Sterility, an Overlooked Health Condition
Abstract
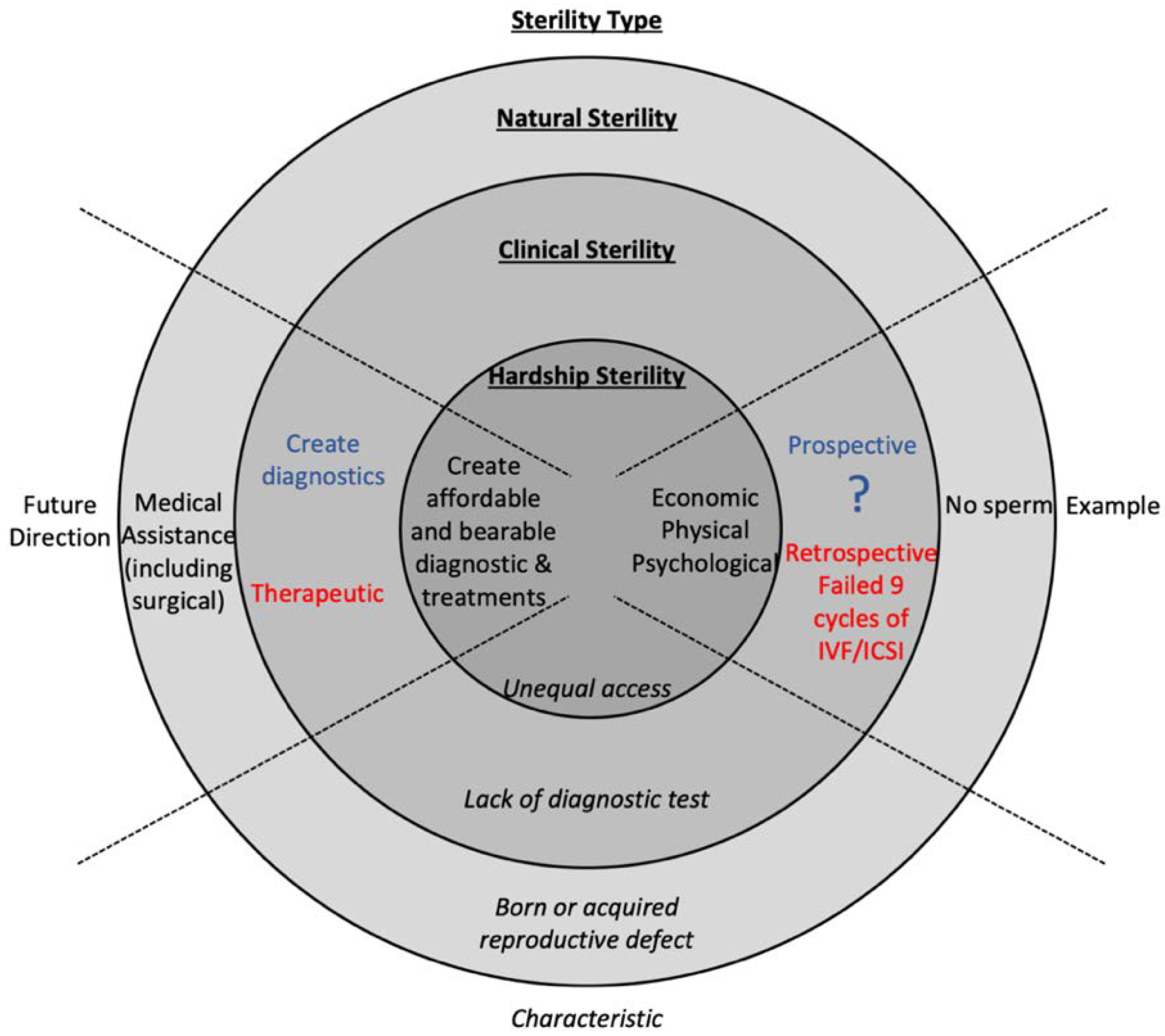
1. Introduction
2. There Is a Need to Diagnose Sterility Prospectively
3. Most Treatments of Infertile Couples Fail to Deliver a Live Birth
3.1. Untreated Infertile Couples
3.2. Treated Infertile Couples
- (1)
- 10% of couples are infertile couples, and more than 4% are involuntarily childless or sterile;
- (2)
- At the least, 64% of infertile couples are not treated, and 70% of them fail to have a live birth;
- (3)
- 7.4% of infertile couples undergo IUI, and 69% of them fail to have a live birth;
- (4)
- ~33% of infertile men undergo varicocele treatment, and 69% of them fail to have a live birth;
- (5)
- ~3.1% of infertile women undergo ICSI, and 47% of them fail to have a live birth.
4. Understanding Sterility Will Benefit the Patient, Physician, and Researchers
5. Economic, Physical, and Psychological Burdens Contribute to Hardship Sterility
6. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Glossary
| Clinical Sterility | The physiological inability of a couple to conceive a child even after medical intervention, including surgical intervention. |
| Clinically Sterile Couple | A couple with 1% or less chance of achieving a live birth with reproductive medicine assistance. |
| Cumulative Live Birth Rate | A couple’s chance to have a live birth after cumulative cycles of fertility treatment. |
| Fertile Couple | A couple that conceives naturally within one year of trying. |
| Hardship Sterility | The physiological inability of a couple to conceive a child even after medical intervention because of economic, psychological, or physical reasons. |
| ICSI | Intracytoplasmic sperm injection; a fertility procedure in which sperm is injected into an egg. |
| Infertility | A difficulty to conceive that is defined in most cases as the inability to conceive a biological child after one year of attempting. |
| IUI | Intrauterine insemination; a fertility procedure in which sperm is placed inside a female’s uterus. |
| IVF | In-vitro fertilization; a fertility treatment that combines the egg and sperm outside the body, and then the embryo is transferred into the uterus. |
| Natural Sterility | The physiological inability of a couple to conceive a child without medical intervention (i.e., “natural means”). |
| Prospective Determination | The ability to diagnose a patient with clinical sterility before beginning treatment. |
| Reproductively Futile Couple | A couple with 1% or less chance of achieving a live birth with reproductive medicine assistance. |
| Retrospective Determination | The ability to diagnose a patient with clinical sterility after treatments have failed. |
| Retrospectively Clinically Sterile Couple | A couple with 1% or less chance of achieving a live birth after failing multiple treatments of reproductive medical assistance. |
| Prospectively Clinically Sterile Couple | A couple with 1% or less chance of achieving a live birth with reproductive medicine assistance. |
| Sterility | The inability to conceive due to natural, clinical, or hardship factors, determined either prospectively or retrospectively. |
| Varicocele Repair | Repair of the enlarged veins within the scrotum |
References
- Gurunath, S.; Pandian, Z.; Anderson, R.A.; Bhattacharya, S. Defining infertility--a systematic review of prevalence studies. Hum. Reprod. Update 2011, 17, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Hadley, R.A. ‘It’s most of my life—going to the pub or the group’: The social networks of involuntarily childless older men. Ageing Soc. 2019, 1–26. [Google Scholar] [CrossRef]
- Miall, C.E. The stigma of involuntary childlessness. Soc. Probl. 1986, 33, 268–282. [Google Scholar] [CrossRef]
- Van Balen, F.; Trimbos-Kemper, T.C. Involuntarily childless couples: Their desire to have children and their motives. J. Psychosom. Obstet. Gynaecol. 1995, 16, 137–144. [Google Scholar] [CrossRef]
- Schwerdtfeger, K.L.; Shreffler, K.M. Trauma of Pregnancy Loss and Infertility for Mothers and Involuntarily Childless Women in the Contemporary United States. J. Loss Trauma 2009, 14, 211–227. [Google Scholar] [CrossRef]
- Khan, A.R.; Iqbal, N.; Afzal, A. Impact of Infertility on Mental Health of Women. Int. J. Indian Psychol. 2019, 7, 804–809. [Google Scholar] [CrossRef]
- Tyuvina, N.; Nikolaevskaya, A. Infertility and mental disorders in women. Communication 1. Neurol. Neuropsychiatry Psychosom. 2019, 11, 117–124. [Google Scholar] [CrossRef][Green Version]
- Miner, S.A.; Daumler, D.; Chan, P.; Gupta, A.; Lo, K.; Zelkowitz, P. Masculinity, Mental Health, and Desire for Social Support. Among Male Cancer and Infertility Patients. Am. J. Men’s Health 2019, 13, 1557988318820396. [Google Scholar] [CrossRef]
- Roy, R.N.; Schumm, W.R.; Britt, S.L. Voluntary versus involuntary childlessness. In Transition to Parenthood; Springer: Berlin, Germany, 2014; pp. 49–68. [Google Scholar]
- Biryukova, S.S.; Tyndik, A.O. Prevalence and determinants of childlessness in Russia and Moscow. Genus 2015, 71, 1–22. [Google Scholar]
- Onyedibe, M.-C.C.; Aliche, C.J.; Ugwu, L.E.; Obi-Keguna, C.N.; Nnama-Okechukwu, C.U.; Okoye, U.O.; Iorfa, S.K.; Ifeagwazi, C.M.; Chukwuorji, J.C.; Osamika, B.E. Self-esteem and psychological distress among involuntary childless couples. Moderating roles of coping strategies. Niger. J. Psychol. Res. 2019, 15, 1–7. [Google Scholar]
- Grube, T. A Grounded Theory Approach to Explore the Experience of Involuntary Childlessness in Couples with Infertility. Ph.D. Thesis, Widener University, Chester, PA, USA, 2019. [Google Scholar]
- Carter, J.; Applegarth, L.; Josephs, L.; Grill, E.; Baser, R.E.; Rosenwaks, Z. A cross-sectional cohort study of infertile women awaiting oocyte donation: The emotional, sexual, and quality-of-life impact. Fertil. Steril. 2011, 95, 711–716.e1. [Google Scholar] [CrossRef] [PubMed]
- Wischmann, T.; Thorn, P. (Male) infertility: What does it mean to men? New evidence from quantitative and qualitative studies. Reprod. Biomed. Online 2013, 27, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Singla, B. Acceptance of donor eggs, donor sperms, or donor embryos in Indian infertile couples. J. Hum. Reprod. Sci. 2018, 11, 169. [Google Scholar] [PubMed]
- Kerckhof, M.; Van Parys, H.; Pennings, G.; De Sutter, P.; Buysse, A.; Provoost, V. Donor insemination disclosure in social networks: Heterosexual couples’ experiences. Cult. Health Sex. 2020, 22, 292–306. [Google Scholar] [CrossRef] [PubMed]
- National Collaborating Centre for Women’s; Children’s Health. National Institute for Health and Clinical Excellence: Guidance. In Fertility: Assessment and Treatment for People with Fertility Problems; Royal College of Obstetricians & Gynaecologists: London, UK, 2013. [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine; Practice Committee of the Society for Assisted Reproductive Technology. Repetitive oocyte donation: A committee opinion. Fertil. Steril. 2014, 102, 964–966. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: A committee opinion. Fertil. Steril. 2020, 113, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Evidence-based treatments for couples with unexplained infertility: A guideline. Fertil. Steril. 2020, 113, 305–322. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: A committee opinion. Fertil. Steril. 2015, 103, e44–e50. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: A committee opinion. Fertil. Steril. 2015, 103, e18–e25. [Google Scholar] [CrossRef]
- Kamel, R.M. Management of the infertile couple: An evidence-based protocol. Reprod. Biol. Endocrinol. 2010, 8, 21. [Google Scholar] [CrossRef]
- Committee on Gynecologic Practice, American Society for Reproductive Medicine. Infertility Workup for the Women’s Health Specialist: ACOG Committee Opinion, Number 781. Obstet. Gynecol. 2019, 133, e377–e384. [Google Scholar] [CrossRef] [PubMed]
- Buckett, W.; Sierra, S. The management of unexplained infertility: An evidence-based guideline from the Canadian Fertility and Andrology Society. Reprod. Biomed. Online 2019, 39, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Warner, L.; Jamieson, D.J.; Barfield, W.D. CDC releases a National Public Health Action Plan. for the Detection, Prevention, and Management of Infertility. J. Womens Health 2015, 24, 548–549. [Google Scholar] [CrossRef]
- Webb, S.; Holman, D. A survey of infertility, surgical sterility and associated reproductive disability in Perth, Western Australia. Aust. J. Public Health 1992, 16, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Hardy, E.; Bahamondes, L.; Osis, M.J.; Costa, R.G.; Faúndes, A. Risk factors for tubal sterilization regret, detectable before surgery. Contraception 1996, 54, 159–162. [Google Scholar] [CrossRef]
- Rubin, I.C.; Zakin, D. Contraception Masking Sterility and Infertility: Tubal and Seminal Factors in 1000 Cases. J. Am. Med Assoc. 1946, 132, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Beke, A. Genetic Causes of Female Infertility. In Genetics of Endocrine Diseases and Syndromes; Igaz, P., Patócs, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 367–383. [Google Scholar]
- Popović, J.; Sulović, V.; Vucetić, D. Laparoscopy treatment of adnexal sterility. Clin. Exp. Obstet. Gynecol. 2005, 32, 31–34. [Google Scholar] [PubMed]
- Mettler, L.; Scheidel, P.; Shirwani, D. Sperm antibody production in female sterility. Int. J. Fertil. 1974, 19, 7–12. [Google Scholar]
- Cohen, M.R. Sterility and Infertility-Medical Views. J. Am. Med Assoc. 1958, 168, 1963–1970. [Google Scholar] [CrossRef]
- Macomber, E.R.a.D. Fertility and Sterility in Human Marriages; W.B. Saunders Company: Philadelphia, PA, USA, 1924. [Google Scholar]
- Guttmacher, A.F. The Role of Artificial Insemination in the Treatment of Human Sterility. Bull. N. Y. Acad. Med. 1943, 19, 573–591. [Google Scholar]
- Eliasson, R. Analysis of Semen in Comprehensive Endocrinology; Burger, H.K.D.D.E., Ed.; Raven Press: New York, NY, USA, 1981. [Google Scholar]
- Leridon, H. A new estimate of permanent sterility by age: Sterility defined as the inability to conceive. Popul. Stud. 2008, 62, 15–24. [Google Scholar] [CrossRef]
- Egozcue, J.; Templado, C.; Vidal, F.; Navarro, J.; Morer-Fargas, F.; Marina, S. Meiotic studies in a series of 1100 infertile and sterile males. Hum. Genet. 1983, 65, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Rochon, M. Sterility and infertility: Two concepts. Cah. Que Demogr. 1986, 15, 27–56. [Google Scholar] [PubMed]
- Brugo-Olmedo, S.; Chillik, C.; Kopelman, S. Definition and causes of infertility. Reprod. Biomed. Online 2001, 2, 173–185. [Google Scholar] [CrossRef]
- Habbema, J.D.F.; Collins, J.; Leridon, H.; Evers, J.L.H.; Lunenfeld, B.; teVelde, E.R. Towards less confusing terminology in reproductive medicine: A proposal. Hum. Reprod. 2004, 19, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Forti, G.; Krausz, C. Evaluation and Treatment of the Infertile Couple1. J. Clin. Endocrinol. Metab. 1998, 83, 4177–4188. [Google Scholar] [CrossRef]
- Show, H.-A.C.D.I.; Decline, S. Prevalence of Infertility and Its Treatment Among Women. US Pharm. 2018, 43, 14. [Google Scholar]
- Abma, J.C.; Martinez, G.M. Childlessness Among Older Women in the United States: Trends and Profiles. J. Marriage Fam. 2006, 68, 1045–1056. [Google Scholar] [CrossRef]
- Gunnell, D.J.; Ewings, P. Infertility prevalence, needs assessment and purchasing. J. Public Health 1994, 16, 29–35. [Google Scholar] [CrossRef]
- Greenhall, E.; Vessey, M. The prevalence of subfertility: A review of the current confusion and a report of two new studies. Fertil. Steril. 1990, 54, 978–983. [Google Scholar] [CrossRef]
- Kreyenfeld, M.; Konietzka, D. Analyzing Childlessness. In Childlessness in Europe: Contexts, Causes, and Consequences; Kreyenfeld, M., Konietzka, D., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 3–5. [Google Scholar]
- Razeghi-Nasrabad, H.B.; Abbasi-Shavazi, M.J.; Moeinifar, M. Are we facing a dramatic increase in infertility and involuntary childlessness that lead to lower fertility. Crescent J. Med. Biol. Sci. 2020, 7, 1–14. [Google Scholar]
- Rowland, D.T. The prevalence of childlessness in cohorts of older women. Australas. J. Ageing 1998, 17, 18–23. [Google Scholar] [CrossRef]
- Ethics Committee of the American Society for Reproductive Medicine. Fertility treatment when the prognosis is very poor or futile: A committee opinion. Fertil. Steril. 2012, 98, e6–e9. [Google Scholar] [CrossRef] [PubMed]
- Spangler, D.B.; Jones, G.S.; Jones, H.W. Infertility due to endometriosis: Conservative surgical therapy. Am. J. Obstet. Gynecol. 1971, 109, 850–857. [Google Scholar] [CrossRef]
- Tanbo, T.; Fedorcsak, P. Endometriosis-associated infertility: Aspects of pathophysiological mechanisms and treatment options. Acta Obstet. Gynecol. Scand. 2017, 96, 659–667. [Google Scholar] [CrossRef]
- Tournaye, H.; Camus, M.; Goossens, A.; Liu, J.; Nagy, P.; Silber, S.; Van Steirteghem, A.C.; Devroey, P. Recent concepts in the management of infertility because of non-obstructive azoospermia. Hum. Reprod. 1995, 10 (Suppl. 1), 115–119. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, J.; Dai, L.; Zhu, Y.; Hu, H.; Tan, L.; Chen, W.; Liang, D.; He, J.; Tu, M. XRCC2 mutation causes meiotic arrest, azoospermia and infertility. J. Med. Genet. 2018, 55, 628–636. [Google Scholar] [CrossRef]
- Schmidt, K.T.; Rosendahl, M.; Ernst, E.; Loft, A.; Andersen, A.N.; Dueholm, M.; Ottosen, C.; Andersen, C.Y. Autotransplantation of cryopreserved ovarian tissue in 12 women with chemotherapy-induced premature ovarian failure: The Danish experience. Fertil. Steril. 2011, 95, 695–701. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Shalaby, S.M.; Abdelaziz, M.; Brakta, S.; Hill, W.D.; Ismail, N.; Al-Hendy, A. Human mesenchymal stem cells partially reverse infertility in chemotherapy-induced ovarian failure. Reprod. Sci. 2018, 25, 51–63. [Google Scholar] [CrossRef]
- Al-Said, S.; Al-Naimi, A.; Al-Ansari, A.; Younis, N.; Shamsodini, A.; A-sadiq, K.; Shokeir, A.A. Varicocelectomy for Male Infertility: A Comparative Study of Open, Laparoscopic and Microsurgical Approaches. J. Urol. 2008, 180, 266–270. [Google Scholar] [CrossRef]
- Lundy, S.D.; Sabanegh, E.S., Jr. Varicocele management for infertility and pain: A systematic review. Arab J. Urol. 2018, 16, 157–170. [Google Scholar] [CrossRef] [PubMed]
- WHO. Sexual and Reproductive Health: Infertility Definitions and Terminology. World Health Organisation: Geneva, Switzerland. Available online: www.who.int/reproductivehealth/topics/infertility/definitions/en (accessed on 27 October 2020).
- WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen; World Health Organisation: Geneva, Switzerland, 2010. [Google Scholar]
- Avidor-Reiss, T.; Carr, A.; Fishman, E.L. The Sperm Centrioles. Mol. Cell. Endocrinol. 2020, 110987. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.A.; Rambhatla, A.; Schon, S.; Agarwal, A.; Krawetz, S.A.; Dupree, J.M.; Avidor-Reiss, T. Male Infertility is a Women’s Health Issue—Research and Clinical Evaluation of Male Infertility Is Needed. Cells 2020, 9, 990. [Google Scholar] [CrossRef] [PubMed]
- Group, C.C.W. The current status and future of andrology: A consensus report from the Cairo workshop group. Andrology 2020, 8, 27–52. [Google Scholar]
- Donnez, J.; Dolmans, M.-M. Transplantation of ovarian tissue. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 1188–1197. [Google Scholar] [CrossRef]
- Pacheco, F.; Oktay, K. Current success and efficiency of autologous ovarian transplantation: A meta-analysis. Reprod. Sci. 2017, 24, 1111–1120. [Google Scholar] [CrossRef]
- Schlegel, P.N.; Palermo, G.D.; Goldstein, M.; Menendez, S.; Zaninovic, N.; Veeck, L.L.; Rosenwaks, Z. Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia. Urology 1997, 49, 435–440. [Google Scholar] [CrossRef]
- Turunc, T.; Gul, U.; Haydardedeoglu, B.; Bal, N.; Kuzgunbay, B.; Peskircioglu, L.; Ozkardes, H. Conventional testicular sperm extraction combined with the microdissection technique in nonobstructive azoospermic patients: A prospective comparative study. Fertil. Steril. 2010, 94, 2157–2160. [Google Scholar] [CrossRef]
- Eken, A.; Gulec, F. Microdissection testicular sperm extraction (micro-TESE): Predictive value of preoperative hormonal levels and pathology in non-obstructive azoospermia. Kaohsiung J. Med Sci. 2018, 34, 103–108. [Google Scholar] [CrossRef]
- Stern, J.E.; Brown, M.B.; Luke, B.; Wantman, E.; Lederman, A.; Missmer, S.A.; Hornstein, M.D. Calculating cumulative live-birth rates from linked cycles of assisted reproductive technology (ART): Data from the Massachusetts SART CORS. Fertil. Steril. 2010, 94, 1334–1340. [Google Scholar] [CrossRef]
- Lechner, L.; Bolman, C.; van Dalen, A. Definite involuntary childlessness: Associations between coping, social support and psychological distress. Hum. Reprod. 2007, 22, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, A. Voluntary or involuntary childlessness? Socio-demographic factors and childlessness intentions among childless Finnish men and women aged 25–44. Finn. Yearb. Popul. Res. 2010, 45, 5–24. [Google Scholar] [CrossRef]
- Parr, N. Childlessness among men in Australia. Popul. Res. Policy Rev. 2010, 29, 319–338. [Google Scholar] [CrossRef]
- Boivin, J.; Bunting, L.; Collins, J.A.; Nygren, K.G. International estimates of infertility prevalence and treatment-seeking: Potential need and demand for infertility medical care. Hum. Reprod. 2007, 22, 1506–1512. [Google Scholar] [CrossRef]
- Chandra, A.; Copen, C.E.; Stephen, E.H. Infertility service use in the United States: Data from the National Survey of Family Growth, 1982–2010. Natl. Health Stat. Rep. 2014, 73, 1–21. [Google Scholar]
- Herbert, D.L.; Lucke, J.C.; Dobson, A.J. Birth outcomes after spontaneous or assisted conception among infertile Australian women aged 28 to 36 years: A prospective, population-based study. Fertil. Steril. 2012, 97, 630–638. [Google Scholar] [CrossRef]
- Steures, P.; van der Steeg, J.W.; Hompes, P.G.A.; Habbema, J.D.F.; Eijkemans, M.J.C.; Broekmans, F.J.; Verhoeve, H.R.; Bossuyt, P.M.M.; van der Veen, F.; Mol, B.W.J. Intrauterine insemination with controlled ovarian hyperstimulation versus expectant management for couples with unexplained subfertility and an intermediate prognosis: A randomised clinical trial. Lancet 2006, 368, 216–221. [Google Scholar] [CrossRef]
- Collins, J.A.; Wrixon, W.; Janes, L.B.; Wilson, E.H. Treatment-Independent Pregnancy among Infertile Couples. N. Engl. J. Med. 1983, 309, 1201–1206. [Google Scholar] [CrossRef]
- Kumar, S.; Awasthi, R.T. Treatment unrelated pregnancies in infertile couples. Med. J. Armed Forces India 1999, 55, 223–225. [Google Scholar] [CrossRef][Green Version]
- Glass, R.H.; Ericsson, R.J. Spontaneous Cure of Male Infertility. Fertil. Steril. 1979, 31, 305–308. [Google Scholar] [CrossRef]
- Dunphy, B.C.; Kay, R.; Robinson, J.N.; Cooke, I.D. The placebo response of subfertile couples to attending a tertiary referral centre **Supported by a Harris Birthright Grant, Royal College of Obstetricians and Gynaecologists, London, United Kingdom. Fertil. Steril. 1990, 54, 1072–1075. [Google Scholar] [CrossRef]
- De Cicco, S.; Tagliaferri, V.; Selvaggi, L.; Romualdi, D.; Di Florio, C.; Immediata, V.; Lanzone, A.; Guido, M. Expectant management may reduce overtreatment in women affected by unexplained infertility confirmed by diagnostic laparoscopy. Arch. Gynecol. Obstet. 2017, 295, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Pinborg, A.; Hougaard, C.O.; Nyboe Andersen, A.; Molbo, D.; Schmidt, L. Prospective longitudinal cohort study on cumulative 5-year delivery and adoption rates among 1338 couples initiating infertility treatment. Hum. Reprod. 2009, 24, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, C.M.; Liu, E.; Armstrong, S.; Arroll, N.; Lensen, S.; Brown, J. Intrauterine insemination with ovarian stimulation versus expectant management for unexplained infertility (TUI): A pragmatic, open-label, randomised, controlled, two-centre trial. Lancet 2018, 391, 441–450. [Google Scholar] [CrossRef]
- Hajder, M.; Hajder, E.; Husic, A. The effects of total motile sperm count on spontaneous pregnancy rate and pregnancy after IUI treatment in couples with male factor and unexplained infertility. Med. Arch. 2016, 70, 39. [Google Scholar] [CrossRef]
- Van Eekelen, R.; van Geloven, N.; van Wely, M.; McLernon, D.J.; Mol, F.; Custers, I.M.; Steures, P.; Bhattacharya, S.; Mol, B.W.; van der Veen, F.; et al. Is IUI with ovarian stimulation effective in couples with unexplained subfertility? Hum. Reprod. 2018, 34, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Cocuzza, M.; Cocuzza, M.A.; Bragais, F.M.P.; Agarwal, A. The role of varicocele repair in the new era of assisted reproductive technology. Clinics 2008, 63, 395–404. [Google Scholar] [CrossRef]
- Evers, J.L.H.; Collins, J.A. Assessment of efficacy of varicocele repair for male subfertility: A systematic review. Lancet 2003, 361, 1849–1852. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, Z.; Cui, W.; Yuan, Y.; Song, W.; Gao, B.; Xin, Z.; Zhu, S. Spontaneous pregnancy rates in Chinese men undergoing microsurgical subinguinal varicocelectomy and possible preoperative factors affecting the outcomes. Fertil. Steril. 2015, 103, 635–639. [Google Scholar] [CrossRef]
- Abdel-Meguid, T.A.; Al-Sayyad, A.; Tayib, A.; Farsi, H.M. Does Varicocele Repair Improve Male Infertility? An. Evidence-Based Perspective from a Randomized, Controlled Trial. Eur. Urol. 2011, 59, 455–461. [Google Scholar] [CrossRef]
- Matthews, G.J.; Matthews, E.D.; Goldstein, M. Induction of spermatogenesis and achievement of pregnancy after microsurgical varicocelectomy in men with azoospermia and severe oligoasthenospermia. Fertil. Steril. 1998, 70, 71–75. [Google Scholar] [CrossRef]
- Ren, W.; Qu, J.; Xue, B.; Hu, J.; Zu, X. Infertility duration and pre-operative sperm progressive motility are significant factors of spontaneous pregnancy after varicocele repair. Am. J. Reprod. Immunol. 2020, e13318. [Google Scholar] [CrossRef]
- Dubin, J.M.; Greer, A.B.; Kohn, T.P.; Masterson, T.A.; Ji, L.; Ramasamy, R. Men with Severe Oligospermia Appear to Benefit From Varicocele Repair: A Cost-effectiveness Analysis of Assisted Reproductive Technology. Urology 2018, 111, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Çayan, S.; Akbay, E. Fate of Recurrent or Persistent Varicocele in the Era of Assisted Reproduction Technology: Microsurgical Subinguinal Redo Varicocelectomy Versus Observation. Urology 2018, 117, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Masterson, T.A.; Greer, A.B.; Ramasamy, R. Time to improvement in semen parameters after microsurgical varicocelectomy in men with severe oligospermia. Can. Urol. Assoc. J. 2019, 13, E66–E69. [Google Scholar] [CrossRef]
- Bahadur, G.; Homburg, R.; Muneer, A.; Racich, P.; Alangaden, T.; Al-Habib, A.; Okolo, S. First line fertility treatment strategies regarding IUI and IVF require clinical evidence. Hum. Reprod. 2016, 31, 1141–1146. [Google Scholar] [CrossRef]
- Tjon-Kon-Fat, R.I.; Bensdorp, A.J.; Scholten, I.; Repping, S.; van Wely, M.; Mol, B.W.J.; van der Veen, F. IUI and IVF for unexplained subfertility: Where did we go wrong? Hum. Reprod. 2016, 31, 2665–2667. [Google Scholar] [CrossRef][Green Version]
- Malchau, S.S.; Henningsen, A.A.; Loft, A.; Rasmussen, S.; Forman, J.; Nyboe Andersen, A.; Pinborg, A. The long-term prognosis for live birth in couples initiating fertility treatments. Hum. Reprod. 2017, 32, 1439–1449. [Google Scholar] [CrossRef]
- Hendin, B.N.; Falcone, T.; Hallak, J.; Nelson, D.R.; Vemullapalli, S.; Goldberg, J.; Thomas, A.J.; Agarwal, A. The Effect of Patient and Semen Characteristics on Live Birth Rates Following Intrauterine Insemination: A Retrospective Study. J. Assist. Reprod. Genet. 2000, 17, 245–252. [Google Scholar] [CrossRef]
- Erdem, A.; Erdem, M.; Atmaca, S.; Korucuoglu, U.; Karabacak, O. Factors affecting live birth rate in intrauterine insemination cycles with recombinant gonadotrophin stimulation. Reprod. Biomed. Online 2008, 17, 199–206. [Google Scholar] [CrossRef]
- Ohannessian, A.; Loundou, A.; Gnisci, A.; Paulmyer-Lacroix, O.; Perrin, J.; Courbiere, B. Unexplained infertility: Live-birth’s prognostic factors to determine the ART management. Minerva Ginecol. 2017, 69, 526–532. [Google Scholar] [PubMed]
- Reindollar, R.H.; Regan, M.M.; Neumann, P.J.; Levine, B.S.; Thornton, K.L.; Alper, M.M.; Goldman, M.B. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: The fast track and standard treatment (FASTT) trial. Fertil. Steril. 2010, 94, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Thoma, M.E.; McLain, A.C.; Louis, J.F.; King, R.B.; Trumble, A.C.; Sundaram, R.; Buck Louis, G.M. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil. Steril. 2013, 99, 1324–1331.e1. [Google Scholar] [CrossRef] [PubMed]
- McLernon, D.J.; Maheshwari, A.; Lee, A.J.; Bhattacharya, S. Cumulative live birth rates after one or more complete cycles of IVF: A population-based study of linked cycle data from 178 898 women. Hum. Reprod. 2016, 31, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Tilling, K.; Nelson, S.M.; Lawlor, D.A. Live-Birth Rate Associated With Repeat In Vitro Fertilization Treatment Cycles. JAMA 2015, 314, 2654–2662. [Google Scholar] [CrossRef]
- Malizia, B.A.; Hacker, M.R.; Penzias, A.S. Cumulative Live-Birth Rates after In Vitro Fertilization. N. Engl. J. Med. 2009, 360, 236–243. [Google Scholar] [CrossRef]
- Chambers, G.M.; Paul, R.C.; Harris, K.; Fitzgerald, O.; Boothroyd, C.V.; Rombauts, L.; Chapman, M.G.; Jorm, L. Assisted reproductive technology in Australia and New Zealand: Cumulative live birth rates as measures of success. Med. J. Aust. 2017, 207, 114–118. [Google Scholar] [CrossRef]
- Yeh, J.S.; Steward, R.G.; Dude, A.M.; Shah, A.A.; Goldfarb, J.M.; Muasher, S.J. Pregnancy rates in donor oocyte cycles compared to similar autologous in vitro fertilization cycles: An analysis of 26,457 fresh cycles from the Society for Assisted Reproductive Technology. Fertil. Steril. 2014, 102, 399–404. [Google Scholar] [CrossRef]
- Baudin, T.; De La Croix, D.; Gobbi, P.E. Fertility and childlessness in the United States. Am. Econ. Rev. 2015, 105, 1852–1882. [Google Scholar] [CrossRef]
- Collins, J. Cost-effectiveness of in vitro fertilization. Semin. Reprod. Med. 2001, 19, 279–289. [Google Scholar] [CrossRef]
- Chambers, G.M.; Sullivan, E.A.; Ishihara, O.; Chapman, M.G.; Adamson, G.D. The economic impact of assisted reproductive technology: A review of selected developed countries. Fertil. Steril. 2009, 91, 2281–2294. [Google Scholar] [CrossRef] [PubMed]
- Katz, P.; Showstack, J.; Smith, J.F.; Nachtigall, R.D.; Millstein, S.G.; Wing, H.; Eisenberg, M.L.; Pasch, L.A.; Croughan, M.S.; Adler, N. Costs of infertility treatment: Results from an 18-month prospective cohort study. Fertil. Steril. 2011, 95, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, S.; Boivin, J.; Peronace, L.; Verhaak, C.M. Why do patients discontinue fertility treatment? A systematic review of reasons and predictors of discontinuation in fertility treatment. Hum. Reprod. Update 2012, 18, 652–669. [Google Scholar] [CrossRef] [PubMed]
- Domar, A.D.; Smith, K.; Conboy, L.; Iannone, M.; Alper, M. A prospective investigation into the reasons why insured United States patients drop out of in vitro fertilization treatment. Fertil. Steril. 2010, 94, 1457–1459. [Google Scholar] [CrossRef] [PubMed]
- Perkins, K.M.; Boulet, S.L.; Jamieson, D.J.; Kissin, D.M. Trends and outcomes of gestational surrogacy in the United States. Fertil. Steril. 2016, 106, 435–442.e2. [Google Scholar] [CrossRef] [PubMed]




| Total Population | Involuntarily Childless Prevalence | Method | Location | Year(s) | Study |
|---|---|---|---|---|---|
| N/A | 1–5% of women * | Data Analysis 1 | N/A | N/A | [37] |
| N/A | 2.1% of women | Surveys | USA | 2011–2015 | [43] |
| N/A | 1–9% of women ** | Data Analysis 2 | USA | 1982, 1988, 1995, 2002 | [44] |
| 3141 | 3% of women | Surveys | UK | 1993 | [45] |
| 1574 | 3% of women | Data Analysis 3 | UK | 1990 | [46] |
| N/A | 5–10% of women | Data Analysis 4 | N/A | N/A | [47] |
| N/A | 3.6% of women | Data Analysis 5 | Iran | 2010 | [48] |
| 3.9% | Average |
| Number of Patients | Successful Pregnancy/Live Birth | Treatment Failure | Location | Study |
|---|---|---|---|---|
| 654 | 343 live births | 48% | Australia | [75] |
| 107 | 35 pregnancies | 67% | Netherlands | [76] |
| 548 | 191 live births | 65% | United States | [77] |
| 108 | 28 pregnancies | 74% | United States | [78] |
| 16 | 4 pregnancies | 75% | United States | [79] |
| 126 | 27 pregnancies | 78% | United Kingdom | [80] |
| 100 | 14 pregnancies | 86% | Germany | [81] |
| 817 | 54 live births | 93% | Denmark | [82] |
| 100 | 9 live births | 91% | New Zealand | [83] |
| 98 | 42 pregnancies | 57% | Bosnia and Herzegovina | [84] |
| 1096 | 386 pregnancies | 65% | Denmark | [85] |
| Total: 3770 | 70% | Weighted Average |
| Patient Number | Treatment: | Successful Pregnancy/Live Birth | Treatment Failure | Location | Study |
|---|---|---|---|---|---|
| 281 | Varicocele Repair | 61 pregnancies | 78% | N/A | [87] |
| 145 | Varicocele Repair | 66 pregnancies | 54% | China | [88] |
| 73 | Varicocele Repair | 24 pregnancies | 67% | N/A | [89] |
| 78 | Varicocele Repair | 15 pregnancies | 81% | USA | [90] |
| 148 | Varicocele Repair | 74 pregnancies | 50% | China | [91] |
| 10 | Varicocele Repair | 1 pregnancy | 90% | USA | [92] |
| 120 | Varicocele Repair | 25 pregnancies | 79% | Turkey | [93] |
| 20 | Varicocele Repair | 5 pregnancies | 75% | USA | [94] |
| Total: 857 | 69% | Weighted Average | |||
| 12,488 | IUI | 4271 live births | 66% | Denmark | [97] |
| 533 | IUI | 111 live births | 79% | USA | [98] |
| 456 | IUI 1 | 96 live births | 79% | Turkey | [99] |
| 133 | IUI | 50 live births | 62% | France | [100] |
| 475 | CC/IUI | 92 live births | 81% | USA | [101] |
| 169 | FSH/IUI | 37 live births | 78% | USA | [101] |
| 817 | IUI | 81 live births | 90% | Denmark | [82] |
| 101 | IUI 2 | 31 live births | 69% | New Zealand | [83] |
| Total: 15,172 | 69% | Weighted Average |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Royfman, R.; Shah, T.A.; Sindhwani, P.; Nadiminty, N.; Avidor-Reiss, T. Sterility, an Overlooked Health Condition. Women 2021, 1, 29-45. https://doi.org/10.3390/women1010003
Royfman R, Shah TA, Sindhwani P, Nadiminty N, Avidor-Reiss T. Sterility, an Overlooked Health Condition. Women. 2021; 1(1):29-45. https://doi.org/10.3390/women1010003
Chicago/Turabian StyleRoyfman, Rachel, Tariq A. Shah, Puneet Sindhwani, Nagalakshmi Nadiminty, and Tomer Avidor-Reiss. 2021. "Sterility, an Overlooked Health Condition" Women 1, no. 1: 29-45. https://doi.org/10.3390/women1010003
APA StyleRoyfman, R., Shah, T. A., Sindhwani, P., Nadiminty, N., & Avidor-Reiss, T. (2021). Sterility, an Overlooked Health Condition. Women, 1(1), 29-45. https://doi.org/10.3390/women1010003








