Optimization of Mono- and Disaccharide Extraction from Cocoa pod Husk
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methodology
2.2. Reagents
2.3. Microwave-Assisted Hydrothermal Pretreatment of CPH and HMC-CPH
2.4. Acquisition of 1H NMR Spectra
2.5. Response Surface Analysis, Box–Behnken Design
3. Results and Discussion
3.1. 1H NMR Spectra Elucidation
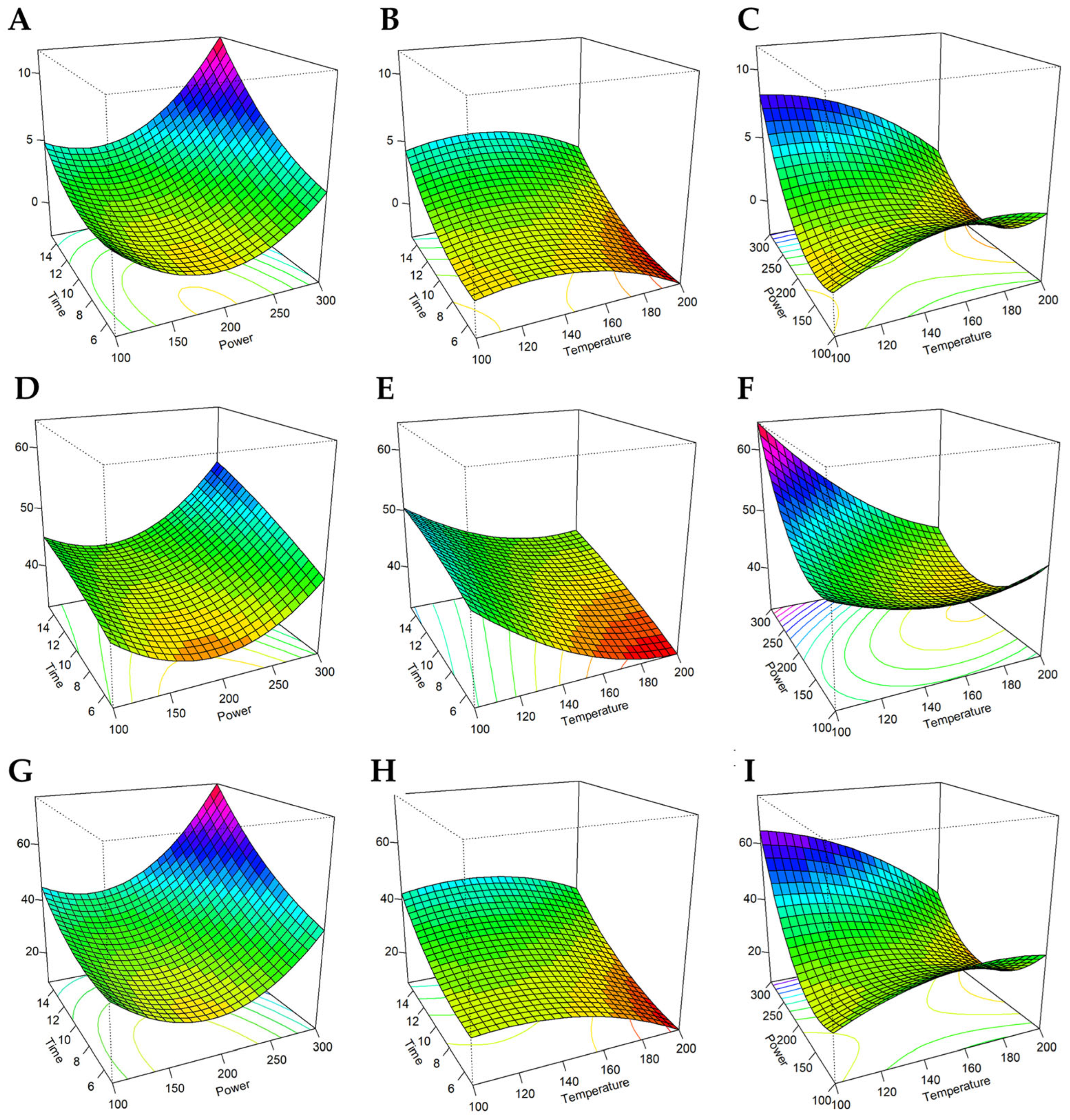
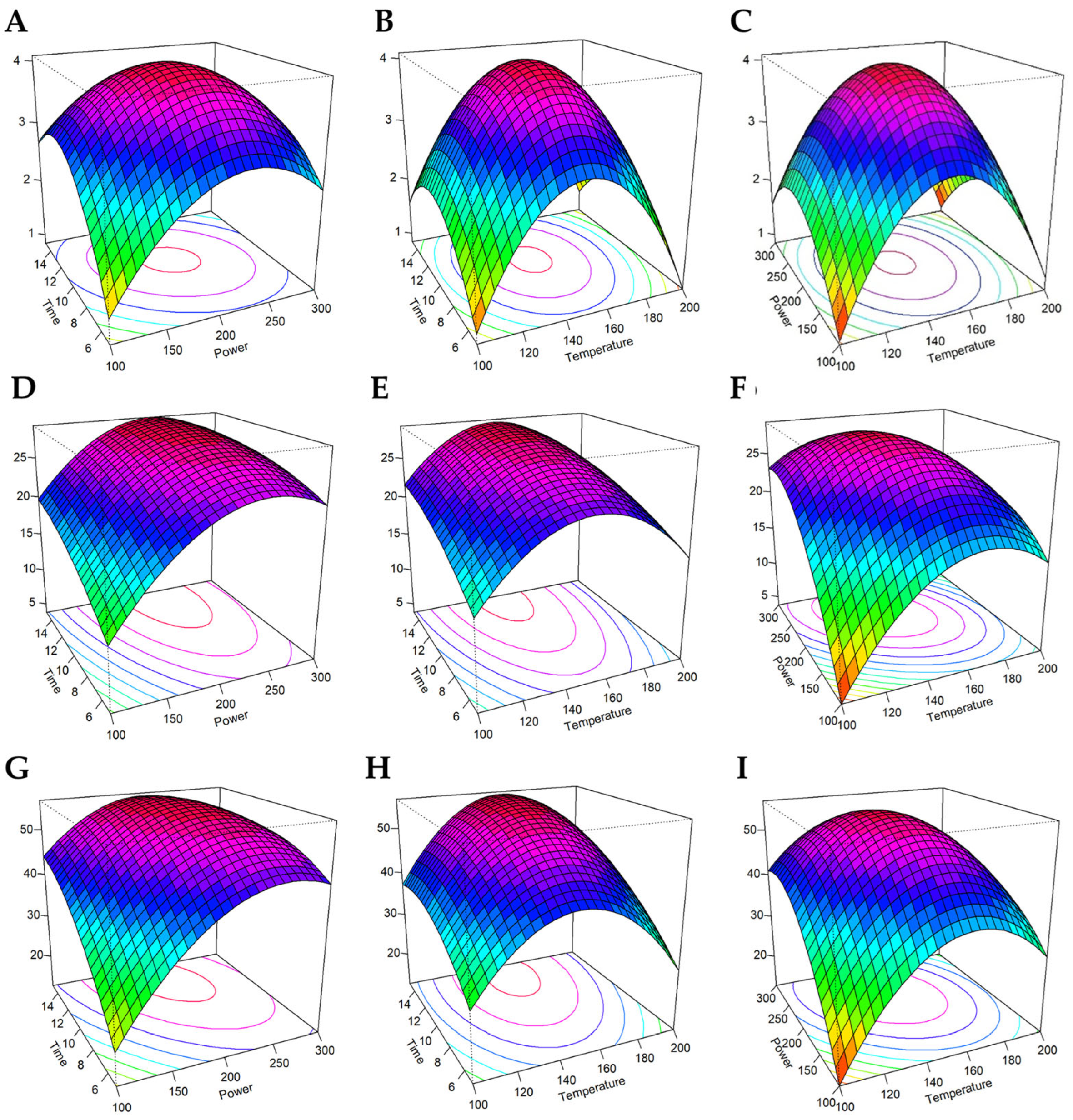
3.2. Optimization of CPH and HMC-CPH MA-HTP Conditions Through RSA-BBD
3.3. RSA—BBD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPH | Cocoa pod husk |
| MA-HTP | Microwave-assisted hydrothermal pretreatment |
| HMC-CPH | Hemicellulose cocoa pod husk |
| RSA | Response surface analysis |
| BBD | Box–Behnken design |
| 1H NMR Qu | Proton nuclear magnetic resonance identification and quantification |
| 1H NMR | Proton nuclear magnetic resonance |
| µM | Micro molar |
| NMR | Nuclear magnetic resonance |
| pH | Hydrogen potential |
References
- Ramos-Escudero, F.; Casimiro-Gonzales, S.; Cádiz-Gurrea, M.d.l.L.; Cancino Chávez, K.; Basilio-Atencio, J.; Ordoñez, E.S.; Muñoz, A.M.; Segura-Carretero, A. Optimizing Vacuum Drying Process of Polyphenols, Flavanols and DPPH Radical Scavenging Assay in Pod Husk and Bean Shell Cocoa. Sci. Rep. 2023, 13, 13900. [Google Scholar] [CrossRef]
- Jarrín-Chacón, J.P.; Núñez-Pérez, J.; Espín-Valladares, R.d.C.; Manosalvas-Quiroz, L.A.; Rodríguez-Cabrera, H.M.; Pais-Chanfrau, J.M. Pectin Extraction from Residues of the Cocoa Fruit (Theobroma cacao L.) by Different Organic Acids: A Comparative Study. Foods 2023, 12, 590. [Google Scholar] [CrossRef]
- Lu, F.; Rodriguez-Garcia, J.; Van Damme, I.; Westwood, N.J.; Shaw, L.; Robinson, J.S.; Warren, G.; Chatzifragkou, A.; McQueen Mason, S.; Gomez, L.; et al. Valorisation Strategies for Cocoa Pod Husk and Its Fractions. Curr. Opin. Green Sustain. Chem. 2018, 14, 80–88. [Google Scholar] [CrossRef]
- Vásquez, Z.S.; de Carvalho Neto, D.P.; Pereira, G.V.M.; Vandenberghe, L.P.S.; de Oliveira, P.Z.; Tiburcio, P.B.; Rogez, H.L.G.; Góes Neto, A.; Soccol, C.R. Biotechnological Approaches for Cocoa Waste Management: A Review. Waste Manag. 2019, 90, 72–83. [Google Scholar] [CrossRef]
- Quiceno-Suarez, A.; Cadena-Chamorro, E.M.; Ciro-Velásquez, H.J.; Arango-Tobón, J.C. By-Products of the Cocoa Agribusiness: High Valueadded Materials Based on Their Bromatological and Chemical Characterization. Rev. Fac. Nac. Agron. Medellin 2024, 77, 10585–10599. [Google Scholar] [CrossRef]
- Priyangini, F.; Walde, S.G.; Chidambaram, R. Extraction Optimization of Pectin from Cocoa Pod Husks (Theobroma cacao L.) with Ascorbic Acid Using Response Surface Methodology. Carbohydr. Polym. 2018, 202, 497–503. [Google Scholar] [CrossRef]
- Soares, T.F.; Oliveira, M.B.P.P. Cocoa By-Products: Characterization of Bioactive Compounds and Beneficial Health Effects. Molecules 2022, 27, 1625. [Google Scholar] [CrossRef]
- Perwitasari, U.; Agustina, N.T.; Pangestu, R.; Amanah, S.; Saputra, H.; Andriani, A.; Fahrurrozi; Juanssilfero, A.B.; Thontowi, A.; Widyaningsih, T.D.; et al. Cacao Pod Husk for Citric Acid Production under Solid State Fermentation Using Response Surface Method. Biomass Convers. Biorefin. 2023, 13, 7165–7173. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Valadez-Carmona, L.; Mendiola, J.A.; Ibáñez, E.; Villamiel, M. Structural Characterisation of Pectin Obtained from Cacao Pod Husk. Comparison of Conventional and Subcritical Water Extraction. Carbohydr. Polym. 2019, 217, 69–78. [Google Scholar] [CrossRef]
- Valladares-Diestra, K.K.; de Souza Vandenberghe, L.P.; Soccol, C.R. A Biorefinery Approach for Pectin Extraction and Second-Generation Bioethanol Production from Cocoa Pod Husk. Bioresour. Technol. 2022, 346, 126635. [Google Scholar] [CrossRef]
- Rogoski, W.; Pereira, G.N.; Cesca, K.; de Oliveira, D.; de Andrade, C.J. An Overview on Pretreatments for the Production of Cassava Peels-Based Xyloligosaccharides: State of Art And Challenges. Waste Biomass Valorization 2023, 14, 2115–2131. [Google Scholar] [CrossRef]
- Fan, X.; Ren, M.; Zhou, C.; Kong, F.; Hua, C.; Fakayode, O.A.; Okonkwo, C.E.; Li, H.; Liang, J.; Wang, X. Total Utilization of Lignocellulosic Biomass with Xylooligosaccharides Production Priority: A Review. Biomass Bioenergy 2024, 181, 107038. [Google Scholar] [CrossRef]
- Lubis, M.A.R.; Lee, S.H.; Mardawati, E.; Rahimah, S.; Antov, P.; Andoyo, R.; Kristak, L.; Nurhadi, B. Biomass Conversion and Sustainable Biorefinery. Biomass Convers. Biorefin. 2024, 12, 4231. [Google Scholar] [CrossRef]
- Muharja, M.; Darmayanti, R.F.; Palupi, B.; Rahmawati, I.; Fachri, B.A.; Setiawan, F.A.; Amini, H.W.; Rizkiana, M.F.; Rahmawati, A.; Susanti, A.; et al. Optimization of Microwave-Assisted Alkali Pretreatment for Enhancement of Delignification Process of Cocoa Pod Husk. Bull. Chem. React. Eng. Catal. 2021, 16, 31–43. [Google Scholar] [CrossRef]
- Pangestu, R.; Amanah, S.; Juanssilfero, A.B.; Yopi; Perwitasari, U. Response Surface Methodology for Microwave-Assisted Extraction of Pectin from Cocoa Pod Husk (Theobroma cacao) Mediated by Oxalic Acid. J. Food Meas. Charact. 2020, 14, 2126–2133. [Google Scholar] [CrossRef]
- Saelee, M.; Sivamaruthi, B.S.; Tansrisook, C.; Duangsri, S.; Chaiyasut, K.; Kesika, P.; Peerajan, S.; Chaiyasut, C. Response Surface Methodological Approach for Optimizing Theobroma cacao L. Oil Extraction. Appl. Sci. 2022, 12, 5482. [Google Scholar] [CrossRef]
- Hennessey-Ramos, L.; Murillo-Arango, W.; Vasco-Correa, J.; Astudillo, I.C.P. Enzymatic Extraction and Characterization of Pectin from Cocoa Pod Husks (Theobroma cacao L.) Using Celluclast® 1.5 L. Molecules 2021, 26, 1473. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken Design: An Alternative for the Optimization of Analytical Methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Aycan Dümenci, N.; Aydın Temel, F.; Turan, N.G. Role of Different Natural Materials in Reducing Nitrogen Loss during Industrial Sludge Composting: Modelling and Optimization. Bioresour. Technol. 2023, 385, 129464. [Google Scholar] [CrossRef]
- Nassef, H.M.; Al-Hazmi, G.A.A.M.; Alayyafi, A.A.A.; El-Desouky, M.G.; El-Bindary, A.A. Synthesis and Characterization of New Composite Sponge Combining of Metal-Organic Framework and Chitosan for the Elimination of Pb(II), Cu(II) and Cd(II) Ions from Aqueous Solutions: Batch Adsorption and Optimization Using Box-Behnken Design. J. Mol. Liq. 2024, 394, 123741. [Google Scholar] [CrossRef]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C. Optimization of Microwave-Assisted Extraction Based on Absorbed Microwave Power and Energy. Chem. Eng. Sci. 2014, 111, 41–47. [Google Scholar] [CrossRef]
- Del Campo, G.; Zuriarrain, J.; Zuriarrain, A.; Berregi, I. Quantitative Determination of Carboxylic Acids, Amino Acids, Carbohydrates, Ethanol and Hydroxymethylfurfural in Honey by 1H NMR. Food Chem. 2016, 196, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.K.; Roy, R. Quantitative 1H NMR Spectroscopy. TrAC Trends Anal. Chem. 2012, 35, 5–26. [Google Scholar] [CrossRef]
- Schievano, E.; Tonoli, M.; Rastrelli, F. NMR Quantification of Carbohydrates in Complex Mixtures. A Challenge on Honey. Anal. Chem. 2017, 89, 13405–13414. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; She, D. Isolation, Structural Characterization, and Potential Applications of Hemicelluloses from Bamboo: A Review. Carbohydr. Polym. 2014, 112, 701–720. [Google Scholar] [CrossRef]
- Hernández-Bolio, G.I.; Fagundo-Mollineda, A.; Caamal-Fuentes, E.E.; Robledo, D.; Freile-Pelegrin, Y.; Hernández-Núñez, E. NMR Metabolic Profiling of Sargassum Species Under Different Stabilization/Extraction Processes. J. Phycol. 2021, 57, 655–663. [Google Scholar] [CrossRef]
- Hou, Z.; Liang, X.; Du, L.; Su, F.; Su, W. Quantitative Determination and Validation of Avermectin B1a in Commercial Products Using Quantitative Nuclear Magnetic Resonance Spectroscopy. Magn. Reson. Chem. 2014, 52, 480–485. [Google Scholar] [CrossRef]
- Al-Mekhlafi, N.A.; Mediani, A.; Ismail, N.H.; Abas, F.; Dymerski, T.; Lubinska-Szczygeł, M.; Vearasilp, S.; Gorinstein, S. Metabolomic and Antioxidant Properties of Different Varieties and Origins of Dragon Fruit. Microchem. J. 2021, 160, 105687. [Google Scholar] [CrossRef]
- Spiteri, M.; Jamin, E.; Thomas, F.; Rebours, A.; Lees, M.; Rogers, K.M.; Rutledge, D.N. Fast and Global Authenticity Screening of Honey Using 1H-NMR Profiling. Food Chem. 2015, 189, 60–66. [Google Scholar] [CrossRef]
- Ouattara, L.Y.; Kouassi, E.K.A.; Soro, D.; Yao, K.B.; Fanou, G.D.; Drogui, A.P.; Tyagi, D.R. Optimization of Thermochemical Hydrolysis of Potassium Hydroxide-Delignified Cocoa (Theobroma cacao L.) Pod Husks under Low Combined Severity Factors (CSF) Conditions. Sci. Afr. 2023, 22, e01908. [Google Scholar] [CrossRef]
- Dallal Chergui, S.; Akretche-Kelfat, S.; Lamoudi, L.; Al-Rshaidat, M.; Boudjelal, F.; Ait-Amar, H. Optimization of Citric Acid Production by Aspergillus niger Using Two Downgraded Algerian Date Varieties. Saudi J. Biol. Sci. 2021, 28, 7134–7141. [Google Scholar] [CrossRef]
- Chauca Espinoza, K.; Grosso Gamboa, C.A.; Cabrera Matara, J.; León Torres, C.; Arellano Barragán, J.; Rodríguez, C.N.; Pretel Sevillano, O. Extracción de azúcares reductores totales por métodos físicos y químicos de planta de Zea mays. Arnaldoa 2017, 24, 82–94. [Google Scholar] [CrossRef][Green Version]
- Vegas Niño, R.M.; Acosta Arteaga, Y.; Fernández Segura, C. Obtención, purificación y aprovechamiento de azúcares reductores a partir de materiales lignocelulósicos. Rev. Boliv. Química 2024, 41, 10–27. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liu, Y.; Yang, X.; McQueen-Mason, S.J.; Gomez, L.D.; Macquarrie, D.J. Comparative Evaluation of Microwave-Assisted Acid, Alkaline, and Salt Pretreatments of Sugarcane Bagasse for Sugar Recovery. Biomass Convers. Biorefin. 2021, 11, 2681–2693. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Y.; Gómez, L.D.; Wei, T.; Yang, X.; Simister, R.; McQueen-Mason, S.J.; Macquarrie, D.J. Thermochemical Pretreatments of Maize Stem for Sugar Recovery: Comparative Evaluation of Microwave and Conventional Heating. Ind. Crops Prod. 2021, 160, 113106. [Google Scholar] [CrossRef]
- Wijaya, Y.P.; Putra, R.D.D.; Widyaya, V.T.; Ha, J.-M.; Suh, D.J.; Kim, C.S. Comparative Study on Two-Step Concentrated Acid Hydrolysis for the Extraction of Sugars from Lignocellulosic Biomass. Bioresour. Technol. 2014, 164, 221–231. [Google Scholar] [CrossRef] [PubMed]
| Essay | Temperature (°C) | Power (W) | Time (min) |
|---|---|---|---|
| 1 | −1 [100] | −1 [100] | 0 [10] |
| 2 | 1 [200] | −1 [100] | 0 [10] |
| 3 | −1 [100] | 1 [300] | 0 [10] |
| 4 | 1 [200] | 1 [300] | 0 [10] |
| 5 | −1 [100] | 0 [200] | −1 [5] |
| 6 | 1 [200] | 0 [200] | −1 [5] |
| 7 | −1 [100] | 0 [200] | 1 [15] |
| 8 | 1 [200] | 0 [200] | 1 [15] |
| 9 | 0 [150] | −1 [100] | −1 [5] |
| 10 | 0 [150] | 1 [300] | −1 [5] |
| 11 | 0 [150] | −1 [100] | 1 [15] |
| 12 | 0 [150] | 1 [300] | 1 [15] |
| 13 | 0 [150] | 0 [200] | 0 [10] |
| 14 | 0 [150] | 0 [200] | 0 [10] |
| 15 | 0 [150] | 0 [200] | 0 [10] |
| D-Fructose, Sucrose, and D-Glucose Standards | CPH and HMC-CPH MA-HTP Extracts | ||||
|---|---|---|---|---|---|
| Assignment | Chemical Shifts Used for Identity Check | Chemical Shift | |||
| δ (ppm) | Multiplicity | Identity Check δ (ppm) | Quantity δ (ppm) | Multiplicity | |
| Fructose | 4.11 | d | 4.11 | d | |
| Fructose | 4.01 | t | 4.01 | t | |
| Fructose | 3.89 | dd | 3.90 | m | |
| Sucrose | 5.40 | d | 5.40 | d | |
| Sucrose | 4.21 | d | 4.20 | d | |
| Sucrose | 4.05 | t | 4.05 | t | |
| Sucrose | 3.76 | t | 3.76 | t | |
| Sucrose | 3.67 | s | 3.67 | s | |
| Sucrose | 3.55 | dd | n/d | n/d | |
| Sucrose | 3.47 | t | 3.47 | t | |
| Glucose | 5.24 | d | 5.24 | d | |
| Glucose | 4.65 | d | 4.65 | d | |
| Glucose | 3.89 | dd | n/d | n/d | |
| Glucose | 3.53 | dd | 3.53 | dd | |
| Glucose | 3.25 | t | 3.25 | t | |
| Independent Variables | Dependent Variable Y | |||||||
|---|---|---|---|---|---|---|---|---|
| Coded −1,0,1 [Uncoded] | Yield CPH | Yield HMC-CPH | ||||||
| Temperature (°C) | Power (W) | Time (min) | Glucose (%) | Sucrose (%) | Fructose (%) | Glucose (%) | Sucrose (%) | Fructose (%) |
| −1 [100] | −1 [100] | 0 [10] | 1.0 | 51.10 | 29.90 | 1.2 | 7.70 | 16.91 |
| 1 [200] | −1 [100] | 0 [10] | 1.0 | 43.17 | 27.80 | 0.4 | 12.00 | 25.16 |
| −1 [100] | 1 [300] | 0 [10] | 9.4 | 68.02 | 70.26 | 2.2 | 26.23 | 44.78 |
| 1 [200] | 1 [300] | 0 [10] | 1.0 | 39.20 | 27.07 | 0.6 | 8.67 | 23.87 |
| −1 [100] | 0 [200] | −1 [5] | 1.2 | 50.39 | 29.56 | 0.5 | 8.15 | 20.20 |
| 1 [200] | 0 [200] | −1 [5] | 0.8 | 43.38 | 26.87 | 1.3 | 17.19 | 27.49 |
| −1 [100] | 0 [200] | 1 [15] | 0.7 | 39.41 | 23.51 | 1.1 | 20.90 | 35.43 |
| 1 [200] | 0 [200] | 1 [15] | 1.1 | 37.91 | 28.08 | 1.7 | 26.92 | 49.61 |
| 0 [150] | −1 [100] | −1 [5] | 0.5 | 34.63 | 20.94 | 1.4 | 13.98 | 21.58 |
| 0 [150] | 1 [300] | −1 [5] | 1.1 | 36.78 | 29.23 | 2.3 | 25.76 | 47.07 |
| 0 [150] | −1 [100] | 1 [15] | 7.5 | 52.79 | 57.48 | 2.8 | 16.42 | 41.23 |
| 0 [150] | 1 [300] | 1 [15] | 14.0 | 61.78 | 88.57 | 1.8 | 22.96 | 44.31 |
| 0 [150] | 0 [200] | 0 [10] | 1.0 | 42.15 | 27.91 | 7.8 | 35.80 | 75.56 |
| 0 [150] | 0 [200] | 0 [10] | 0.8 | 35.13 | 23.10 | 2.3 | 28.57 | 51.86 |
| 0 [150] | 0 [200] | 0 [10] | 1.4 | 43.38 | 31.83 | 2.1 | 17.45 | 34.10 |
| Source | Carbohydrate | Threshold (0.01) | ||
|---|---|---|---|---|
| Temperature (°C) | Power (Watt) | Time (Min) | ||
| CPH | Glucose | 135.4 | 180.6 | 5.8 |
| CPH | Sucrose | 154.3 | 256.3 | 20.2 |
| CPH | Fructose | 129.5 | 173.8 | 5.2 |
| HMC-CPH | Glucose | 142.2 | 204.4 | 10.5 |
| HMC-CPH | Sucrose | 148.8 | 215.6 | 14.3 |
| HMC-CPH | Fructose | 151.6 | 231.6 | 13.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez-Patlán, E.E.; Espinosa-Solares, T.; Herbert-Pucheta, J.E.; Zuleta-Prada, H.; Hernández-Núñez, E. Optimization of Mono- and Disaccharide Extraction from Cocoa pod Husk. Polysaccharides 2025, 6, 105. https://doi.org/10.3390/polysaccharides6040105
Suárez-Patlán EE, Espinosa-Solares T, Herbert-Pucheta JE, Zuleta-Prada H, Hernández-Núñez E. Optimization of Mono- and Disaccharide Extraction from Cocoa pod Husk. Polysaccharides. 2025; 6(4):105. https://doi.org/10.3390/polysaccharides6040105
Chicago/Turabian StyleSuárez-Patlán, Edna Elena, Teodoro Espinosa-Solares, José Enrique Herbert-Pucheta, Holber Zuleta-Prada, and Emanuel Hernández-Núñez. 2025. "Optimization of Mono- and Disaccharide Extraction from Cocoa pod Husk" Polysaccharides 6, no. 4: 105. https://doi.org/10.3390/polysaccharides6040105
APA StyleSuárez-Patlán, E. E., Espinosa-Solares, T., Herbert-Pucheta, J. E., Zuleta-Prada, H., & Hernández-Núñez, E. (2025). Optimization of Mono- and Disaccharide Extraction from Cocoa pod Husk. Polysaccharides, 6(4), 105. https://doi.org/10.3390/polysaccharides6040105








