mills, K.L.; et al. Lifetime prevalence of gender-based violence in women and the relationship with mental disorders and psychosocial function. JAMA 2011, 306, 513–521. [Google Scholar] [CrossRef] [Green Version]
- Webster, K. A Preventable Burden: Measuring and Addressing the Prevalence and Health Impacts of Intimate Partner Violence in Australian Women: Key Findings and Future Directions; Australia’s National Research Organisation for Women’s Safety: Sydney, Australia, 2016. [Google Scholar]
- E Troeger, C.; Blacker, B.F.; Khalil, I.A.; Zimsen, S.R.M.; Albertson, S.B.; Abate, D.; Abdela, J.; Adhikari, T.B.; Aghayan, S.; Agrawal, S.; et al. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: An analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2018, 7, 69–89. [Google Scholar] [CrossRef] [Green Version]
- Yudin, M.H. Risk management of seasonal influenza during pregnancy: Current perspectives. Int. J. Womens Health 2014, 6, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Beydoun, H.A.; Beydoun, M.A.; Kaufman, J.S.; Lo, B.; Zonderman, A.B. Intimate partner violence against adult women and its association with major depressive disorder, depressive symptoms and postpartum depression: A systematic review and meta-analysis. Soc. Sci. Med. 2012, 75, 959–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Underwood, L.; Waldie, K.; D’Souza, S.; Peterson, E.R.; Morton, S. A review of longitudinal studies on antenatal and postnatal depression. Arch. Womens Ment. Health 2016, 19, 711–720. [Google Scholar] [CrossRef]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Fagundes, C.P.; Glaser, R.; Kiecolt-Glaser, J.K. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav. Immun. 2013, 27, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Herbert, T.B.; Cohen, S. Depression and immunity: A meta-analytic review. Psychol. Bull. 1993, 113, 472–486. [Google Scholar] [CrossRef]
- Petralia, M.C.; Mazzon, E.; Fagone, P.; Falzone, L.; Bramanti, P.; Nicoletti, F.; Basile, M.S. Retrospective follow-up analysis of the transcriptomic patterns of cytokines, cytokine receptors and chemokines at preconception and during pregnancy, in women with post-partum depression. Exp. Ther. Med. 2019, 18, 2055–2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalokhe, A.S.; Ibegbu, C.C.; Kaur, S.P.; Amara, R.R.; Kelley, M.E.; Del Rio, C.; Stephenson, R. Intimate Partner Violence is Associated with Increased CD4+ T-Cell Activation Among HIV-Negative High-Risk Women. Pathog. Immun. 2016, 1, 193–213. [Google Scholar] [CrossRef]
- Campbell, J.C.; Baty, M.L.; Ghandour, R.M.; Stockman, J.K.; Francisco, L.; Wagman, J. The intersection of intimate partner violence against women and HIV/AIDS: A review. Int. J. Inj. Contr. Saf. Promot. 2008, 15, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Lorente, S.; Blasco-Ros, C.; Coe, C.L.; Martinez, M. Recovery of immune control over herpes simplex virus type 1 in female victims of intimate partner violence. Psychosom. Med. 2010, 72, 97–106. [Google Scholar] [CrossRef]
- Garcia-Linares, M.I.; Sanchez-Lorente, S.; Coe, C.L.; Martinez, M. Intimate male partner violence impairs immune control over herpes simplex virus type 1 in physically and psychologically abused women. Psychosom. Med. 2014, 66, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, M.; Broome, M.; Smith, B.; Bowden, H. A bad case of the flu? The comparative phenomenology of depression and somatic illness. J. Conscious Stud. 2013, 20, 198–218. [Google Scholar]
- Heim, E.; Wegmann, I.; Maercker, A. Cultural values and the prevalence of mental disorders in 25 countries: A secondary data analysis. Soc. Sci. Med. 2017, 189, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Rees, S.J.; Fisher, J.R.; Steel, Z.; Mohsin, M.; Nadar, N.; Moussa, B.; Hassoun, F.; Yousif, M.; Krishna, Y.; Khalil, B.; et al. Prevalence and Risk Factors of Major Depressive Disorder Among Women at Public Antenatal Clinics From Refugee, Conflict-Affected, and Australian-Born Backgrounds. JAMA Netw. Open 2019, 2, e193442. [Google Scholar] [CrossRef] [Green Version]
- Dillon, G.; Hussain, R.; Loxton, D.; Rahman, S. Mental and Physical Health and Intimate Partner Violence against Women: A Review of the Literature. Int. J. Family Med. 2013, 2013, 313909. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.E.; VonKorff, M.; Piccinelli, M.; Fullerton, C.; Ormel, J. An international study of the relation between somatic symptoms and depression. N. Engl. J. Med. 1999, 341, 1329–1335. [Google Scholar] [CrossRef]
- Glover, R.; van Schalkwyk, M.C.; Akl, E.A.; Kristjannson, E.; Lotfi, T.; Petkovic, J.; Petticrew, M.P.; Pottie, K.; Tugwell, P.; Welch, V. A framework for identifying and mitigating the equity harms of COVID-19 policy interventions. J. Clin. Epidemiol. 2020, 128, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Darcy, J.M.; Grzywacz, J.G.; Stephens, R.L.; Leng, I.; Clinch, C.R.; Arcury, T.A. Maternal Depressive Symptomatology: 16-Month Follow-up of Infant and Maternal Health-Related Quality of Life. J. Am. Board Fam. Med. 2011, 24, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Orr, S.T.; Blazer, D.G.; James, S.A.; Reiter, J.P. Depressive Symptoms and Indicators of Maternal Health Status during Pregnancy. J. Women’s Health 2007, 16, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Hewagama, S.; Walker, S.P.; Stuart, R.L.; Gordon, C.; Johnson, P.D.R.; Friedman, N.D.; O’Reilly, M.; Cheng, A.C.; Giles, M.L. 2009 H1N1 Influenza A and Pregnancy Outcomes in Victoria, Australia. Clin. Infect. Dis. 2010, 50, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.R.; Dorey, R.B.; Warricker, F.D.M.; Alwan, N.A.; Jones, C.E. The effectiveness of influenza vaccination in pregnancy in relation to child health outcomes: Systematic review and meta-analysis. Vaccine 2020, 38, 1601–1613. [Google Scholar] [CrossRef]
- Mendez-Figueroa, H.; Raker, C.; Anderson, B.L. Neonatal characteristics and outcomes of pregnancies complicated by influenza infection during the 2009 pandemic. Am. J. Obstet. Gynecol. 2011, 204, S58–S63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Sociodemographic Characteristics | All Women | Trauma Events at T1 | Depression at T1 | IPV at T1 | ILI during Pregnancy at T2 |
|---|---|---|---|---|---|
| n (%) | n (%) 3 or More | n (%) Yes | n (%) Yes | n (%) Yes | |
| All women | 1102 | 225 (20) | 216 (19) | 390 (35) | 111 (10) |
| Age group at T1 | |||||
| <25 | 213 (19) | 39 (18) | 51 (24) | 105 (48) | 19 (8.9) |
| 25–35 | 678 (62) | 112 (16) | 122 (18) | 219 (32) | 72 (10.6) |
| >35 | 211 (19) | 74 (35) | 43 (20) | 66 (31) | 20 (9.5) |
| p value | <0.001 | 0.175 | <0.001 | 0.734 | |
| Marital Status at T1 | |||||
| Married/domestic partnership | 1021 (93) | 204 (20) | 191 (19) | 350 (34) | 103 (10.1) |
| Separated/divorced/other | 81 (7) | 21 (26) | 25 (31) | 40 (49) | 8 (7.2) |
| p value | 0.443 | 0.009 | 0.007 | 0.951 | |
| Highest level of educational attainment at T1 | |||||
| No postschool qualification | 508 (46) | 110 (49) | 114 (53) | 230 (59) | 51 (10) |
| Diploma and vocational education | 234 (21) | 49 (22) | 44 (20) | 68 (17) | 23 (10) |
| University degree | 360 (33) | 66 (29) | 58 (27) | 92 (24) | 37 (10) |
| p value | 0.228 | 0.063 | <0.001 | 0.984 | |
| Country of Origin | |||||
| Women born in Australia | 524 (48) | 106 (47) | 74 (34) | 137 (35) | 35 (7) |
| Women from refugee background | 578 (52) | 119 (53) | 142 (66) | 253 (65) | 76 (13) |
| Significant findings are in bold | 0.452 | <0.001 | <0.001 | <0.001 |
| All Women | ILI at T2 | |
|---|---|---|
| n (%) | n (%) Yes | |
| All women | 1102 | 111 (10) |
| Trauma events at T1 | ||
| none | 545 | 39 (7) |
| 1–2 | 333 | 42 (13) |
| 3 or more | 224 | 30 (13) |
| p value | 0.006 | |
| IPV at T1 | ||
| No IPV and/or low respect | 717 (65) | 61 (9) |
| Severe Psychological and/or Physical IPV | 385 (35) | 50 (13) |
| p value | 0.018 | |
| Depression at T1 | ||
| No | 886 (80) | 78 (9) |
| Yes | 214 (20) | 33 (15) |
| p value | 0.004 | |
| PTSD at T1 | ||
| Yes | 1038 (94) | 100 (10) |
| No | 64 (6) | 11 (17) |
| p value | 0.051 |
| Relationship | Standardized Path Co-Efficient (Beta) | Standard Error (SE) | p-Value | |
|---|---|---|---|---|
| Step 1–ILI at T2 on IPV at T1, IPV at T1 on Trauma at T1 | ||||
| ILI at T2 on | ||||
| IPV atT1 | 0.181 | 0.065 | 0.005 | |
| IPV at T1 on | ||||
| Age at T1 | −0.145 | 0.036 | <0.001 | |
| Trauma events at T1 | 0.199 | 0.044 | <0.001 | |
| Trauma events at T1 on | ||||
| Age at T1 | 0.134 | 0.033 | <0.001 | |
| Step 2–ILI at T2 on IPV at T1 mediated by depression, IPV on Trauma | ||||
| ILI at T2 on | ||||
| Depression at T1 | 0.256 | 0.07 | <0.001 | |
| Depression at T1 on | ||||
| IPV at T1 | 0.358 | 0.049 | <0.001 | |
| Trauma events | 0.229 | 0.047 | <0.001 | |
| IPV at T1 on | ||||
| Age | −0.151 | 0.036 | <0.001 | |
| Trauma events | 0.186 | 0.044 | <0.001 | |
| Trauma events at T1 on | ||||
| Age | 0.131 | 0.033 | <0.001 | |
| Indirect effect | ||||
| ILI at T2, Depression at T1, IPV at T1 | 0.092 | 0.029 | 0.001 | |
| ILI at T2, Depression at T1, Trauma at T1 | 0.059 | 0.020 | 0.004 | |
| ILI at T2, Depression at T1, IPV at T1 Trauma at T1 | 0.017 | 0.007 | 0.011 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rees, S.J.; Wells, R.; Mohsin, M.; Nadar, N.; Moussa, B.; Hassoun, F.; Yousif, M.; Khalil, B.; Krishna, Y.; Nancarrow, H.; et al. The Association between Intimate Partner Violence, Depression and Influenza-like Illness Experienced by Pregnant Women in Australia. Women 2021, 1, 192-203. https://doi.org/10.3390/women1040017
Rees SJ, Wells R, Mohsin M, Nadar N, Moussa B, Hassoun F, Yousif M, Khalil B, Krishna Y, Nancarrow H, et al. The Association between Intimate Partner Violence, Depression and Influenza-like Illness Experienced by Pregnant Women in Australia. Women. 2021; 1(4):192-203. https://doi.org/10.3390/women1040017
Chicago/Turabian StyleRees, Susan J., Ruth Wells, Mohammed Mohsin, Nawal Nadar, Batool Moussa, Fatima Hassoun, Mariam Yousif, Batoul Khalil, Yalini Krishna, Heather Nancarrow, and et al. 2021. "The Association between Intimate Partner Violence, Depression and Influenza-like Illness Experienced by Pregnant Women in Australia" Women 1, no. 4: 192-203. https://doi.org/10.3390/women1040017
APA StyleRees, S. J., Wells, R., Mohsin, M., Nadar, N., Moussa, B., Hassoun, F., Yousif, M., Khalil, B., Krishna, Y., Nancarrow, H., Silove, D., & Fisher, J. (2021). The Association between Intimate Partner Violence, Depression and Influenza-like Illness Experienced by Pregnant Women in Australia. Women, 1(4), 192-203. https://doi.org/10.3390/women1040017






