Development and Application of Biodegradable Pectin/Carboxymethylcellulose Films with Cinnamon Essential Oil and Cold Plasma Modification for Chicken Meat Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Film-Forming Solution
2.3. Treatment of the Film-Forming Solution with Cold Plasma (CP)
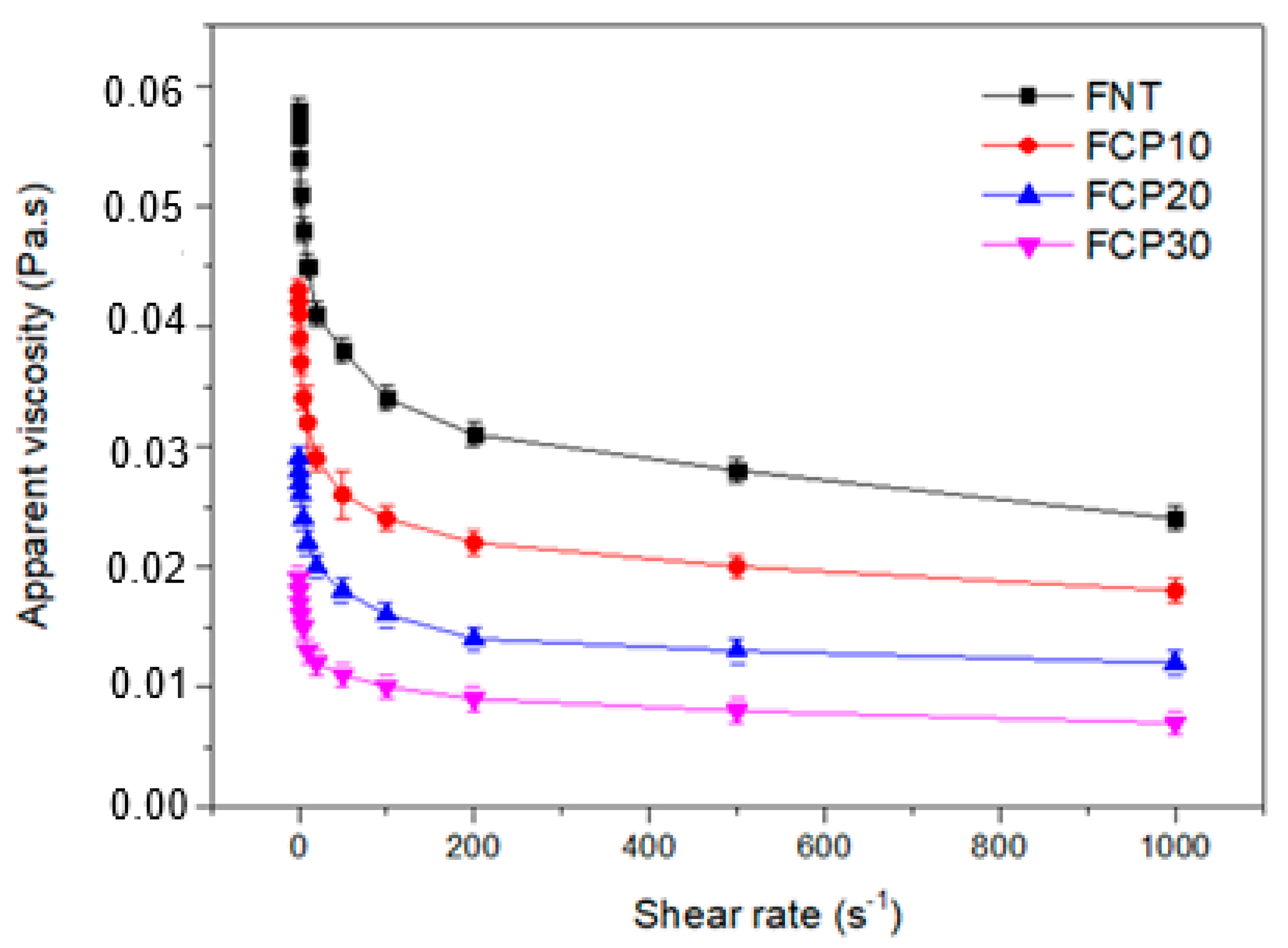
Shear Rate and Apparent Viscosity Measurements
2.4. Film Production
2.5. Film Characterization
2.5.1. Thickness
2.5.2. Moisture Content
2.5.3. Water Vapor Permeability (WVP)
2.5.4. Solubility
2.5.5. Contact Angle
2.5.6. Tensile Strength and Elongation at Break
2.5.7. Color
2.5.8. Surface Morphology
2.5.9. Biodegradability
2.6. Application of the Films in the Storage of Chicken Meat Fillets
2.6.1. Total Volatile Basic Nitrogen (TVB-N)
2.6.2. Peroxide Value
2.6.3. Thiobarbituric Acid Reactive Substances (TBARS)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Apparent Viscosity of the Film-Forming Solution
3.2. Physical, Barrier, and Mechanical Properties of the Films
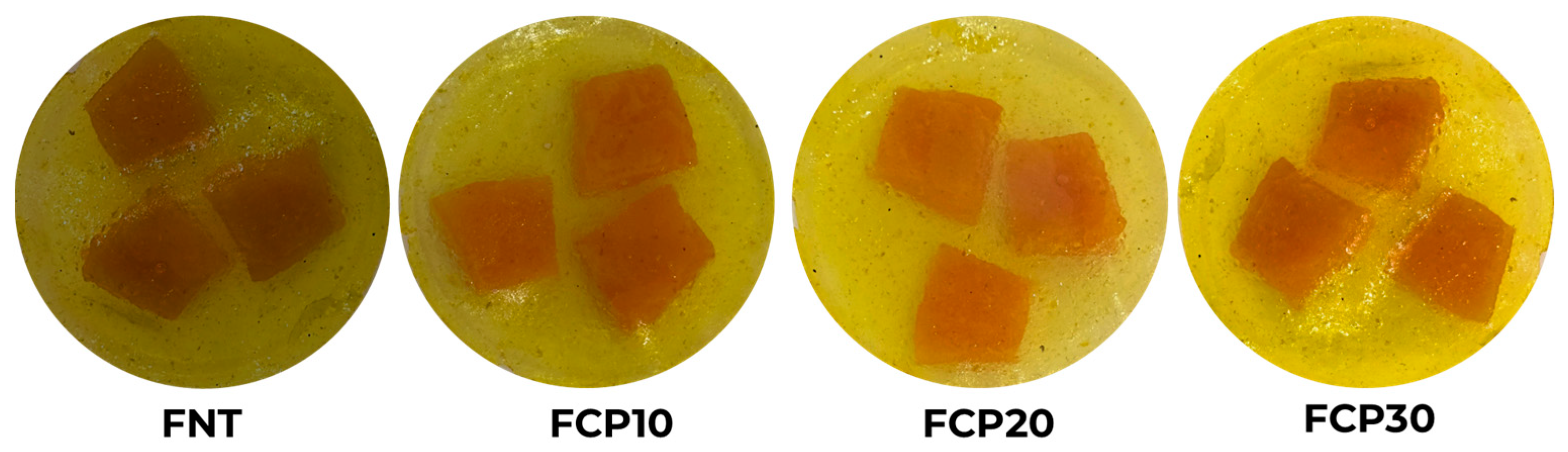
3.3. Color
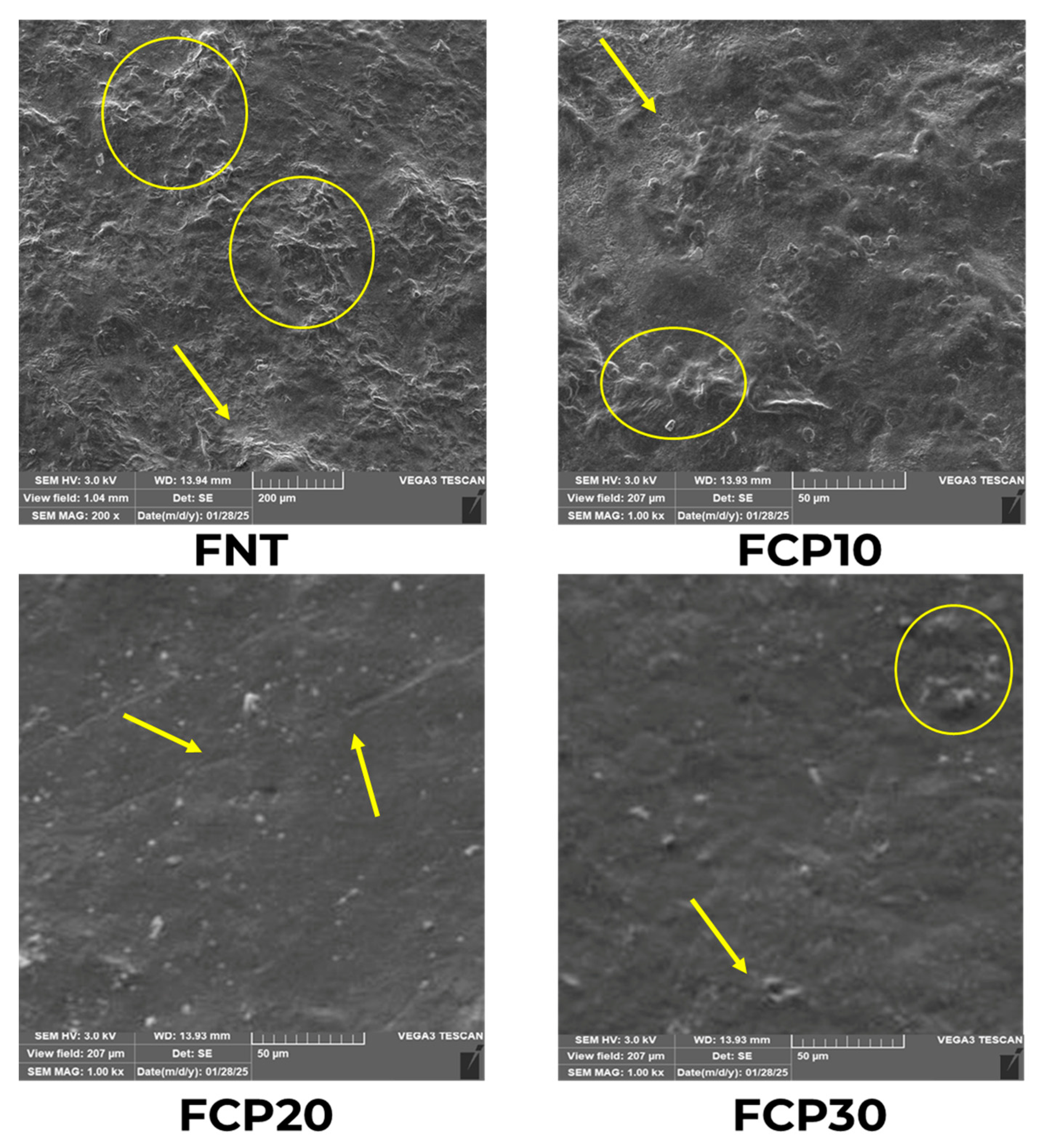
3.4. Morphology by SEM
3.5. Biodegradability
3.6. Storage of Chicken Meat Fillets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hlaing, K.S.S.; Fall, M.; Tristanto, N.A.; Carole, N.V.; Kaharso, V.C.; Golshany, H.; Xia, W. Preparation of edible films from pectin/carboxymethyl chitosan incorporating polyphenol-rich roselle leaf extracts for food packaging applications. Int. J. Biol. Macromol. 2025, 310, 143351. [Google Scholar] [CrossRef]
- Syarifuddin, A.; Muflih, M.H.; Izzah, N.; Fadillah, U.; Ainani, A.F.; Dirpan, A. Pectin-based edible films and coatings: From extraction to application on food packaging towards circular economy—A review. Carbohydr. Polym. Technol. Appl. 2025, 9, 100680. [Google Scholar] [CrossRef]
- Jesus, G.A.; Castro, M.C.; Souza, P.R.; Monteiro, J.P.; Sabino, R.M.; Sablani, S.S.; Bonafé, E.G. Antioxidant, UV-blocking, and biodegradable pectin films containing selenium nanoparticles for sustainable food packaging. Food Hydrocoll. 2025, 167, 111449. [Google Scholar] [CrossRef]
- Liu, C.; Li, N.; Niu, L.; Li, X.; Feng, J.; Liu, Z. The preparation, physicochemical characterization and antibacterial activity of pectin/zinc alginate composite film. Carbohydr. Res. 2025, 550, 109412. [Google Scholar] [CrossRef] [PubMed]
- Wichaphian, A.; Yasan, P.; Pathom-aree, W.; Lumyong, S.; Suwannarach, N.; Kumla, J.; Srinuanpan, S. From agricultural waste to active films: Enhanced crystallinity of spent mushroom substrate-derived cellulose via deep eutectic solvent-based microwave-assisted pretreatment and its application in reinforcing CMC-based composite films. J. Agric. Food Res. 2025, 20, 101759. [Google Scholar] [CrossRef]
- Sasaki, J.C.S.; Su, Y.; Spinosa, W.A.; de Lima Lopes Filho, P.E.; Burd, B.S.; Scontri, M.; Herculano, R.D. Eco-sustainable, edible, biodegradable and antioxidant pectin and bacterial cellulose films loaded with coconut oil for strawberry preservation. Int. J. Biol. Macromol. 2025, 308, 142701. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.W.; Zhang, X.; Little, E.M.; Zaldivar, L.R.; White, S.A.; Campbell, Y.L.; Schilling, M.W. Efficacy of a carboxymethylcellulose (CMC)-based edible film with propylene glycol to control ham mite populations that infest dry cured ham. J. Stored Prod. Res. 2023, 103, 102162. [Google Scholar] [CrossRef]
- Rincón, E.; De Haro-Niza, J.; Morcillo-Martín, R.; Espinosa, E.; Rodríguez, A. Boosting functional properties of active-CMC films reinforced with agricultural residues-derived cellulose nanofibres. RSC Adv. 2023, 13, 24755–24766. [Google Scholar] [CrossRef] [PubMed]
- Spinei, M.; Oroian, M.; Ursachi, V.F. Characterization of biodegradable films based on carboxymethyl cellulose and citrus pectin films enriched with bee bread oil and thyme oil. LWT 2024, 214, 117088. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Osei, P.O.; Gao, F.; Wu, X.; Liao, X. Preparation of pectin/carboxymethyl cellulose composite films via high-pressure processing and enhancement of antimicrobial packaging. Int. J. Biol. Macromol. 2024, 281, 136462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, W.; Zhang, S.; Tang, J.; Shi, X.; Qin, S.; Xiao, H. Enhancing the applicability of gelatin-carboxymethyl cellulose films by cold plasma modification for the preservation of fruits. LWT 2023, 178, 114612. [Google Scholar] [CrossRef]
- Santos, N.C.; Almeida, R.L.J.; da Silva, G.M.; Monteiro, S.S.; de Alcântara Ribeiro, V.H.; de França Silva, A.P.; de Almeida Mota, M.M. Influence of high hydrostatic pressure (HHP) pretreatment on plum (Prunus salicina) drying: Drying approach, physical, and morpho-structural properties of the powder and total phenolic compounds. J. Food Process. Preserv. 2022, 46, e16968. [Google Scholar] [CrossRef]
- Goiana, M.L.; Rosa, M.D.F.; Mattos, A.L.A.; Fernandes, F.A.N. Development of plasma-treated corn-starch-based film incorporated with acerola and grape pomace extract possessing pH-sensing capability. Polymers 2025, 17, 938. [Google Scholar] [CrossRef] [PubMed]
- Monyela, N.F.; Belay, Z.A.; Pfukwa, H.; Caleb, O.J. Cold plasma activated edible coatings: A status review on the impact on edible coating properties, fresh produce quality, and the sustainable development goal paradigm. Food Packag. Shelf Life 2025, 49, 101486. [Google Scholar] [CrossRef]
- Costa, E.S.; dos Santos Moreira, I.; Gomes, J.P.; do Socorro Rocha Bastos, M.; Mattos, A.L.A.; Santos, N.C.; Matsui, K.N. Barrier, mechanical and antimicrobial properties of yam starch film with cássia cinnamon essential oil and its application in strawberry storage. Packag. Technol. Sci. 2025, 68, 681–697. [Google Scholar] [CrossRef]
- Almeida, R.L.J.; Santos, N.C.; Feitoza, J.V.F.; de Alcântara Ribeiro, V.H.; de Alcântara Silva, V.M.; de Figueiredo, M.J.; de Sousa Muniz, C.E. The impact of the pulsed electric field on the structural, morphological, functional, textural, and rheological properties of red rice starch (Oryza sativa). J. Food Process Eng. 2022, 45, e14145. [Google Scholar] [CrossRef]
- Eskandari, Z.; Osanloo, M.; Mazloomi, S.M.; Zarenezhad, E.; Mollakhalili-Meybodi, N.; Nematollahi, A. Bioactive edible films for chicken breast packaging: Integration of chia seed mucilage with nanoemulsions of Cinnamomum zeylanicum and Satureja khuzestanica. Appl. Food Res. 2025, 5, 100959. [Google Scholar] [CrossRef]
- Tahsiri, Z.; Hedayati, S.; Niakousari, M. Improving the techno-functionality of wild almond protein isolate-based films by its hydrolysates and cold plasma treatment. Food Hydrocoll. Health 2025, 7, 100199. [Google Scholar] [CrossRef]
- Santos, N.C.; Almeida, R.L.J.; Albuquerque, J.C.; de Lima, T.L.B.; de Sousa, F.M.; de Alcântara Silva, V.M.; Melo, M.O.P.; Leite Filho, M.T.; dos Santos Silva, R.; Pinheiro, L.S.S.; et al. Effect of ultrasound and freeze-drying to enhance the extraction of phenolic compounds in dragon fruit peels and apply them in edible starch-based films. Packag. Technol. Sci. 2024, 37, 901–916. [Google Scholar] [CrossRef]
- Monteiro, S.S.; de Araujo Queiroz, J.V.S.; Gomes, H.M.; Santos, L.; Moreira, J.C.F.; Gelain, D.P.; de Bittencourt Pasquali, M.A. Characterization of mucilage from Opuntia cochenillifera cladodes: Rheological behavior, cytotoxicity, and antioxidant potential. Colloids Surf. A Physicochem. Eng. Asp. 2025, 707, 135824. [Google Scholar] [CrossRef]
- Vityazev, F.V.; Khramova, D.S.; Saveliev, N.Y.; Ipatova, E.A.; Burkov, A.A.; Beloserov, V.S.; Popov, S.V. Pectin–glycerol gel beads: Preparation, characterization and swelling behaviour. Carbohydr. Polym. 2020, 238, 116166. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.K.Q.; Felipe, K.H.; de Souza Silva, J.D.; Santos, N.C.; de Farias Araújo, M.S.; Vieira, P.P.F.; Gusmão, T.A.S.; Gouveia, D.S. Development of Nile tilapia gelatin films with grape pomace extract for fish fillet storage: Hydrophobic, functional properties, and biodegradability. Food Biosci. 2025, 68, 106675. [Google Scholar] [CrossRef]
- Medina-Jaramillo, C.; Ochoa-Yepes, O.; Bernal, C.; Famá, L. Active and smart biodegradable packaging based on starch and natural extracts. Carbohydr. Polym. 2017, 176, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Wen, H.; Xu, Z.; Zou, J.; Liu, X.; Zhang, D.; Wang, X. Preservation mechanism of chitosan/gelatin films modified by deep eutectic solvent on chilled chicken breast at 4 °C storage. Food Control 2025, 174, 111237. [Google Scholar] [CrossRef]
- ISO 20200:2015; Plastics—Determination of the Degree of Disintegration of Plastic Materials Under Simulated Composting Conditions in a Laboratory-Scale Test. ISO: Geneva, Switzerland, 2015.
- Janowicz, M.; Sitkiewicz, I.; Ciurzyńska, A.; Galus, S. Rheological properties of film-forming dispersions of selected biopolymers used for packaging films or food coating. Coatings 2022, 12, 1704. [Google Scholar] [CrossRef]
- Amorim, T.A.; Carvalho, A.J.D.B.A.; da Silva Figueiredo, L.; dos Santos Lima, M.; Sarinho, A.M.; Santos, N.C.; de Pereira Gusmão, R. Structure-function relationship and antioxidant mechanisms of pectin from red and white pitaya peels for functional food applications. Food Hydrocoll. 2025, 167, 111455. [Google Scholar] [CrossRef]
- Huo, J.; Zhu, B.; Ma, C.; You, L.; Cheung, P.C.K.; Pedisić, S.; Hileuskaya, K. Effects of chemically reactive species generated in plasma treatment on the physico-chemical properties and biological activities of polysaccharides: An overview. Carbohydr. Polym. 2024, 342, 122361. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.; Kooshki, S. Innovative method for water-in-oil emulsion treatment using atmospheric nonthermal-plasma technology. Langmuir 2023, 39, 14459–14473. [Google Scholar] [CrossRef] [PubMed]
- Luque-Agudo, V.; Hierro-Oliva, M.; Gallardo-Moreno, A.M.; González-Martín, M.L. Effect of plasma treatment on the surface properties of polylactic acid films. Polym. Test. 2021, 96, 107097. [Google Scholar] [CrossRef]
- Romani, V.P.; Olsen, B.; Collares, M.P.; Oliveira, J.R.M.; Prentice, C.; Martins, V.G. Cold plasma and carnauba wax as strategies to produce improved bi-layer films for sustainable food packaging. Food Hydrocoll. 2020, 108, 106087. [Google Scholar] [CrossRef]
- Sani, I.K.; Aminoleslami, L.; Mirtalebi, S.S.; Sani, M.A.; Mansouri, E.; Eghbaljoo, H.; Kazemzadeh, B. Cold plasma technology: Applications in improving edible films and food packaging. Food Packag. Shelf Life 2023, 37, 101087. [Google Scholar] [CrossRef]
- Santhosh, R.; Babu, D.M.; Thakur, R.; Nath, D.; Hoque, M.; Gaikwad, K.K.; Sarkar, P. Effect of atmospheric cold plasma treatment on structural, thermal, and mechanical properties of pea protein isolate edible films. Sustain. Chem. Pharm. 2024, 37, 101398. [Google Scholar] [CrossRef]
- Li, Q.; Zhi, A.; He, X.; Shen, F.; Fang, Y.; Hu, Q.; Liu, X. Effect of modified atmosphere packaging assisted cold plasma on performance of edible soybean protein isolate films. Food Biosci. 2023, 56, 103382. [Google Scholar] [CrossRef]
- Santos, N.C.; Almeida, R.L.; de Lima, T.L.; da Silva Figueira, J.G.; Martins, A.N.A.; da Silva, L.A.; Rocha, A.P.T. Cold plasma and pulsed electric field combined preserve functional properties, bioaccessibility, and polyphenol stability in lychee peels during storage. Food Biosci. 2025, 68, 106719. [Google Scholar] [CrossRef]
- Gupta, R.K.; Guha, P.; Srivastav, P.P. Natural polymers in biodegradable/edible film: A review on environmental concerns, cold plasma technology and nanotechnology application on food packaging-a recent trend. Food Chem. Adv. 2022, 1, 100135. [Google Scholar] [CrossRef]
- Sheerzad, S.; Khorrami, R.; Khanjari, A.; Gandomi, H.; Basti, A.A.; Khansavar, F. Improving chicken meat shelf-life: Coating with whey protein isolate, nanochitosan, bacterial nanocellulose, and cinnamon essential oil. LWT 2024, 197, 115912. [Google Scholar] [CrossRef]
- Bharti, S.K.; Pathak, V.; Alam, T.; Arya, A.; Singh, V.K.; Verma, A.K.; Rajkumar, V. Materialization of novel composite bio-based active edible film functionalized with essential oils on antimicrobial and antioxidative aspect of chicken nuggets during extended storage. J. Food Sci. 2020, 85, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Oyom, W.; Strange, J.; Nowlin, K.; Tukur, P.; Ferdaus, M.J.; Faraji, H.; Tahergorabi, R. Development and characterization of bigel systems as carriers for thyme essential oil utilizing hydrogel from chicken processing by-products for food applications. Int. J. Biol. Macromol. 2025, 292, 139222. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, X.; Huang, M.; Xu, Y. UV responded modified polyvinyl alcohol bio-active films with oregano essential oil microcapsules: Microbial control and sensory quality preservation for ready-to-eat chicken breast. Food Control 2025, 175, 111325. [Google Scholar] [CrossRef]


| Parameters | FNT | FCP10 | FCP20 | FCP30 |
|---|---|---|---|---|
| Thickness (mm) | 0.14 ± 0.00 b | 0.15 ± 0.001 a | 0.14 ± 0.001 b | 0.13 ± 0.00 c |
| Moisture content (%) | 14.20 ± 0.23 a | 13.41 ± 0.09 b | 12.75 ± 0.13 c | 10.03 ± 0.22 d |
| WVP (g·mm/kPa·h·m2) | 1.33 ± 0.15 d | 2.80 ± 0.11 c | 3.25 ± 0.19 b | 4.61 ± 0.34 a |
| Solubility (%) | 80.66 ± 0.25 a | 74.10 ± 0.16 b | 69.53 ± 0.23 c | 65.24 ± 0.18 d |
| Contact angle (°) | 51.15 ± 0.40 d | 53.29 ± 0.12 c | 57.50 ± 0.20 b | 62.38 ± 0.43 a |
| Tensile strenght (Mpa) | 3.29 ± 0.21 d | 4.32 ± 0.19 c | 5.91 ± 0.15 b | 6.74 ± 0.22 a |
| Elongation at break (%) | 0.18 ± 0.05 d | 0.26 ± 0.04 c | 0.35 ± 0.11 b | 0.47 ± 0.16 a |
| Films | L* | a* | b* | |
|---|---|---|---|---|
| FNT |  | 40.77 ± 0.14 d | 12.43 ± 0.30 a | 48.79 ± 0.15 d |
| FCP10 |  | 46.72 ± 0.21 c | 1.19 ± 0.19 c | 52.11 ± 0.28 c |
| FCP20 |  | 61.05 ± 0.17 b | 4.47 ± 0.42 b | 64.87 ± 0.22 a |
| FCP30 |  | 63.71 ± 0.12 a | 2.70 ± 0.10 bc | 63.41 ± 0.19 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, N.C.; Almeida, R.L.J.; Silva, G.M.d.; Fonseca, M.T.S.d.; Farias, C.M.S.; Silva, V.M.d.A.; Teles, F.G.; Ribeiro, V.H.d.A.; Alves, K.d.A.; Araújo, R.H.C.R.; et al. Development and Application of Biodegradable Pectin/Carboxymethylcellulose Films with Cinnamon Essential Oil and Cold Plasma Modification for Chicken Meat Preservation. Polysaccharides 2025, 6, 64. https://doi.org/10.3390/polysaccharides6030064
Santos NC, Almeida RLJ, Silva GMd, Fonseca MTSd, Farias CMS, Silva VMdA, Teles FG, Ribeiro VHdA, Alves KdA, Araújo RHCR, et al. Development and Application of Biodegradable Pectin/Carboxymethylcellulose Films with Cinnamon Essential Oil and Cold Plasma Modification for Chicken Meat Preservation. Polysaccharides. 2025; 6(3):64. https://doi.org/10.3390/polysaccharides6030064
Chicago/Turabian StyleSantos, Newton Carlos, Raphael L. J. Almeida, Gabriel M. da Silva, Maria T. S. da Fonseca, Cosme M. S. Farias, Virgínia M. de A. Silva, Fábio G. Teles, Victor H. de A. Ribeiro, Kalinny de A. Alves, Railene H. C. R. Araújo, and et al. 2025. "Development and Application of Biodegradable Pectin/Carboxymethylcellulose Films with Cinnamon Essential Oil and Cold Plasma Modification for Chicken Meat Preservation" Polysaccharides 6, no. 3: 64. https://doi.org/10.3390/polysaccharides6030064
APA StyleSantos, N. C., Almeida, R. L. J., Silva, G. M. d., Fonseca, M. T. S. d., Farias, C. M. S., Silva, V. M. d. A., Teles, F. G., Ribeiro, V. H. d. A., Alves, K. d. A., Araújo, R. H. C. R., Andrade, R. O. d., Gusmão, R. P. d., Gomes, J. P., & Rocha, A. P. T. (2025). Development and Application of Biodegradable Pectin/Carboxymethylcellulose Films with Cinnamon Essential Oil and Cold Plasma Modification for Chicken Meat Preservation. Polysaccharides, 6(3), 64. https://doi.org/10.3390/polysaccharides6030064









