Thermoplastic and Biocompatible Materials Based on Block Copolymers of Chitosan and Poly(ε-caprolactone)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Consumables
2.2. Methodology for Preparation of Block Copolymers
2.3. Gel Permeation Chromatography
2.4. Copolymer Characterizations
2.5. Study of Biocompatibility of Films
3. Results and Discussion
3.1. Synthesis of Block Copolymers
3.2. Molecular Weight Studies
3.3. XRD Analysis
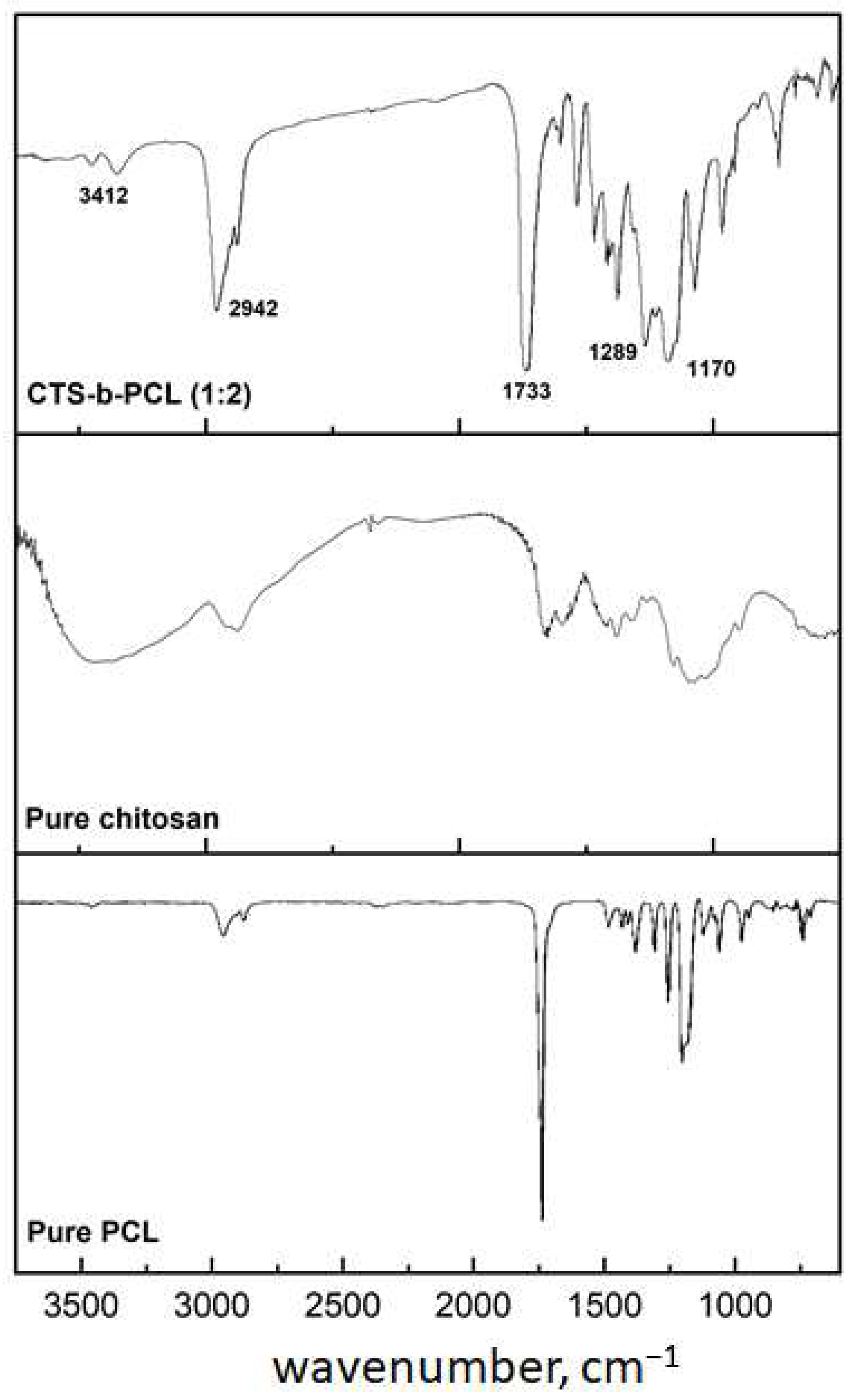
3.4. FTIR Analysis
3.5. Melt Flow Studies
3.6. Scanning Electron Microscopy (SEM) Studies
3.7. Mechanical Properties
3.8. Water Contact Angle and Swelling Capacity Test
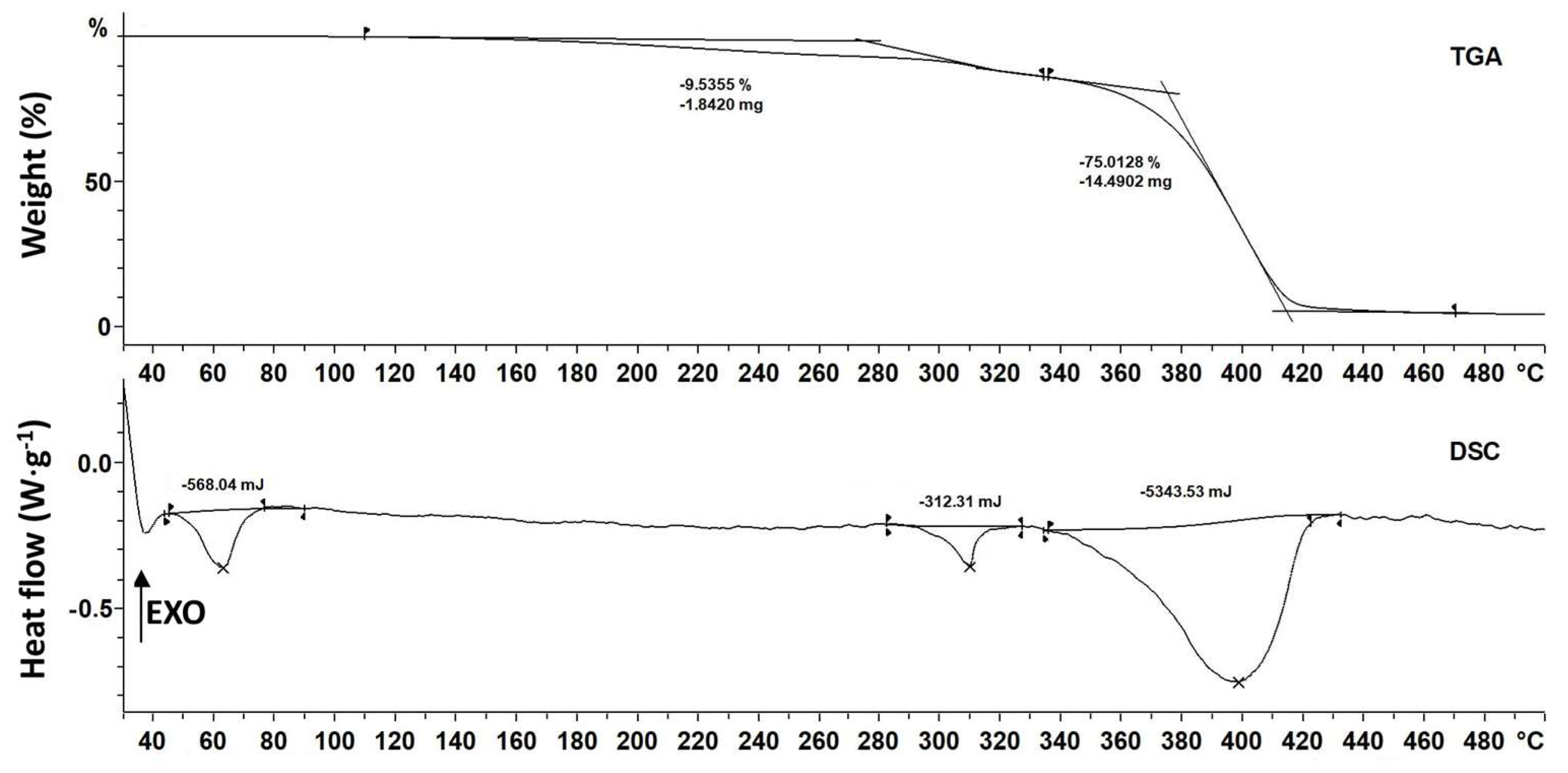
3.9. Thermal Properties
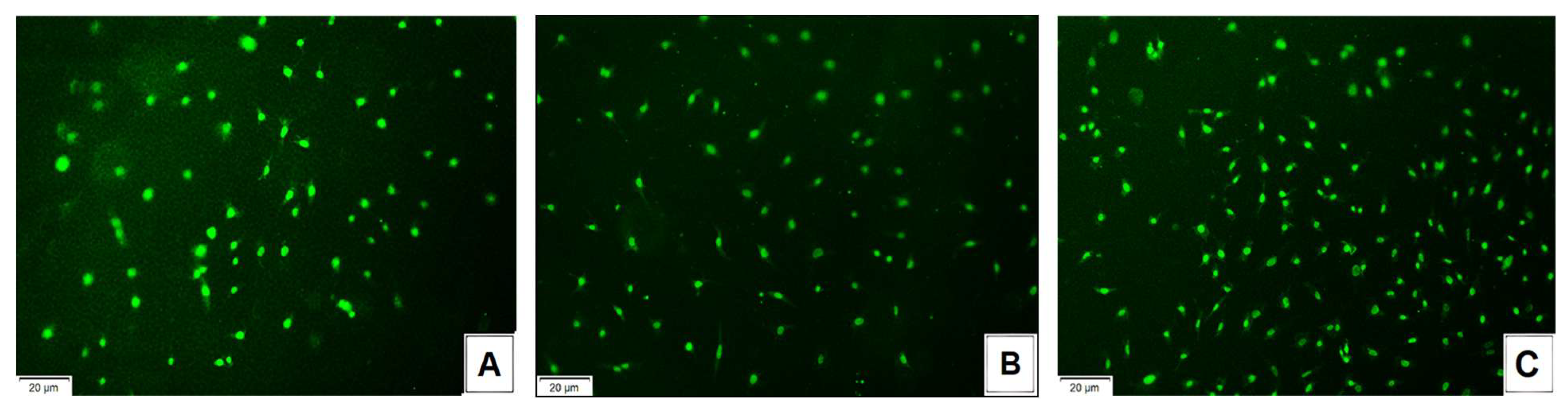
3.10. Biological Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTS | Chitosan |
| PCL | Polycaprolactone |
| DMSO | Dimethylsulfoxide |
References
- Melčová, V.; Svoradová, K.; Menčík, P.; Kontárová, S.; Rampichová, M.; Hedvičáková, V.; Sovková, V.; Přikryl, R.; Vojtová, L. FDM 3D Printed Composites for Bone Tissue Engineering Based on Plasticized Poly(3-Hydroxybutyrate)/Poly(d,l-Lactide) Blends. Polymers 2020, 12, 2806. [Google Scholar] [CrossRef] [PubMed]
- Alagoz, A.S.; Hasirci, V. 3D Printing of Polymeric Tissue Engineering Scaffolds Using Open-Source Fused Deposition Modeling. Emergent Mater. 2020, 3, 429–439. [Google Scholar] [CrossRef]
- Tchobanian, A.; Van Oosterwyck, H.; Fardim, P. Polysaccharides for Tissue Engineering: Current Landscape and Future Prospects. Carbohydr. Polym. 2019, 205, 601–625. [Google Scholar] [CrossRef] [PubMed]
- Zennifer, A.; Senthilvelan, P.; Sethuraman, S.; Sundaramurthi, D. Key Advances of Carboxymethyl Cellulose in Tissue Engineering & 3D Bioprinting Applications. Carbohydr. Polym. 2021, 256, 117561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chang, S.J.; Jing, Y.; Wang, L.; Chen, C.-J.; Liu, J.-T. Application of Chitosan with Different Molecular Weights in Cartilage Tissue Engineering. Carbohydr. Polym. 2023, 314, 120890. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Xu, B.; Zhang, R.; Fan, Y.; Xie, H.; Li, X. Applications of Decellularized Materials in Tissue Engineering: Advantages, Drawbacks and Current Improvements, and Future Perspectives. J. Mater. Chem. B 2020, 8, 10023–10049. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Gupta, A.; Agrawal, G. Recent Advances in Polysaccharides Based Biomaterials for Drug Delivery and Tissue Engineering Applications. Carbohydr. Polym. Technol. Appl. 2021, 2, 100067. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels As Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Biswal, T. Biopolymers for Tissue Engineering Applications: A Review. Mater. Today Proc. 2021, 41, 397–402. [Google Scholar] [CrossRef]
- Okamoto, M.; John, B. Synthetic Biopolymer Nanocomposites for Tissue Engineering Scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Haider, A.; Khan, S.; Iqbal, D.N.; Khan, S.U.; Haider, S.; Mohammad, K.; Mustfa, G.; Rizwan, M.; Haider, A. Chitosan as a Tool for Tissue Engineering and Rehabilitation: Recent Developments and Future Perspectives—A Review. Int. J. Biol. Macromol. 2024, 278, 134172. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Sharma, S.; Jabin, S.; Jadoun, S. Chitosan Nanocomposite for Tissue Engineering and Regenerative Medicine: A Review. Int. J. Biol. Macromol. 2024, 254, 127660. [Google Scholar] [CrossRef] [PubMed]
- Gholap, A.D.; Rojekar, S.; Kapare, H.S.; Vishwakarma, N.; Raikwar, S.; Garkal, A.; Mehta, T.A.; Jadhav, H.; Prajapati, M.K.; Annapure, U. Chitosan Scaffolds: Expanding Horizons in Biomedical Applications. Carbohydr. Polym. 2024, 323, 121394. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan Chemistry and Pharmaceutical Perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Santos-Carballal, B.; Fernández Fernández, E.; Goycoolea, F.M. Chitosan in Non-Viral Gene Delivery: Role of Structure, Characterization Methods, and Insights in Cancer and Rare Diseases Therapies. Polymers 2018, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Uchegbu, I.F.; Carlos, M.; McKay, C.; Hou, X.; Schätzlein, A.G. Chitosan Amphiphiles Provide New Drug Delivery Opportunities. Polym. Int. 2014, 63, 1145–1153. [Google Scholar] [CrossRef]
- Pan, A.-D.; Zeng, H.-Y.; Foua, G.B.; Alain, C.; Li, Y.-Q. Enzymolysis of Chitosan by Papain and Its Kinetics. Carbohydr. Polym. 2016, 135, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Wee, C.E.; Wai, L.K.; Zin, N.M.; Azmi, F. Biomimetic Amphiphilic Chitosan Nanoparticles: Synthesis, Characterization and Antimicrobial Activity. Carbohydr. Polym. 2021, 254, 117299. [Google Scholar] [CrossRef] [PubMed]
- Deka, B.C.; Bhattacharyya, P.K. Reactivity of Chitosan Derivatives and Their Interaction with Guanine: A Computational Study. J. Chem. Sci. 2016, 128, 589–598. [Google Scholar] [CrossRef]
- Cohn, D.; Hotovely Salomon, A. Designing Biodegradable Multiblock PCL/PLA Thermoplastic Elastomers. Biomaterials 2005, 26, 2297–2305. [Google Scholar] [CrossRef] [PubMed]
- Imre, B.; García, L.; Puglia, D.; Vilaplana, F. Reactive Compatibilization of Plant Polysaccharides and Biobased Polymers: Review on Current Strategies, Expectations and Reality. Carbohydr. Polym. 2019, 209, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rahman, A.; Arifin, M.A.B.; Ahmed, S. A Review on Chitosan and Chitosan-Based Bionanocomposites: Promising Material for Combatting Global Issues and Its Applications. Int. J. Biol. Macromol. 2021, 185, 832–848. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.; Peters, L.M.; Mucalo, M.R. Chitosan: A Review of Sources and Preparation Methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Brzeziński, M.; Biela, T. Micro- and Nanostructures of Polylactide Stereocomplexes and Their Biomedical Applications. Polym. Int. 2015, 64, 1667–1675. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent Advances in 3D-Printed Polylactide and Polycaprolactone-Based Biomaterials for Tissue Engineering Applications. Int. J. Biol. Macromol. 2022, 218, 930–968. [Google Scholar] [CrossRef] [PubMed]
- Backes, E.H.; Harb, S.V.; Beatrice, C.A.G.; Shimomura, K.M.B.; Passador, F.R.; Costa, L.C.; Pessan, L.A. Polycaprolactone Usage in Additive Manufacturing Strategies for Tissue Engineering Applications: A Review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 1479–1503. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer—Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical Properties and Cell Cultural Response of Polycaprolactone Scaffolds Designed and Fabricated via Fused Deposition Modeling. J. Biomed. Mater. Res. 2001, 55, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.E.; San, W.Y.; Wei, F.; Li, S.; Huang, M.-H.; Vert, M.; Hutmacher, D.W. Processing of Polycaprolactone and Polycaprolactone-Based Copolymers into 3D Scaffolds, and Their Cellular Responses. Tissue Eng. Part A 2009, 15, 3013–3024. [Google Scholar] [CrossRef] [PubMed]
- Thaore, V.; Chadwick, D.; Shah, N. Sustainable Production of Chemical Intermediates for Nylon Manufacture: A Techno-Economic Analysis for Renewable Production of Caprolactone. Chem. Eng. Res. Des. 2018, 135, 140–152. [Google Scholar] [CrossRef]
- Christen, M.-O.; Vercesi, F. Polycaprolactone: How a Well-Known and Futuristic Polymer Has Become an Innovative Collagen-Stimulator in Esthetics. Clin. Cosmet. Investig. Dermatol. 2020, 13, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Prabhath, A.; Vernekar, V.N.; Vasu, V.; Badon, M.; Avochinou, J.-E.; Asandei, A.D.; Kumbar, S.G.; Weber, E.; Laurencin, C.T. Kinetic Degradation and Biocompatibility Evaluation of Polycaprolactone-Based Biologics Delivery Matrices for Regenerative Engineering of the Rotator Cuff. J. Biomed. Mater. Res. Part A 2021, 109, 2137–2153. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, L.A.; Downes, S. Physicochemical Characterisation of Degrading Polycaprolactone Scaffolds. Polym. Degrad. Stab. 2010, 95, 2269–2276. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation Mechanisms of Polycaprolactone in the Context of Chemistry, Geometry and Environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Sarasam, A.; Madihally, S.V. Characterization of Chitosan–Polycaprolactone Blends for Tissue Engineering Applications. Biomaterials 2005, 26, 5500–5508. [Google Scholar] [CrossRef] [PubMed]
- García Cruz, D.M.; Gomez Ribelles, J.L.; Salmerón Sánchez, M. Blending Polysaccharides with Biodegradable Polymers. I. Properties of Chitosan/Polycaprolactone Blends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 85B, 303–313. [Google Scholar] [CrossRef] [PubMed]
- García Cruz, D.M.; Coutinho, D.F.; Costa Martinez, E.; Mano, J.F.; Gómez Ribelles, J.L.; Salmerón Sánchez, M. Blending Polysaccharides with Biodegradable Polymers. II. Structure and Biological Response of Chitosan/Polycaprolactone Blends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87B, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-S. A Comparison of the Structure, Thermal Properties, and Biodegradability of Polycaprolactone/Chitosan and Acrylic Acid Grafted Polycaprolactone/Chitosan. Polymer 2005, 46, 147–155. [Google Scholar] [CrossRef]
- Wan, Y.; Wu, H.; Yu, A.; Wen, D. Biodegradable Polylactide/Chitosan Blend Membranes. Biomacromolecules 2006, 7, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Tsverova, N.E.; Mochalova, A.E.; Morozov, A.G.; Yunin, P.A.; Smirnova, L.A.; Grishin, I.D. Synthesis and Properties of Chitosan-Polylactide Compositions Produced with the Use of Compatibilizers. Polym. Sci. Ser. B 2015, 57, 239–243. [Google Scholar] [CrossRef]
- Demina, T.S.; Akopova, T.A.; Zelenetsky, A.N. Materials Based on Chitosan and Polylactide: From Biodegradable Plastics to Tissue Engineering Constructions. Polym. Sci. Ser. C 2021, 63, 219–226. [Google Scholar] [CrossRef]
- Demina, T.S.; Akopova, T.A.; Vladimirov, L.V.; Zelenetskii, A.N.; Markvicheva, E.A.; Grandfils, C. Polylactide-Based Microspheres Prepared Using Solid-State Copolymerized Chitosan and d,l-Lactide. Mater. Sci. Eng. C 2016, 59, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Akopova, T.A.; Demina, T.S.; Shchegolikhin, A.N.; Kurkin, T.S.; Grandfils, C.; Perov, N.S.; Kechekyan, A.S.; Zelenetskii, A.N. A Novel Approach to Design Chitosan-Polyester Materials for Biomedical Applications. Int. J. Polym. Sci. 2012, 2012, 827967. [Google Scholar] [CrossRef]
- Peesan, M.; Rujiravanit, R.; Supaphol, P. Electrospinning of Hexanoyl Chitosan/Polylactide Blends. J. Biomater. Sci. Polym. Ed. 2006, 17, 547–565. [Google Scholar] [CrossRef] [PubMed]
- Sarasam, A.R.; Samli, A.I.; Hess, L.; Ihnat, M.A.; Madihally, S.V. Blending Chitosan with Polycaprolactone: Porous Scaffolds and Toxicity. Macromol. Biosci. 2007, 7, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Sarasam, A.R.; Krishnaswamy, R.K.; Madihally, S.V. Blending Chitosan with Polycaprolactone: Effects on Physicochemical and Antibacterial Properties. Biomacromolecules 2006, 7, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Lednev, I.; Salomatina, E.; Ilyina, S.; Zaitsev, S.; Kovylin, R.; Smirnova, L. Development of Biodegradable Polymer Blends Based on Chitosan and Polylactide and Study of Their Properties. Materials 2021, 14, 4900. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Wang, G.; Lei, M.; Guo, Y.; Song, Y.; Lu, T.; Wang, Y. Multi-Scale Investigation on the Phase Miscibility of Polylactic Acid/o-Carboxymethyl Chitosan Blends. Polymer 2019, 176, 159–167. [Google Scholar] [CrossRef]
- Meng, Q.; Heuzey, M.-C.; Carreau, P.J. Hierarchical Structure and Physicochemical Properties of Plasticized Chitosan. Biomacromolecules 2014, 15, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, J.; Gao, W.; Liang, H.; Wang, H.; Li, J. Preparation of Chitosan/PLA Blend Micro/Nanofibers by Electrospinning. Mater. Lett. 2009, 63, 658–660. [Google Scholar] [CrossRef]
- Dorati, R.; Pisani, S.; Maffeis, G.; Conti, B.; Modena, T.; Chiesa, E.; Bruni, G.; Musazzi, U.M.; Genta, I. Study on Hydrophilicity and Degradability of Chitosan/Polylactide-Co-Polycaprolactone Nanofibre Blend Electrospun Membrane. Carbohydr. Polym. 2018, 199, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.-C.; Teng, K.-W.; Huang, N.-C.; Kang, L.-Y.; Fu, K.-Y.; Hsieh, P.-S.; Dai, L.-G.; Dai, N.-T. Evaluation of Polycaprolactone/Gelatin/Chitosan Electrospun Membrane for Peritoneal Adhesion Reduction. Ann. Plast. Surg. 2020, 84, S116–S122. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shi, A.; Guo, S.; Fang, Y.; Chen, S.; Li, J. Preparation of Chitosan-g-Polylactide Graft Copolymers via Self-Catalysis of Phthaloylchitosan and Their Complexation with DNA. React. Funct. Polym. 2010, 70, 301–305. [Google Scholar] [CrossRef]
- Grande, R.; Pessan, L.A.; Carvalho, A.J.F. Ternary Melt Blends of Poly(Lactic Acid)/Poly(Vinyl Alcohol)-Chitosan. Ind. Crops Prod. 2015, 72, 159–165. [Google Scholar] [CrossRef]
- Tylingo, R.; Kempa, P.; Banach-Kopeć, A.; Mania, S. A Novel Method of Creating Thermoplastic Chitosan Blends to Produce Cell Scaffolds by FDM Additive Manufacturing. Carbohydr. Polym. 2022, 280, 119028. [Google Scholar] [CrossRef] [PubMed]
- Siparsky, G.L.; Voorhees, K.J.; Miao, F. Hydrolysis of Polylactic Acid (PLA) and Polycaprolactone (PCL) in Aqueous Acetonitrile Solutions: Autocatalysis. J. Environ. Polym. Degrad. 1998, 6, 31–41. [Google Scholar] [CrossRef]
- Ma, G.; Yang, D.; Kennedy, J.F.; Nie, J. Synthesize and Characterization of Organic-Soluble Acylated Chitosan. Carbohydr. Polym. 2009, 75, 390–394. [Google Scholar] [CrossRef]
- Katsogiannis, K.A.G.; Vladisavljević, G.T.; Georgiadou, S. Porous Electrospun Polycaprolactone (PCL) Fibres by Phase Separation. Eur. Polym. J. 2015, 69, 284–295. [Google Scholar] [CrossRef]
- Karim, M.; Boikess, R.S.; Schwartz, R.A.; Cohen, P.J. Dimethyl Sulfoxide (DMSO): A Solvent That May Solve Selected Cutaneous Clinical Challenges. Arch. Dermatol. Res. 2023, 315, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Joodaki, H.; Panzer, M.B. Skin Mechanical Properties and Modeling: A Review. Proc. Inst. Mech. Eng. H 2018, 232, 323–343. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.; Negi, Y.S.; Pradhan, S.; Dash, M.; Samal, S.K. 3—Wettability and Contact Angle of Polymeric Biomaterials. In Characterization of Polymeric Biomaterials; Tanzi, M.C., Farè, S., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 57–81. ISBN 978-0-08-100737-2. [Google Scholar]
- Baier, R.E. Surface Behaviour of Biomaterials: The Theta Surface for Biocompatibility. J. Mater. Sci. Mater. Med. 2006, 17, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Fu, W.; Xing, Y.; Ma, X.; Chen, C. Advances in Versatile Anti-Swelling Polymer Hydrogels. Mater. Sci. Eng. C 2021, 127, 112208. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, E.; Hassan, M.H.; Omar, A.M.; Acar, A.A.; Fallah, A.; Cooper, G.; Weightman, A.; Blunn, G.; Koc, B.; Bartolo, P. Accelerated Degradation of Poly-ε-Caprolactone Composite Scaffolds for Large Bone Defects. Polymers 2023, 15, 670. [Google Scholar] [CrossRef] [PubMed]
- Udoetok, I.A.; Mohamed, M.H.; Wilson, L.D. Hybrid Chitosan Biosorbents: Tunable Adsorption at Surface and Micropore Domains. Biomimetics 2024, 9, 725. [Google Scholar] [CrossRef] [PubMed]



| Initial Composition of Homopolymer Mixture | Copolymer Yield After 10 min of Irradiation, % | Copolymer Yield After 20 min of Irradiation, % | Copolymer Yield After 30 min of Irradiation, % |
|---|---|---|---|
| CTS:PCL (1:1) | 24.7 ± 0.4 | 60.8 ± 0.3 | 99.0 ± 0.2 |
| CTS:PCL (1:2) | 26.1 ± 0.1 | 58.2 ± 0.2 | 98.5 ± 0.1 |
| CTS:PCL (1:3) | 25.9 ± 0.1 | 61.7 ± 0.3 | 98.7 ± 0.2 |
| CTS:PCL (1:4) | 24.4 ± 0.2 | 63.0 ± 0.1 | 98.0 ± 0.2 |
| Substance | Mw | Mn | Mw/Mn |
|---|---|---|---|
| Initial PCL | 84,000 | 52,000 | 1.60 |
| PCL after ultrasonic treatment t = 30 min | 30,700 | 20,000 | 1.57 |
| Initial chitosan | 250,000 | 140,000 | 1.8 |
| CTS after ultrasonic treatment t = 30 min | 60,000 | 39,000 | 1.53 |
| PCL from CTS – b – PCL (1:1) | 23,500 | 16,800 | 1.40 |
| PCL from CTS – b – PCL (1:2) | 31,000 | 20,000 | 1.55 |
| PCL from CTS – b – PCL (1:3) | 30,000 | 22,000 | 1.40 |
| PCL from CTS – b – PCL (1:4) | 32,000 | 23,000 | 1.45 |
| Composition | Melt Flow Rate, g/min |
|---|---|
| CTS | - |
| PCL | 17 ± 0.7 |
| CTS:PCL (1:1) blend | - |
| CTS:PCL (1:2) blend | 5.5 ± 1.3 (with phase separation) |
| CTS:PCL (1:3) blend | 8.3 ± 1.7 (with phase separation) |
| CTS:PCL (1:4) blend | 11 ± 1.4 (with phase separation) |
| CTS – b – PCL (1:1) | - |
| CTS – b – PCL (1:2) | 7.2 ± 0.6 |
| CTS – b – PCL (1:3) | 9.5 ± 0.3 |
| CTS – b – PCL (1:4) | 14.2 ± 1.1 |
| Composition | C (%) | O (%) | N (%) |
|---|---|---|---|
| CTS – b – PCL (1:2) before extrusion | 65.62 ± 3.25 | 31.86 ± 1.63 | 2.52 ± 0.08 |
| CTS – b – PCL (1:2) after extrusion | 65.58 ± 3.43 | 31.83 ± 1.56 | 2.59 ± 0.12 |
| CTS:PCL (1:2) theoretically calculated | 65.42 | 31.92 | 2.66 |
| Composition | Tensile Strength, σ (MPa) | Elongation at Break, ε (%) |
|---|---|---|
| CTS | 14.4 ± 0.8 | 1.2 ± 0.1 |
| PCL | 32.3 ± 1.5 | 45.9 ± 2.0 |
| Films by solvent casting method | ||
| CTS – b – PCL (1:1) | 42.1 ± 2.1 | 15.2 ± 1.1 |
| CTS – b – PCL (1:2) | 67.3 ± 2.8 | 34.1 ± 2.0 |
| CTS – b – PCL (1:3) | 55.3 ± 1.4 | 38.5 ± 1.9 |
| CTS – b – PCL (1:4) | 43.4 ± 2.6 | 39.3 ± 2.2 |
| Filaments by extrusion | ||
| CTS – b – PCL (1:2) | 24.5 ± 1.2 | 38.3 ± 1.5 |
| CTS – b – PCL (1:3) | 35.1 ± 3.9 | 45.1 ± 3.3 |
| CTS – b – PCL (1:4) | 41.8 ± 1.5 | 52.3 ± 2.0 |
| Composition | Contact Angle, Deg | Water Uptake, % |
|---|---|---|
| CTS | 96 ± 1.5 | 160 ± 11 |
| PCL | 131 ± 0.4 | 0 |
| CTS – b – PCL (1:1) | 110 ± 0.7 | 92 ± 3 |
| CTS – b – PCL (1:2) | 118 ± 0.3 | 62 ± 5 |
| CTS – b – PCL (1:3) | 123 ± 1.0 | 45 ± 7 |
| CTS – b – PCL (1:4) | 125 ± 0.5 | 36 ± 4 |
| Series | Parameters | Composition | ||
|---|---|---|---|---|
| Pure CTS | CTS:PCL (1:2) Blend | CTS – b – PCL (1:2) | ||
| Control (n = 8) | OD | 0.583 ± 0.088 | 0.479 ± 0.082 | 0.521 ± 0.084 |
| V, % | 100 | 100 | 100 | |
| Cytotoxicity rank | 0 | 0 | 0 | |
| Extract (n = 8) | OD | 0.710 ± 0.033 | 0.454 ± 0.031 | 0.620 ± 0.049 |
| V, % | 122 | 95 | 119 | |
| Cytotoxicity rank | 0 | 1 | 0 | |
| Extract 1:1 (n = 8) | OD | 0.630 ± 0.042 | 0.473 ± 0.084 | 0.588 ± 0.017 |
| V, % | 109 | 100 | 113 | |
| Cytotoxicity rank | 0 | 0 | 0 | |
| Extract 1:2 (n = 8) | OD | 0.618 ± 0.016 | 0.466 ± 0.047 | 0.575 ± 0.022 |
| V, % | 106 | 97 | 110 | |
| Cytotoxicity rank | 0 | 1 | 0 | |
| Extract 1:4 (n = 8) | OD | 0.650 ± 0.028 | 0.440 ± 0.038 | 0.610 ± 0.087 |
| V, % | 111 | 91 | 117 | |
| Cytotoxicity rank | 0 | 1 | 0 | |
| Series | Parameters | Composition | ||
|---|---|---|---|---|
| Pure CTS | CTS:PCL (1:2) Blend | CTS – b – PCL (1:2) | ||
| Control (n = 8) | OD | 0.494 ± 0.014 | 0.405 ± 0.021 | 0.427 ± 0.011 |
| V, % | 100 | 100 | 100 | |
| Cytotoxicity rank | 0 | 0 | 0 | |
| Extract (n = 8) | OD | 0.510 ± 0.023 | 0.340 ± 0.010 | 0.470 ± 0.034 |
| V, % | 104 | 84 | 110 | |
| Cytotoxicity rank | 0 | 1 | 0 | |
| Extract 1:1 (n = 8) | OD | 0.507 ± 0.014 | 0.425 ± 0.029 | 0.429 ± 0.012 |
| V, % | 103 | 105 | 100 | |
| Cytotoxicity rank | 0 | 0 | 0 | |
| Extract 1:2 (n = 8) | OD | 0.550 ± 0.035 | 0.376 ± 0.043 | 0.447 ± 0.038 |
| V, % | 112 | 93 | 105 | |
| Cytotoxicity rank | 0 | 1 | 0 | |
| Extract 1:4 (n = 8) | OD | 0.580 ± 0.023 | 0.383 ± 0.033 | 0.455 ± 0.022 |
| V, % | 118 | 95 | 106 | |
| Cytotoxicity rank | 0 | 1 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lednev, I.; Zaitsev, S.; Maltseva, E.; Kovylin, R.; Smirnova, L. Thermoplastic and Biocompatible Materials Based on Block Copolymers of Chitosan and Poly(ε-caprolactone). Polysaccharides 2025, 6, 63. https://doi.org/10.3390/polysaccharides6030063
Lednev I, Zaitsev S, Maltseva E, Kovylin R, Smirnova L. Thermoplastic and Biocompatible Materials Based on Block Copolymers of Chitosan and Poly(ε-caprolactone). Polysaccharides. 2025; 6(3):63. https://doi.org/10.3390/polysaccharides6030063
Chicago/Turabian StyleLednev, Ivan, Sergey Zaitsev, Ekaterina Maltseva, Roman Kovylin, and Larisa Smirnova. 2025. "Thermoplastic and Biocompatible Materials Based on Block Copolymers of Chitosan and Poly(ε-caprolactone)" Polysaccharides 6, no. 3: 63. https://doi.org/10.3390/polysaccharides6030063
APA StyleLednev, I., Zaitsev, S., Maltseva, E., Kovylin, R., & Smirnova, L. (2025). Thermoplastic and Biocompatible Materials Based on Block Copolymers of Chitosan and Poly(ε-caprolactone). Polysaccharides, 6(3), 63. https://doi.org/10.3390/polysaccharides6030063






