Gallic Acid Functionalization Improves the Pharmacological Profile of Fucoidan B: A Polysaccharide with Antioxidant Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Collection and Extraction of FucB from Spatoglossum Schröederi
2.3. Conjugation with Gallic Acid
2.4. Physicochemical Analyses
2.4.1. Quantification of Sulfate, Protein, and Phenolic Compounds
2.4.2. Molecular Weight Determination
2.4.3. Agarose Gel Electrophoresis Analyses
2.4.4. Infrared Spectroscopy (FT-IR)
2.4.5. Nuclear Magnetic Resonance (NMR)
2.4.6. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray Spectroscopy (EDS)
2.4.7. X-Ray Diffraction (XRD)
2.4.8. Thermogravimetric Analysis (TGA)
2.5. Antioxidant Activity
2.5.1. Hydrogen Peroxide Scavenging Assay
2.5.2. Superoxide Anion Scavenging Assay
2.5.3. Ferrous Ion (Fe2+) Chelation Assay
2.5.4. Copper (Cu2+) Chelation Assay
2.5.5. Total Antioxidant Capacity (TAC) Assay
2.5.6. Reducing Power Assay
2.6. Language Editing
2.7. Statistical Analysis
3. Results and Discussion
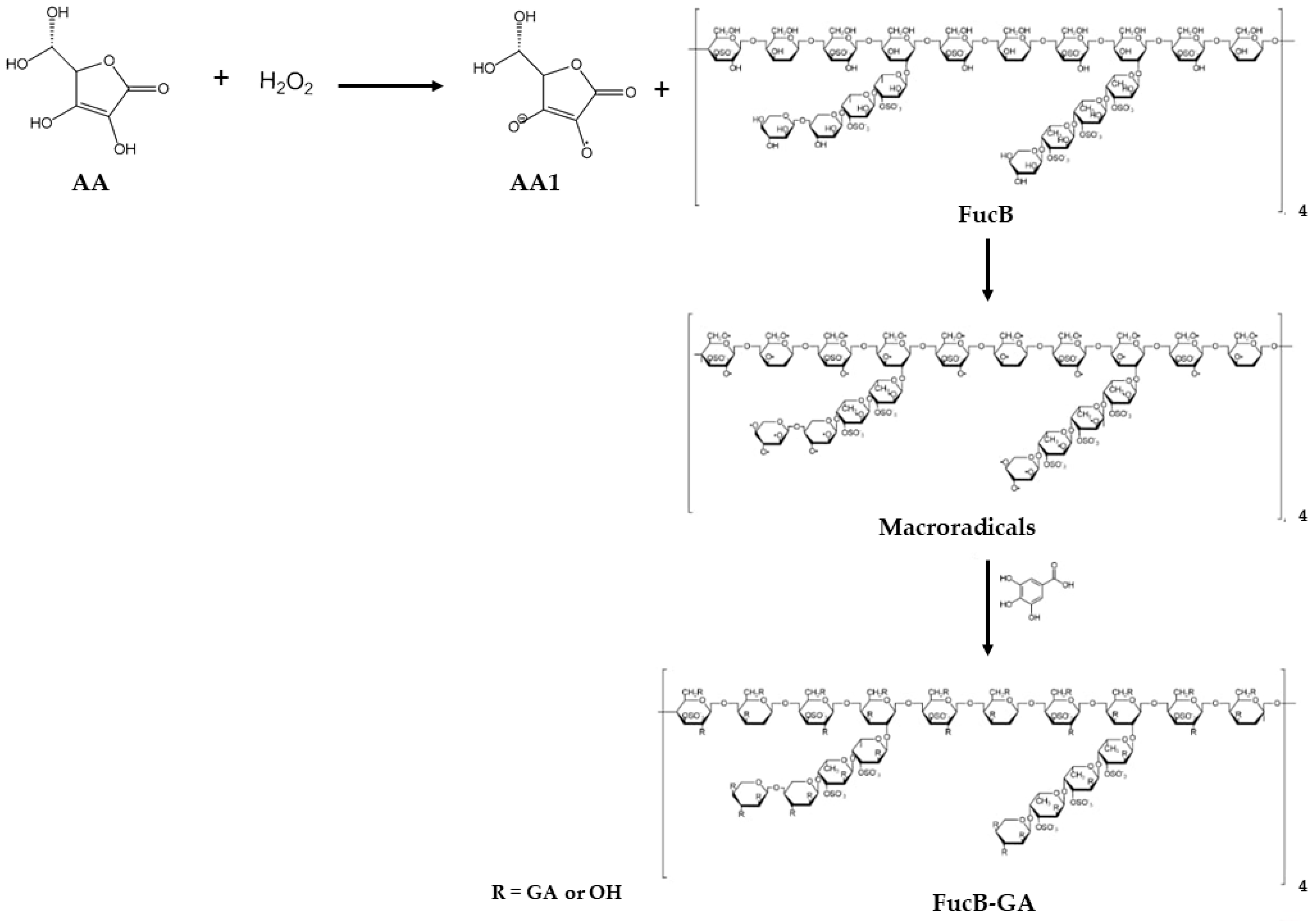
3.1. Method for Modifying FucB with GA
3.2. Physicochemical Characterization of FucB and FucB-GA
3.2.1. Chemical Composition
| Samples | Protein (%) | Phenolic Compounds (%) | Sulfate (%) | Molecular Mass (kDa) |
|---|---|---|---|---|
| FucB | nd | * 0.1 ± 0.1 | # 23.8 ± 1.6 | 21.5 |
| FucB-GA | nd | * 2.8 ± 0.1 | # 26.8 ± 1.5 | 22.8 |
3.2.2. Agarose Gel Electrophoresis
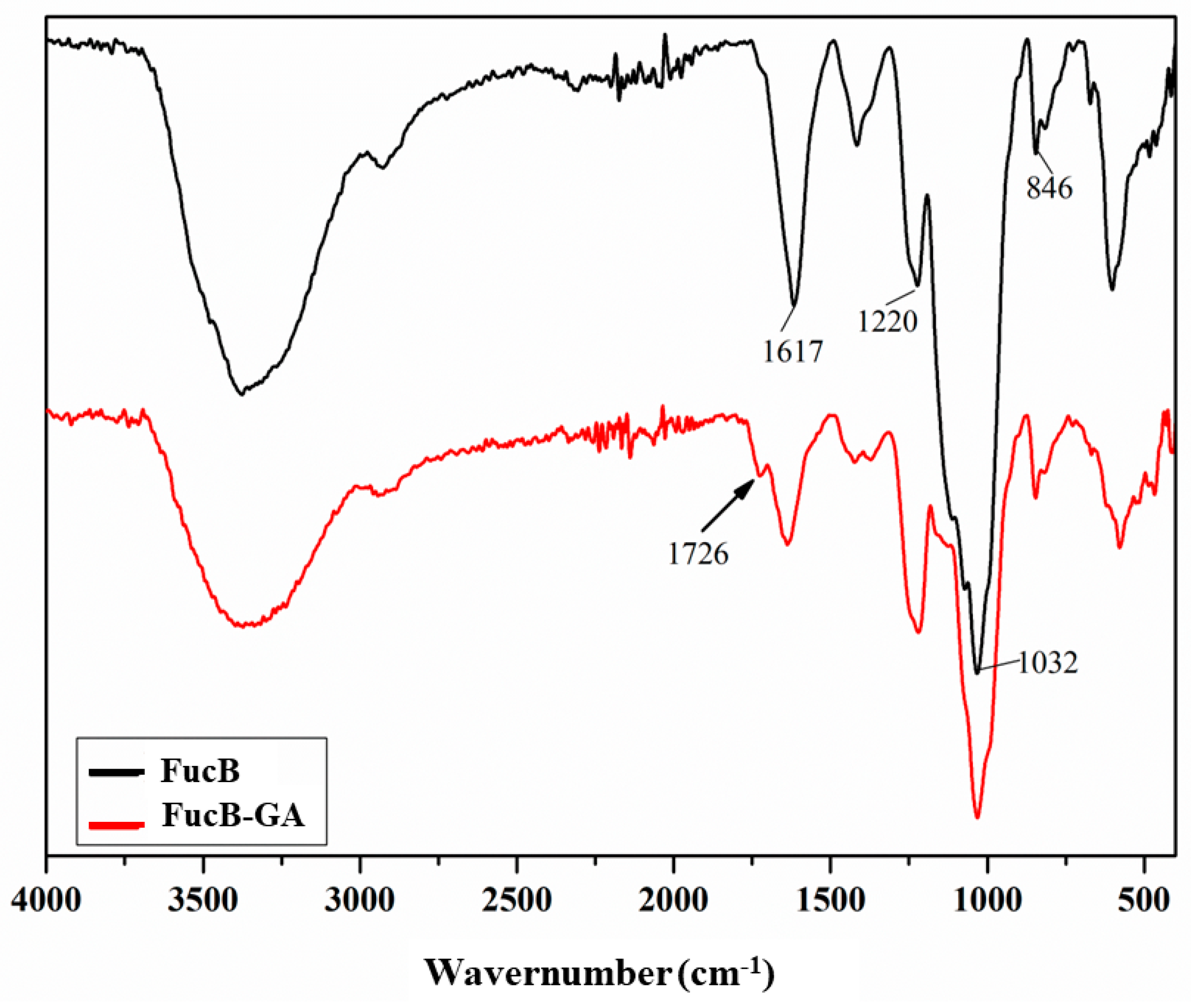
3.2.3. Infrared Spectroscopy (FTIR)
3.2.4. Nuclear Magnetic Resonance (NMR)
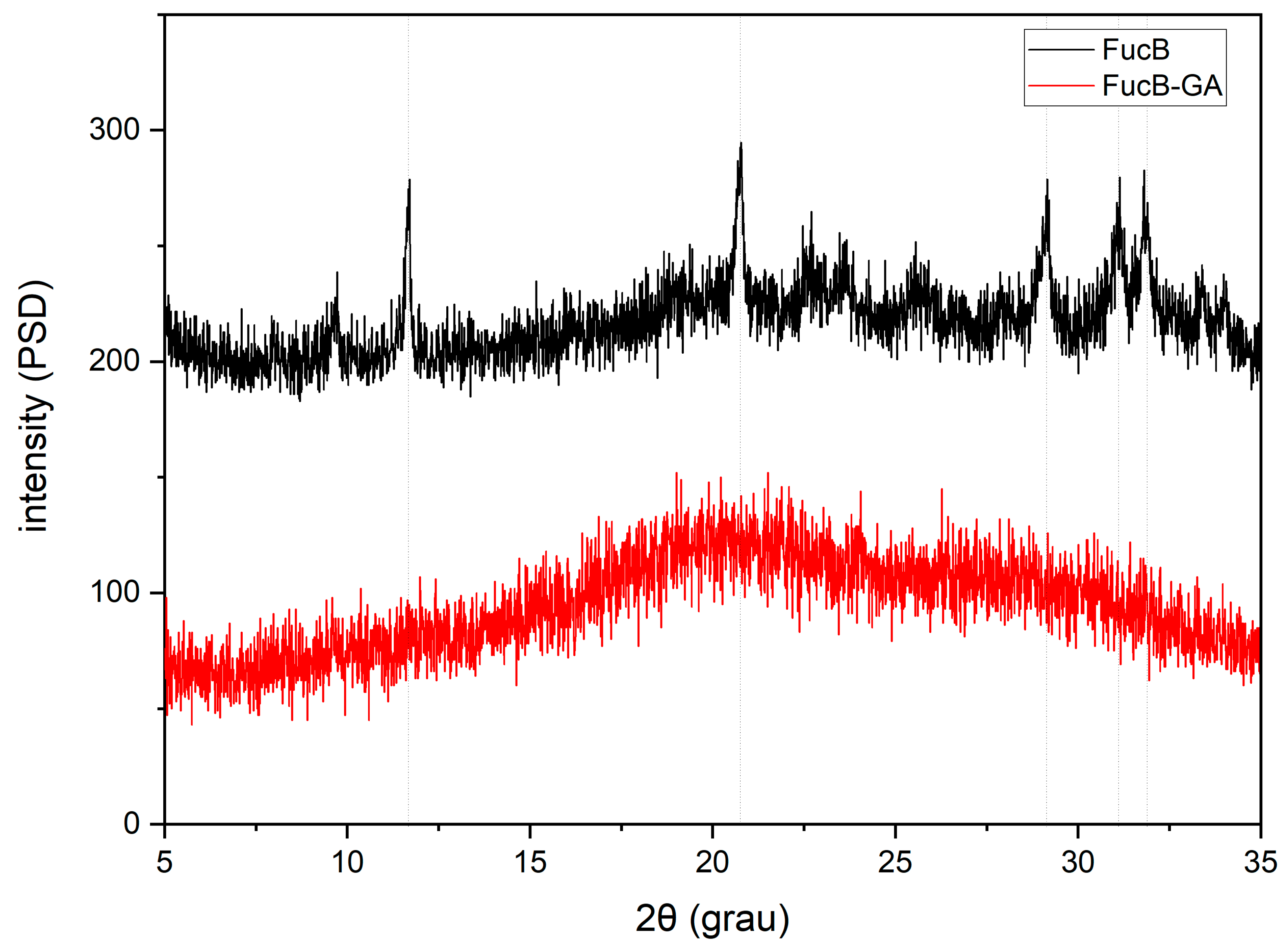
3.2.5. X-Ray Diffraction (XRD)
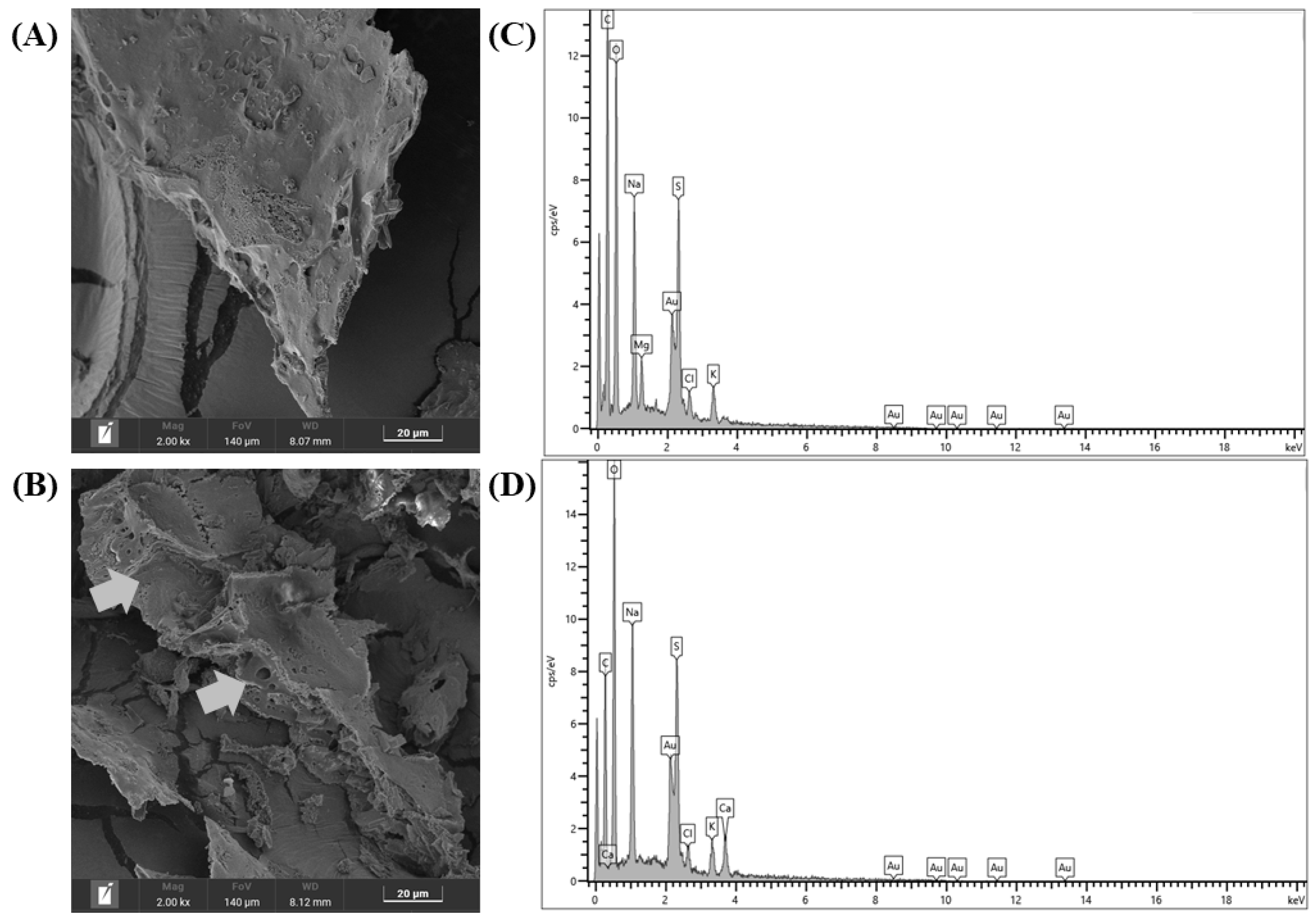
3.2.6. SEM and EDS Analyses
3.2.7. Thermogravimetric Analysis (TG/DTG)
3.3. In Vitro Antioxidant Activities
3.3.1. Hydrogen Peroxide Scavenging
3.3.2. Superoxide Radical Scavenging
3.3.3. Chelation of Ferric and Cupric Ions
3.3.4. Total Antioxidant Capacity (TAC)
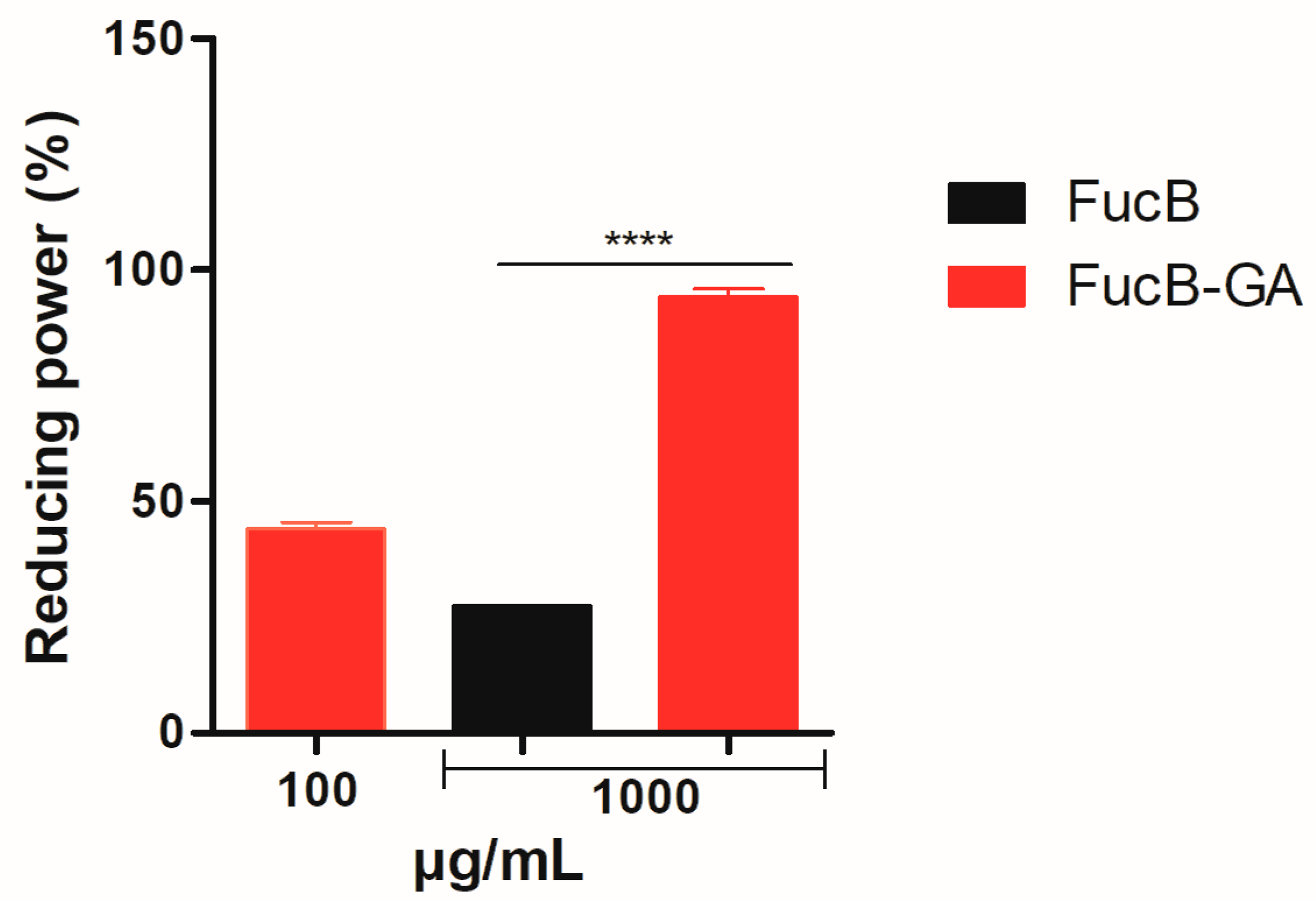
3.3.5. Reducing Power
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| XRD | X-ray diffraction |
| FTIR | Fourier-transform infrared spectroscopy |
| TG/DTG | Thermogravimetric analysis |
| EDS | Energy-dispersive X-ray spectroscopy |
| FucB | Fucoidan purified from brown seaweed Spatoglossum schröederi |
| FucB-GA | Fucoidan–gallic acid conjugate |
| NMR | Nuclear magnetic resonance |
| ROS | Reactive oxygen species |
| SP | Sulfated polysaccharide |
| GA | Gallic acid |
| SEM | Scanning electron microscopy |
| NBT | Nitro blue tetrazolium |
| EDTA | 2,2′,2″,2′′′-(Ethane-1,2-diyldinitrilo) tetra-acetic acid |
| PDA | 1,3-diaminopropane-acetate buffer |
| UFRN | Federal University of Rio Grande do Norte |
| TAC | Total antioxidant capacity |
| ANOVA | Analysis of variance |
References
- Zhang, L.; Wang, X.; Cueto, R.; Effi, C.; Zhang, Y.; Tan, H.; Qin, X.; Yang, X.; Wang, H. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 2019, 26, 101284. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Song, X.; Wang, Y.; Wang, J.; Su, S.; Zhu, J.; Geng, Y. Studies on protection of astaxanthin from oxidative damage induced by H2O2 in RAW 264.7 cells based on 1H NMR metabolomics. J. Agric. Food Chem. 2019, 67, 13568–13576. [Google Scholar] [CrossRef] [PubMed]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A brief overview on antioxidant activity determination of silver nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Begum, R.; Howlader, S.; Mamun-Or-Rashid, A.N.M.; Rafiquzzaman, S.M.; Ashraf, G.M.; Albadrani, G.M.; Sayed, A.A.; Peluso, I.; Abdel-Daim, M.M.; Uddin, S. Antioxidant and signal-modulating effects of brown seaweed-derived compounds against oxidative stress-associated pathology. Oxid. Med. Cell. Longev. 2021, 2021, 9974890. [Google Scholar] [CrossRef]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic effects of fucoidan: A review on recent studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [PubMed]
- Deniaud-Bouët, E.; Hardouin, K.; Potin, P.; Kloareg, B.; Hervé, C. A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carb. Polym. 2017, 175, 395–408. [Google Scholar] [CrossRef]
- Rocha, H.A.O.; Franco, C.R.; Trindade, E.S.; Carvalho, L.C.; Veiga, S.S.; Leite, E.L.; Dietrich, C.P.; Nader, H.B. A fucan from the brown seaweed Spatoglossum schröederi inhibits chinese hamster ovary cell adhesion to several extracellular matrix proteins. Braz. J. Med. Biol. Res. 2001, 34, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Gamal-Eldeen, A.M.; Ahmed, E.F.; Abo-Zeid, M.A. In vitro cancer chemopreventive properties of polysaccha-ride extract from the brown alga, Sargassum latifolium. Food Chem. Toxicol. 2009, 47, 1378–1384. [Google Scholar] [CrossRef]
- Silva, T.M.A.; Alves, L.G.; Queiroz, K.C.S.; Santos, M.G.L.; Marques, C.T.; Chavante, S.F.; Rocha, H.A.O.; Leite, E.L. Partial characterization and anticoagulant activity of a heterofucan from the brown seaweed Padina gymnospora. Braz. J. Med. Biol. Res. 2005, 38, 523–533. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman Al, E.; Bilian, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef]
- Sun, Y.; Hou, S.; Song, S.; Zhang, B.; Ai, C.; Chen, X.; Liu, N. Impact of acidic, water and alkaline extraction on structural features, antioxidant activities of Laminaria japonica polysaccharides. Int. J. Biol. Macromol. 2018, 112, 985–995. [Google Scholar] [CrossRef]
- Zhao, T.; Yang, M.; Ma, L.; Liu, X.; Ding, Q.; Chai, G.; Lu, Y.; Wei, H.; Zhang, S.; Ding, C. Structural modifi-cation and biological activity of polysaccharides. Molecules 2023, 28, 5416. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Yong, H.; Kan, J.; Jin, C. Recent advances in flavonoid-grafted polysaccharides: Synthesis, struc-tural characterization, bioactivities and potential applications. Int. J. Biol. Macromol. 2018, 116, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Boundaoui, K.; Le Cerf, D.; Dulong, V. Functionalisation and behaviours of polysaccharides conjugated with phenolic compounds by oxidoreductase catalysis: A review. Int. J. Biol. Macromol. 2024, 283, 137660. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, J.; Li, Q.; Liu, C.; Niu, F.; Yue, R.; Zhang, Y.; Zhu, H.; Ma, C.; Deng, S. Free radical-mediated grafting of natural polysaccharides such as chitosan, starch, inulin, and pectin with some polyphenols: Synthesis, structural characterization, bioactivities, and applications–A review. Foods 2023, 12, 3688. [Google Scholar] [CrossRef]
- Curcio, M.; Puoci, F.; Iemma, F.; Parisi, O.I.; Cirillo, G.; Spizzirri, U.G.; Picci, N. Covalent insertion of antioxidant molecules on chitosan by a free radical grafting procedure. J. Agric. Food Chem. 2009, 57, 5933–5938. [Google Scholar] [CrossRef] [PubMed]
- Melo, K.D.C.M.; Lisboa, L.S.; Queiroz, M.F.; Paiva, W.S.; Luchiari, A.C.; Camara, R.B.G.; Costa, L.S.; Rocha, H.A.O. Antioxidant activity of fucoidan modified with gallic acid using the redox method. Mar. Drugs 2022, 20, 490. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.F.; Zhang, H.Y.; Shen, L. Proton dissociation is important to understanding structure–activity relationships of gallic acid antioxidants. Bioorg. Med. Chem. Lett. 2006, 16, 4095–4098. [Google Scholar] [CrossRef]
- Kim, Y.J. Antimelanogenic and antioxidant properties of gallic acid. Biol. Pharm. Bull. 2007, 30, 1052–1055. [Google Scholar] [CrossRef]
- Couto, A.; Kassuya, C.; Calixto, J.B.; Petrovick, P. R Anti-inflammatory, antiallodynic effects and quantitative analysis of gallic acid in spray dried powders from Phyllanthus niruri leaves, stems, roots and whole plant. Rev. Bras. Farmacogn. 2013, 23, 124–131. [Google Scholar] [CrossRef]
- Xiang, Z.; Guan, H.; Zhao, X.; Xie, Q.; Xie, Z.; Cai, F.; Dang, R.; Li, M.; Wang, C. Dietary gallic acid as an antioxidant: A review of its food industry applications, health benefits, bioavailability, nano-delivery systems, and drug interactions. Food Res. Int. 2021, 180, 114068. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Negreiros, M.M.; Batista, L.A.N.C.; Viana, R.L.S.; Sabry, D.A.; Paiva, A.A.O.; Paiva, W.S.; Machado, R.I.A.; Sousa Junior, F.L.; Pontes, D.L.; Vitoriano, J.O.; et al. Gallic acid-laminarin conjugate is a better antioxidant than sulfated or carboxylated laminarin. Antioxidants 2020, 9, 1192. [Google Scholar] [CrossRef]
- Rocha, H.A.O.; Bezerra, L.C.L.M.; Albuquerque, I.R.L.; Costa, L.S.; Guerra, C.M.P.; Abreu, L.D.; Nader, H.B.; Leite, E.L. A xylogalactofucan from the brown seaweed Spatoglossum schröederi stimulates the synthesis of an an-tithrombotic heparan sulfate from endothelial cells. Planta Med. 2005, 71, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Wynne, M.J. A checklist of benthic marine algae of the tropical and subtropical western Atlantic. Can. J. Bot. 1986, 64, 2239–2281. [Google Scholar] [CrossRef]
- Rocha, H.A.O.; Morass, F.A.; Trindade, E.S.; Franco, C.R.C.; Torquato, R.J.S.; Veiga, S.S.; Valente, A.P.; Mourão, P.A.S.; Leite, E.L.; Nader, H.B.; et al. Structural and hemostatic activities of a sulfated galactofucan from the brown alga Spatoglossum schroederi. J. Biol. Chem. 2005, 280, 41278–41288. [Google Scholar] [CrossRef]
- Paiva, W.S.; Queiroz, M.F.; Araujo Sabry, D.; Santiago, A.L.C.M.A.; Sassaki, G.L.; Batista, A.C.L.; Rocha, H.A.O. Preparation, structural characterization, and property investigation of gallic acid-grafted fungal chitosan conjugate. J. Fungi 2021, 7, 812. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitiv. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Dietrich, C.P.; Dietrich, S.M.C. Electrophoretic behaviour of acidic mucopolysaccharides in diamine buffers. Anal. Biochem. 1976, 70, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Melo, K.R.T.; Camara, R.B.G.; Queiroz, M.F.; Vidal, A.A.J.; Lima, C.R.M.; Melo-Silveira, R.F.; Almeida-Lima, L.; Rocha, H.A.O. Evaluation of sulfated polysaccharides from the brown seaweed Dictyopteris justii as antioxidant agents and as inhibitors of the formation of calcium oxalate crystals. Molecules 2013, 18, 14543–14563. [Google Scholar] [CrossRef]
- Soeiro, V.C.; Melo, K.R.T.; Alves, M.G.C.F.; Medeiros, M.J.C.; Grilo, M.L.P.M.; Almeida-Lima, J.; Pontes, D.L.; Costa, L.S.; Rocha, H.A.O. Dextran: Influence of molecular weight in antioxidant properties and immunomodulatory potential. Int. J. Mol. Sci. 2016, 17, 1340. [Google Scholar] [CrossRef] [PubMed]
- Arizmendi-Cotero, D.; Gómez-Espinosa, R.M.; García, O.D.; Gómez-Vidales, V.; Dominguez-Lopez, A. Electron paramagnetic resonance study of hydrogen peroxide/ascorbic acid ratio as initiator redox pair in the inulin-gallic acid molecular grafting reaction. Carbohydr. Polym. 2016, 136, 350–357. [Google Scholar] [CrossRef]
- Liu, J.; Lu, J.; Kan, J.; Jin, C. Synthesis of chitosan-gallic acid conjugate: Structure characterization and in vitro anti-diabetic potential. Int. J. Biol. Macromol. 2013, 62, 321–329. [Google Scholar] [CrossRef]
- Liu, J.; Pu, H.; Chen, C.; Liu, Y.; Bai, R.; Kan, J.; Jin, C. Reaction mechanisms and structural and physicochemical properties of caffeic acid grafted chitosan synthesized in ascorbic acid and hydroxyl peroxide redox system. J. Agric. Food Chem. 2018, 66, 279–289. [Google Scholar] [CrossRef]
- Liu, T.; Ren, Q.; Wang, S.; Gao, J.; Shen, C.; Zhang, S.; Wang, Y.; Guan, F. Chemical modification of polysaccharides: A review of synthetic approaches, biological activity and the structure–activity relationship. Molecules 2023, 28, 6073. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, L.; Fang, Z.; Hu, Y.; Chen, S.; Sugawara, T.; Ye, X. The effect of the molecular architecture on the antioxidant properties of chitosan gallate. Mar. Drugs 2016, 14, 95. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O.; Costa, L.S. Gallic acid-dextran conjugate: Green syn-thesis of a novel antioxidant molecule. Antioxidants 2019, 8, 478. [Google Scholar] [CrossRef] [PubMed]
- Presa, F.B.; Marques, M.L.M.; Viana, R.L.S.; Nobre, L.T.D.B.; Costa, L.S.; Rocha, H.A.O. The protective role of sulfated polysaccharides from green seaweed Udotea flabellum in cells exposed to oxidative damage. Mar. Drugs 2018, 16, 135. [Google Scholar] [CrossRef]
- Rabenstein, D.L. Heparin and heparan sulfate: Structure and function. Nat. Prod. Rep. 2002, 19, 312–331. [Google Scholar] [CrossRef]
- Zheng, J.; Li, B.; Ji, Y.; Chen, Y.; Lv, X.; Zhang, X.; Linhardt, R.J. Prolonged release and shelf-life of anticoagulant sulfated polysaccharides encapsulated with ZIF-8. Int. J. Biol. Macromol. 2021, 183, 1174–1183. [Google Scholar] [CrossRef]
- He, W.; Sun, H.; Su, L.; Zhou, D.; Zhang, X.; Shanggui, D.; Chen, Y. Structure and anticoagulant activity of a sulfated fucan from the sea cucumber Acaudina leucoprocta. Int. J. Biol. Macromol. 2020, 164, 87–94. [Google Scholar] [CrossRef]
- Hou, L.; Wu, P. Two-dimensional correlation infrared spectroscopy of heat-induced esterification of cellulose with 1,2,3,4-butanetetracarboxylic acid in the presence of sodium hypophosphite. Cellulose 2019, 26, 2759–2769. [Google Scholar] [CrossRef]
- Yang, T.; Liu, T.; Lin, I. Functionalities of chitosan conjugated with stearic acid and gallic acid and application of the modified chitosan in stabilizing labile aroma compounds in an oil-in-water emulsion. Food Chem. 2017, 228, 541–549. [Google Scholar] [CrossRef]
- Bai, R.; Yong, H.; Zhang, X.; Liu, J.; Liu, J. Structural characterization and protective effect of gallic acid grafted O-carboxymethyl chitosan against hydrogen peroxide-induced oxidative damage. Int. J. Biol. Macromol. 2020, 143, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Kim, S.; Ahn, C.; Je, J. Preparation, characterization, and antioxidant properties of gallic acid-grafted-chitosans. Carbohydr. Polym. 2011, 83, 1617–1622. [Google Scholar] [CrossRef]
- Pasanphan, W.; Chirachanchai, S. Conjugation of gallic acid onto chitosan: An approach for green and water-based antioxidante. Carbohydr. Polym. 2008, 72, 169–177. [Google Scholar] [CrossRef]
- Ali, A.; Chiang, Y.W.; Santos, R.M. X-ray diffraction techniques for mineral characterization: A review for engineers of the fundamentals, applications, and research directions. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Daub, C.D.; Mabate, B.; Malgas, S.; Pletschke, B.I. Fucoidan from Ecklonia maxima is a powerful inhibitor of the diabetes-related enzyme, α-glucosidase. Int. J. Biol. Macromol. 2020, 151, 412–420. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, Y.-J.; Park, Y.-B.; Woo, H.-C.; Chun, B.-S. Structural, antioxidant, and emulsifying activities of fucoidan from Saccharina japonica using pressurized liquid extraction. Carbohydr. Polym. 2016, 153, 518–525. [Google Scholar] [CrossRef]
- Puhari, S.S.M.; Yuvaraj, S.; Vasudevan, V.; Ramprasath, T.; Rajkumar, P.; Arunkumar, K.; Amutha, C.; Selvam, G.S. Isolation and characterization of fucoidan from four brown algae and study of the cardioprotective effect of fucoidan from Sargassum wightii against high glucose-induced oxidative stress in H9c2 cardiomyoblast cells. J. Food Biochem. 2022, 46, 14412. [Google Scholar] [CrossRef]
- Govindan, S.; Nivethaa, E.A.K.; Saravanan, R.; Narayanan, V.; Stephen, A. Synthesis and characterization of chi-tosan–silver nanocomposite. Appl. Nanosci. 2012, 2, 299–303. [Google Scholar] [CrossRef]
- Mishra, R.K.; Majeed, A.B.A.; Banthia, A.K. Development and characterization of pectin/gelatin hydrogel mem-branes for wound dressing. Int. J. Plast. Technol. 2011, 15, 82–95. [Google Scholar] [CrossRef]
- Liu, J.; Lu, J.; Kan, J.; Tang, Y.; Jin, C. Preparation, characterization and antioxidant activity of phenolic acids grafted carboxymethyl chitosan. Preparation, characterization and antioxidant activity of phenolic acids grafted carboxymethyl chitosan. Int. J. Biol. Macromol. 2013, 62, 85–93. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, H.; Zhang, L.; Sun, Q. Horseradish peroxidase-mediated synthesis of an antioxidant gallic acid-g-chitosan derivative and its preservation application in cherry tomatoes. RSC Adv. 2018, 8, 20363–20371. [Google Scholar] [CrossRef] [PubMed]
- Arab, K.; Ghanbarzadeh, B.; Ayaseh, A.; Jahanbin, K. Extraction, purification, physicochemical properties and antioxidant activity of a new polysaccharide from Ocimum album L. seed. Int. J. Biol. Macromol. 2021, 180, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wang, T.; Zhou, M.; Xue, J.; Lu, Y. In vitro antioxidant-activity evaluation of gallic-acid-grafted chitosan conjugate synthesized by free-radical-induced grafting method. J. Agric. Food Chem. 2016, 64, 5893–5900. [Google Scholar] [CrossRef]
- Hadidi, M.; Amoli, P.I.; Jelyani, A.Z.; Hasiri, Z.; Rouhafza, A.; Ibars, A.; Khaksar, F.B.; Tabrizi, S.T. Polysaccha-rides from pineapple core as a canning by-product: Extraction optimization, chemical structure, antioxidant and functional properties. Int. J. Biol. Macromol. 2020, 163, 2357–2364. [Google Scholar] [CrossRef]
- Castaño, J.; Rodríguez-Llamazares, S.; Carrasco, C.; Bouza, R. Physical, chemical and mechanical properties of pehuen cellulosic husk and its pehuenstarch based composites. Carbohydr. Polym. 2012, 90, 1550–1556. [Google Scholar] [CrossRef]
- Hajji, M.; Hamdi, M.; Sellimi, S.; Ksouda, G.; Laouer, H.; Li, S.; Nasri, M. Structural characterization, antioxidant and antibacterial activities of a novel polysaccharide from Periploca laevigata root barks. Carbohydr. Polym. 2019, 206, 380–388. [Google Scholar] [CrossRef]
- Liu, H.; Tang, W.; Lei, S.; Zhang, Y.; Cheng, M.; Liu, Q.; Wang, W. Extraction optimization, characterization and biological activities of polysaccharide extracts from nymphaea hybrid. Int. J. Mol. Sci. 2023, 24, 8974. [Google Scholar] [CrossRef]
- Pongali, P.; Kasim, N.; Hanibah, H.; Rosli, N.H.A. Glutaryl kappa-carrageenan: Synthesis, characterization and investigation of potential as gel-based biopolymer electrolyte. Polym. Bull. 2025, 82, 5693–5715. [Google Scholar] [CrossRef]
- Goudar, N.; Vanjeri, V.N.; Dixit, S.; Hiremani, V.; Sataraddi, S.; Gasti, T.; Vootla, S.K.; Masti, S.P.; Chougale, R.B. Evaluation of multifunctional properties of gallic acid crosslinked poly (vinyl alcohol)/tragacanth gum blend films for food packaging applications. Int. J. Biol. Macromol. 2020, 158, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive oxygen species: Drivers of physiological and pathological processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Jesumani, V.; Du, H.; Pei, P.; Aslam, M.; Huang, N. Comparative study on skin protection activity of polyphenol-rich extract and polysaccharide-rich extract from Sargassum vachellianum. PLoS ONE 2020, 15, e0227308. [Google Scholar] [CrossRef]
- Silva, M.M.C.L.; Lisboa, L.S.; Paiva, W.S.; Batista, L.A.N.C.; Luchiari, A.C.; Rocha, H.A.O.; Camara, R.B.G. Comparison of in vitro and in vivo antioxidant activities of commercial fucoidans from Macrocystis pyrifera, Undaria pinnatifida, and Fucus vesiculosus. Int. J. Biol. Macromol. 2022, 216, 757–767. [Google Scholar] [CrossRef]
- Caruso, F.; Incerpi, S.; Pedersen, J.; Belli, S.; Kaur, S.; Rossi, M. Aromatic polyphenol π-π interactions with super-oxide radicals contribute to radical scavenging and can make polyphenols mimic superoxide dismutase activity. Curr. Issues Mol. Biol. 2022, 44, 5209–5220. [Google Scholar] [CrossRef]
- Wu, J. Tackle the free radicals damage in COVID-19. Nitric Oxide 2020, 102, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hong, K.; Yang, T. Functionalities of tremella fuciformis polysaccharides modified with gallic acid. Molecules 2024, 29, 5890. [Google Scholar] [CrossRef]
- Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic. Biol. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef]
- Xie, M.; Hu, B.; Wang, Y.; Zeng, X. Grafting of gallic acid onto chitosan enhances antioxidant activities and alters rheological properties of the copolymer. J. Agric. Food Chem. 2014, 62, 9128–9136. [Google Scholar] [CrossRef] [PubMed]
- Gromadzka, G.; Tarnacka, B.; Flaga, A.; Adamczyk, A. copper dyshomeostasis in neurodegenerative diseases—Therapeutic implications. Int. J. Mol. Sci. 2020, 21, 9259. [Google Scholar] [CrossRef]
- Møller, L.B.; Lenartowicz, M.; Zabot, M.T.; Josiane, A.; Burglen, L.; Bennett, C.; Riconda, D.; Fisher, R.; Janssens, S.; Mohammed, S.; et al. Clinical expression of Menkes disease in females with normal karyotype. Orphanet J. Rare Dis. 2012, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Shribman, S.; Marjot, T.; Sharif, A.; Vimalesvaran, S.; Ala, A.; Alexander, G.; Dhawan, A.; Dooley, J.; Gillett, G.T.; Kelly, D.; et al. Investigation and management of Wilson’s disease: A practical guide from the British Association for the study of the liver. Lancet Gastroenterol. Hepatol. 2022, 7, 560–575. [Google Scholar] [CrossRef]
- Brewer, G.J. Novel therapeutic approaches to the treatment of Wilson’s disease. Expert Opin. Pharmacother. 2006, 7, 317–324. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O.; Costa, L.S. Gallic acid-chitosan conjugate inhibits the formation of calcium oxalate crystals. Molecules 2019, 24, 2074. [Google Scholar] [CrossRef] [PubMed]
- Camara, R.B.G.; Costa, L.S.; Fidelis, G.P.; Nobre, L.T.D.B.; Dantas-Santos, N.; Cordeiro, S.L.; Costa, M.S.S.P.; Alves, L.G.; Rocha, H.A.O. Heterofucans from the brown seaweed Canistrocarpus cervicornis with anticoagulant and antioxidant activities. Mar. Drugs 2011, 9, 124–138. [Google Scholar] [CrossRef]
- Rodrigues-Souza, I.; Pessatti, J.B.K.; da Silva, L.R.; Bellan, D.d.L.; de Souza, I.R.; Cestari, M.M.; de Assis, H.C.S.; Rocha, H.A.O.; Simas, F.F.; Trindade, E.d.S.; et al. Protective potential of sulfated polysaccharides from tropical seaweeds against alkylating- and oxidizing-induced genotoxicity. Int. J. Biol. Macromol. 2022, 211, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Petchev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and anti-inflammatory effects of fucoidan: A review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.R.d.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Gallic Acid Functionalization Improves the Pharmacological Profile of Fucoidan B: A Polysaccharide with Antioxidant Properties. Polysaccharides 2025, 6, 89. https://doi.org/10.3390/polysaccharides6040089
Santos JRd, Sabry DA, Sassaki GL, Rocha HAO. Gallic Acid Functionalization Improves the Pharmacological Profile of Fucoidan B: A Polysaccharide with Antioxidant Properties. Polysaccharides. 2025; 6(4):89. https://doi.org/10.3390/polysaccharides6040089
Chicago/Turabian StyleSantos, Joicy Ribeiro dos, Diego Araujo Sabry, Guilherme Lanzi Sassaki, and Hugo Alexandre Oliveira Rocha. 2025. "Gallic Acid Functionalization Improves the Pharmacological Profile of Fucoidan B: A Polysaccharide with Antioxidant Properties" Polysaccharides 6, no. 4: 89. https://doi.org/10.3390/polysaccharides6040089
APA StyleSantos, J. R. d., Sabry, D. A., Sassaki, G. L., & Rocha, H. A. O. (2025). Gallic Acid Functionalization Improves the Pharmacological Profile of Fucoidan B: A Polysaccharide with Antioxidant Properties. Polysaccharides, 6(4), 89. https://doi.org/10.3390/polysaccharides6040089








