Development and Evaluation of Modified Dioscorea hispida Starch as a Sustainable Super-Disintegrant for Immediate-Release Tablets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Starch Modification
2.2.1. Preparation of Natural Starch (NS)
2.2.2. Preparation of Carboxymethyl Starch (CMS)
2.2.3. Preparation of Modified Starch (MS)
2.3. Physicochemical Properties and Characterization
2.3.1. Thermal Analysis
2.3.2. Fourier Transform Infrared Spectroscopy—Attenuated Total Reflectance (FTIR–ATR) Characterization
2.3.3. Water Uptake and Bulk Swelling
2.3.4. Sedimentation Volume
2.3.5. Bulk Density and Tapped Density
2.3.6. Viscosity
2.3.7. Morphology
2.4. Evaluation of Disintegrating Properties
2.4.1. Determination of Optimal Disintegrant Concentration
2.4.2. Swelling Behavior of Starch Tablets
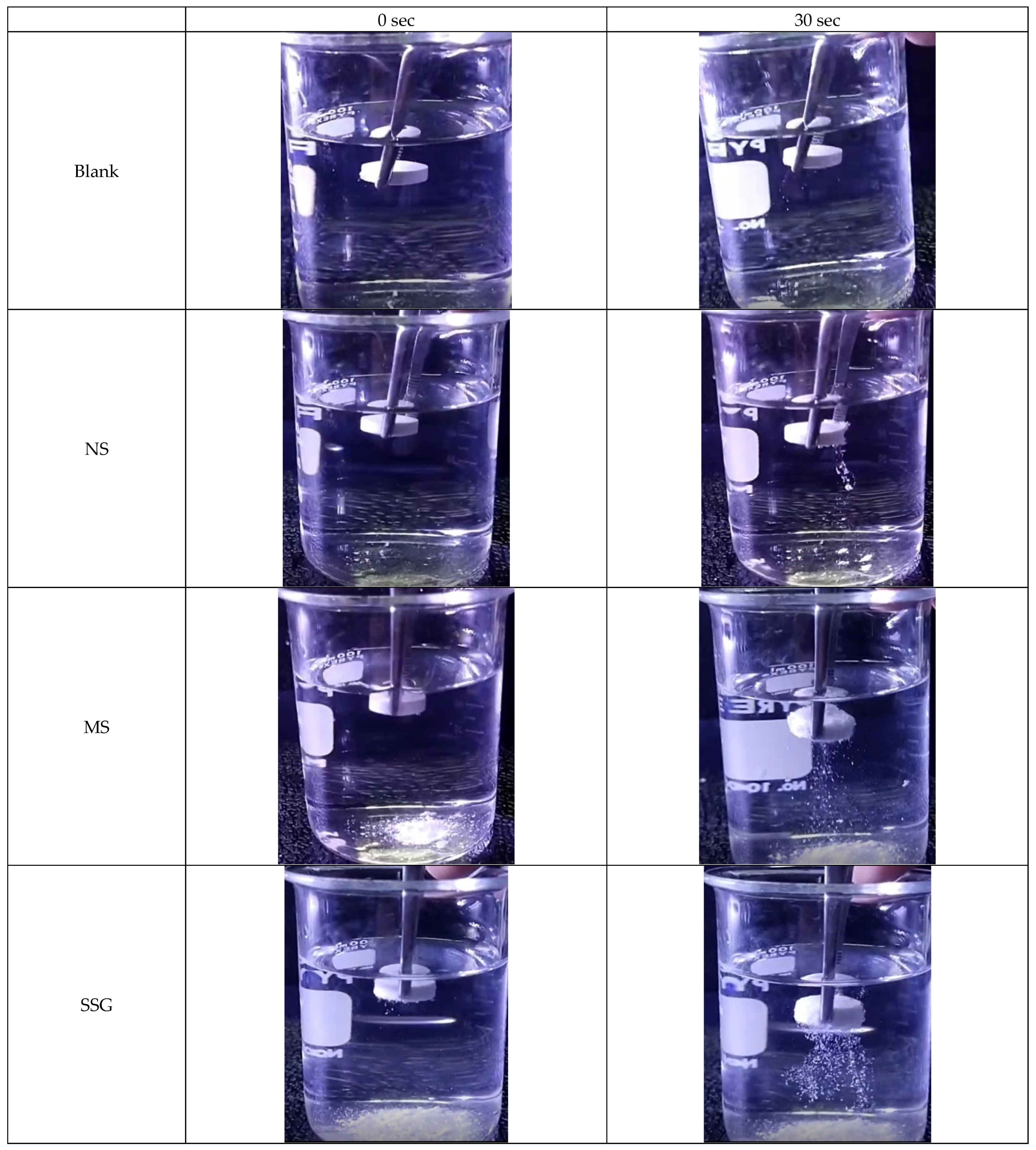
2.4.3. Efficacy of Disintegration in Paracetamol Tablets
2.4.4. Tablet Morphology
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties and Characterizations
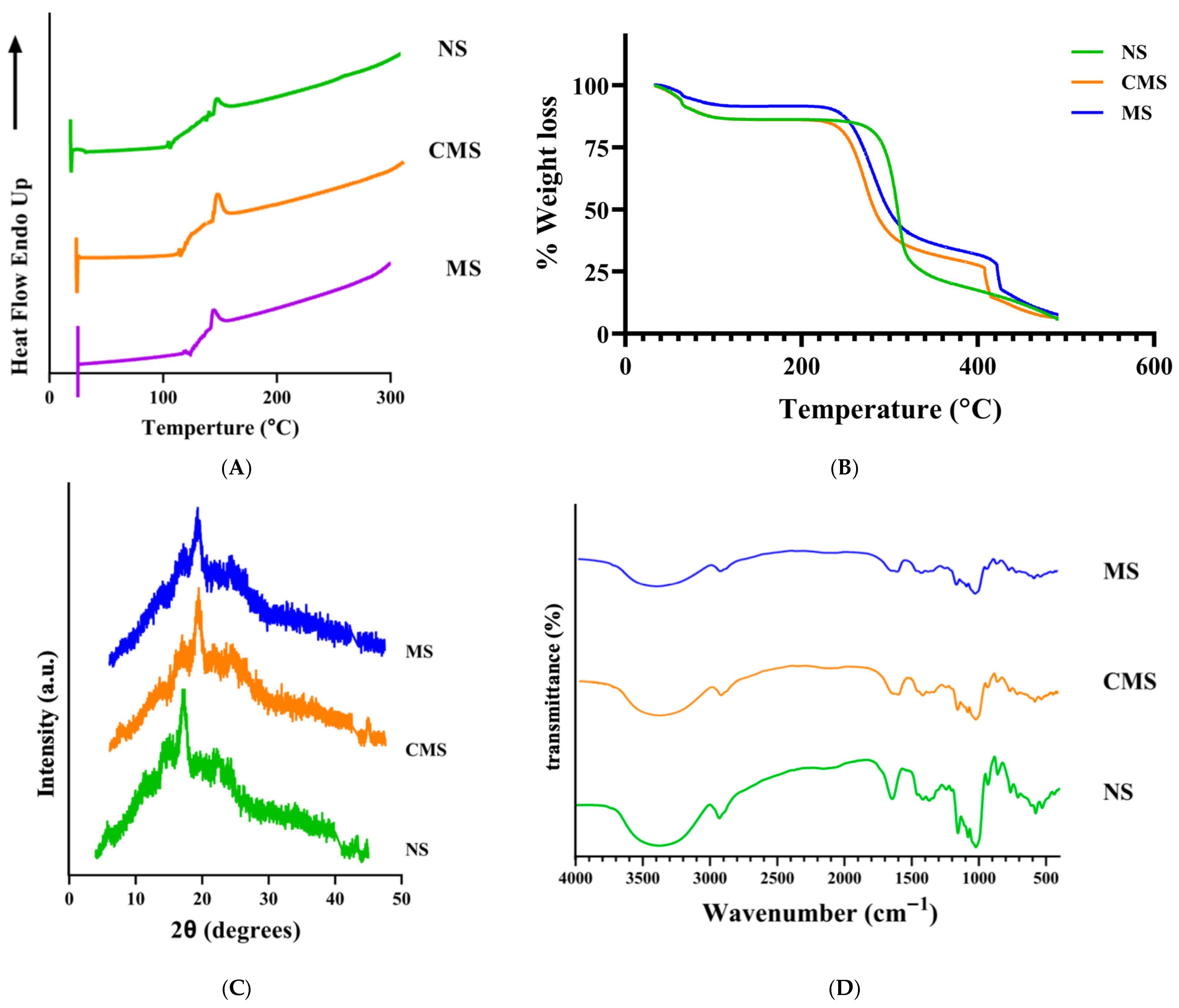
3.1.1. DSC, TGA, XRD, and FTIR–ATR Characterizations
3.1.2. Hydration Behavior
3.1.3. Density Evaluation
3.1.4. Viscosity of Starch Dispersions
3.1.5. Microscopic Morphology
3.2. Evaluation of Disintegration in Tablet
3.2.1. Determination of the Amount of Disintegrating
3.2.2. Characteristics of Swelling in Starch Tablet
3.3. Paracetamol Tablets Formulation Properties
3.3.1. Hardness, Thickness, Friability, Weight Variation, and Disintegration
3.3.2. Dissolution Rate of Paracetamol Tablets
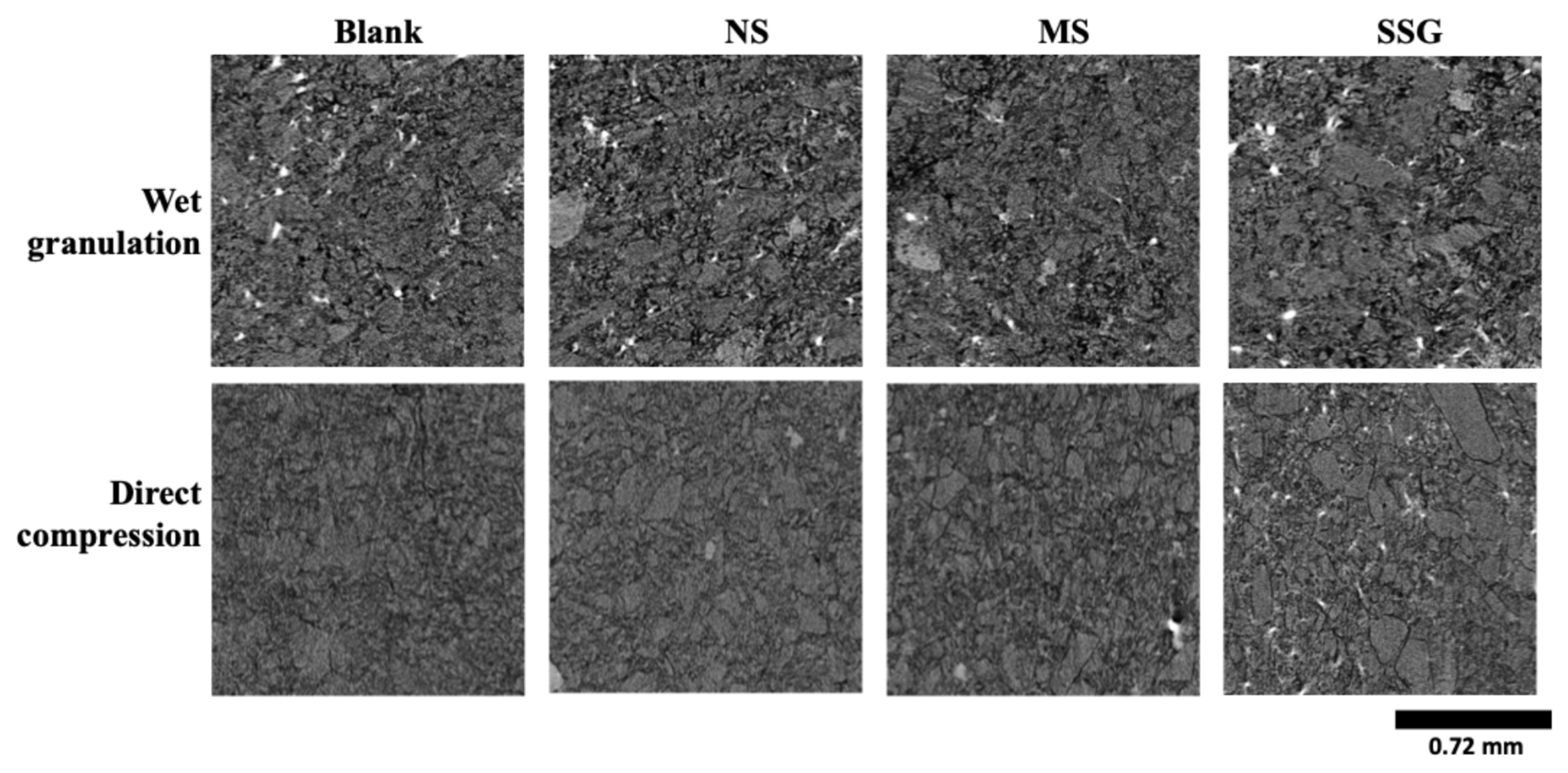
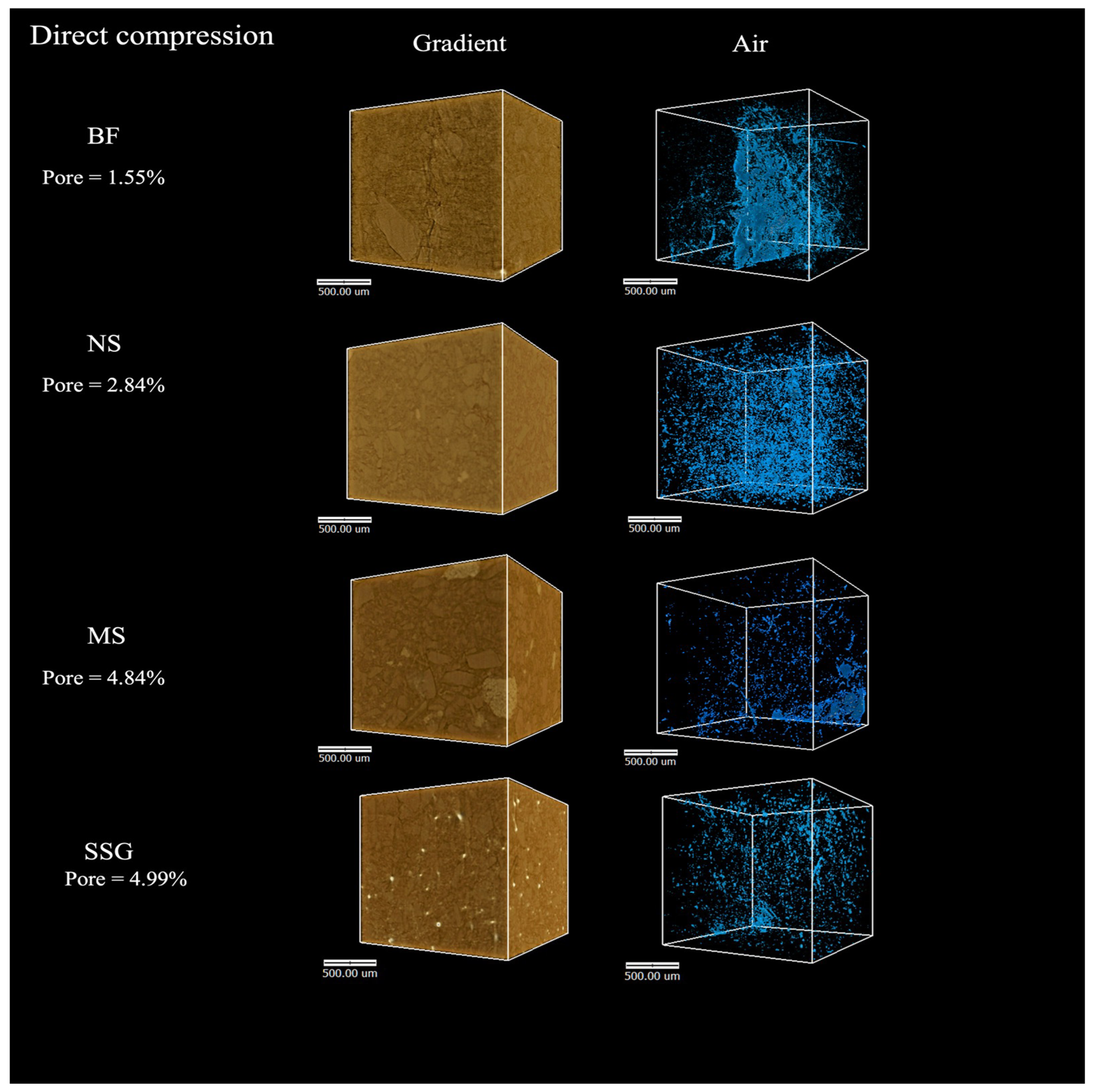
3.3.3. Tablet Morphology and Porosity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NS | Native Starch |
| CMS | Carboxymethyl starch |
| MS | Modified starch |
| SEM | Scanning electron microscopy |
| SSG | Sodium starch glycolate |
| SR-XTM | Synchrotron radiation X-ray tomography |
| DSC | Differential scanning calorimetry |
| TGA | Thermal gravimetric analysis |
| PXRD | Powder X-ray diffraction |
| FTIR-ATR | Fourier transform infrared spectroscopy—attenuated total reflectance |
References
- Augsburger, L.L.; Hoag, S.W. Pharmaceutical Dosage Forms–Tablets; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Sheskey, P.J.; Cook, W.G.; Cable, C.G. Handbook of Pharmaceutical Excipients Eight Edition; Pharmaceutical Press: London, UK, 2017. [Google Scholar]
- Rani, N.; Dev, D.; Prasad, D.N. Recent Trends in Developments of Superdisintegrants: An Overview. J. Drug Deliv. Ther. 2022, 12, 163–169. [Google Scholar] [CrossRef]
- Dilebo, J.; Gabriel, T. An Overview of Factors Affecting Superdisintegrants Functionalities. Int. J. Pharm. Sci. Nanotechnol. 2019, 12, 4355–4361. [Google Scholar] [CrossRef]
- Desai, P.M.; Liew, C.V.; Heng, P.W.S. Review of Disintegrants and the Disintegration Phenomena. J. Pharm. Sci. 2016, 105, 2545–2555. [Google Scholar] [CrossRef]
- Wei, Q.; Yang, F.; Luan, L. Preparation and in Vitro/in Vivo Evaluation of a Ketoprofen Orally Disintegrating/Sustained Release Tablet. Drug Dev. Ind. Pharm. 2013, 39, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Chowdary, K.P.R.; Enturi, V.; Sandhya Rani, A. Preparation and Evaluation of Starch Phosphate—A New Modified Starch as a Disintegrant in Tablet Formulations. Int. J. Chem. Sci. 2011, 9, 3241–3243. [Google Scholar]
- Tran, P.H.L.; Tran, T.T.D. Recent Strategic Developments in the Use of Superdisintegrants for Drug Delivery. Curr. Pharm. Des. 2020, 26, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Quodbach, J.; Kleinebudde, P. A Critical Review on Tablet Disintegration. Pharm. Dev. Technol. 2016, 21, 763–774. [Google Scholar] [CrossRef]
- Markl, D.; Zeitler, J.A. A Review of Disintegration Mechanisms and Measurement Techniques. Pharm. Res. 2017, 34, 890–917. [Google Scholar] [CrossRef]
- Wisudyaningsih, B.; Wijiani, N.; Anggraeni, V. Modification of Purple Sweet Potato Starch (Ipomoea batatas L. Poir) with Pragelatination and Acetylation Methods as Disintegrant of Paracetamol Tablets. Pharm. Educ. 2023, 23, 207–211. [Google Scholar] [CrossRef]
- Aanisah, N.; Wardhana, Y.W.; Chaerunisaa, A.Y.; Budiman, A. Review on Modification of Glucomannan as an Excipient in Solid Dosage Forms. Polymers 2022, 14, 2550. [Google Scholar] [CrossRef]
- Pal, M.S.; Waikar, D.S.B. Natural Excipients Used for Formulation of Pharmaceutical Beads. Int. J. Pharm. Sci. Rev. Res. 2023, 81, 6–12. [Google Scholar] [CrossRef]
- Debotton, N.; Dahan, A. Applications of Polymers as Pharmaceutical Excipients in Solid Oral Dosage Forms. Med. Res. Rev. 2017, 37, 52–97. [Google Scholar] [CrossRef]
- Jiang, Q.; Gao, W.; Li, X.; Xia, Y.; Wang, H.; Wu, S.; Huang, L.; Liu, C.; Xiao, P. Characterizations of Starches Isolated from Five Different Dioscorea L. Species. Food Hydrocoll. 2012, 29, 35–41. [Google Scholar] [CrossRef]
- Estiasih, T.; Ahmadi, K.; Sari, I.N.I.; Kuliahsari, D.E.; Martati, E. Traditional Detoxification of Wild Yam (Dioscorea hispida Dennst) Tuber in Chips Processing at East Java, Indonesia. J. Ethn. Foods 2022, 9, 49. [Google Scholar] [CrossRef]
- Hoover, R. Composition, Molecular Structure, and Physicochemical Properties of Tuber and Root Starches: A Review. Carbohydr. Polym. 2001, 45, 253–267. [Google Scholar] [CrossRef]
- Okunlola, A.; Odeku, O.A. Compressional Characteristics and Tableting Properties of Starches Obtained from Four Dioscorea Species. Farmacia 2009, 57, 756–770. [Google Scholar]
- Amani, N.G.; Buléon, A.; Kamenan, A.; Colonna, P. Variability in Starch Physicochemical and Functional Properties of Yam (Dioscorea Sp) Cultivated in Ivory Coast. J. Sci. Food Agric. 2004, 84, 2085–2096. [Google Scholar] [CrossRef]
- Oluwasina, O.O.; Wahab, O.; Umunna, Q.; Nwosa, O.C. Dioscorea dumetorum Pax as an Alternative Starch Source for Industrial Applications. IOSR J. Appl. Chem. 2017, 10, 5–13. [Google Scholar] [CrossRef]
- Okunlola, A.; Odeku, O.A. Comparative Evaluation of Starches Obtained from Dioscorea Species as Intragranular Tablet Disintegrant. J. Drug Deliv. Sci. Technol. 2008, 18, 445–447. [Google Scholar] [CrossRef]
- Obadi, M.; Xu, B. Review on the Physicochemical Properties, Modifications, and Applications of Starches and Its Common Modified Forms Used in Noodle Products. Food Hydrocoll. 2021, 112, 106286. [Google Scholar] [CrossRef]
- Arueya, G.L.; Ojesanmi, A.A. Evaluation of Effects of Increasing Molar Substitution of Hydroxypropylene on Physicochemical, Functional and Morphological Properties of Starch from Water Yam (Dioscorea alata). J. Food Res. 2019, 8, 58. [Google Scholar] [CrossRef]
- Chen, Y.F.; Kaur, L.; Singh, J. Chemical Modification of Starch. In Starch in Food: Structure, Function and Applications; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- Estiasih, T.; Kuliahsari, D.E.; Martati, E.; Ahmadi, K. Cyanogenic Compounds Removal and Characteristics of Non-and Pregelatinized Traditional Detoxified Wild Yam (Dioscorea hispida) Tuber Flour. Food Sci. Technol. 2022, 42, e1191. [Google Scholar] [CrossRef]
- Kumoro, A.C.; Retnowati, D.S.; Budiyati, C.S. Removal of Cyanides from Gadung (Dioscorea hispida Dennst.) Tuber Chips Using Leaching and Steaming Techniques. J. Appl. Sci. Res. 2011, 7, 2140–2146. [Google Scholar]
- Thi, L.; Thuy, H.; Nguyen, L.; Thanh, P.; Quynh Nhu, N.; Thao, N.T.; Thi, H.; Thao, T.; Luong, N.T.; Van Khoi, N.; et al. Evaluation of Reaction Conditions for Carboxymethylation of Mung Bean Starch Using Monochloroacetic Acid. J. Sci. Technol. Food 2020, 20, 37–46. [Google Scholar]
- Anutrakulchai, P.; Kittipongpatana, O.S. Effect of Dual Hydroxypropyl-Carboxymethyl Modification on the Physicochemical Properties of Mung Bean Starch. Chiang Mai Univ. J. Nat. Sci. 2011, 10, 57–69. [Google Scholar]
- Kittipongpatana, O.S.; Kittipongpatana, N. Physicochemical, in Vitro Digestibility and Functional Properties of Carboxymethyl Rice Starch Cross-Linked with Epichlorohydrin. Food Chem. 2013, 141, 1438–1444. [Google Scholar] [CrossRef]
- Lakshmi, B.S.; Ramu, A.; Vidyadhara, S.; Sailaja, Y.; Keerthana, R.S. Formulation and Evaluation of Ornidazole Oral Re-Constitutable Suspension. Res. J. Pharm. Technol. 2023, 16, 3289–3294. [Google Scholar] [CrossRef]
- Vlassenbroeck, J.; Masschaele, B.; Cnudde, V.; Dierick, M.; Pieters, K.; Van Hoorebeke, L.; Jacobs, P. Octopus 8: A High Performance Tomographic Reconstruction Package for X-Ray Tube and Synchrotron Micro-CT. In Advances in X-Ray Tomography for Geomaterials; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Limaye, A. Drishti: A Volume Exploration and Presentation Tool. In Proceedings of the Developments in X-Ray Tomography VIII; SPIE: Bellingham, WA, USA, 2012; Volume 8506. [Google Scholar]
- Krstic, M.; Maksimovic, Z.; Ibric, S.; Bakic, T.; Prodanovic, J.; Razic, S. Lignocellulosic Biomass as a Source of Microcrystalline Cellulose—Chemical and Technological Characterization and Future Perspectives. Cellul. Chem. Technol. 2018, 52, 577–588. [Google Scholar]
- Dome, K.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Changes in the Crystallinity Degree of Starch Having Different Types of Crystal Structure after Mechanical Pretreatment. Polymers 2020, 12, 641. [Google Scholar] [CrossRef]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared Spectroscopy as a Tool to Characterise Starch Ordered Structure—A Joint FTIR-ATR, NMR, XRD and DSC Study. Carbohydr. Polym. 2016, 139, 35–42. [Google Scholar] [CrossRef]
- Ahmad, M.; Gani, A.; Hassan, I.; Huang, Q.; Shabbir, H. Production and Characterization of Starch Nanoparticles by Mild Alkali Hydrolysis and Ultra-Sonication Process. Sci. Rep. 2020, 10, 3533. [Google Scholar] [CrossRef]
- Wurm, F.; Mann, K.J.; Seidl, B.; Kozich, M.; Pham, T.; Bechtold, T. Single Fibre Coating of Cellulose Acetate Fibres for Hydrophilic Modification Using Carboxymethylated Starch. Carbohydr. Polym. Technol. Appl. 2023, 6, 100379. [Google Scholar] [CrossRef]
- Basri, S.N.; Zainuddin, N.; Hashim, K.; Yusof, N.A. Preparation and Characterization of Irradiated Carboxymethyl Sago Starch-Acid Hydrogel and Its Application as Metal Scavenger in Aqueous Solution. Carbohydr. Polym. 2016, 138, 34–40. [Google Scholar] [CrossRef]
- Zhang, J.; Li, A.; Wang, A. Study on Superabsorbent Composite. VI. Preparation, Characterization and Swelling Behaviors of Starch Phosphate-Graft-Acrylamide/Attapulgite Superabsorbent Composite. Carbohydr. Polym. 2006, 65, 150–158. [Google Scholar] [CrossRef]
- Lawal, M.V. Modified Starches as Direct Compression Excipients—Effect of Physical and Chemical Modifications on Tablet Properties: A Review. Starch-Staerke 2019, 71, 1800040. [Google Scholar] [CrossRef]
- Marta, H.; Hasya, H.N.L.; Lestari, Z.I.; Cahyana, Y.; Arifin, H.R.; Nurhasanah, S. Study of Changes in Crystallinity and Functional Properties of Modified Sago Starch (Metroxylon sp.) Using Physical and Chemical Treatment. Polymers 2022, 14, 4845. [Google Scholar] [CrossRef]
- Ashogbon, A.O. Dual Modification of Various Starches: Synthesis, Properties and Applications. Food Chem. 2021, 342, 128325. [Google Scholar] [CrossRef]
- Atuchukwu, E.; Adedokun, M.; Emeje, M. Synthesis, Characterization, and Functional Properties of a Novel Sodium Carboxymethyl Starch Obtained from Matured Seeds of Brachystegia eurycoma. Egypt. Pharm. J. 2021, 20, 145–156. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, Q.; Chen, Y.; Li, X.; Mao, X.; Chen, X.; Huang, L.; Gao, W. Preparation, Physico-Chemical Characterization and Biological Activities of Two Modified Starches from Yam (Dioscorea opposita Thunb.). Food Hydrocoll. 2016, 55, 244–253. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Tuszynska, M.; Olewnik-Kruszkowska, E. Comparative Study of Gelatin Hydrogels Modified by Various Cross-Linking Agents. Materials 2021, 14, 396. [Google Scholar] [CrossRef]
- Ma, X.; Cheng, Y.; Qin, X.; Guo, T.; Deng, J.; Liu, X. Hydrophilic Modification of Cellulose Nanocrystals Improves the Physicochemical Properties of Cassava Starch-Based Nanocomposite Films. LWT 2017, 86, 318–326. [Google Scholar] [CrossRef]
- Subroto, E.; Cahyana, Y.; Indiarto, R.; Rahmah, T.A. Modification of Starches and Flours by Acetylation and Its Dual Modifications: A Review of Impact on Physicochemical Properties and Their Applications. Polymers 2023, 15, 2990. [Google Scholar] [CrossRef]
- Punia, S. Barley Starch Modifications: Physical, Chemical and Enzymatic—A Review. Int. J. Biol. Macromol. 2020, 144, 578–585. [Google Scholar] [CrossRef]
- Kodili, E.G.; Elechi, O.N. Evaluation and Optimization of the Physical and Sensory Properties of Enhanced Bread Produced from Wheat Flour and Chemically Modified African Yam Bean and Cassava Starches. Int. J. Res. Sci. Innov. 2022, 9, 110–123. [Google Scholar] [CrossRef]
- Jia, R.; Cui, C.; Gao, L.; Qin, Y.; Ji, N.; Dai, L.; Wang, Y.; Xiong, L.; Shi, R.; Sun, Q. A Review of Starch Swelling Behavior: Its Mechanism, Determination Methods, Influencing Factors, and Influence on Food Quality. Carbohydr. Polym. 2023, 321, 121260. [Google Scholar] [CrossRef]
- Ashogbon, A.O. The Recent Development in the Syntheses, Properties, and Applications of Triple Modification of Various Starches. Starch-Staerke 2021, 73, 2000125. [Google Scholar] [CrossRef]
- Kumari, B.; Sit, N. Comprehensive Review on Single and Dual Modification of Starch: Methods, Properties and Applications. Int. J. Biol. Macromol. 2023, 253, 126952. [Google Scholar] [CrossRef]
- Janssen, P.H.M.; Berardi, A.; Kok, J.H.; Thornton, A.W.; Dickhoff, B.H.J. The Impact of Lactose Type on Disintegration: An Integral Study on Porosity and Polymorphism. Eur. J. Pharm. Biopharm. 2022, 180, 251–259. [Google Scholar] [CrossRef]
- Martinez Villadiego, K.; Arias Tapia, M.J.; Useche, J.; Ledesma, Y.; Leyton, A. Thermal and Morphological Characterization of Native and Plasticized Starches of Sweet Potato (Ipomoea batatas) and Diamante Yam (Dioscorea rotundata). J. Polym. Environ. 2021, 29, 871–880. [Google Scholar] [CrossRef]
- Zou, J.; Li, Y.; Wang, F.; Su, X.; Li, Q. Relationship between Structure and Functional Properties of Starch from Different Cassava (Manihot esculenta Crantz) and Yam (Dioscorea opposita Thunb) Cultivars Used for Food and Industrial Processing. LWT 2023, 173, 114261. [Google Scholar] [CrossRef]
- Alamu, E.O.; Maziya-Dixon, B.; Okonkwo, C.C.; Asiedu, R. Physicochemical and Bioactive Properties of Selected White Yam (Dioscorea rotundata) Varieties Adapted to Riverine Areas of Nigeria. Afr. J. Food Sci. 2014, 8, 402–409. [Google Scholar] [CrossRef]
- Schomberg, A.K.; Diener, A.; Wünsch, I.; Finke, J.H.; Kwade, A. The Use of X-Ray Microtomography to Investigate the Microstructure of Pharmaceutical Tablets: Potentials and Comparison to Common Physical Methods. Int. J. Pharm. X 2021, 3, 100090. [Google Scholar] [CrossRef]
- Veronica, N.; Lee, E.S.M.; Heng, P.W.S.; Liew, C.V. Functionality of Wet-Granulated Disintegrant in Comparison to Directly Incorporated Disintegrant in a Poorly Water-Soluble Tablet Matrix. Int. J. Pharm. 2024, 661, 124467. [Google Scholar] [CrossRef]
- Šantl, M.; Ilić, I.; Vrečer, F.; Baumgartner, S. A Compressibility and Compactibility Study of Real Tableting Mixtures: The Impact of Wet and Dry Granulation versus a Direct Tableting Mixture. Int. J. Pharm. 2011, 414, 131–139. [Google Scholar] [CrossRef]
- Karehill, P.G.; Glazer, M.; Nyström, C. Studies on Direct Compression of Tablets. XXIII. The Importance of Surface Roughness for the Compactability of Some Directly Compressible Materials with Different Bonding and Volume Reduction Properties. Int. J. Pharm. 1990, 64, 35–43. [Google Scholar] [CrossRef]
- Johansson, B.; Alderborn, G. The Effect of Shape and Porosity on the Compression Behaviour and Tablet Forming Ability of Granular Materials Formed from Microcrystalline Cellulose. Eur. J. Pharm. Biopharm. 2001, 52, 347–357. [Google Scholar] [CrossRef]
- Mohylyuk, V. Effect of Roll Compaction Pressure on the Properties of High Drug-Loaded Piracetam Granules and Tablets. Drug Dev. Ind. Pharm. 2022, 48, 425–437. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, Y.; Ni, N.; Zhao, L.; Lin, X.; Wang, Y.; Du, R.; Shen, L. A New Parameter for Characterization of Tablet Friability Based on a Systematical Study of Five Excipients. Int. J. Pharm. 2022, 611, 121339. [Google Scholar] [CrossRef]
- Markl, D.; Strobel, A.; Schlossnikl, R.; Bøtker, J.; Bawuah, P.; Ridgway, C.; Rantanen, J.; Rades, T.; Gane, P.; Peiponen, K.E.; et al. Characterisation of Pore Structures of Pharmaceutical Tablets: A Review. Int. J. Pharm. 2018, 538, 188–214. [Google Scholar] [CrossRef]
- Sun, C.C. Microstructure of Tablet—Pharmaceutical Significance, Assessment, and Engineering. Pharm. Res. 2017, 34, 918–928. [Google Scholar] [CrossRef]
- Bauhuber, S.; Warnke, G.; Berardi, A. Disintegrant Selection in Hydrophobic Tablet Formulations. J. Pharm. Sci. 2021, 110, 2028–2037. [Google Scholar] [CrossRef]
- Rojas, J.; Guisao, S.; Ruge, V. Functional Assessment of Four Types of Disintegrants and Their Effect on the Spironolactone Release Properties. AAPS PharmSciTech 2012, 13, 1054–1062. [Google Scholar] [CrossRef]







| Ingredients | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Paracetamol (mg) | 500 | 500 | 500 | 500 |
| NS | - | 4% | - | - |
| MS | - | - | 4% | - |
| SSG | - | - | - | 4% |
| 10% PVP K90 | qs. | qs. | qs. | qs. |
| Talcum | 2.4% | 2.4% | 2.4% | 2.4% |
| Magnesium stearate | 0.5% | 0.5% | 0.5% | 0.5% |
| Properties | Blank | 4%NS | 4%MS | 4%SSG |
|---|---|---|---|---|
| Hardness (Kg/cm2) | ||||
| Range | 7.0–8.0 | 7.0–7.5 | 7.0–7.5 | 7.0–7.5 |
| Average | 7.33 ± 0.48 | 7.10 ± 0.32 | 7.30 ± 0.31 | 7.30 ± 0.27 |
| Thickness (mm) | ||||
| Range | 5.33–5.63 | 5.34–5.52 | 5.32–5.48 | 5.43–5.57 |
| Average | 5.54 ± 0.21 | 5.45 ± 0.12 | 5.43 ± 0.14 | 5.50 ± 0.17 |
| % Friability | 0.19 ± 0.02 | 0.37 ± 0.07 | 0.36 ± 0.01 | 0.71 ± 0.02 |
| Weight variation | 529.24 ± 4.04 | 551.65 ± 5.03 | 549.85 ± 4.02 | 552.30 ± 5.23 |
| k | 2.4 | 2.4 | 2.4 | 2.4 |
| Acceptance value (AV) | 11.58 | 13.71 | 11.57 | 14.07 |
| Disintegration time (s) | 60 ± 2.32 | 44.82 ± 5.67 | 9.42 ± 2.42 | 3.32 ± 1.14 |
| Reference Formulation | Test Formulation | f1 (Difference) | f2 (Similarity) | Profile Similarity |
|---|---|---|---|---|
| 4% SSG | 4% MS | 7 | 63 | Equivalent |
| 4% SSG | 4% NS | 33 | 32 | Not equivalent |
| 4% SSG | Blank | 46 | 27 | Not equivalent |
| Samples | Average of Porosity (%) | Average of Pore in Volume (mm3) |
|---|---|---|
| W1 | 11.40 ± 0.48 | 0.34 ± 0.03 |
| W2 | 13.42 ± 0.47 | 0.39 ± 0.03 |
| W3 | 13.90 ± 0.92 | 0.41 ± 0.05 |
| W4 | 15.81 ± 0.97 | 0.49 ± 0.05 |
| D1 | 1.55 ± 0.04 | 0.05 ± 0.01 |
| D2 | 2.84 ± 0.02 | 0.09 ± 0.01 |
| D3 | 4.84 ± 0.05 | 0.15 ± 0.01 |
| D4 | 4.99 ± 0.07 | 0.15 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanpramukkun, N.; Teruya, T.; Charoenwattanasatien, R.; Pakawanit, P.; Limsitthichaikoon, S. Development and Evaluation of Modified Dioscorea hispida Starch as a Sustainable Super-Disintegrant for Immediate-Release Tablets. Polysaccharides 2025, 6, 109. https://doi.org/10.3390/polysaccharides6040109
Hanpramukkun N, Teruya T, Charoenwattanasatien R, Pakawanit P, Limsitthichaikoon S. Development and Evaluation of Modified Dioscorea hispida Starch as a Sustainable Super-Disintegrant for Immediate-Release Tablets. Polysaccharides. 2025; 6(4):109. https://doi.org/10.3390/polysaccharides6040109
Chicago/Turabian StyleHanpramukkun, Nuntachai, Thavisak Teruya, Ratana Charoenwattanasatien, Phakkhananan Pakawanit, and Sucharat Limsitthichaikoon. 2025. "Development and Evaluation of Modified Dioscorea hispida Starch as a Sustainable Super-Disintegrant for Immediate-Release Tablets" Polysaccharides 6, no. 4: 109. https://doi.org/10.3390/polysaccharides6040109
APA StyleHanpramukkun, N., Teruya, T., Charoenwattanasatien, R., Pakawanit, P., & Limsitthichaikoon, S. (2025). Development and Evaluation of Modified Dioscorea hispida Starch as a Sustainable Super-Disintegrant for Immediate-Release Tablets. Polysaccharides, 6(4), 109. https://doi.org/10.3390/polysaccharides6040109






