Propolis-Functionalized Biomaterials for Wound Healing: A Systematic Review with Emphasis on Polysaccharide-Based Platforms
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
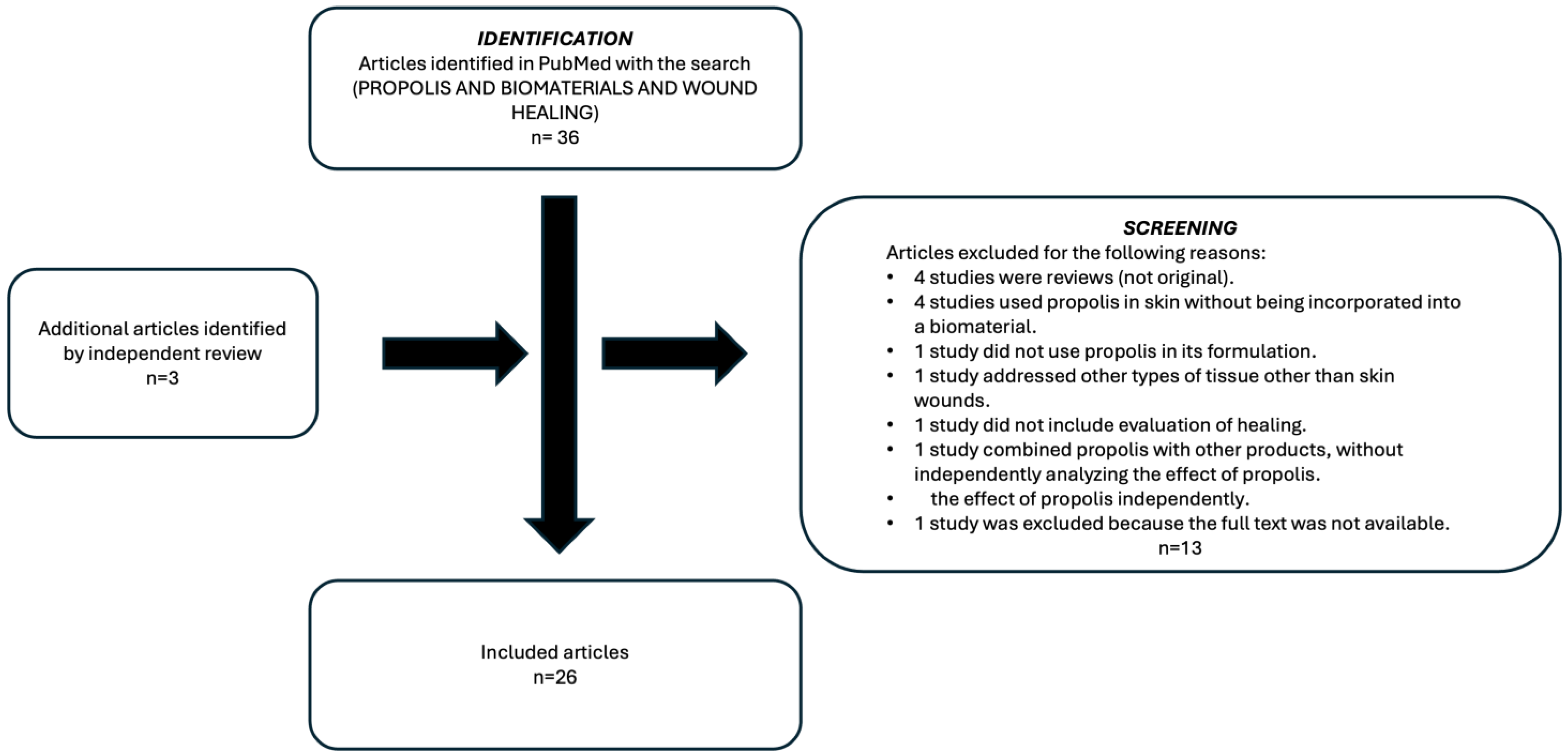
2.4. Study Selection Process
2.5. Data Extraction and Analysis
2.6. Methodological Quality Assessment
2.7. Analysis of the Results
2.8. Use of Generative Artificial Intelligence
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Apoptotic Factor |
| AQP3 | Aquaporin 3 |
| ARRIVE | Animal Research: Reporting of In Vivo Experiments |
| ATP | Adenosine Triphosphate |
| ATR | Attenuated Total Reflectance |
| BC | Bacterial Cellulose |
| BWD | Bilayer Wound Dressing |
| CA | Cellulose Acetate |
| CAM | Chorioallantoic Membrane |
| CAPE | Caffeic Acid Phenethyl Ester |
| CS | Chitosan |
| DLS | Dynamic Light Scattering |
| DNA | Deoxyribonucleic Acid |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DSC | Differential Scanning Calorimetry |
| DTZ | Diltiazem |
| ECM | Extracellular Matrix |
| EDX | Energy-Dispersive X-ray Spectroscopy |
| EEP | Ethanolic Extract of Propolis |
| EPP | Standardized Extract of Propolis |
| FE-SEM | Field Emission Scanning Electron Microscopy |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| GPC | Gel Permeation Chromatography |
| GPT | Glutamate Pyruvate Transaminase |
| HA | Hyaluronic Acid |
| HFB4 | Human Fibroblasts |
| HNT | Halloysite Nanotubes |
| HPLC | High-Performance Liquid Chromatography |
| HPCS | Hydroxypropyl Chitosan |
| HRMS | High-Resolution Mass Spectrometry |
| IL | Interleukin |
| MEF | Mouse Embryonic Fibroblasts |
| MIC | Minimum Inhibitory Concentration |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| MS | Mass Spectrometry |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MyD88 | Myeloid Differentiation Primary Response 88 |
| NF | Nanofibers |
| NF-κB | Nuclear Factor kappa-B |
| NIH | National Institutes of Health |
| NL | Nanolignin |
| NLRP | NOD-, LRR-, and Pyrin Domain-Containing Protein (Inflammasome Family) |
| NMR | Nuclear Magnetic Resonance |
| NPs | Nanoparticles |
| NRL | Natural Rubber Latex |
| PP | Propolis |
| PCL | Polycaprolactone |
| PEC | Pectin |
| PLGA | Poly(lactic-co-glycolic acid) |
| EMP | Propolis Extracted with Methylal |
| PMN | Polymorphonuclear Cells |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PU | Polyurethane |
| PULMA | Methacrylated Pullulan |
| PVA | Polyvinyl Alcohol |
| QCS | Quaternized Chitosan |
| RNA | Ribonucleic Acid |
| SEM | Scanning Electron Microscopy |
| SFO | Silk Fibroin Oxidized |
| SPI | Soy Protein Isolate |
| TAC | Total Antioxidant Capacity |
| TEM | Transmission Electron Microscopy |
| TGA | Thermogravimetric Analysis |
| TLR4 | Toll-Like Receptor 4 |
| TNF-α | Tumor Necrosis Factor alpha |
| TRIF | TIR-domain-containing Adapter-Inducing Interferon-β |
| UHPLC | Ultra-High-Performance Liquid Chromatography |
| UV–Vis | Ultraviolet–Visible Spectroscopy |
| WEP | Water Extract of Propolis |
| WVP | Water Vapor Permeability |
| WVTR | Water Vapor Transmission Rate |
| XRD | X-ray Diffraction |
| ZnO-NPs | Zinc Oxide Nanoparticles |
References
- Celleno, L.; Tamburi, F. Structure and Function of the Skin. In Nutritional Cosmetics; Elsevier: Amsterdam, The Netherlands, 2009; pp. 3–45. [Google Scholar]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef]
- Iversen, A.K.S.; Lichtenberg, M.; Fritz, B.G.; Díaz-Pinés Cort, I.; Al-Zoubaidi, D.F.; Gottlieb, H.; Kirketerp-Møller, K.; Bjarnsholt, T.; Jakobsen, T.H. The Chronic Wound Characterisation Study and Biobank: A Study Protocol for a Prospective Observational Cohort Investigation of Bacterial Community Composition, Inflammatory Responses and Wound-Healing Trajectories in Non-Healing Wounds. BMJ Open 2024, 14, e084081. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lin, F.; Thalib, L.; Chaboyer, W. Global Prevalence and Incidence of Pressure Injuries in Hospitalised Adult Patients: A Systematic Review and Meta-Analysis. Int. J. Nurs. Stud. 2020, 105, 103546. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Britto, E.J.; Nazewic, T.A.; Popowicz, P.; Robins, M. Wound Dressings [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024; Available online: https://www.ncbi.nlm.nih (accessed on 15 August 2025).
- Tatarusanu, S.-M.; Lupascu, F.-G.; Profire, B.-S.; Szilagyi, A.; Gardikiotis, I.; Iacob, A.-T.; Caluian, I.; Herciu, L.; Giscă, T.-C.; Baican, M.-C.; et al. Modern Approaches in Wounds Management. Polymers 2023, 15, 3648. [Google Scholar] [CrossRef]
- Gushiken, L.F.S.; Beserra, F.P.; Bastos, J.K.; Jackson, C.J.; Pellizzon, C.H. Cutaneous Wound Healing: An Update from Physiopathology to Current Therapies. Life 2021, 11, 665. [Google Scholar] [CrossRef]
- Prete, S.; Dattilo, M.; Patitucci, F.; Pezzi, G.; Parisi, O.I.; Puoci, F. Natural and Synthetic Polymeric Biomaterials for Application in Wound Management. J. Funct. Biomater. 2023, 14, 455. [Google Scholar] [CrossRef]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical Aspects of Propolis Research in Modern Times. Evid.-Based Complement. Altern. Med. 2013, 2013, 964149. [Google Scholar] [CrossRef]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and Functional Properties of Propolis (Bee Glue): A Review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef]
- Zhang, W.; Margarita, G.E.; Wu, D.; Yuan, W.; Yan, S.; Qi, S.; Xue, X.; Wang, K.; Wu, L. Antibacterial Activity of Chinese Red Propolis against Staphylococcus Aureus and MRSA. Molecules 2022, 27, 1693. [Google Scholar] [CrossRef]
- Bouchelaghem, S. Propolis Characterization and Antimicrobial Activities against Staphylococcus Aureus and Candida Albicans: A Review. Saudi J. Biol. Sci. 2022, 29, 1936–1946. [Google Scholar] [CrossRef]
- Zulhendri, F.; Lesmana, R.; Tandean, S.; Christoper, A.; Chandrasekaran, K.; Irsyam, I.; Suwantika, A.A.; Abdulah, R.; Wathoni, N. Recent Update on the Anti-Inflammatory Activities of Propolis. Molecules 2022, 27, 8473. [Google Scholar] [CrossRef] [PubMed]
- Lesmana, R.; Tandean, S.; Christoper, A.; Suwantika, A.A.; Wathoni, N.; Abdulah, R.; Fearnley, J.; Bankova, V.; Zulhendri, F. Propolis as an Autophagy Modulator in Relation to Its Roles in Redox Balance and Inflammation Regulation. Biomed. Pharmacother. 2024, 175, 116745. [Google Scholar] [CrossRef]
- Malekahmadi, M.; Pahlavani, N.; Heshmati, J.; Clayton, Z.S.; Beigmohammadi, M.T.; Navashenaq, J.G.; Alavi-Naeini, A. Effect of Propolis Supplementation on Oxidative Stress Markers: A Systematic Review of Randomized Controlled Trials. J. Herb. Med. 2023, 40, 100679. [Google Scholar] [CrossRef]
- Popova, M.; Trusheva, B.; Chimshirova, R.; Antonova, D.; Gechovska, K.; Thanh, L.N.; Lien, N.T.P.; Phuong, D.T.L.; Bankova, V. Chemical Profile and Antioxidant Capacity of Propolis from Tetragonula, Lepidotrigona, Lisotrigona and Homotrigona Stingless Bee Species in Vietnam. Molecules 2022, 27, 7834. [Google Scholar] [CrossRef]
- Papp, Z.; Bouchelaghem, S.; Szekeres, A.; Meszéna, R.; Gyöngyi, Z.; Papp, G. The Scent of Antifungal Propolis. Sensors 2021, 21, 2334. [Google Scholar] [CrossRef]
- Cerqueira, P.; Cunha, A.; Almeida-Aguiar, C. Potential of Propolis Antifungal Activity for Clinical Applications. J. Appl. Microbiol. 2022, 133, 1207–1228. [Google Scholar] [CrossRef]
- Berretta, A.A.; Silveira, M.A.D.; Cóndor Capcha, J.M.; De Jong, D. Propolis and Its Potential against SARS-CoV-2 Infection Mechanisms and COVID-19 Disease. Biomed. Pharmacother. 2020, 131, 110622. [Google Scholar] [CrossRef]
- Ożarowski, M.; Karpiński, T.M. The Effects of Propolis on Viral Respiratory Diseases. Molecules 2023, 28, 359. [Google Scholar] [CrossRef] [PubMed]
- Asfaram, S.; Fakhar, M.; Keighobadi, M.; Akhtari, J. Promising Anti-Protozoan Activities of Propolis (Bee Glue) as Natural Product: A Review. Acta Parasitol. 2021, 66, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Forma, E.; Bryś, M. Anticancer Activity of Propolis and Its Compounds. Nutrients 2021, 13, 2594. [Google Scholar] [CrossRef]
- Altabbal, S.; Athamnah, K.; Rahma, A.; Wali, A.F.; Eid, A.H.; Iratni, R.; Al Dhaheri, Y. Propolis: A Detailed Insight of Its Anticancer Molecular Mechanisms. Pharmaceuticals 2023, 16, 450. [Google Scholar] [CrossRef] [PubMed]
- da Rosa, C.; Bueno, I.L.; Quaresma, A.C.M.; Longato, G.B. Healing Potential of Propolis in Skin Wounds Evidenced by Clinical Studies. Pharmaceuticals 2022, 15, 1143. [Google Scholar] [CrossRef]
- Yang, J.; Pi, A.; Yan, L.; Li, J.; Nan, S.; Zhang, J.; Hao, Y. Research Progress on Therapeutic Effect and Mechanism of Propolis on Wound Healing. Evid.-Based Complement. Altern. Med. 2022, 2022, 5798941. [Google Scholar] [CrossRef]
- Stojko, M.; Wolny, D.; Włodarczyk, J. Nonwoven Releasing Propolis as a Potential New Wound Healing Method—A Review. Molecules 2021, 26, 5701. [Google Scholar] [CrossRef]
- Bal-Öztürk, A.; Torkay, G.; İdil, N.; Akar, R.O.; Özbaş, Z.; Özkahraman, B. Propolis-Loaded Photocurable Methacrylated Pullulan Films: Evaluation of Mechanical, Antibacterial, Biocompatibility, Wound Healing and pro-Angiogenic Abilities. Int. J. Biol. Macromol. 2024, 282, 137071. [Google Scholar] [CrossRef]
- El-Ghoul, Y.; Altuwayjiri, A.S.; Alharbi, G.A. Synthesis and Characterization of New Electrospun Medical Scaffold-Based Modified Cellulose Nanofiber and Bioactive Natural Propolis for Potential Wound Dressing Applications. RSC Adv. 2024, 14, 26183–26197. [Google Scholar] [CrossRef]
- Ferreira, L.M.D.M.C.; Cruz, N.F.D.; Lynch, D.G.; Costa, P.F.D.; Salgado, C.G.; Silva-Júnior, J.O.C.; Rossi, A.; Ribeiro-Costa, R.M. Hydrogel Containing Propolis: Physical Characterization and Evaluation of Biological Activities for Potential Use in the Treatment of Skin Lesions. Pharmaceuticals 2024, 17, 1400. [Google Scholar] [CrossRef]
- Jaberifard, F.; Almajidi, Y.Q.; Arsalani, N.; Ghorbani, M. A Self-Healing Crosslinked-Xanthan Gum/Soy Protein Based Film Containing Halloysite Nanotube and Propolis with Antibacterial and Antioxidant Activity for Wound Healing. Int. J. Pharm. 2024, 656, 124073. [Google Scholar] [CrossRef] [PubMed]
- Mehdikhani, M.; Yilgör, P.; Poursamar, S.A.; Etemadi, N.; Gokyer, S.; Navid, S.; Farzan, M.; Farzan, M.; Babaei, M.; Rafienia, M. A Hybrid 3D-Printed and Electrospun Bilayer Pharmaceutical Membrane Based on Polycaprolactone/Chitosan/Polyvinyl Alcohol for Wound Healing Applications. Int. J. Biol. Macromol. 2024, 282, 136692. [Google Scholar] [CrossRef]
- Tayfeh-Ebrahimi, R.; Amniattalab, A.; Mohammadi, R. Evaluation of Effect of Biologically Synthesized Ethanolic Extract of Propolis-Loaded Poly(-Lactic-Co-Glycolic Acid) Nanoparticles on Wound Healing in Diabetic Rats. Int. J. Low. Extrem. Wounds 2024, 23, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Vaseghi, A.; Sadeghizadeh, M.; Herb, M.; Grumme, D.; Demidov, Y.; Remmler, T.; Maleki, H.H. 3D Printing of Biocompatible and Antibacterial Silica–Silk–Chitosan-Based Hybrid Aerogel Scaffolds Loaded with Propolis. ACS Appl. Bio Mater. 2024, 7, 7917–7935. [Google Scholar] [CrossRef]
- Zayed, H.S.; Saleh, S.; Omar, A.E.; Saleh, A.K.; Salama, A.; Tolba, E. Development of Collagen–Chitosan Dressing Gel Functionalized with Propolis–Zinc Oxide Nanoarchitectonics to Accelerate Wound Healing. Int. J. Biol. Macromol. 2024, 261, 129665. [Google Scholar] [CrossRef] [PubMed]
- Kapare, H.S.; Giram, P.S.; Raut, S.S.; Gaikwad, H.K.; Paiva-Santos, A.C. Formulation Development and Evaluation of Indian Propolis Hydrogel for Wound Healing. Gels 2023, 9, 375. [Google Scholar] [CrossRef] [PubMed]
- Phonrachom, O.; Charoensuk, P.; Kiti, K.; Saichana, N.; Kakumyan, P.; Suwantong, O. Potential Use of Propolis-Loaded Quaternized Chitosan/Pectin Hydrogel Films as Wound Dressings: Preparation, Characterization, Antibacterial Evaluation, and in Vitro Healing Assay. Int. J. Biol. Macromol. 2023, 241, 124633. [Google Scholar] [CrossRef]
- de Figueiredo, A.C.; Anaya-Mancipe, J.M.; de Barros, A.O.D.S.; Santos-Oliveira, R.; Dias, M.L.; Thiré, R.M.D.S.M. Nanostructured Electrospun Polycaprolactone—Propolis Mats Composed of Different Morphologies for Potential Use in Wound Healing. Molecules 2022, 27, 5351. [Google Scholar] [CrossRef]
- Shie Karizmeh, M.; Poursamar, S.A.; Kefayat, A.; Farahbakhsh, Z.; Rafienia, M. An in Vitro and in Vivo Study of PCL/Chitosan Electrospun Mat on Polyurethane/Propolis Foam as a Bilayer Wound Dressing. Biomater. Adv. 2022, 135, 112667. [Google Scholar] [CrossRef]
- Ceylan, S. Propolis Loaded and Genipin-Crosslinked PVA/Chitosan Membranes; Characterization Properties and Cytocompatibility/Genotoxicity Response for Wound Dressing Applications. Int. J. Biol. Macromol. 2021, 181, 1196–1206. [Google Scholar] [CrossRef]
- Pahlevanneshan, Z.; Deypour, M.; Kefayat, A.; Rafienia, M.; Sajkiewicz, P.; Esmaeely Neisiany, R.; Enayati, M.S. Polyurethane-Nanolignin Composite Foam Coated with Propolis as a Platform for Wound Dressing: Synthesis and Characterization. Polymers 2021, 13, 3191. [Google Scholar] [CrossRef]
- Sharaf, S.M.; Al-Mofty, S.E.-D.; El-Sayed, E.-S.M.; Omar, A.; Abo Dena, A.S.; El-Sherbiny, I.M. Deacetylated Cellulose Acetate Nanofibrous Dressing Loaded with Chitosan/Propolis Nanoparticles for the Effective Treatment of Burn Wounds. Int. J. Biol. Macromol. 2021, 193, 2029–2037. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Doostan, M.; Khorramizadeh, M.R. Antibacterial and Antioxidant Assessment of Cellulose Acetate/Polycaprolactone Nanofibrous Mats Impregnated with Propolis. Int. J. Biol. Macromol. 2019, 140, 1260–1268. [Google Scholar] [CrossRef]
- Krupp, T.; dos Santos, B.D.; Gama, L.A.; Silva, J.R.; Arrais-Silva, W.W.; de Souza, N.C.; Américo, M.F.; de Souza Souto, P.C. Natural Rubber-Propolis Membrane Improves Wound Healing in Second--Degree Burning Model. Int. J. Biol. Macromol. 2019, 131, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, W.A.; Azzazy, H.M. Apitherapeutics and Phage-Loaded Nanofibers As Wound Dressings with Enhanced Wound Healing and Antibacterial Activity. Nanomedicine 2017, 12, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- Zancanela, D.C.; Funari, C.S.; Herculano, R.D.; Mello, V.M.; Rodrigues, C.M.; Borges, F.A.; de Barros, N.R.; Marcos, C.M.; Almeida, A.M.F.; Guastaldi, A.C. Natural Rubber Latex Membranes Incorporated with Three Different Types of Propolis: Physical-Chemistry and Antimicrobial Behaviours. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Eskandarinia, A.; Kefayat, A.; Rafienia, M.; Agheb, M.; Navid, S.; Ebrahimpour, K. Cornstarch-Based Wound Dressing Incorporated with Hyaluronic Acid and Propolis: In Vitro and in Vivo Studies. Carbohydr. Polym. 2019, 216, 25–35. [Google Scholar] [CrossRef]
- Marquele-Oliveira, F.; da Silva Barud, H.; Torres, E.C.; Machado, R.T.A.; Caetano, G.F.; Leite, M.N.; Frade, M.A.C.; Ribeiro, S.J.L.; Berretta, A.A. Development, Characterization and Pre-Clinical Trials of an Innovative Wound Healing Dressing Based on Propolis (EPP-AF®)-Containing Self-Microemulsifying Formulation Incorporated in Biocellulose Membranes. Int. J. Biol. Macromol. 2019, 136, 570–578. [Google Scholar] [CrossRef]
- Barud, H.D.S.; de Araújo Júnior, A.M.; Saska, S.; Mestieri, L.B.; Campos, J.A.D.B.; de Freitas, R.M.; Ferreira, N.U.; Nascimento, A.P.; Miguel, F.G.; Vaz, M.M.d.O.L.L.; et al. Antimicrobial Brazilian Propolis (EPP-AF) Containing Biocellulose Membranes as Promising Biomaterial for Skin Wound Healing. Evid.-Based Complement. Altern. Med. 2013, 2013, 703024. [Google Scholar] [CrossRef]
- Picolotto, A.; Pergher, D.; Pereira, G.P.; Machado, K.G.; da Silva Barud, H.; Roesch-Ely, M.; Gonzalez, M.H.; Tasso, L.; Figueiredo, J.G.; Moura, S. Bacterial Cellulose Membrane Associated with Red Propolis as Phytomodulator: Improved Healing Effects in Experimental Models of Diabetes Mellitus. Biomed. Pharmacother. 2019, 112, 108640. [Google Scholar] [CrossRef] [PubMed]
- Khodabakhshi, D.; Eskandarinia, A.; Kefayat, A.; Rafienia, M.; Navid, S.; Karbasi, S.; Moshtaghian, J. In Vitro and in Vivo Performance of a Propolis-Coated Polyurethane Wound Dressing with High Porosity and Antibacterial Efficacy. Colloids Surf. B Biointerfaces 2019, 178, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Eskandarinia, A.; Kefayat, A.; Gharakhloo, M.; Agheb, M.; Khodabakhshi, D.; Khorshidi, M.; Sheikhmoradi, V.; Rafienia, M.; Salehi, H. A Propolis Enriched Polyurethane-Hyaluronic Acid Nanofibrous Wound Dressing with Remarkable Antibacterial and Wound Healing Activities. Int. J. Biol. Macromol. 2020, 149, 467–476. [Google Scholar] [CrossRef]
- Eskandarinia, A.; Kefayat, A.; Agheb, M.; Rafienia, M.; Amini Baghbadorani, M.; Navid, S.; Ebrahimpour, K.; Khodabakhshi, D.; Ghahremani, F. A Novel Bilayer Wound Dressing Composed of a Dense Polyurethane/Propolis Membrane and a Biodegradable Polycaprolactone/Gelatin Nanofibrous Scaffold. Sci. Rep. 2020, 10, 3063. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Trusheva, B.; Bankova, V. Propolis of Stingless Bees: A Phytochemist’s Guide through the Jungle of Tropical Biodiversity. Phytomedicine 2021, 86, 153098. [Google Scholar] [CrossRef]
- Economy Desk. Union Head: Iran Ranks 3rd in Global Honey Production. Iran Daily, 26 January 2025. [Google Scholar]
- Afrouzan, H.; Tahghighi, A.; Zakeri, S.; Es-Haghi, A. Chemical Composition and Antimicrobial Activities of Iranian Propolis. Iran. Biomed. J. 2018, 22, 50–65. [Google Scholar] [CrossRef]
- Fathi Hafshejani, S.; Lotfi, S.; Rezvannejad, E.; Mortazavi, M.; Riahi-Madvar, A. Correlation between Total Phenolic and Flavonoid Contents and Biological Activities of 12 Ethanolic Extracts of Iranian Propolis. Food Sci. Nutr. 2023, 11, 4308–4325. [Google Scholar] [CrossRef]
- Asgharpour, F.; Moghadamnia, A.A.; Kazemi, S.; Nouri, H.R.; Pouramir, M.; Mousavi, S.N.; Motallebnejad, M. Chemical Composition Analysis and In Vitro Investigation of Cytotoxic and Antioxidative Activities of Iranian Propolis against Breast Cancer Cell Line, MCF-7. ChemistrySelect 2018, 3, 10857–10863. [Google Scholar] [CrossRef]
- Berretta, A.A.; Arruda, C.; Miguel, F.G.; Baptista, N.; Nascimento, A.P.; Marquele-Oliveira, F.; Hori, J.I.; Barud, H.D.S.; Damaso, B.; Ramos, C.; et al. Functional Properties of Brazilian Propolis: From Chemical Composition Until the Market. In Superfood and Functional Food—An Overview of Their Processing and Utilization; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Silva, M.P.; Silva, T.M.; Mengarda, A.C.; Salvadori, M.C.; Teixeira, F.S.; Alencar, S.M.; Luz Filho, G.C.; Bueno-Silva, B.; de Moraes, J. Brazilian Red Propolis Exhibits Antiparasitic Properties in Vitro and Reduces Worm Burden and Egg Production in an Mouse Model Harboring Either Early or Chronic Schistosoma Mansoni Infection. J. Ethnopharmacol. 2021, 264, 113387. [Google Scholar] [CrossRef]
- Gomes, K.O.; Messias da Silva, L.C.F.; dos Santos, R.D.; Prado, B.A.; da Silva Montes, P.; Silva Rodrigues, L.F.; de Araújo, M.O.; Bilac, C.A.; Freire, D.O.; Gris, E.F.; et al. Chemical Characterization and Antibacterial Activities of Brazilian Propolis Extracts from Apis Mellifera Bees and Stingless Bees (Meliponini). PLoS ONE 2024, 19, e0307289. [Google Scholar] [CrossRef]
- Salatino, A.; Salatino, M.L.F.; Negri, G. How Diverse Is the Chemistry and Plant Origin of Brazilian Propolis? Apidologie 2021, 52, 1075–1097. [Google Scholar] [CrossRef]
- Iqbal, Y.; Ahmed, I.; Irfan, M.F.; Chatha, S.A.S.; Zubair, M.; Ullah, A. Recent Advances in Chitosan-Based Materials; The Synthesis, Modifications and Biomedical Applications. Carbohydr. Polym. 2023, 321, 121318. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Sun, H.; Pan, L.; Wang, D.; Sun, M.; Zeng, Z.; Li, S.; Dong, Q.; Su, F. Chitosan and Its Derivatives as Potential Biomaterials for Biomedical and Pharmaceutical Applications: A Comprehensive Review on Green Extraction Approaches, Recent Progresses, and Perspectives. Eur. Polym. J. 2025, 229, 113882. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of Xanthan Gum as Polysaccharide in Tissue Engineering: A Review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef]
- Jadav, M.; Pooja, D.; Adams, D.J.; Kulhari, H. Advances in Xanthan Gum-Based Systems for the Delivery of Therapeutic Agents. Pharmaceutics 2023, 15, 402. [Google Scholar] [CrossRef]
- Huang, J.; Li, L. 3D Printing of Cellulose-Based Biomaterials: A Review. BME Horiz. 2024, 3, 138. [Google Scholar] [CrossRef]
- Joseph, A.; Umamaheswari, S.; Vassou, M.C. Bacterial Cellulose: A Versatile Biomaterial for Biomedical Application. Carbohydr. Res. 2025, 552, 109350. [Google Scholar] [CrossRef]
- Saadan, R.; Ihammi, A.; Chigr, M.; Fatimi, A. A Short Overview of the Formulation of Cellulose-Based Hydrogels and Their Biomedical Applications. In Proceedings of the IOCBE 2024, Online, 16–18 October 2024; MDPI: Basel, Switzerland, 2024; p. 3. [Google Scholar]
- Marques, P.A.C.; Guerra, N.B.; dos Santos, L.S.; Mussagy, C.U.; Pegorin Brasil, G.S.; Burd, B.S.; Su, Y.; da Silva Sasaki, J.C.; Scontri, M.; de Lima Lopes Filho, P.E.; et al. Natural Rubber Latex-Based Biomaterials for Drug Delivery and Regenerative Medicine: Trends and Directions. Int. J. Biol. Macromol. 2024, 267, 131666. [Google Scholar] [CrossRef]
- Andrade, K.L.; Ramlow, H.; Floriano, J.F.; Acosta, E.D.; Faita, F.L.; Machado, R.A.F. Latex and Natural Rubber: Recent Advances for Biomedical Applications. Polímeros 2022, 32, e2022015. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Deng, X.; Gould, M.; Ali, M.A. A Review of Current Advancements for Wound Healing: Biomaterial Applications and Medical Devices. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2542–2573. [Google Scholar] [CrossRef]
- Sharma, A.; Kokil, G.R.; He, Y.; Lowe, B.; Salam, A.; Altalhi, T.A.; Ye, Q.; Kumeria, T. Inorganic/Organic Combination: Inorganic Particles/Polymer Composites for Tissue Engineering Applications. Bioact. Mater. 2023, 24, 535–550. [Google Scholar] [CrossRef]
- Pang, S.; Wu, D.; Yang, H.; Kamutzki, F.; Kurreck, J.; Gurlo, A.; Hanaor, D.A.H. Enhanced Mechanical Performance and Bioactivity in Strontium/Copper Co-Substituted Diopside Scaffolds. Biomater. Adv. 2023, 145, 213230. [Google Scholar] [CrossRef]
- Wang, W.; Liu, M.; Shafiq, M.; Li, H.; Hashim, R.; EL-Newehy, M.; EL-Hamshary, H.; Morsi, Y.; Mo, X. Synthesis of Oxidized Sodium Alginate and Its Electrospun Bio-Hybrids with Zinc Oxide Nanoparticles to Promote Wound Healing. Int. J. Biol. Macromol. 2023, 232, 123480. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cai, J.; Liu, D.; Liu, S.; Lei, D.; Zheng, L.; Wei, Q.; Gao, M. Zinc-Based Metal Organic Framework with Antibacterial and Anti-Inflammatory Properties for Promoting Wound Healing. Regen. Biomater. 2022, 9, rbac019. [Google Scholar] [CrossRef] [PubMed]
- Shiva, S.; Asuwin Prabu, R.G.; Bajaj, G.; John, A.E.; Chandran, S.; Kumar, V.V.; Ramakrishna, S. A Review on the Recent Applications of Synthetic Biopolymers in 3D Printing for Biomedical Applications. J. Mater. Sci. Mater. Med. 2023, 34, 62. [Google Scholar] [CrossRef] [PubMed]
- Almasi, H.; Jahanbakhsh Oskouie, M.; Saleh, A. A Review on Techniques Utilized for Design of Controlled Release Food Active Packaging. Crit. Rev. Food Sci. Nutr. 2021, 61, 2601–2621. [Google Scholar] [CrossRef]
- Ferreira, P.S.; Ribeiro, S.M.; Pontes, R.; Nunes, J. Production Methods and Applications of Bioactive Polylactic Acid: A Review. Environ. Chem. Lett. 2024, 22, 1831–1859. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Wrona, M.; Nerin, C.; Grabska-Zielińska, S. Polylactide-Based Films with the Addition of Poly(Ethylene Glycol) and Extract of Propolis—Physico-Chemical and Storage Properties. Foods 2022, 11, 1488. [Google Scholar] [CrossRef]
- Tavares, L.; Smaoui, S.; Lima, P.S.; de Oliveira, M.M.; Santos, L. Propolis: Encapsulation and Application in the Food and Pharmaceutical Industries. Trends Food Sci. Technol. 2022, 127, 169–180. [Google Scholar] [CrossRef]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An Overview of Encapsulation Technologies for Food Applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent Advances in 3D Printing of Biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef]
- Sarapa, A.; Peter, A.; Buettner, A.; Loos, H.M. Organoleptic and Chemical Properties of Propolis: A Review. Eur. Food Res. Technol. 2025, 251, 1331–1352. [Google Scholar] [CrossRef]
- Mitić, Ž.; Stolić, A.; Stojanović, S.; Najman, S.; Ignjatović, N.; Nikolić, G.; Trajanović, M. Instrumental Methods and Techniques for Structural and Physicochemical Characterization of Biomaterials and Bone Tissue: A Review. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 930–949. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, T.C.; Salzer, E.; Crispim, J.F.; Fabra, G.T.; LeVisage, C.; Pandit, A.; Tryfonidou, M.; Maitre, C.L.; Ito, K. Characterization of Biomaterials Intended for Use in the Nucleus Pulposus of Degenerated Intervertebral Discs. Acta Biomater. 2020, 114, 1–15. [Google Scholar] [CrossRef]
- Mugnaini, G.; Resta, C.; Poggi, G.; Bonini, M. Photopolymerizable Pullulan: Synthesis, Self-Assembly and Inkjet Printing. J. Colloid. Interface Sci. 2021, 592, 430–439. [Google Scholar] [CrossRef]
- Alminderej, F.M. Study of New Cellulosic Dressing with Enhanced Antibacterial Performance Grafted with a Biopolymer of Chitosan and Myrrh Polysaccharide Extract. Arab. J. Chem. 2020, 13, 3672–3681. [Google Scholar] [CrossRef]
- Alminderej, F.M.; El-Ghoul, Y. Synthesis and Study of a New Biopolymer-based Chitosan/Hematoxylin Grafted to Cotton Wound Dressings. J. Appl. Polym. Sci. 2019, 136, 47625. [Google Scholar] [CrossRef]
- Cao, H.; Wang, M.; Ding, J.; Lin, Y. Hydrogels: A Promising Therapeutic Platform for Inflammatory Skin Diseases Treatment. J. Mater. Chem. B 2024, 12, 8007–8032. [Google Scholar] [CrossRef]
- Nazhat, S.N. Thermal Analysis of Biomaterials. In Principles and Applications of Thermal Analysis; Wiley: Hoboken, NJ, USA, 2008; pp. 256–285. [Google Scholar]
- Erra Díaz, F.; Dantas, E.; Geffner, J. Unravelling the Interplay between Extracellular Acidosis and Immune Cells. Mediat. Inflamm. 2018, 2018, 1218297. [Google Scholar] [CrossRef]
- Kohane, D.S.; Langer, R. Biocompatibility and Drug Delivery Systems. Chem. Sci. 2010, 1, 441–446. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Evaluación Biológica de Dispositivos Médicos. Parte 5: Pruebas de Citotoxicidad in Vitro. Organización Internacional de Normalización: Geneva, Switzerland, 2009.
- Yilmaz, S.; Sova, M.; Ergün, S. Antimicrobial Activity of Trans-Cinnamic Acid and Commonly Used Antibiotics against Important Fish Pathogens and Nonpathogenic Isolates. J. Appl. Microbiol. 2018, 125, 1714–1727. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Fernandez Junior, A.; Lopes, C.A.M.; Funari, S.R.C.; Bankova, V. Seasonal Effect of Brazilian Propolis on Candida Albicans and Candida Tropicalis. J. Venom. Anim. Toxins 2001, 7, 139–144. [Google Scholar] [CrossRef]
- Zhang, L.; Kong, Y.; Wu, D.; Zhang, H.; Wu, J.; Chen, J.; Ding, J.; Hu, L.; Jiang, H.; Shen, X. Three Flavonoids Targeting the Β-hydroxyacyl-acyl Carrier Protein Dehydratase from Helicobacter pylori: Crystal Structure Characterization with Enzymatic Inhibition Assay. Protein Sci. 2008, 17, 1971–1978. [Google Scholar] [CrossRef]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Šolmajer, T.; Jerala, R. Characterization of Quercetin Binding Site on DNA Gyrase. Biochem. Biophys. Res. Commun. 2003, 306, 530–536. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial Mechanisms of Cinnamon and Its Constituents: A Review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Orsi, R.O.; Fernandes, A.; Bankova, V.; Sforcin, J.M. The Effects of Brazilian and Bulgarian Propolis in Vitro against Salmonella Typhi and Their Synergism with Antibiotics Acting on the Ribosome. Nat. Prod. Res. 2012, 26, 430–437. [Google Scholar] [CrossRef]
- Eumkeb, G.; Chukrathok, S. Synergistic Activity and Mechanism of Action of Ceftazidime and Apigenin Combination against Ceftazidime-Resistant Enterobacter Cloacae. Phytomedicine 2013, 20, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Harakeh, S.; Khan, I.; Almasaudi, S.B.; Azhar, E.I.; Al-Jaouni, S.; Niedzweicki, A. Role of Nutrients and Phyto-Compounds in the Modulation of Antimicrobial Resistance. Curr. Drug Metab. 2017, 18, 858–867. [Google Scholar] [CrossRef]
- Siriwong, S.; Teethaisong, Y.; Thumanu, K.; Dunkhunthod, B.; Eumkeb, G. The Synergy and Mode of Action of Quercetin plus Amoxicillin against Amoxicillin-Resistant Staphylococcus Epidermidis. BMC Pharmacol. Toxicol. 2016, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wang, Y.; Yin, X.; Liu, X.; Xuan, H. Ethanol Extract of Propolis and Its Constituent Caffeic Acid Phenethyl Ester Inhibit Breast Cancer Cells Proliferation in Inflammatory Microenvironment by Inhibiting TLR4 Signal Pathway and Inducing Apoptosis and Autophagy. BMC Complement. Altern. Med. 2017, 17, 471. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Liu, X.; Hao, S.; Zhang, Z.; Xuan, H. Chinese Poplar Propolis Inhibits MDA-MB-231 Cell Proliferation in an Inflammatory Microenvironment by Targeting Enzymes of the Glycolytic Pathway. J. Immunol. Res. 2021, 2021, 6641341. [Google Scholar] [CrossRef]
- Bhaskar, S.; Shalini, V.; Helen, A. Quercetin Regulates Oxidized LDL Induced Inflammatory Changes in Human PBMCs by Modulating the TLR-NF-ΚB Signaling Pathway. Immunobiology 2011, 216, 367–373. [Google Scholar] [CrossRef]
- Comalada, M.; Camuesco, D.; Sierra, S.; Ballester, I.; Xaus, J.; Gálvez, J.; Zarzuelo, A. In Vivo Quercitrin Anti-inflammatory Effect Involves Release of Quercetin, Which Inhibits Inflammation through Down-regulation of the NF-κB Pathway. Eur. J. Immunol. 2005, 35, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Veiga, R.S.; De Mendonça, S.; Mendes, P.B.; Paulino, N.; Mimica, M.J.; Lagareiro Netto, A.A.; Lira, I.S.; López, B.G.-C.; Negrão, V.; Marcucci, M.C. Artepillin C and Phenolic Compounds Responsible for Antimicrobial and Antioxidant Activity of Green Propolis and Baccharis dracunculifolia DC. J. Appl. Microbiol. 2017, 122, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wu, Y.; Chen, X.; Jiang, X.; Wang, K.; Hu, F. Chinese Propolis Exerts Anti-Proliferation Effects in Human Melanoma Cells by Targeting NLRP1 Inflammatory Pathway, Inducing Apoptosis, Cell Cycle Arrest, and Autophagy. Nutrients 2018, 10, 1170. [Google Scholar] [CrossRef]
- Lv, S.; Qu, Q. Mechanism of Propolis Affecting Oral Flora Structure and Immune Microenvironment of Oral Mucositis in Patients with Leukemia Undergoing Chemotherapy. Indian J. Pharm. Sci. 2021, 83, 268–274. [Google Scholar] [CrossRef]
- Amin, M.U.; Khurram, M.; Khattak, B.; Khan, J. Antibiotic Additive and Synergistic Action of Rutin, Morin and Quercetin against Methicillin Resistant Staphylococcus Aureus. BMC Complement. Altern. Med. 2015, 15, 59. [Google Scholar] [CrossRef]
- He, M.; Wu, T.; Pan, S.; Xu, X. Antimicrobial Mechanism of Flavonoids against Escherichia Coli ATCC 25922 by Model Membrane Study. Appl. Surf. Sci. 2014, 305, 515–521. [Google Scholar] [CrossRef]
- Majiene, D.; Trumbeckaite, S.; Pavilonis, A.; Savickas, A.; Martirosyan, D. Antifungal and Antibacterial Activity of Propolis. Curr. Nutr. Food Sci. 2007, 3, 304–308. [Google Scholar] [CrossRef]
- Rajendran, N.; Subramaniam, S.; Christena, L.R.; Muthuraman, M.S.; Subramanian, N.S.; Pemiah, B.; Sivasubramanian, A. Antimicrobial Flavonoids Isolated from Indian Medicinal Plant Scutellaria oblonga Inhibit Biofilms Formed by Common Food Pathogens. Nat. Prod. Res. 2016, 30, 2002–2006. [Google Scholar] [CrossRef]
- Olczyk, P.; Ramos, P.; Komosinska-Vassev, K.; Stojko, J.; Pilawa, B. Positive Effect of Propolis on Free Radicals in Burn Wounds. Evid.-Based Complement. Altern. Med. 2013, 2013, 356737. [Google Scholar] [CrossRef]
- Parihar, A.; Parihar, M.S.; Milner, S.; Bhat, S. Oxidative Stress and Anti-Oxidative Mobilization in Burn Injury. Burns 2008, 34, 6–17. [Google Scholar] [CrossRef]
- Boufadi, M.Y.; Soubhye, J.; Van Antwerpen, P. Anti-inflammatory, Antioxidant Effects, and Bioaccessibility of Tigzirt Propolis. J. Food Biochem. 2021, 45, e13663. [Google Scholar] [CrossRef]
- Touzani, S.; Imtara, H.; Katekhaye, S.; Mechchate, H.; Ouassou, H.; Alqahtani, A.S.; Noman, O.M.; Nasr, F.A.; Fearnley, H.; Fearnley, J.; et al. Determination of Phenolic Compounds in Various Propolis Samples Collected from an African and an Asian Region and Their Impact on Antioxidant and Antibacterial Activities. Molecules 2021, 26, 4589. [Google Scholar] [CrossRef] [PubMed]
- Aytekin, A.A.; Tuncay Tanrıverdi, S.; Aydın Köse, F.; Kart, D.; Eroğlu, İ.; Özer, Ö. Propolis Loaded Liposomes: Evaluation of Antimicrobial and Antioxidant Activities. J. Liposome Res. 2020, 30, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Pellavio, G.; Laforenza, U.; Ranzato, E. Propolis Induces AQP3 Expression: A Possible Way of Action in Wound Healing. Molecules 2019, 24, 1544. [Google Scholar] [CrossRef] [PubMed]
- Noli, C.; Miolo, A. The Mast Cell in Wound Healing. Vet. Dermatol. 2001, 12, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Van De Water, L.; Varney, S.; Tomasek, J.J. Mechanoregulation of the Myofibroblast in Wound Contraction, Scarring, and Fibrosis: Opportunities for New Therapeutic Intervention. Adv. Wound Care 2013, 2, 122–141. [Google Scholar] [CrossRef]
- Wulff, B.C.; Parent, A.E.; Meleski, M.A.; DiPietro, L.A.; Schrementi, M.E.; Wilgus, T.A. Mast Cells Contribute to Scar Formation during Fetal Wound Healing. J. Investig. Dermatol. 2012, 132, 458–465. [Google Scholar] [CrossRef]
- Chen, L.; Schrementi, M.E.; Ranzer, M.J.; Wilgus, T.A.; DiPietro, L.A. Blockade of Mast Cell Activation Reduces Cutaneous Scar Formation. PLoS ONE 2014, 9, e85226. [Google Scholar] [CrossRef]
- Nader, M.A. Caffeic Acid Phenethyl Ester Attenuates IgE-Induced Immediate Allergic Reaction. Inflammopharmacology 2013, 21, 169–176. [Google Scholar] [CrossRef]
- Cho, M.S.; Park, W.S.; Jung, W.-K.; Qian, Z.; Lee, D.-S.; Choi, J.-S.; Lee, D.-Y.; Park, S.-G.; Seo, S.-K.; Kim, H.-J.; et al. Caffeic Acid Phenethyl Ester Promotes Anti-Inflammatory Effects by Inhibiting MAPK and NF-ΚB Signaling in Activated HMC-1 Human Mast Cells. Pharm. Biol. 2014, 52, 926–932. [Google Scholar] [CrossRef]
- Barroso, P.R.; Lopes-Rocha, R.; Pereira, E.M.F.; Marinho, S.A.; de Miranda, J.L.; Lima, N.L.; Verli, F.D. Effect of Propolis on Mast Cells in Wound Healing. Inflammopharmacology 2012, 20, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Nakamura, R.; Watanabe, K.; Oka, K.; Ohta, S.; Mishima, S.; Teshima, R. Effects of Propolis from Different Areas on Mast Cell Degranulation and Identification of the Effective Components in Propolis. Int. Immunopharmacol. 2010, 10, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Lee, S.; Kim, S.-H. Chrysin Suppresses Mast Cell-Mediated Allergic Inflammation: Involvement of Calcium, Caspase-1 and Nuclear Factor-ΚB. Toxicol. Appl. Pharmacol. 2011, 254, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Miłek, M.; Franke, G.; Tomczyk, M.; Górecki, M.; Cwiková, O.; Jarošová, A.; Dżugan, M. The Influence of Geographical Origin on Poplar Propolis Composition and the Impact of Human Microbiota. Pharmaceuticals 2024, 17, 768. [Google Scholar] [CrossRef]


| Reference | Country | Base Biomaterial | Biomaterial Form | Type and Concentration of Propolis |
|---|---|---|---|---|
| [31] | Turkey | Methacrylated pullulan (PULMAN) | Photocurable hydrogel-type film | Ethanolic propolis extract (Turkey), used at 0.5%, 0.1%, and 2% (w/w of solid polymer) |
| [32] | Saudi Arabia | Microcrystalline cellulose and chitosan (CS) | Electrospun nanofibers mats | Propolis powder (from northern Tunisia), incorporated at 2% w/v into polymer solution. |
| [33] | Brazil | Polyacrylamide and methylcellulose hydrogel | Hydrogel | Propolis extract (from Brazil) incorporated at 1.0% and 2.5% w/v. |
| [34] | Iran | Dialdehyde xanthan gum and soy protein isolate | Crosslinked self-healing nanocomposite film | Ethanolic extract of propolis (Mashhad, Iran), 1 wt% and 3 w/w% |
| [35] | Iran | Bilayer scaffold consisting of 3D-printed PCL coated with propolis and electrospun PVA/CS/PCL nanofibers loaded with diltiazem hydrochloride. | Bilayer membrane: 3D-printed scaffold with electrospun nanofiber overlay. | Ethanolic propolis extract (from hives in Isfahan, Iran) applied as a coating in 30% ethanol (no exact concentration on scaffold reported). |
| [36] | Iran | Poly(lactic-co-glycolic acid) (PLGA) | Nanoparticles | Ethanolic extract of propolis (exact concentration not specified.) |
| [37] | Iran | Silica–oxidized silk fibroin–chitosan–propolis (silica–SFO–CS–PP) scaffold. | 3D-printed aerogel scaffold | Propolis extracted with ethanol (EEP) and methylal (EMP), including EEP-CS and EMP-CS nanoparticle forms. Specific mass concentration not reported. |
| [38] | Egypt | Marine collagen–chitosan gel functionalized with zinc oxide nanoparticles (ZnO–NPs) and propolis. | Bioactive gel | Raw propolis from Mansoura (Egypt), used at 0.25 g (PP2) and 0.5 g (PP1) in ZnO–NPs synthesis; final gel concentrations 1.67% and 3.3% w/v. |
| [39] | India | Polyvinyl alcohol (PVA) | Hydrogel | Indian propolis extract; 4 g incorporated into hydrogel (concentration not expressed as % w/w). |
| [40] | Thailand | Quaternized chitosan (QCS) and pectin | Hydrogel film | Green propolis (Chiang Mai, Thailand), 0.5%w/v |
| [41] | Brazil | Polycaprolactone (PCL) | Electrospun nanomats (fiber, beaded fiber, beads only) | Ethanolic extract of Brazilian green propolis (Apis Flora, São Paulo, Brazil); 15% v/v added to polymer solution. |
| [42] | Iran | Polycaprolactone (PCL), Chitosan (CS), and Polyurethane (PU) coated with ethanolic extract of propolis (EEP). | Electrospun fibrous sublayer + PU foam top layer = bilayer wound dressing (BWD).) | Ethanolic extract of propolis (EEP) from Isfahan, Iran; concentration not specified. |
| [43] | Turkey | Polyvinyl alcohol (PVA) and chitosan crosslinked with genipin. | Membranes (PVA/C, PVA/C-P1, PVA/C-P2) | Ethanolic extract of propolis from Istanbul, Turkey; 0.25% and 0.50% v/v. |
| [44] | Iran | Polyurethane (PU) foam with nanolignin (NL), coated with ethanolic extract of propolis (EEP). | Porous coated foam | Ethanolic extract of propolis from Shahr-e Kord (Iran); used as coating (exact concentration not specified). |
| [45] | Egypt | Deacetylated cellulose acetate nanofibers loaded with chitosan/propolis nanoparticles. | Electrospun nanofibers | Ethanolic extract of propolis (Egypt); 10 mg used in nanoparticle formulation. |
| [46] | Iran | Cellulose acetate (CA) and polycaprolactone (PCL). | Electrospun nanofibrous | Ethanolic extract of propolis from Khansar, Iran. Concentration not explicitly quantified. |
| [47] | Brazil | Natural rubber (Hevea brasiliensis) and aqueous extract of propolis. | Membrane (cast film). | Aqueous extract of propolis from Scaptotrigona polysticta, Brazil; 10% w/w relative to natural rubber. |
| [48] | Egypt | Polyvinyl alcohol, chitosan, and honey | Electrospun nanofibers | Aqueous extract of propolis (PP), loaded at 10% w/w in the HPCS nanofiber matrix. |
| [49] | Brazil | Natural rubber latex (NRL) | Cast membranes | Three types: green, red, and poplar propolis; incorporated as ethanolic extract (1 mL of extract in 3 mL of NRL). |
| [50] | Iran | Cornstarch blended with hyaluronic acid (HA) and ethanolic extract of propolis (EEP) | Solvent-cast film | Ethanolic extract of propolis from Isfahan, Iran; used at 0.25%, 0.5%, and 1% w/w. |
| [51] | Brazil | Bacterial cellulose (BC) | Membrane (BC/PP membrane) | Propolis EPP-AF® soft extract in self-microemulsifying formulation (SMEF); final amount in BC/PP membrane: 5 mg/cm2 (dry matter), with 84.25 µg/cm2 of p-coumaric acid and 306.25 µg/cm2 of artepillin C. |
| [52] | Brazil | Bacterial cellulose (BC) | Membrane | Brazilian green propolis standardized extract (EPP-AF); incorporated via ethanol solutions at 1.2%, 2.4%, and 3.6% w/v. |
| [53] | Brazil | Bacterial cellulose | Membrane impregnated with extracts | Red propolis from Northeastern Brazil; ethyl acetate and butanol extracts at 1% (w/v) concentration. |
| [54] | Iran | Polyurethane (PU), coated with water extract of propolis (WEP). | Porous foam wound | Water extract of propolis (WEP) from Isfahan, Iran; used at 10%, 20%, and 30% v/v. |
| [55] | Iran | Polyurethane (PU) and hyaluronic acid (HA), enriched with ethanolic extract of propolis (EEP). | Electrospun nanofibers | Ethanolic extract of propolis (EEP) from Isfahan, Iran; used at 0.5%, 1%, and 2% w/w. |
| [56] | Iran | Polyurethane (PU) membrane with ethanolic extract of propolis (EEP) and electrospun Polycaprolactone/Gelatin (PCL/Gel) nanofibers. | Bilayer wound dressing (PU/EEP membrane as top layer, PCL/Gel scaffold as sublayer). | Iranian ethanolic extract of propolis; 0.5% w/w in PU layer. |
| Reference | Chemical and Morphological Analysis | Mechanical Properties | Thermal Properties | Degradation Behavior | Fluid Interaction, Swelling, Propolis Release, and Vapor Transmission | Main Findings |
|---|---|---|---|---|---|---|
| [31] | FTIR and NMR confirmed chemical structure; homogeneous dispersion with propolis. | Modulus 2.55 MPa, strength 2.48 MPa, elongation 848%. | TGA/DSC showed stability, degradation ~225 °C. | 50–60% weight loss in PBS over 2 weeks. | Swelling decreased with propolis; release equilibrium at 24 h. | Good mechanical, thermal and fluid properties; homogeneous structure. |
| [32] | FTIR and SEM confirmed crosslinking and uniform fiber formation. | Strength 7.42–8.27 MPa, modulus 174.61–199.08 MPa. | Not reported | Not reported | Swelling 436–438%; WVP 1698–1735 g/m2/day. | Hydrophilic scaffold; good fiber morphology and moisture transmission. |
| [33] | XRD confirmed amorphous structure; porosity increased with 2.5% propolis. | Not reported | Not reported | Not reported | Swelling up to 1541%; high solubility at 2.5%. | Excellent fluid interaction; high swelling and porosity. |
| [34] | FTIR, XRD, SEM and EDX confirmed chemical bonding and uniform structure. | Strength 16.9 MPa, elongation 14.2%; improved with HNTs. | TGA: three stages; higher residue with HNTs. | 24% loss with 7% HNTs in 10 days. | Increased swelling with PP; no release data. | Good mechanical and thermal behavior; tunable degradation and swelling. |
| [35] | FTIR and SEM confirmed hydrogen bonding; uniform nanofiber distribution. | Strength 2.05–4.86 MPa, modulus 3.35–6.58 MPa. | Not reported. | Up to 68.75% loss in 3 days; pH dependent. | Swelling ~496%; DTZ release controlled in bilayer. | Balanced mechanical and degradation profile; pH-responsive system. |
| [36] | FTIR, TEM and FE-SEM showed spherical nanoparticles and propolis inclusion. | Not reported | Not reported | Not reported | Not reported | Nanoparticles with confirmed structure; no performance data. |
| [37] | FTIR, SEM and UV–Vis confirmed interactions and hierarchical structure. | Not reported | Degradation above 575 °C; improved stability. | Varied by formulation over 6 days. | Good swelling; release studied at pH 2 and 7. | High thermal stability; functional swelling and release. |
| [38] | XRD, TEM and DLS confirmed nanoparticle morphology and propolis loading. | Not reported | Not reported. | Not reported | Not reported | Confirmed nanostructure; no release data. |
| [39] | FTIR and DSC confirmed uniform dispersion and molecular interaction. | Strength 198.18 g; comparable to povidone-iodine. | DSC showed absence of propolis peaks, indicating dispersion. | Not reported | Biphasic release: 40% in 40 min, sustained 4 h. | Stable hydrogel with good mechanical strength and release profile. |
| [40] | FTIR and SEM confirmed smooth, homogeneous surfaces with good compatibility. | Strength 33.96 MPa, modulus 20.21 MPa; elongation decreased. | Not reported | Higher degradation in QCS/Pec than QCS. | Swelling ~600%; 64% release in 48 h. | Robust mechanical performance; good release and antioxidant profile. |
| [41] | FTIR and SEM confirmed morphology and phytochemicals; GPC showed polymer degradation. | Not reported | DSC: dual melting peaks; TGA: single degradation ~383–404 °C. | Not reported | Burst release in 30 min; equilibrium at 2 h. | Controlled release and thermal behavior; propolis stability confirmed. |
| [42] | SEM and FTIR confirmed successful incorporation of functional groups. | Strength ~6 MPa; elongation ~350%. | Not reported | 28-day weight loss ranged 3–28%. | Swelling varied; contact angle ~58°. | Enhanced hydrophilicity and swelling; validated incorporation. |
| [43] | FTIR, SEM, and XRD confirmed propolis crosslinking and reduced crystallinity. | Strength 12.72 MPa; elongation 58.68%. | Not reported | Weight loss increased over 9 days. | WVTR 75.16–106.15 g/m2/day; angle ~45°. | Improved mechanical performance; good degradation and hydrophilicity. |
| [44] | FTIR, SEM and DLS confirmed chemical interactions and nanostructure. | Strength ~0.91 MPa; elongation ~73–96%. | Not reported | Not reported | Absorption ~267%; angle ~50°. | Meets tensile requirements; good hydrophilicity and absorption. |
| [45] | FTIR, SEM and TEM confirmed deacetylation and nanofiber formation. | Not reported | DSC: 325 °C exothermic peak confirms incorporation. | Slow dissolution over 7 days. | 36.2% at 48 h; 51.6% at 264 h. | Stable nanofibers; slow release and reduced dissolution. |
| [46] | FTIR and SEM confirmed propolis coating; smooth nanofibers observed. | Not reported | Not reported | Not reported | Absorption 400%; treated mats fully hydrophilic. | Hydrophilic nanofibers; high water retention. |
| [47] | Microscopy showed propolis modified surface and porosity. | Not reported | Not reported | Not reported | Angle decreased to ~65°, more wettability. | Wettability improved with propolis. |
| [48] | FTIR and SEM confirmed stable incorporation of propolis. | Not reported | Not reported | Not reported | Not reported. | Stable matrix; data lacking for behavior. |
| [49] | UHPLC and FTIR confirmed classification and no new bonds. | Strength increased with red and poplar propolis. | Not reported | Not reported | Release: Green 54.2%, Red 41.5%, Poplar 49.0%. | Safe profiles; different release by propolis type. |
| [50] | FTIR, SEM and GC-MS confirmed bioactive compound integration. | Strength decreased with EEP; elongation increased. | Not reported | Hydrolytic: 31% in 1 week; enzymatic: 87% in 24 h. | EEP release up to 74% in 48 h. | Bioactive integration; sustained degradation and release. |
| [51] | HPLC and laser diffraction confirmed compound incorporation and droplet size. | Not reported | Not reported | Not reported | 90% p-coumaric acid, 24% artepillin C in 48 h. | Efficient compound release over time. |
| [52] | SEM, XRD, FTIR and TGA confirmed surface and thermal changes. | Not reported | TGA: extended degradation up to 450 °C. | Not reported | 24 h: p-coumaric 60.2%, isosakuranetin 53.9%. | Effective delivery of bioactive compounds. |
| [53] | HRMS and UFLC confirmed flavonoid quantification. | Not reported | Not reported | Not reported | Progressive decline of compound over 14 days. | High flavonoid content; release data confirms activity. |
| [54] | FTIR and SEM confirmed coating; pore structure remained unchanged. | Strength 2.99 MPa; elongation 434%. | Not reported | Not reported | Uptake decreased with WEP; angle 35.53°. | High hydrophilicity; mechanical compromise with WEP. |
| [55] | FTIR and SEM confirmed porous fibers; porosity > 80%. | Strength decreased with EEP; elongation increased. | Reduced stability with EEP; onset 300–460 °C. | Not reported | Sustained release over 48 h; 43% total. | Thermally stable; effective propolis release and absorption. |
| [56] | FTIR, SEM and GC-MS confirmed EEP incorporation and nanofiber formation. | Strength ~5.8 MPa; elongation ~340%. | TGA: lower temp with EEP; mass loss from gelatin moisture. | Enzymatic: 78% loss in 14 days. | Hydrophilic sublayer; release up to 48 h. | Good hydrophilicity and sustained release in bilayers. |
| Reference | Antibacterial Assay | MTT Assay | In Vivo Biological Properties | |
|---|---|---|---|---|
| Technique Used | Results | |||
| [31] | Agar disk diffusion | S. aureus ATCC 25923: 20.6–26.2 mm with propolis, compared 23.1 mm. E. coli ATCC 25923: 10.5–13.7 mm with propolis compared 11.3 mm without propolis. | NIH/3T3 fibroblasts. Viability: 93.73%, 90.47%, 81.74%, 61.04% for increasing PRO; p < 0.05. Biocompatible; dose-dependent cytotoxicity; optimal at 90.47% | Not reported |
| [32] | Agar disk diffusion | M. luteus: 15.0–25.8 mm with propolis, compared to 25.8 mm without propolis. S. aureus: 14.0–19.3 mm with propolis, compared to 23.8 mm without propolis. E. coli: 11.0–15.2 mm with propolis, compared to 18.0 mm without propolis. P. aeruginosa: 12.0–17.5 mm with propolis, compared to 20.0 mm without propolis | HepG2 (human hepatocellular carcinoma), fibroblasts. Improved metabolic activity over 24–72 h; 70/30 CA/CS blend > 90/10; p < 0.05. No cytotoxicity; excellent biocompatibility; improved fibroblast activity due to propolis | Not reported |
| [33] | Microdilution in plates | S. aureus: 93–97% with propolis; ~100% with standard drug. P. aeruginosa: >80–100% with propolis; ~90% with propolis hydrogel; 100% only at high drug concentrations. E. coli: ~80–90% with propolis extract; no inhibition with hydrogels; >90% only at high drug concentrations | Not reported | Not reported |
| [34] | Agar disk diffusion | The activity was more potent against Staphylococcus aureus than against Escherichia coli. It does not provide specific numerical values (in millimeters) for the diameter of these inhibition zones | NIH3T3 fibroblasts. All samples promoted proliferation; no dead cells. High cytocompatibility; synergistic effect of PP and HNTs. | Not reported |
| [35] | Well diffusion and Kirby–Bauer | S. aureus. Propolis: 10–17 mm (well); membrane 2–15 mm (Kirby–Bauer) | L929 fibroblasts. >80% viability (P1/P2); 99.89% for PCP-10; <30% for PCP-12. Safe up to 10% DTZ; hydrophilic scaffolds promote attachment. | Wistar rats; full-thickness skin defect. 95.3% closure at 14 days (vs 75.3% control); reduced IL-1β, TNF-α; enhanced collagen, granulation, and epidermis formation |
| [36] | Not reported | Not reported | Not reported | Rats; 20 mm full-thickness wound. EEPNPs enhanced angiogenesis, fibroblast growth, and contraction; reduced inflammation and infection |
| [37] | Agar disk diffusion | Post-printed modified scaffolds reached up to 2.5 cm against E. coli and 1.5 cm against S. aureus, while pre-printed scaffolds showed no obvious bactericidal properties or ZOI. | L929 fibroblasts. Viability: up to 120% (SFO-PM-CH); PE scaffold toxic at high propolis. Optimal at 200 μg/mL; post-printing modification improved performance | Not reported |
| [38] | Agar disk diffusion | The gel loaded with 5% PP1/ZnO–NPs showed inhibition zones of 9 ± 0.08 mm (E. coli), 10 ± 0.84 mm (S. typhimurium), 10 ± 1.05 mm (S. mutans), and 7 ± 0.05 mm (C. albicans). The 10% PP1/ZnO–NPs gel was the most effective overall, with inhibition zones of 26 ± 2.31 mm (E. coli), 25 ± 2.73 mm (S. typhimurium), 20 ± 1.99 mm (S. mutans), and 12 ± 0.91 mm (C. albicans). In comparison, gels with 5% PP2/ZnO–NPs produced inhibition zones of 14 ± 1.42 mm, 15 ± 1.88 mm, 16 ± 1.76 mm, and 8 ± 0.07 mm for the same microorganisms, respectively. The 10% PP2/ZnO–NPs gel showed increased activity, with inhibition zones of 21 ± 1.51 mm (E. coli), 19 ± 1.92 mm (S. typhimurium), 19 ± 1.79 mm (S. mutans), and 10 ± 1.01 mm (C. albicans). | Not reported | Sprague-Dawley rats; 2 × 2 cm2 full-thickness incision. G6 group had 94.31% contraction by day 14; high collagen, capillaries, low inflammation |
| [39] | Not reported | Not reported | Not reported | Wistar rats; burn, excision, incision models. Propolis hydrogel showed 91.45% contraction by day 21 (excision); enhanced tensile strength and collagen formation. |
| [40] | Surface antibacterial assay (CFU/mL) | Pec, QCS, propolis-loaded Pec, and propolis-loaded QCS films demonstrated complete growth inhibition for all three tested bacteria (S. aureus, S. epidermidis, S. pyogenes), as no colonies were detected (ND) | L929 fibroblasts. >70% viability for all hydrogels. Non-toxic; suitable for wound dressing | Not reported |
| [41] | Not reported | Not reported | Not reported | Not reported |
| [42] | Agar disk diffusion | S. aureus PU (0 mm), PCL2:1CS-PU (0 mm), PU/EEP (4.65 ± 0.44 mm), and BWD (0.53 ± 0.19 mm). E coli PU (0 mm), PCL2:1CS-PU (0.58 ± 0.19 mm), PU/EEP (1.32 ± 0.39 mm), and BWD (1.54 ± 0.41 mm | L929 fibroblasts. No significant viability difference PCL/CS vs. BWD; PCL/CS > PU/EEP. Good biocompatibility; PCL/CS mat enhanced viability | Wistar rats; 11 mm punch wound. BWD nearly healed by day 15; better dermis, hair follicles, and sebaceous glands |
| [43] | Not reported | Not reported | MEF (mouse embryonic fibroblasts). Proliferation with PVA/C-P2: 176%, 775%, 853% at 24, 72, 120 h. Enhanced proliferation; good cytocompatibility; suitable for wound healing. | Not reported |
| [44] | Agar disk diffusion | S. aureus, (PU-NL/WEP):10.5 ± 0.7 mm, (PU-NL/EEP): 11.2 ± 0.6 mm. Against E. coli, PU-NL/WEP: 4.3 ± 0.3 mm, and PU-NL/EEP films: 7.2 ± 0.4 mm. | L929 fibroblasts. All samples biocompatible; PU-NL/EEP highest viability EEP non-toxic; supports fibroblast growth | Wistar rats; 11 mm dorsal excision. PU-NL/EEP wounds nearly closed by day 10; better epidermis and dermis vs. control; p < 0.05 |
| [45] | Not reported | Not reported | HFB4 fibroblasts. IC50: 116 μg/mL (NPs), 137.5 μg/mL (free); 89.46% viability at 25 μg/mL. Safe below 25 μg/mL; no cytotoxicity at tested dose | Mice; 2 × 2 cm2 burn wound. NFMTs reduced wound size by day 21; induced regeneration of epidermis, follicles, glands |
| [46] | Minimum Inhibitory Concentration (MIC) Assay. | The MIC against Staphylococcus aureus was 270 µg/mL. The MIC against Staphylococcus epidermidis was 340 µg/mL. Furthermore, the MIC against Escherichia coli was 900 µg/mL, and against Pseudomonas aeruginosa, it was 1200 µg/mL | Not reported | Not reported |
| [47] | Not reported | Not reported | Not reported | Wistar rats; second-degree burns. NRP60 group had 60.18% lesion retraction at day 10 vs. 19.46% control; enhanced collagen, epithelium, and angiogenesis |
| [48] | Viable cell count technique (Log CFU) | HPCS-Pr nanofibers demonstrated enhanced antibacterial activity against S. aureus and MRSA compared to the commercial Aquacel Ag dressing1. However, they exhibited “nearly no” antibacterial activity against E. coli and multidrug-resistant (MDR) P. aeruginosa | Human fibroblasts. Lower proliferation in HPCS-Pr vs. HPCS-BV and Aquacel Ag. Biocompatible despite reduced proliferation | Mice; 9 mm dorsal wound. HPCS-Pr had superior closure vs. Aquacel Ag; strong collagen deposition and early granulation |
| [49] | Minimum Inhibitory Concentration (MIC) Assay. | MIC: red propolis (78 µg/mL), poplar propolis (312.5 µg/mL), green propolis (1250 µg/mL) | 3T3 fibroblasts. Red propolis eluate: 104.13% (30%), 91.64% (50%); green toxic at 50–100%. Concentration-dependent toxicity; red propolis best performance | Not reported |
| [50] | Agar disk diffusion | S. aureus ATCC 25923: 0.93–4.68 mm with propolis, 0 mm without propolis E. coli ATCC 25922: 1.21–4.33 mm with propolis, 0 mm without propolis S. epidermidis ATCC 25925: 1.02–2.92 mm with propolis, 0 mm without propolis P. aeruginosa ATCC 27853: 0 mm in all formulations | L929 fibroblasts. EEP > 0.5% reduced proliferation at 7 days; 0.5% EEP optimal. CS/HA/0.5%EEP most biocompatible; higher EEP reduced cell growth | Wistar rats; 12 mm excised skin. CS/HA/0.5%EEP accelerated healing by day 14; induced fibroblast proliferation and HA synthesis |
| [51] | Agar disk diffusion | S. aureus (22.3–23.7 mm), K. pneumoniae (23.0–25.0 mm), P. aeruginosa (14.7–16.7 mm), and E. coli (16.7 mm) over 0–24 hours1. Notably, the zone for S. epidermidis significantly increased from 21.7 ± 1.2 mm at 0 h to 40.0 ± 0.0 mm after 24 h | Not reported | Wistar rats; 1.5 cm full-thickness wounds. BC/PP group nearly healed by day 14; reduced neutrophil infiltration; no difference in angiogenesis vs. BC |
| [52] | Agar disk diffusion | Staphylococcus aureus ATCC 25923: 8.0–10.0 mm with propolis; resistant with BC without propolis. Staphylococcus aureus ATCC 43,300 (MRSA): 7.0–9.0 mm with propolis; resistant without propolis. Staphylococcus epidermidis ATCC 14990: 7.0–9.0 mm with propolis; resistant without propolis | Not reported | Rattus Norvegicus; 6 mm circular incisions. All wounds fully repaired by 30 days; no significant difference among groups; confirmed by histology. |
| [53] | Not reported | Not reported | Not reported | Diabetic mice; 10 mm dorsal wound. Red propolis groups had better healing by day 14; increased MPO and cytokine activity at day 7 |
| [54] | Agar disk diffusion | S. aureus (0.93–3.89 mm) and E. coli (1.21–3.55 mm) showed increasing inhibition with higher propolis concentration. No antimicrobial activity was observed against S. epidermidis or P. aeruginosa in any of the formulations. | L929 fibroblasts. All WEP dressings > 100% viability on day 7; p < 0.05. Propolis enhanced proliferation; no cytotoxicity | Wistar rats; 11 mm excised skin. PU/30WEP group had best contraction (5.68%) at day 15 vs. 20.97% control; improved dermis and follicles |
| [55] | Agar disk diffusion | Staphylococcus aureus (ATCC 25923): 0.35–5.63 mm. Escherichia coli (ATCC 25922):0.43–3.18 mm | L929 fibroblasts. Highest viability in PU-HA; 2% EEP reduced viability on day 7. All samples biocompatible; optimal EEP concentration needed | Wistar rats; 11 mm dorsal wound. PU-HA/1%EEP showed complete closure by day 21; superior dermis and collagen development |
| [56] | Agar disk diffusion | Staphylococcus aureus (ATCC 25923): 5.4 ± 0.3 mm. Escherichia coli (ATCC 25922): 1.9 ± 0.4 mm. Staphylococcus epidermidis (ATCC 25925): 1.0 ± 0.2 mm. Pseudomonas aeruginosa (ATCC 27853): 0 mm (resistant strain). Klebsiella pneumoniae (ATCC 27553): 0 mm (resistant strain) | L929 fibroblasts. No toxicity; higher proliferation in PCL/Gel vs. PU/EEP after day 4. Good biocompatibility; PCL/Gel enhanced proliferation | Wistar rats; 11 mm punch biopsy. Wound area 2.6% by day 15 in PU/EEP-PCL/Gel vs. 17.9% control; enhanced collagen, dermis, and hair follicle formation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loya-Hernández, L.P.; Arzate-Quintana, C.; Castillo-González, A.R.; Camarillo-Cisneros, J.; Romo-Sáenz, C.I.; Favila-Pérez, M.A.; Quiñonez-Flores, C.M. Propolis-Functionalized Biomaterials for Wound Healing: A Systematic Review with Emphasis on Polysaccharide-Based Platforms. Polysaccharides 2025, 6, 74. https://doi.org/10.3390/polysaccharides6030074
Loya-Hernández LP, Arzate-Quintana C, Castillo-González AR, Camarillo-Cisneros J, Romo-Sáenz CI, Favila-Pérez MA, Quiñonez-Flores CM. Propolis-Functionalized Biomaterials for Wound Healing: A Systematic Review with Emphasis on Polysaccharide-Based Platforms. Polysaccharides. 2025; 6(3):74. https://doi.org/10.3390/polysaccharides6030074
Chicago/Turabian StyleLoya-Hernández, Lydia Paulina, Carlos Arzate-Quintana, Alva Rocío Castillo-González, Javier Camarillo-Cisneros, César Iván Romo-Sáenz, María Alejandra Favila-Pérez, and Celia María Quiñonez-Flores. 2025. "Propolis-Functionalized Biomaterials for Wound Healing: A Systematic Review with Emphasis on Polysaccharide-Based Platforms" Polysaccharides 6, no. 3: 74. https://doi.org/10.3390/polysaccharides6030074
APA StyleLoya-Hernández, L. P., Arzate-Quintana, C., Castillo-González, A. R., Camarillo-Cisneros, J., Romo-Sáenz, C. I., Favila-Pérez, M. A., & Quiñonez-Flores, C. M. (2025). Propolis-Functionalized Biomaterials for Wound Healing: A Systematic Review with Emphasis on Polysaccharide-Based Platforms. Polysaccharides, 6(3), 74. https://doi.org/10.3390/polysaccharides6030074








