Chitosan Mixtures from Marine Sources: A Comparative Study of Biological Responses and Practical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chitosan Types
2.3. Determination of Physicochemical Characteristics
2.4. Preparation of Chitosan Solutions and Binary Mixtures
2.5. MM and DDA Calculation of the Mixtures
2.6. In Vitro Assessment
2.6.1. Preparation of Bacterial Suspensions
2.6.2. Micro-Well Dilution Assay
2.6.3. Evaluation of the Fractional Inhibitory Index of Chitosan in the Mixtures
2.7. In Vivo Testing—Brine Shrimp Lethality Assay (BSLA) Testing Protocol
2.7.1. Experimental Design for Larvae
2.7.2. Structural Modifications and Ingestion Assessment of Polymers
2.7.3. Larval Behavior and Observations on Growth and Development
2.8. Statistical Interpretation
3. Results
3.1. Characteristics of the Chitosan Samples Used
3.2. In Vitro Antibacterial and Antifungal Activity
3.3. In Vivo Testing (BSLA)
3.3.1. Larval Survival 24 h After Exposure
3.3.2. Incorporation and Accumulation of Chitosan Particles in the Body of Larvae, 48 h After Exposure
3.3.3. Larval Behavior and Observations on Growth and Development
3.3.4. Cytological Effects
3.3.5. Comparative Analysis of the Effects Induced by Chitosan on the Two Biological Systems
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ul-Islam, M.; Alabbosh, K.F.; Manan, S.; Khan, S.; Ahmad, F.; Ullah, M.W. Chitosan-Based Nanostructured Biomaterials: Synthesis, Properties, and Biomedical Applications. Adv. Ind. Eng. Polym. Res. 2024, 7, 79–99. [Google Scholar] [CrossRef]
- Banks, I.R.; Specht, C.A.; Donlin, M.J.; Gerik, K.J.; Levitz, S.M.; Lodge, J.K. A Chitin Synthase and Its Regulator Protein Are Critical for Chitosan Production and Growth of the Fungal Pathogen Cryptococcus Neoformans. Eukaryot. Cell 2005, 4, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Araki, Y.; Ito, E. A Pathway of Chitosan Formation in Mucor Rouxii: Enzymatic Deacetylation of Chitin. Eur. J. Biochem. 1974, 55, 71–78. [Google Scholar] [CrossRef]
- Baker, L.G.; Specht, C.A.; Donlin, M.J.; Lodge, J.K. Chitosan, the Deacetylated Form of Chitin, Is Necessary for Cell Wall Integrity in Cryptococcus Neoformans. Eukaryot. Cell 2007, 6, 855–867. [Google Scholar] [CrossRef]
- Adams, D.J. Fungal Cell Wall Chitinases and Glucanases. Microbiology 2004, 150, 2029–2035. [Google Scholar] [CrossRef]
- Elsoud, M.M.A.; El Kady, E.M. Current Trends in Fungal Biosynthesis of Chitin and Chitosan. Bull. Natl. Res. Cent. 2019, 43, 59. [Google Scholar] [CrossRef]
- Bellich, B.; D’agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef]
- Li, B.; Elango, J.; Wu, W. Recent Advancement of Molecular Structure and Biomaterial Function of Chitosan from Marine Organisms for Pharmaceutical and Nutraceutical Application. Appl. Sci. 2020, 10, 4719. [Google Scholar] [CrossRef]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Jóźwik-Pruska, J.; Wiśniewska-Wrona, M. Chitin and Chitosan as Polymers of the Future—Obtaining, Modification, Life Cycle Assessment and Main Directions of Application. Polymers 2023, 15, 793. [Google Scholar] [CrossRef]
- Jung, H.S.; Kim, M.H.; Shin, J.Y.; Park, S.R.; Jung, J.Y.; Park, W.H. Electrospinning and Wound Healing Activity of β-Chitin Extracted from Cuttlefish Bone. Carbohydr. Polym. 2018, 193, 205–211. [Google Scholar] [CrossRef]
- Krishnan, S.; Chakraborty, K.; Dhara, S. Biomedical Potential of β-Chitosan from Cuttlebone of Cephalopods. Carbohydr. Polym. 2021, 273, 118591. [Google Scholar] [CrossRef]
- Schröder, V.; Gherghel, D.; Apetroaei, M.R.; Gîjiu, C.L.; Isopescu, R.; Dinculescu, D.; Apetroaei, M.-M.; Enache, L.E.; Mihai, C.-T.; Rău, I.; et al. α-Chitosan and β-Oligochitosan Mixtures-Based Formula for In Vitro Assessment of Melanocyte Cells Response. Int. J. Mol. Sci. 2024, 25, 6768. [Google Scholar] [CrossRef] [PubMed]
- De Lima Batista, A.C.; De Souza Neto, F.E.; De Souza Paiva, W. Review of Fungal Chitosan: Past, Present and Perspectives in Brazil. Polímeros 2018, 28, 275–283. [Google Scholar] [CrossRef]
- Kozma, M.; Acharya, B.; Bissessur, R. Chitin, Chitosan, and Nanochitin: Extraction, Synthesis, and Applications. Polymers 2022, 14, 3989. [Google Scholar] [CrossRef] [PubMed]
- Vaz, L.M.; Branco, R.; Morais, P.V.; Guiomar, A.J. Sterilized Polyhexanide-Releasing Chitosan Membranes with Potential for Use in Antimicrobial Wound Dressings. Membranes 2023, 13, 877. [Google Scholar] [CrossRef]
- Abdelsalam, K.M.; Shaltout, N.A.; Ibrahim, H.A.; Tadros, H.R.Z.; Aly-Eldeen, M.A.E.; Beltagy, E.A. A Comparative Study of Biosynthesized Marine Natural-Product Nanoparticles as Antifouling Biocides. Oceanologia 2022, 64, 35–49. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Leung, S.W.; Cheng, P.C.; Chou, C.M.; Lin, C.; Kuo, Y.C.; Lee, Y.L.A.; Liu, C.Y.; Mi, F.L.; Cheng, C.H. A Novel Low-Molecular-Weight Chitosan/Gamma-Polyglutamic Acid Polyplexes for Nucleic Acid Delivery into Zebrafish Larvae. Int. J. Biol. Macromol. 2022, 194, 384–394. [Google Scholar] [CrossRef]
- Solairaj, D.; Rameshthangam, P.; Arunachalam, G. Anticancer Activity of Silver and Copper Embedded Chitin Nanocomposites against Human Breast Cancer (MCF-7) Cells. Int. J. Biol. Macromol. 2017, 105, 608–619. [Google Scholar] [CrossRef]
- Bhoopathy, S.; Inbakandan, D.; Rajendran, T.; Chandrasekaran, K.; Kasilingam, R.; Gopal, D. Curcumin Loaded Chitosan Nanoparticles Fortify Shrimp Feed Pellets with Enhanced Antioxidant Activity. Mater. Sci. Eng. C 2021, 120, 111737. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Sultan, Y.Y.; Seif, M.M.; Marrez, D.A. Bio-Evaluation of Crustacean and Fungal Nano-Chitosan for Applying as Food Ingredient. Toxicol. Rep. 2018, 5, 348–356. [Google Scholar] [CrossRef]
- Iancu, I.M.; Schröder, V.; Apetroaei, M.-R.; Crețu, R.M.; Mireșan, H.; Honcea, A.; Iancu, V.; Bucur, L.A.; Mitea, G.; Atodiresei-Pavalache, G. Biocompatibility of Membranes Based on a Mixture of Chitosan and Lythri Herba Aqueous Extract. Appl. Sci. 2023, 13, 8023. [Google Scholar] [CrossRef]
- Panneer, D.S.; Tirunavukkarasu, S.; Sadaiyandi, V.; Rajendiran, N.; Mohammad, F.; Oh, W.C.; Sagdevan, S. Antiproliferative Potentials of Chitin and Chitosan Encapsulated Gold Nanoparticles Derived from Unhatched Artemia Cysts. Chem. Phys. Lett. 2022, 790, 139345. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.M.; Nemeş, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef] [PubMed]
- Masselin, A.; Rousseau, A.; Pradeau, S.; Fort, L.; Gueret, R.; Buon, L.; Armand, S.; Cottaz, S.; Choisnard, L.; Fort, S. Optimizing Chitin Depolymerization by Lysozyme to Long-Chain Oligosaccharides. Mar. Drugs 2021, 19, 320. [Google Scholar] [CrossRef]
- Manimohan, M.; Rahaman, M.; Pandiaraj, S.; Thiruvengadam, M.; Pugalmani, S. Exploring Biological Activity and In-Vitro Anticancer Effects of a New Biomaterial Derived from Schiff Base Isolated from Homarus americanus (Lobster) Shell Waste. Sustain. Chem. Pharm. 2024, 37, 101363. [Google Scholar] [CrossRef]
- Kandasamy, G.; Manisekaran, R.; Arthikala, M.K. Chitosan Nanoplatforms in Agriculture for Multi-Potential Applications—Adsorption/Removal, Sustained Release, Sensing of Pollutants & Delivering Their Alternatives—A Comprehensive Review. Environ. Res. 2024, 240, 117447. [Google Scholar]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked Ionic Polysaccharides for Stimuli-Sensitive Drug Delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef]
- Polk, A.E.; Amsden, B.; Scarratt, D.J.; Gonzal, A.; Okhamafe, A.O.; Goosen, M.F. Oral Delivery in Aquaculture: Controlled Release of Proteins from Chitosan-Alginate Microcapsules. Aquac. Eng. 1994, 13, 311–323. [Google Scholar] [CrossRef]
- Stie, M.B.; Thoke, H.S.; Issinger, O.G.; Hochscherf, J.; Guerra, B.; Olsen, L.F. Delivery of Proteins Encapsulated in Chitosan-Tripolyphosphate Nanoparticles to Human Skin Melanoma Cells. Colloids Surf. B Biointerfaces 2019, 174, 216–223. [Google Scholar] [CrossRef]
- Zhou, W.; He, Y.; Liu, F.; Liao, L.; Huang, X.; Li, R.; Zou, Y.; Zhou, L.; Zou, L.; Liu, Y.; et al. Carboxymethyl Chitosan-Pullulan Edible Films Enriched with Galangal Essential Oil: Characterization and Application in Mango Preservation. Carbohydr. Polym. 2021, 256, 117579. [Google Scholar] [CrossRef]
- Mahmoud, D.E.; Billa, N. Physicochemical Modifications in Microwave-Irradiated Chitosan: Biopharmaceutical and Medical Applications. J. Biomater. Sci. Polym. Ed. 2024, 35, 898–915. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.S.; Anjum, M.A.; Naz, S.; Ali, S.; Hussain, S.; Azam, M.; Sardar, H.; Khaliq, G.; Canan, İ.; Ejaz, S. Incorporation of Ascorbic Acid in Chitosan-Based Edible Coating Improves Postharvest Quality and Storability of Strawberry Fruits. Int. J. Biol. Macromol. 2021, 189, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Indumathi, M.P.; Sarojini, K.S.; Rajarajeswari, G.R. Antimicrobial and Biodegradable Chitosan/Cellulose Acetate Phthalate/ZnO Nano Composite Films with Optimal Oxygen Permeability and Hydrophobicity for Extending the Shelf Life of Black Grape Fruits. Int. J. Biol. Macromol. 2019, 132, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Amirani, E.; Hallajzadeh, J.; Asemi, Z.; Mansournia, M.A.; Yousefi, B. Effects of Chitosan and Oligochitosans on the Phosphatidylinositol 3-Kinase-AKT Pathway in Cancer Therapy. Int. J. Biol. Macromol. 2020, 164, 456–467. [Google Scholar] [CrossRef]
- Yi, G.; Ling, J.; Jiang, Y.; Lu, Y.Q.; Yang, L.Y.; Ouyang, X.K. Fabrication, Characterization, and in Vitro Evaluation of Doxorubicin-Coupled Chitosan Oligosaccharide Nanoparticles. J. Mol. Struct. 2022, 1268, 133688. [Google Scholar] [CrossRef]
- Ye, L.; Chen, H. Characterization of the Interactions between Chitosan/Whey Protein at Different Conditions. Food Sci. Technol. 2019, 39, 163–169. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of Chitosan in Food, Pharmaceuticals, Medicine, Cosmetics, Agriculture, Textiles, Pulp and Paper, Biotechnology, and Environmental Chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, Biodistribution and Toxicity of Chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems—A Review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef]
- Dhavale, R.P.; Sahoo, S.C.; Kollu, P.; Jadhav, S.U.; Patil, P.S.; Dongale, T.D.; Chougale, A.D.; Patil, P.B. Chitosan Coated Magnetic Nanoparticles as Carriers of Anticancer Drug Telmisartan: PH-Responsive Controlled Drug Release and Cytotoxicity Studies. J. Phys. Chem. Solids 2021, 148, 109749. [Google Scholar] [CrossRef]
- Rostami, E. Progresses in Targeted Drug Delivery Systems Using Chitosan Nanoparticles in Cancer Therapy: A Mini-Review. J. Drug Deliv. Sci. Technol. 2020, 58, 101813. [Google Scholar] [CrossRef]
- Huang, S.-J.; Wang, T.-H.; Chou, Y.-H.; Wang, H.-M.D.; Hsu, T.-C.; Yow, J.-L.; Tzang, B.-S.; Chiang, W.-H. Hybrid PEGylated Chitosan/PLGA Nanoparticles Designed as PH-Responsive Vehicles to Promote Intracellular Drug Delivery and Cancer Chemotherapy. Int. J. Biol. Macromol. 2022, 210, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent Advances on Chitosan-Based Micro- and Nanoparticles in Drug Delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef]
- Sreekumar, S.; Goycoolea, F.M.; Moerschbacher, B.M.; Rivera-Rodriguez, G.R. Parameters Influencing the Size of Chitosan-TPP Nano- and Microparticles. Sci. Rep. 2018, 8, 4695. [Google Scholar] [CrossRef]
- Wu, T.; Wu, H.; Wang, Q.; He, X.; Shi, P.; Yu, B.; Cong, H.; Shen, Y. Current Status and Future Developments of Biopolymer Microspheres in the Field of Pharmaceutical Preparation. Adv. Colloid Interface Sci. 2024, 334, 103317. [Google Scholar] [CrossRef]
- Shah, B.R.; Dvořák, P.; Velíšek, J.; Mráz, J. Opening a New Gateway towards the Applications of Chitosan Nanoparticles Stabilized Pickering Emulsion in the Realm of Aquaculture. Carbohydr. Polym. 2021, 265, 118096. [Google Scholar] [CrossRef]
- Carvalho, S.G.; Silvestre, A.L.P.; dos Santos, A.M.; Fonseca-Santos, B.; Rodrigues, W.D.; Gremião, M.P.D.; Chorilli, M.; Villanova, J.C.O. Polymeric-Based Drug Delivery Systems for Veterinary Use: State of the Art. Int. J. Pharm. 2021, 604, 120756. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef]
- Kipkoech, C.; Kinyuru, J.N.; Imathiu, S.; Meyer-Rochow, V.B.; Roos, N. In Vitro Study of Cricket Chitosan’s Potential as a Prebiotic and a Promoter of Probiotic Microorganisms to Control Pathogenic Bacteria in the Human Gut. Foods 2021, 10, 2310. [Google Scholar] [CrossRef]
- Elsherbiny, A.S.; Galal, A.; Ghoneem, K.M.; Salahuddin, N.A. Novel Chitosan-Based Nanocomposites as Ecofriendly Pesticide Carriers: Synthesis, Root Rot Inhibition and Growth Management of Tomato Plants. Carbohydr. Polym. 2022, 282, 119111. [Google Scholar] [CrossRef]
- Gao, K.; Zhan, J.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.H.; Chen, X.; Li, P. Synthesis and Effects of the Selective Oxidation of Chitosan in Induced Disease Resistance against Botrytis cinerea. Carbohydr. Polym. 2021, 265, 118073. [Google Scholar] [CrossRef]
- Delgado-Cedeño, A.; Hernández-Martínez, S.P.; Ramos-Zayas, Y.; Marroquín-Cardona, A.G.; Méndez-Zamora, G.; Franco-Molina, M.A.; Kawas, J.R. Insoluble Chitosan Complex as a Potential Adsorbent for Aflatoxin B1 in Poultry Feed. Front. Mater. 2022, 9, 1044495. [Google Scholar] [CrossRef]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for Food Packaging: Recent Advances in Active and Intelligent Films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Gutiérrez-Martínez, P.; Ramos-Guerrero, A.; Rodríguez-Pereida, C.; Coronado-Partida, L.; Angulo-Parra, J.; González-Estrada, R. Chitosan for Postharvest Disinfection of Fruits and Vegetables. In Postharvest Disinfection of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2018; pp. 231–241. [Google Scholar]
- Khanmohammadi, M.; Elmizadeh, H.; Ghasemi, K. Investigation of Size and Morphology of Chitosan Nanoparticles Used in Drug Delivery System Employing Chemometric Technique. Iran. J. Pharm. Res. IJPR 2015, 14, 665–675. [Google Scholar] [PubMed]
- Jang, M.-K.; Kong, B.-G.; Jeong, Y.-I.; Lee, C.H.; Nah, J.-W. Physicochemical Characterization of α-Chitin, β-Chitin, and γ-Chitin Separated from Natural Resources. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the Potential of Hydrogels for Advanced Therapeutic Applications: Current Achievements and Future Directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Wang, C.; Song, Z.; Ning, X.; Wang, Y.; Xiao, J. Solid Lipid–Threshold Guided Chitosan Coating of NLCs for Improved Gastrointestinal Stability and Curcumin Bioavailability. Colloids Surf. A Physicochem. Eng. Asp. 2025, 723, 137388. [Google Scholar] [CrossRef]
- Schröder, V.; Rău, I.; Dobrin, N.; Stefanov, C.; Mihali, C.V.; Pădureţu, C.C.; Apetroaei, M.R. Micromorphological Details and Identification of Chitinous Wall Structures in Rapana venosa (Gastropoda, Mollusca) Egg Capsules. Sci. Rep. 2020, 10, 14550. [Google Scholar] [CrossRef]
- Supernak, M.; Makurat-Kasprolewicz, B.; Kaczmarek-Szczepańska, B.; Pałubicka, A.; Sakowicz-Burkiewicz, M.; Ronowska, A.; Wekwejt, M. Chitosan-Based Membranes as Gentamicin Carriers for Biomedical Applications—Influence of Chitosan Molecular Weight. Membranes 2023, 13, 542. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Boki, H.; Kamijo, H.; Nakajima, R.; Oka, T.; Shishido-Takahashi, N.; Suga, H.; Sugaya, M.; Sato, S.; Miyagaki, T. YKL-40 Promotes Proliferation of Cutaneous T-Cell Lymphoma Tumor Cells through Extracellular Signal–Regulated Kinase Pathways. J. Investig. Dermatol. 2020, 140, 860–868.e3. [Google Scholar] [CrossRef]
- Chang, M.C.; Chen, C.T.; Chiang, P.F.; Chiang, Y.C. The Role of Chitinase-3-like Protein-1 (YKL40) in the Therapy of Cancer and Other Chronic-Inflammation-Related Diseases. Pharmaceuticals 2024, 17, 307. [Google Scholar] [CrossRef] [PubMed]
- Böckelmann, L.C.; Felix, T.; Calabrò, S.; Schumacher, U. YKL-40 Protein Expression in Human Tumor Samples and Human Tumor Cell Line Xenografts: Implications for Its Use in Tumor Models. Cell. Oncol. 2021, 44, 1183–1195. [Google Scholar] [CrossRef]
- Mazur, M.; Zielińska, A.; Grzybowski, M.M.; Olczak, J.; Fichna, J. Chitinases and Chitinase-like Proteins as Therapeutic Targets in Inflammatory Diseases, with a Special Focus on Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6966. [Google Scholar] [CrossRef]
- Maraksa, K.; Suyotha, W.; Cheirsilp, B. Production of Alpha-and Beta-Chitin, Chitosan and Protein Hydrolysate from Seafood Processing Wastes Using an Integration of Lactic Acid and Digestive Protease from Fish Viscera as Alternative Green Extraction. Biocatal. Agric. Biotechnol. 2025, 64, 103496. [Google Scholar] [CrossRef]
- Dinculescu, D.D.; Apetroaei, M.R.; Gîjiu, C.L.; Anton, M.; Enache, L.; Schröder, V.; Isopescu, R.; Rău, I. Simultaneous Optimization of Deacetylation Degree and Molar Mass of Chitosan from Shrimp Waste. Polymers 2024, 16, 170. [Google Scholar] [CrossRef]
- Dinculescu, D.; Gîjiu, C.L.; Apetroaei, M.R.; Isopescu, R.; Rău, I.; Schröder, V. Optimization of Chitosan Extraction Process from Rapana Venosa Egg Capsules Waste Using Experimental Design. Materials 2023, 16, 525. [Google Scholar] [CrossRef]
- Pădurețu, C.-C.; Isopescu, R.; Rău, I.; Apetroaei, M.R.; Schröder, V. Influence of the Parameters of Chitin Deacetylation Process on the Chitosan Obtained from Crab Shell Waste. Korean J. Chem. Eng. 2019, 36, 1890–1899. [Google Scholar] [CrossRef]
- Apetroaei, M.; Manea, A.M.; Tihan, G.; Zgârian, R.; Schroder, V.; Rǎu, I. Improved Method of Chitosan Extraction from Different Crustacean Species of Romanian Black Sea Coast. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2017, 79, 25–36. [Google Scholar]
- Berkow, E.L.; Lockhart, S.R.; Ostrosky-Zeichner, L. Antifungal Susceptibility Testing: Current Approaches. Clin. Microbiol. Rev. 2020, 33, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Melcón, C.; Alonso-Calleja, C.; García-Fernández, C.; Carballo, J.; Capita, R. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) for Twelve Antimicrobials (Biocides and Antibiotics) in Eight Strains of Listeria Monocytogenes. Biology 2021, 11, 46. [Google Scholar] [CrossRef]
- Hazen, K.C. Fungicidal versus Fungistatic Activity of Terbinafine and Itraconazole: An in Vitro Comparison. J. Am. Acad. Dermatol. 1998, 38, S37–S41. [Google Scholar] [CrossRef] [PubMed]
- Jalalifar, S.; Razavi, S.; Mirzaei, R.; Irajian, G.; Bagheri, K.P. A Hope for Ineffective Antibiotics to Return to Treatment: Investigating the Anti-Biofilm Potential of Melittin Alone and in Combination with Penicillin and Oxacillin against Multidrug Resistant-MRSA and -VRSA. Front. Microbiol. 2024, 14, 1269392. [Google Scholar] [CrossRef] [PubMed]
- White, R.L.; Burgess, D.S.; Manduru, M.; Bosso, J.A. Comparison of Three Different in Vitro Methods of Detecting Synergy: Time-Kill, Checkerboard, and E Test. Antimicrob. Agents Chemother. 1996, 40, 1914–1918. [Google Scholar] [CrossRef]
- Manfra, L.; Savorelli, F.; Di Lorenzo, B.; Libralato, G.; Comin, S.; Conti, D.; Floris, B.; Francese, M.; Gallo, M.L.; Gartner, I.; et al. Intercalibration of Ecotoxicity Testing Protocols with Artemia Franciscana. Ecol. Indic. 2015, 57, 41–47. [Google Scholar] [CrossRef]
- Libralato, G.; Prato, E.; Migliore, L.; Cicero, A.M.; Manfra, L. A Review of Toxicity Testing Protocols and Endpoints with Artemia spp. Ecol. Indic. 2016, 69, 35–49. [Google Scholar] [CrossRef]
- Apetroaei, M.R.; Pădureţu, C.; Rău, I.; Schroder, V. New-Chitosan Characterization and Its Bioassay in Different Salinity Solutions Using Artemia Salina as Bio Tester. Chem. Pap. 2018, 72, 1853–1860. [Google Scholar] [CrossRef]
- Hassan, U.A.; Hussein, M.Z.; Alitheen, N.B.; Ariff, S.A.Y.; Masarudin, M.J. In Vitro Cellular Localization and Efficient Accumulation of Fluorescently Tagged Biomaterials from Monodispersed Chitosan Nanoparticles for Elucidation of Controlled Release Pathways for Drug Delivery Systems. Int. J. Nanomed. 2018, 13, 5075–5095. [Google Scholar] [CrossRef]
- Kuen, C.Y.; Masarudin, M.J. Chitosan Nanoparticle-Based System: A New Insight into the Promising Controlled Release System for Lung Cancer Treatment. Molecules 2022, 27, 473. [Google Scholar] [CrossRef]
- Roy, S.; Chakraborty, T.; Begum, J.; Hasnain, M.S.; Nayak, A.K. Chitosan. In Chitosan in Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–11. [Google Scholar]
- Popescu, R. Development and Preliminary Evaluation of Intranasal Hydrocolloidal Systems Based on Chitosan and PVA with Insulin, for Central Nervous System-Associated Diseases. Farmacia 2024, 72, 963–974. [Google Scholar] [CrossRef]
- Liu, A.; Song, H.; Jia, P.; Lin, Y.; Song, Q.; Gao, J. Supramolecular Assembly and Reversible Transition and of Chitosan Fluorescent Micelles by Noncovalent Modulation. Adv. Polym. Technol. 2021, 2021, 5175473. [Google Scholar] [CrossRef]
- Mohite, P.; Shah, S.R.; Singh, S.; Rajput, T.; Munde, S.; Ade, N.; Prajapati, B.G.; Paliwal, H.; Mori, D.D.; Dudhrejiya, A.V. Chitosan and Chito-Oligosaccharide: A Versatile Biopolymer with Endless Grafting Possibilities for Multifarious Applications. Front. Bioeng. Biotechnol. 2023, 11, 1190879. [Google Scholar] [CrossRef]
- Apetroaei, M.-M. Integrating Nutraceuticals in the One Health Framework: A Path to Holistic Health Solutions. Farmacia 2024, 72, 719–729. [Google Scholar] [CrossRef]
- Merz, C.R. Physicochemical and Colligative Investigation of α (Shrimp Shell)- and β (Squid Pen)-Chitosan Membranes: Concentration-Gradient-Driven Water Flux and Ion Transport for Salinity Gradient Power and Separation Process Operations. ACS Omega 2019, 4, 21027–21040. [Google Scholar] [CrossRef] [PubMed]
- Seidi, F.; Yazdi, M.K.; Jouyandeh, M.; Dominic, M.; Naeim, H.; Nezhad, M.N.; Bagheri, B.; Habibzadeh, S.; Zarrintaj, P.; Saeb, M.R.; et al. Chitosan-Based Blends for Biomedical Applications. Int. J. Biol. Macromol. 2021, 183, 1818–1850. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.A.; Onzi, G.R.; Morawski, A.S.; Pohlmann, A.R.; Guterres, S.S.; Contri, R.V. Chitosan as a Coating Material for Nanoparticles Intended for Biomedical Applications. React. Funct. Polym. 2020, 147, 104459. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Chang, S.-H.; Lin, H.-T.V.; Wu, G.-J.; Tsai, G.J. PH Effects on Solubility, Zeta Potential, and Correlation between Antibacterial Activity and Molecular Weight of Chitosan. Carbohydr. Polym. 2015, 134, 74–81. [Google Scholar] [CrossRef]
- Akdaşçi, E.; Duman, H.; Eker, F.; Bechelany, M.; Karav, S. Chitosan and Its Nanoparticles: A Multifaceted Approach to Antibacterial Applications. Nanomaterials 2025, 15, 126. [Google Scholar] [CrossRef]
- Pădurețu, C.-C.; Apetroaei, M.R.; Rău, I.; Schroder, V. Characterization of chitosan extracted from different romanian black sea crustaceans. Chem. Mater. Sci. 2018, 80, 13–24. [Google Scholar]
- Petroni, S.; Tagliaro, I.; Antonini, C.; D’Arienzo, M.; Orsini, S.; Mano, J.; Brancato, V.; Borges, J.; Cipolla, L. Chitosan-Based Biomaterials: Insights into Chemistry, Properties, Devices, and Their Biomedical Applications. Mar. Drugs 2023, 21, 147. [Google Scholar] [CrossRef] [PubMed]
- Mirbagheri, V.S.; Alishahi, A.; Ahmadian, G.; Petroudi, S.H.H.; Ojagh, S.M.; Romanazzi, G. Toward Understanding the Antibacterial Mechanism of Chitosan: Experimental Approach and in Silico Analysis. Food Hydrocoll. 2024, 147, 109382. [Google Scholar] [CrossRef]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial Activity of Chitosans and Chitosan Oligomers with Different Molecular Weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sbeih, K.A.; Al-Mazaideh, G.M.; Al-Zereini, W.A. Production of Medium-Sized Chitosan Oligomers Using Molecular Sieves and Their Antibacterial Activity. Carbohydr. Polym. 2022, 295, 119889. [Google Scholar] [CrossRef]
- Fernández-Pan, I.; Maté, J.I.; Gardrat, C.; Coma, V. Effect of Chitosan Molecular Weight on the Antimicrobial Activity and Release Rate of Carvacrol-Enriched Films. Food Hydrocoll. 2015, 51, 60–68. [Google Scholar] [CrossRef]
- Guan, Z.; Feng, Q. Chitosan and Chitooligosaccharide: The Promising Non-Plant-Derived Prebiotics with Multiple Biological Activities. Int. J. Mol. Sci. 2022, 23, 6761. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018, 2018, 1708172. [Google Scholar] [CrossRef]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial Properties of Chitosan from Different Developmental Stages of the Bioconverter Insect Hermetia Illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar] [CrossRef]
- Khan, H.; Andleeb, S.; Nisar, T.; Latif, Z.; Raja, S.A.; Awan, U.A.; Maqbool, K.; Khurshid, S. Interactions of Chitosan-Coated Green Synthesized Silver Nanoparticles Using Mentha Spicata and Standard Antibiotics against Bacterial Pathogens. Curr. Pharm. Biotechnol. 2023, 24, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.K.; Kim, S.W. Recent Advances in Polymeric Drug Delivery Systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Palma-Guerrero, J.; Lopez-Jimenez, J.A.; Pérez-Berná, A.J.; Huang, I.-C.; Jansson, H.-B.; Salinas, J.; Villalaín, J.; Read, N.D.; Lopez-Llorca, L.V. Membrane Fluidity Determines Sensitivity of Filamentous Fungi to Chitosan. Mol. Microbiol. 2010, 75, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Robles-Martínez, L.; Guerra-Sánchez, M.G.; Hernández-Lauzardo, A.N.; Pardo, J.P.; Velázquez-del Valle, M.G. Effects of Chitosan and Oligochitosan on Development and Mitochondrial Function of Rhizopus stolonifer. J. Basic Microbiol. 2014, 54, S42–S49. [Google Scholar] [CrossRef]
- Balusamy, S.R.; Rahimi, S.; Sukweenadhi, J.; Sunderraj, S.; Shanmugam, R.; Thangavelu, L.; Mijakovic, I.; Perumalsamy, H. Chitosan, Chitosan Nanoparticles and Modified Chitosan Biomaterials, a Potential Tool to Combat Salinity Stress in Plants. Carbohydr. Polym. 2022, 284, 119189. [Google Scholar] [CrossRef]
- Moenne, A.; González, A. Chitosan-, Alginate- Carrageenan-Derived Oligosaccharides Stimulate Defense against Biotic and Abiotic Stresses, and Growth in Plants: A Historical Perspective. Carbohydr. Res. 2021, 503, 108298. [Google Scholar] [CrossRef]
- Motta, C.M.; Simoniello, P.; Arena, C.; Capriello, T.; Panzuto, R.; Vitale, E.; Agnisola, C.; Tizzano, M.; Avallone, B.; Ferrandino, I. Effects of Four Food Dyes on Development of Three Model Species, Cucumis Sativus, Artemia Salina and Danio Rerio: Assessment of Potential Risk for the Environment. Environ. Pollut. 2019, 253, 1126–1135. [Google Scholar] [CrossRef]
- Soltanian, S.; François, J.M.; Dhont, J.; Arnouts, S.; Sorgeloos, P.; Bossier, P. Enhanced Disease Resistance in Artemia by Application of Commercial β-Glucans Sources and Chitin in a Gnotobiotic Artemia Challenge Test. Fish Shellfish. Immunol. 2007, 23, 1304–1314. [Google Scholar] [CrossRef]
- Riddle, M.R.; Baxter, B.K.; Avery, B.J. Molecular Identification of Microorganisms Associated with the Brine Shrimp Artemia franciscana. Aquat. Biosyst. 2013, 9, 7. [Google Scholar] [CrossRef]
- Richardson, S.; Kolbe, H.V.; Dunca, R. Potential of Low Molecular Mass Chitosan as a DNA Delivery System: Biocompatibility, Body Distribution and Ability to Complex and Protect DNA. Int. J. Pharm. 1999, 178, 231–243. [Google Scholar] [CrossRef]
- Opanasopit, P.; Aumklad, P.; Kowapradit, J.; Ngawhiranpat, T.; Apirakaramwong, A.; Rojanarata, T.; Puttipipatkhachorn, S. Effect of Salt Forms and Molecular Weight of Chitosans on In Vitro Permeability Enhancement in Intestinal Epithelial Cells (Caco-2). Pharm. Dev. Technol. 2007, 12, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qu, G.; Sun, Y.; Yang, T.; Yao, Z.; Shen, W.; Shen, Z.; Ding, Q.; Zhou, H.; Ping, Q. Biological Evaluation of N-Octyl-O-Sulfate Chitosan as a New Nano-Carrier of Intravenous Drugs. Eur. J. Pharm. Sci. 2008, 33, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.V.; Nanduri, L.S.Y. Interaction of Chitin/Chitosan with Salivary and Other Epithelial Cells—An Overview. Int. J. Biol. Macromol. 2017, 104, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Au, S.Y.; Lee, C.M.; Weinstein, J.E.; van den Hurk, P.; Klaine, S.J. Trophic Transfer of Microplastics in Aquatic Ecosystems: Identifying Critical Research Needs. Integr. Environ. Assess. Manag. 2017, 13, 505–509. [Google Scholar] [CrossRef]
- Bucci, K.; Tulio, M.; Rochman, C.M. What is Known and Unknown about the Effects of Plastic Pollution: A Meta-Analysis and Systematic Review. Ecol. Appl. 2020, 30, e02044. [Google Scholar] [CrossRef]
- Granek, E.F.; Brander, S.M.; Holland, E.B. Microplastics in Aquatic Organisms: Improving Understanding and Identifying Research Directions for the next Decade. Limnol. Oceanogr. Lett. 2020, 5, 1–4. [Google Scholar] [CrossRef]
- Fogliano, C.; Carotenuto, R.; Agnisola, C.; Motta, C.M.; Avallone, B. Impact of Benzodiazepine Delorazepam on Growth and Behaviour of Artemia salina Nauplii. Biology 2024, 13, 808. [Google Scholar] [CrossRef]
- Ahmadzadeh, P.; Naeemi, A.S.; Mansouri, B. Toxicity of Polystyrene Nanoplastic and Copper Oxide Nanoparticle in Artemia Salina: Single and Combined Effects on Stress Responses. Mar. Environ. Res. 2025, 203, 106831. [Google Scholar] [CrossRef]
- Lima, L.R.; Andrade, F.K.; Alves, D.R.; de Morais, S.M.; Vieira, R.S. Anti-Acetylcholinesterase and Toxicity against Artemia salina of Chitosan Microparticles Loaded with Essential Oils of Cymbopogon flexuosus, Pelargonium × ssp. and Copaifera officinalis. Int. J. Biol. Macromol. 2021, 167, 1361–1370. [Google Scholar] [CrossRef]
- Lopalco, P.; Lobasso, S.; Lopes-Dos-Santos, R.M.A.; Van Stappen, G.; Corcelli, A. Lipid Profile Changes During the Development of Artemia franciscana, from Cysts to the First Two Naupliar Stages. Front. Physiol. 2019, 9, 1872. [Google Scholar] [CrossRef]
- Wydro, P.; Krajewska, B.; Ha̧c-Wydro, K. Chitosan as a Lipid Binder: A Langmuir Monolayer Study of Chitosan−Lipid Interactions. Biomacromolecules 2007, 8, 2611–2617. [Google Scholar] [CrossRef]
- Chae, S.Y.; Jang, M.-K.; Nah, J.-W. Influence of Molecular Weight on Oral Absorption of Water Soluble Chitosans. J. Control. Release 2005, 102, 383–394. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Bai, B.; Song, G.; Zhang, J.; Yu, H.; Huang, S.; Wang, Z.; Lu, G. Topical Drug Delivery Strategies for Enhancing Drug Effectiveness by Skin Barriers, Drug Delivery Systems and Individualized Dosing. Front. Pharmacol. 2024, 14, 1333986. [Google Scholar] [CrossRef]
- Simion, D.; Apetroaei, M.; Gaidau, C.; Simion, M.; Vasile, G.; Cruceru, L.; Pascu, L.; Ciprian, C.; Apetroaei, M.; Schroder, V. Advanced Biotechnologies for Obtaining Biodegradable Collagen Based “Core-Shell/Hollow” Structural Nano—SiO2 Composite and Its Applications for Drug. In Proceedings of the International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, Albena, Bulgaria, 17–26 June 2014; SGEM: Vienna, Austria, 2014; Volume 1. [Google Scholar]



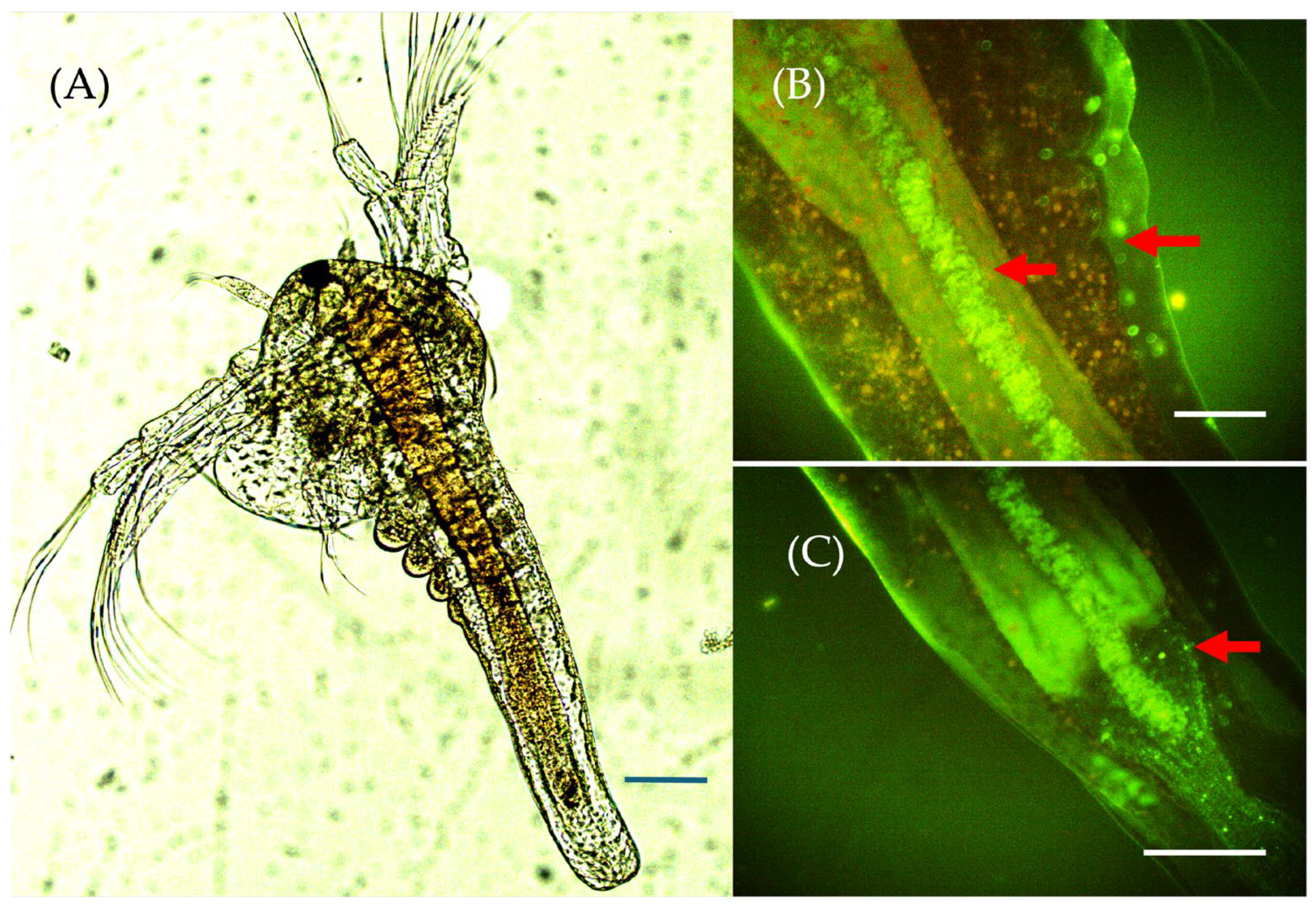


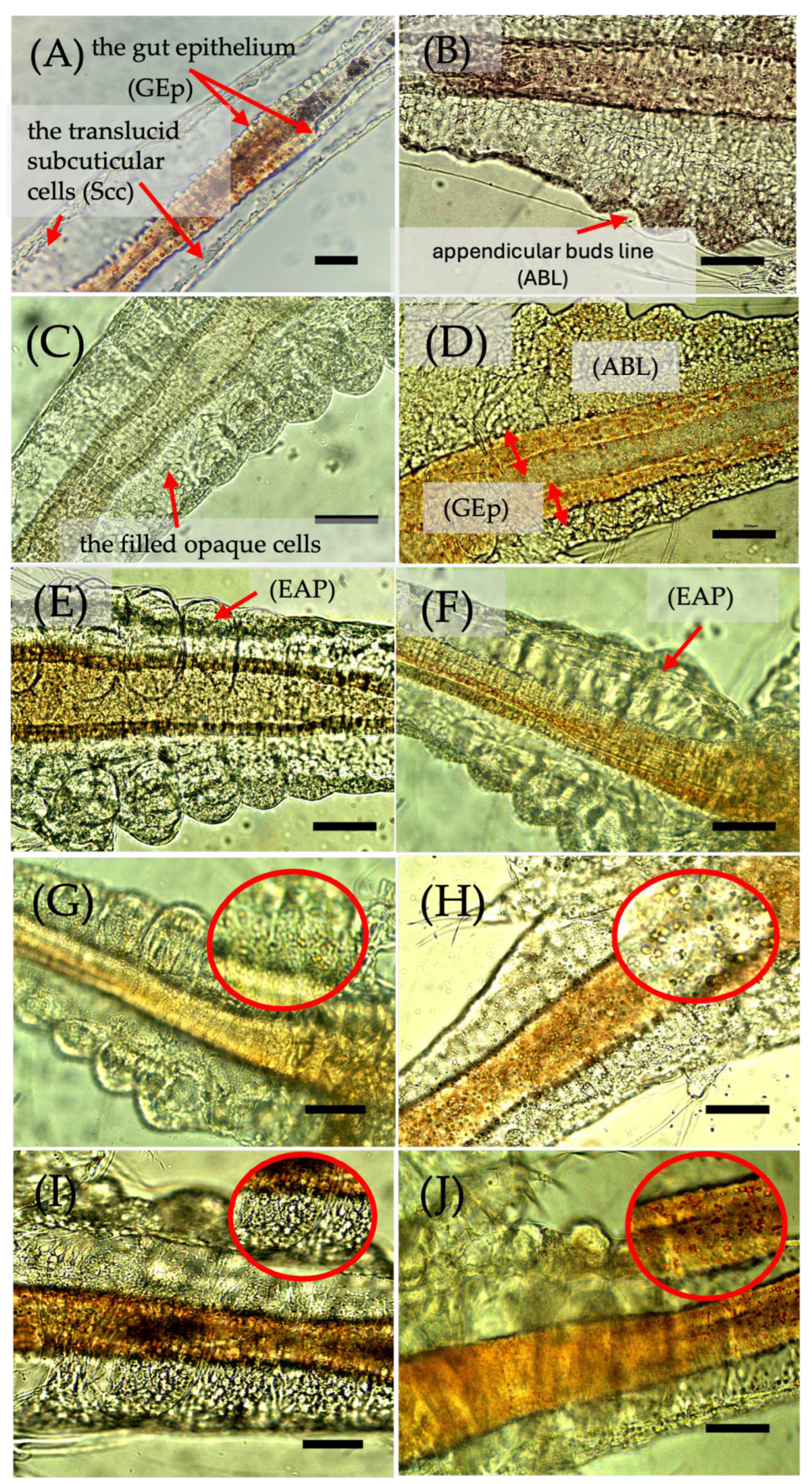

| Sample | CNaOH (%) | Chitin Mass/NaOH Solution Volume | Time (min) | MM (kDa) | DDA (%) |
|---|---|---|---|---|---|
| α-oligoCH | 35 | 01:13 | 150 | 26.39 | 75.50 |
| α-CH (polymer) | 45 | 01:18 | 120 | 804.33 | 86.65 |
| Samples | CH1 | CH2 | CH3 | Mixture 1 CH1:CH2 | Mixture 2 CH1:CH3 | Control | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | K1 | K2 | K3 | M | ||||
| Chitosan type | β-oligoCH | α-oligo CH | α-CH | 1:1 | 2:1 | 3:1 | 1:1 | 2:1 | 3:1 | Water (35 ppt) |
| Total larvae | 89 | 67 | 61 | 76 | 78 | 85 | 100 | 94 | 80 | 45 |
| Average number of larvae/cuvette | 14 | 10 | 10 | 10 | 10 | 11 | 14 | 11 | 11 | 10 |
| Samples | CH1 | CH2 | CH3 | Mixture 1 | Mixture 2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| CH1:CH2 | CH1:CH3 | ||||||||
| C1 | C2 | C3 | K1 | K2 | K3 | ||||
| Chitosan type | β-oligoCH | α-oligoCH | α-CH | 1:1 | 2:1 | 3:1 | 1:1 | 2:1 | 3:1 |
| MM (kDa) | 1.5 | 26.39 | 804.33 | 2.84 | 2.19 | 1.96 | 2.99 | 2.25 | 2 |
| DDA (%) | 70 | 75.5 | 86.65 | 71.83 | 71.1 | 70.79 | 75.55 | 73.33 | 72.38 |
| Sample | Staphylococcus aureus ATCC 23235 (Gram-Positive) | Ratio Value | Escherichia coli ATCC 25922 (Gram-Negative) | Ratio Value | Candida parapsilosis ATCC 22019 | Ratio Value | |||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | MIC | MFC | MFC/MIC | |
| CH1 | 0.16 | 0.32 | 2.00 | 2.50 | 5.00 | 2.00 | 0.31 | 0.31 | 1.00 |
| CH2 | 0.50 | 1.00 | 2.00 | 0.60 | 5.00 | 2.00 | 0.80 | 1.70 | 2.00 |
| CH3 | 0.80 | 1.70 | 2.00 | 1.70 | 3.50 | 2.00 | 1.70 | 3.00 | 2.00 |
| C1 | 2.50 | 5.00 | 2.00 | 2.50 | 2.50 | 1.00 | 2.50 | 5.00 | 2.00 |
| C2 | 2.50 | 5.00 | 2.00 | 2.50 | 2.50 | 1.00 | 2.50 | 5.00 | 2.00 |
| C3 | 2.50 | 5.00 | 2.00 | 2.50 | 2.50 | 1.00 | 2.50 | 5.00 | 2.00 |
| K1 | 1.25 | 2.50 | 2.00 | 2.50 | 2.50 | 1.00 | 2.50 | 5.00 | 2.00 |
| K2 | 1.25 | 2.50 | 2.00 | 2.50 | 2.50 | 1.00 | 2.50 | 5.00 | 2.00 |
| K3 | 1.25 | 2.50 | 2.00 | 2.50 | 2.50 | 1.00 | 2.50 | 5.00 | 2.00 |
| ceftriaxone | 0.16 | 0.30 | 2.00 | 0.16 | 0.16 | 1.00 | - | 2.00 | |
| fluconazole | - | - | 0.62 | 1.25 | 2.00 | ||||
| Microorganisms | Mixture 1 | Mixture 2 | ||||
|---|---|---|---|---|---|---|
| FICICH2 | FICICH1 | FICI * | FICCH3 | FICCH1 | FICI ** | |
| Staphylococcus aureus | 5 | 15.6 | 20.6 | 1.6 | 7.8 | 9.4 |
| Escherichia coli | 4 | 1 | 5.2 | 1.5 | 1 | 2.5 |
| Candida parapsilosis | 3.1 | 8.1 | 11.2 | 1.5 | 8.1 | 9.5 |
| VAR vs. VAR | R | N | p-Value |
|---|---|---|---|
| Naupliar stages vs. Survival 70 | 0.94 | 9 | 0.000068 |
| Organogenesis vs. Naupliar stages | 0.89 | 9 | 0.000608 |
| Motility vs. Naupliar stages | 0.88 | 9 | 0.000783 |
| Organogenesis vs. Survival 70 | 0.81 | 9 | 0.003800 |
| Organogenesis vs. Motility | 0.8 | 9 | 0.004580 |
| Cellular inclusions vs. Naupliar stages | 0.78 | 9 | 0.006800 |
| Cellular inclusions vs. Survival 35 | −0.78 | 9 | 0.006850 |
| Cellular inclusions vs. Survival 70 | 0.77 | 9 | 0.007280 |
| Motility vs. Survival 70 | 0.72 | 9 | 0.01 |
| Cellular inclusions vs. Organogenesis | 0.60 | 9 | 0.04 |
| Cellular inclusions vs. Motility | 0.55 | 9 | 0.06 |
| Naupliar stages vs. Survival 35 | −0.39 | 9 | 0.15 |
| Organogenesis vs. Survival 35 | −0.39 | 9 | 0.15 |
| MFC/MIC m vs. Cellular inclusions | −0.35 | 9 | 0.18 |
| Survival 70 vs. Survival 35 | −0.31 | 9 | 0.21 |
| MFC/MIC m vs. Organogenesis | 0.29 | 9 | 0.23 |
| Motility vs. Survival 35 | −0.28 | 9 | 0.23 |
| MFC/MIC m vs. Survival 70 | −0.23 | 9 | 0.28 |
| MFC/MIC m vs. Naupliar stages | −0.18 | 9 | 0.32 |
| MFC/MIC m vs. Motility | −0.12 | 9 | 0.37 |
| MFC/MIC m vs. Survival 35 | −0.02 | 9 | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schröder, V.; Mitea, G.; Rău, I.; Apetroaei, M.R.; Iancu, I.M.; Apetroaei, M.-M. Chitosan Mixtures from Marine Sources: A Comparative Study of Biological Responses and Practical Applications. Polysaccharides 2025, 6, 80. https://doi.org/10.3390/polysaccharides6030080
Schröder V, Mitea G, Rău I, Apetroaei MR, Iancu IM, Apetroaei M-M. Chitosan Mixtures from Marine Sources: A Comparative Study of Biological Responses and Practical Applications. Polysaccharides. 2025; 6(3):80. https://doi.org/10.3390/polysaccharides6030080
Chicago/Turabian StyleSchröder, Verginica, Gabriela Mitea, Ileana Rău, Manuela Rossemary Apetroaei, Irina Mihaela Iancu, and Miruna-Maria Apetroaei. 2025. "Chitosan Mixtures from Marine Sources: A Comparative Study of Biological Responses and Practical Applications" Polysaccharides 6, no. 3: 80. https://doi.org/10.3390/polysaccharides6030080
APA StyleSchröder, V., Mitea, G., Rău, I., Apetroaei, M. R., Iancu, I. M., & Apetroaei, M.-M. (2025). Chitosan Mixtures from Marine Sources: A Comparative Study of Biological Responses and Practical Applications. Polysaccharides, 6(3), 80. https://doi.org/10.3390/polysaccharides6030080









