The Stability of Four Kinds of Cellulose Pickering Emulsions and Optimization of the Properties of Mayonnaise by a Soybean Byproduct Pickering Emulsion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Cellulose from Soybean Byproduct and Other Waste Residue
2.3. Experimental Study on the Microstructure of Cellulose
2.4. Determination of the Physicochemical Properties of Cellulose
2.4.1. Water-Holding Capacity (WHC)
2.4.2. Oil-Holding Capacity (OHC)
2.4.3. Swelling Capacity
2.4.4. Angle of Repose
2.4.5. Bulk Density (BD)
2.5. Preparation of the Pickering Emulsions
2.6. Determination of Pickering Emulsion Stability
2.6.1. The Effects of Ionic Strength and pH
2.6.2. The Effects of Centrifugation, Storage and Temperature
2.6.3. The Effect of Solid Emulsifier and Oil Phase Ratio
2.7. Application Effect of the Cellulose Pickering Emulsion
2.7.1. Production of Mayonnaise
2.7.2. Determination of Fat, Moisture and Dietary Fiber Content in Mayonnaise
2.7.3. Determination of Some Indicators Affecting the Quality of Mayonnaise
2.8. Data Analysis
3. Results and Discussions
3.1. Microstructure of Cellulose
3.2. Physicochemical Properties of Cellulose
3.3. Pickering Emulsion Stability
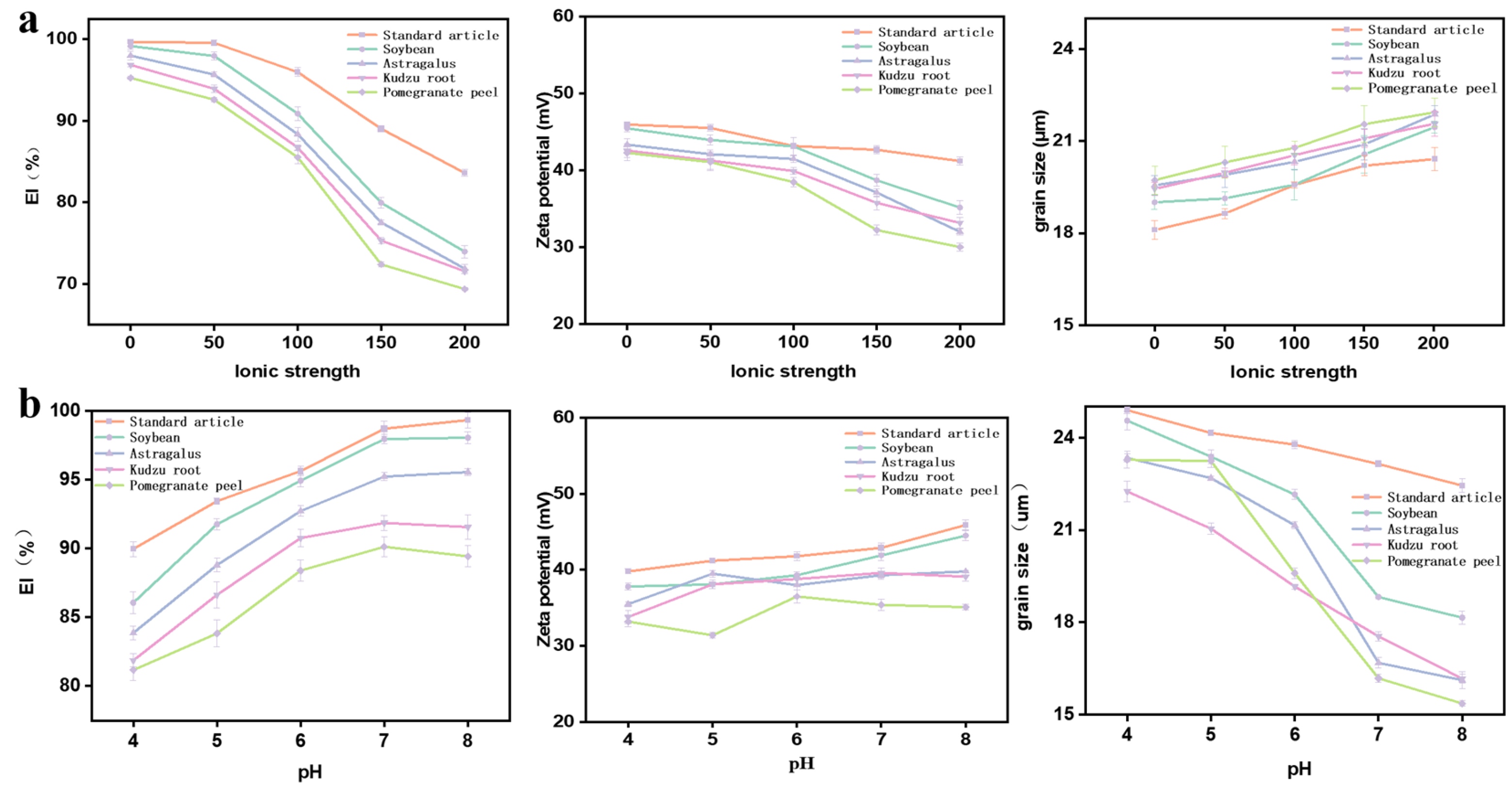
3.3.1. The Effects of Ionic Strength and pH on Stability
3.3.2. The Effects of Centrifugation, Storage and Temperature on Stability
3.3.3. The Effect of Solid Emulsifier Concentration and Oil Phase Ratio on Stability
3.4. The Application of a Pickering Emulsion Immobilized by Soybean Byproduct Cellulose in Mayonnaise
3.4.1. Contents of Fat, Moisture and Dietary Fiber in Mayonnaise
3.4.2. Mayonnaise Zeta Potential and Particle Size
3.4.3. The Acid Value and Peroxide Value of Mayonnaise
3.4.4. Swelling Rate of the Mayonnaise
3.4.5. Centrifugal Oiling Rate of Mayonnaise
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Usman, M.; Li, Q.; Luo, D.; Xing, Y.; Dong, D. Valorization of soybean by-products for sustainable waste processing with health benefits. J. Sci. Food Agric. 2025, 105, 5150–5162. [Google Scholar] [CrossRef]
- Zhu, B.; Huang, F.; Guo, J.; Song, K.; He, J.; Liu, S.; Zhou, X. Unveiling the hidden potential of Pueraria lobata: A comprehensive analysis based on fiber morphology and physicochemical properties. J. Mater. Sci. 2024, 59, 20824–20839. [Google Scholar] [CrossRef]
- Asghar, A.; Afzaal, M.; Saeed, F.; Ahmed, A.; Ateeq, H.; Shah, Y.A.; Islam, F.; Hussain, M.; Akram, N.; Shah, M.A. Valorization and food applications of okara (soybean residue): A concurrent review. Food Sci. Nutr. 2023, 11, 3631–3640. [Google Scholar] [CrossRef]
- Liu, Z. A review on the emerging conversion technology of cellulose, starch, lignin, protein and other organics from vegetable-fruit-based waste. Int. J. Biol. Macromol. 2023, 242, 124804. [Google Scholar] [CrossRef] [PubMed]
- Schröder, A.; Laguerre, M.; Tenon, M.; Schroën, K.; Berton-Carabin, C.C. Natural particles can armor emulsions against lipid oxidation and coalescence. Food Chem. 2021, 347, 129003. [Google Scholar] [CrossRef] [PubMed]
- Sitnikov, P.; Legki, P.; Torlopov, M.; Druz, Y.; Mikhaylov, V.; Tarabukin, D.; Vaseneva, I.; Markarova, M.; Ushakov, N.; Udoratina, E. Efficient (bio) emulsification/degradation of crude oil using cellulose nanocrystals. Polysaccharides 2023, 4, 402–420. [Google Scholar] [CrossRef]
- Xia, T.; Xue, C.; Wei, Z. Physicochemical characteristics, applications and research trends of edible Pickering emulsions. Trends Food Sci. Technol. 2021, 107, 1–15. [Google Scholar] [CrossRef]
- Geng, S.; Jiang, Z.; Ma, H.; Pu, P.; Liu, B.; Liang, G. Fabrication and characterization of novel edible Pickering emulsion gels stabilized by dihydromyricetin. Food Chem. 2021, 343, 128486. [Google Scholar] [CrossRef]
- Souza, A.G.D.; Ferreira, R.R.; Aguilar, E.S.F.; Zanata, L.; Rosa, D.D.S. Cinnamon essential oil nanocellulose-based pickering emulsions: Processing parameters effect on their formation, stabilization, and antimicrobial activity. Polysaccharides 2021, 2, 608–625. [Google Scholar] [CrossRef]
- Liu, F.; Tang, C.H. Soy protein nanoparticle aggregates as pickering stabilizers for oil-in-water emulsions. J. Agric. Food Chem. 2013, 61, 8888–8898. [Google Scholar] [CrossRef]
- Taslikh, M.; Mollakhalili-Meybodi, N.; Alizadeh, A.M.; Mousavi, M.M.; Nayebzadeh, K.; Mortazavian, A.M. Mayonnaise main ingredients influence on its structure as an emulsion. J. Food Sci. Technol. 2022, 59, 2108–2116. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.; Jeon, S.; Jeong, S.; Mun, S. Development of egg yolk-free mayonnaise using rice protein with xanthan gum. Food Sci. Biotechnol. 2025, 34, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Li, J.; Li, X.; Wang, C.; Zhou, B.; Su, Y.; Yang, Y. Effect of protein microparticle and pectin on properties of light mayonnaise. LWT-Food Sci. Technol. 2017, 82, 8–14. [Google Scholar] [CrossRef]
- Bano, S.; Negi, Y.S. Studies on cellulose nanocrystals isolated from groundnut shells. Carbohydr. Polym. 2017, 157, 1041–1049. [Google Scholar] [CrossRef]
- Luo, M.; Hu, K.; Zeng, Q.; Yang, X.; Wang, Y.; Dong, L.; Huang, F.; Zhang, R.; Su, D. Comparative analysis of the morphological property and chemical composition of soluble and insoluble dietary fiber with bound phenolic compounds from different algae. J. Food Sci. 2020, 85, 3843–3851. [Google Scholar] [CrossRef]
- Kan, J.; Liu, J.; Yong, H.; Liu, Y.; Qin, Y.; Liu, J. Development of active packaging based on chitosan-gelatin blend films functionalized with Chinese hawthorn (Crataegus pinnatifida) fruit extract. Int. J. Biol. Macromol. 2019, 140, 384–392. [Google Scholar] [CrossRef]
- Song, X.; Liu, L.; Wu, X.; Liu, Y.; Yuan, J. Chitosan-based functional films integrated with magnolol: Characterization, antioxidant and antimicrobial activity and pork preservation. Int. J. Mol. Sci. 2021, 22, 7769. [Google Scholar] [CrossRef]
- Lu, H.; Gui, Y.; Zheng, L.; Liu, X. Morphological, crystalline, thermal and physicochemical properties of cellulose nanocrystals obtained from sweet potato residue. Food Res. Int. 2013, 50, 121–128. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, H.; Li, Y.; Huang, Q. Fabrication of milled cellulose particles-stabilized Pickering emulsions. Food Hydrocoll. 2018, 77, 427–435. [Google Scholar] [CrossRef]
- Yu, J.; Yun, M.; Li, J.; Gao, Y.; Mao, L. Development of Oleogel-in-Water High Internal Phase Emulsions with Improved Physicochemical Stability and Their Application in Mayonnaise. Foods 2024, 13, 2738. [Google Scholar] [CrossRef]
- Ma, M.M.; Mu, T.H. Effects of extraction methods and particle size distribution on the structural, physicochemical, and functional properties of dietary fiber from deoiled cumin. Food Chem. 2016, 194, 237–246. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Hou, Q.; Li, X. Isolation and characterization of microfibrillated cellulose from agro-industrial soybean residue (okara). BioResources 2018, 13, 7944–7956. [Google Scholar] [CrossRef]
- Aldosari, O.F.; Jabli, M.; Morad, M.H. Chemical extraction of cellulose from Ligno-cellulosic Astragalus armatus pods: Characterization, and application to the biosorption of methylene blue. Arab. J. Chem. 2023, 16, 105019. [Google Scholar] [CrossRef]
- Razzak, A.; Khiari, R.; Moussaoui, Y.; Belgacem, M.N. Cellulose nanofibers from Schinus molle: Preparation and characterization. Molecules 2022, 27, 6738. [Google Scholar] [CrossRef] [PubMed]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Song, S.; Li, Y.; Zhu, Q.; Zhang, X.; Wang, Y.; Tao, L.; Yu, L. Structure and properties of Pickering emulsions stabilized solely with novel buckwheat protein colloidal particles. Int. J. Biol. Macromol. 2023, 226, 61–71. [Google Scholar] [CrossRef]
- Wei, Z.; Cheng, J.; Huang, Q. Food-grade Pickering emulsions stabilized by ovotransferrin fibrils. Food Hydrocoll. 2019, 94, 592–602. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Q.; Wang, B.; Feng, X.; Xu, H.; Mao, Z.; You, C.; Sui, X. Synthetic semicrystalline cellulose oligomers as efficient Pickering emulsion stabilizers. Carbohydr. Polym. 2021, 254, 117445. [Google Scholar] [CrossRef]
- Wen, C.; Yuan, Q.; Liang, H.; Vriesekoop, F. Preparation and stabilization of d-limonene Pickering emulsions by cellulose nanocrystals. Carbohydr. Polym. 2014, 112, 695–700. [Google Scholar] [CrossRef]
- Jiang, F.; Zhu, Y.; Hu, W.X.; Li, M.; Liu, Y.; Feng, J.; Lv, X.; Yu, X.; Du, S.K. Characterization of quinoa starch nanoparticles as a stabilizer for oil in water Pickering emulsion. Food Chem. 2023, 427, 136697. [Google Scholar] [CrossRef]
- Laca, A.; Sáenz, M.C.; Paredes, B.; Díaz, M. Rheological properties, stability and sensory evaluation of low-cholesterol mayonnaises prepared using egg yolk granules as emulsifying agent. J. Food Eng. 2010, 97, 243–252. [Google Scholar] [CrossRef]
- An, D.; Zhai, S.; Li, L. Characteristics of soy protein hydrolysate nanofibrils and their stabilization mechanism for Pickering emulsion: Interfacial properties, Rheology and stability. LWT 2023, 189, 115473. [Google Scholar] [CrossRef]
- Liu, X.; Sun, H.; Mu, T.; Gontard, N. Exploring the potential of potato products: Puree and cellulose nanofibers, to improve the nutritional value of mayonnaise. Food Chem. 2024, 437, 137864. [Google Scholar] [CrossRef]
- Xu, W.; Yin, Y.; Cao, B.; Sun, H.; Zhu, X.; Zang, J.; Kang, M.; Luo, D. Fabrication and characterization of mayonnaise based on xanthan gum/lysozyme nanoparticles and konjac glucomannan as egg yolk substitutes under different temperature stresses. LWT 2024, 210, 116832. [Google Scholar] [CrossRef]





| Specimen | Moisture | Fat | Dietary Fiber |
|---|---|---|---|
| 1 | 8.32 b | 85.62 ± 0.76 c | / |
| 2 | 5.12 a | 88.34 ± 0.66 d | / |
| 3 | 9.31 c | 81.78 ± 0.15 b | / |
| Pickering-2% | 8.78 bc | 83.21 ± 0.65 bc | 1.02 ± 0.05 a |
| Pickering-4% | 11.16 d | 81.44 ± 0.78 b | 1.15 ± 0.03 b |
| Pickering-6% | 14.14 e | 79.61 ± 0.31 a | 1.08 ± 0.03 ab |
| Pickering-8% | 18.84 f | 76.21 ± 0.36 a | 1.11 ± 0.01 ab |
| Pickering-10% | 20.45 g | 74.19 ± 0.57 a | 1.18 ± 0.03 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Feng, Z.; Liu, L.; Zhang, Y.; Li, J.; Wu, X. The Stability of Four Kinds of Cellulose Pickering Emulsions and Optimization of the Properties of Mayonnaise by a Soybean Byproduct Pickering Emulsion. Polysaccharides 2025, 6, 77. https://doi.org/10.3390/polysaccharides6030077
Zheng Z, Feng Z, Liu L, Zhang Y, Li J, Wu X. The Stability of Four Kinds of Cellulose Pickering Emulsions and Optimization of the Properties of Mayonnaise by a Soybean Byproduct Pickering Emulsion. Polysaccharides. 2025; 6(3):77. https://doi.org/10.3390/polysaccharides6030077
Chicago/Turabian StyleZheng, Zhanxin, Ziwei Feng, Liu Liu, Yuhuan Zhang, Jianke Li, and Xiaoxia Wu. 2025. "The Stability of Four Kinds of Cellulose Pickering Emulsions and Optimization of the Properties of Mayonnaise by a Soybean Byproduct Pickering Emulsion" Polysaccharides 6, no. 3: 77. https://doi.org/10.3390/polysaccharides6030077
APA StyleZheng, Z., Feng, Z., Liu, L., Zhang, Y., Li, J., & Wu, X. (2025). The Stability of Four Kinds of Cellulose Pickering Emulsions and Optimization of the Properties of Mayonnaise by a Soybean Byproduct Pickering Emulsion. Polysaccharides, 6(3), 77. https://doi.org/10.3390/polysaccharides6030077






