A Direct Preparation of Cellulose Nanocrystals by ZnCl2-Based Deep Eutectic Solvent
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
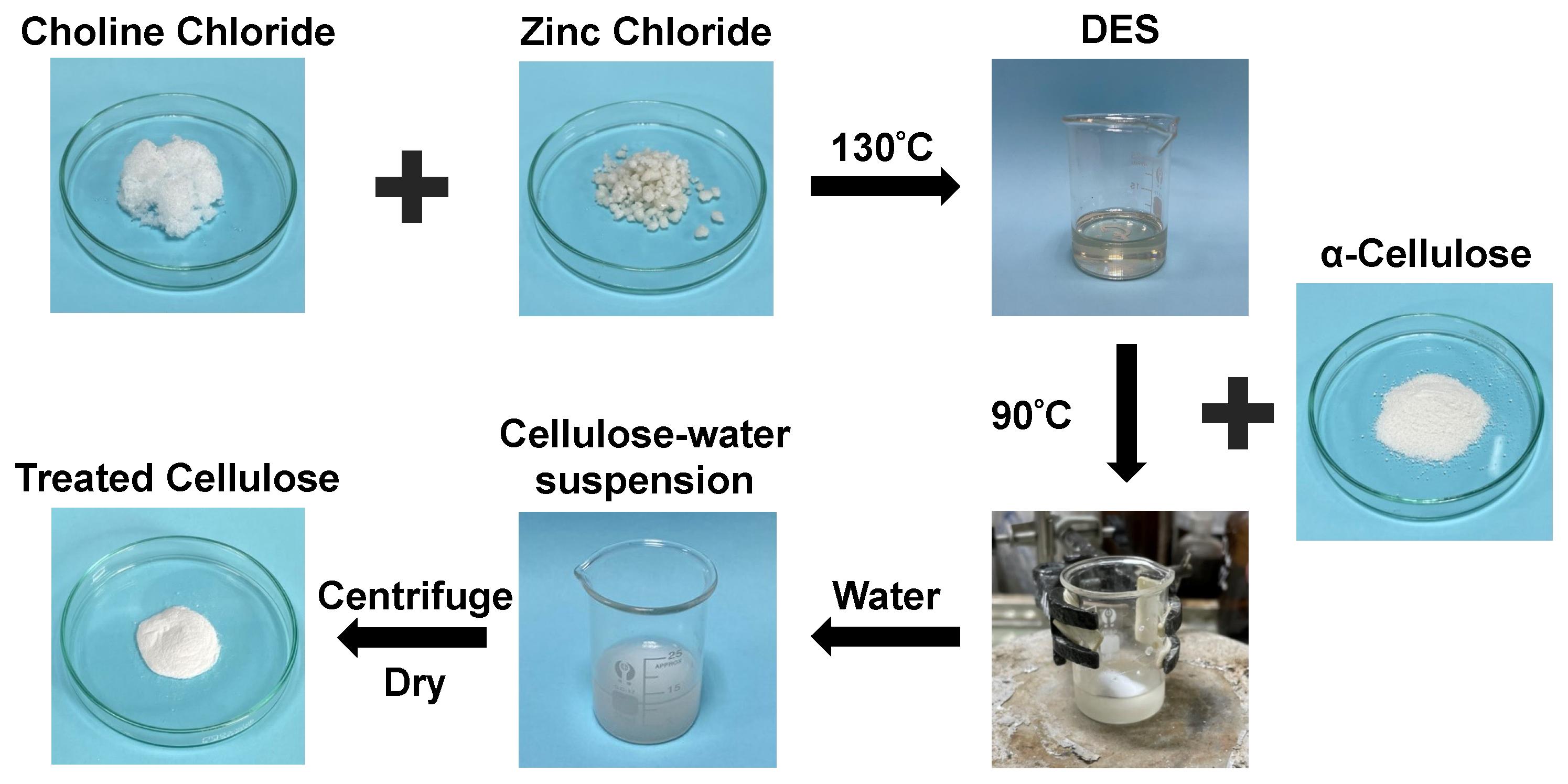
2.2. Preparation of Deep Eutectic Solvents (DESs)
2.3. Extraction of Nanocrystals Cellulose
2.4. Material Characterization
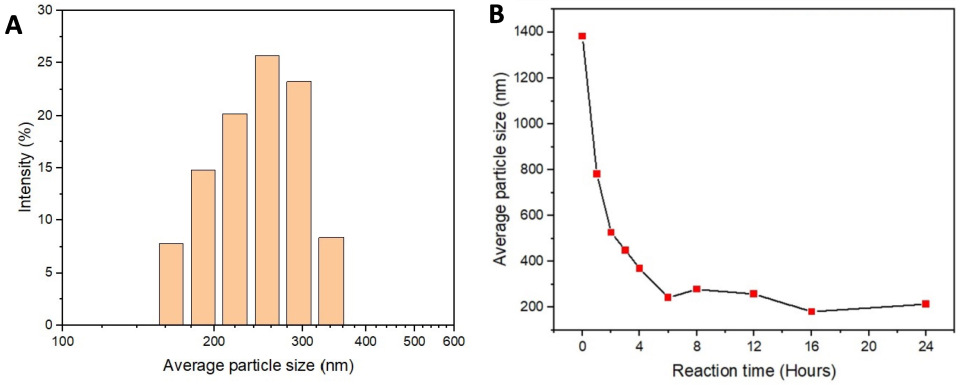
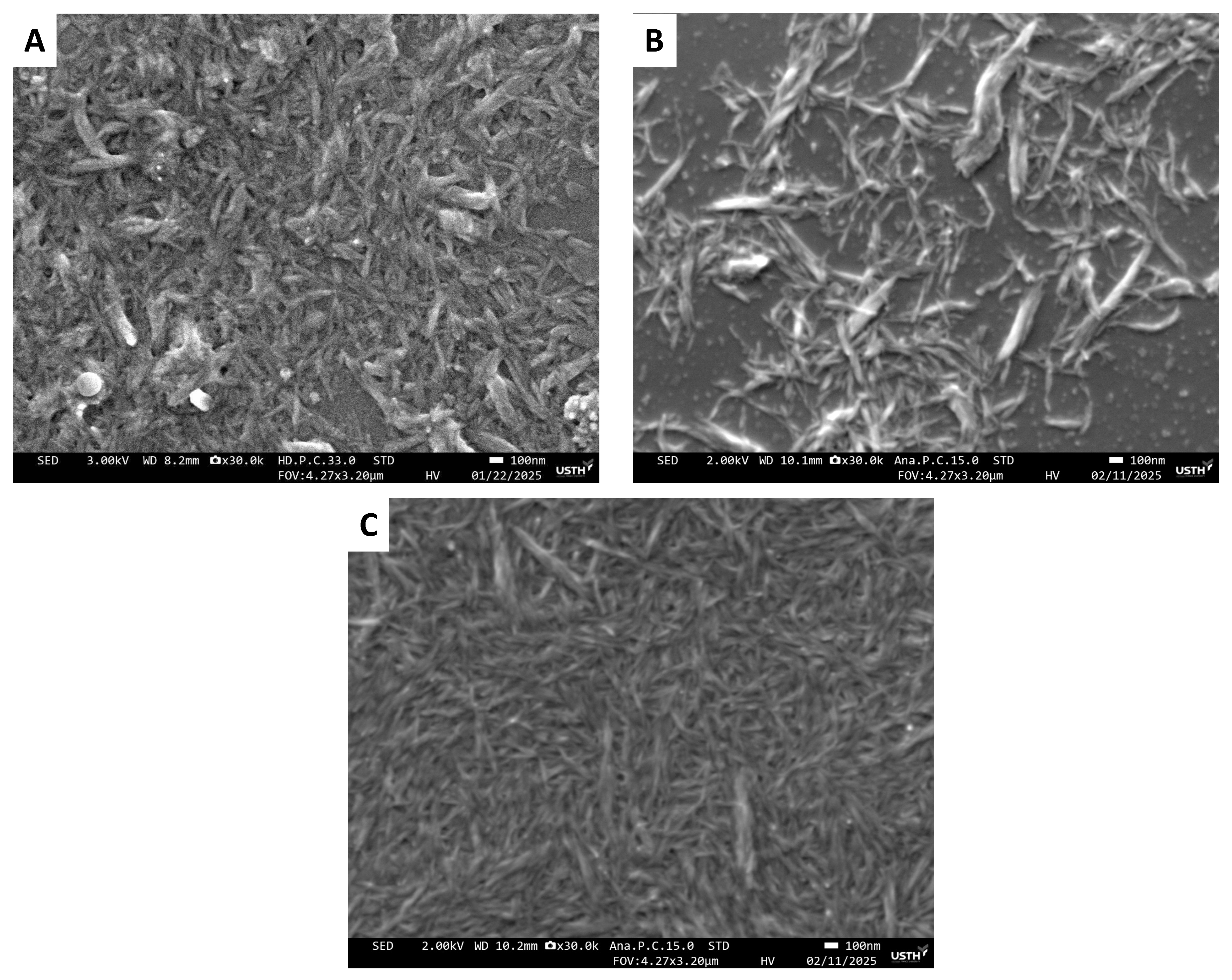
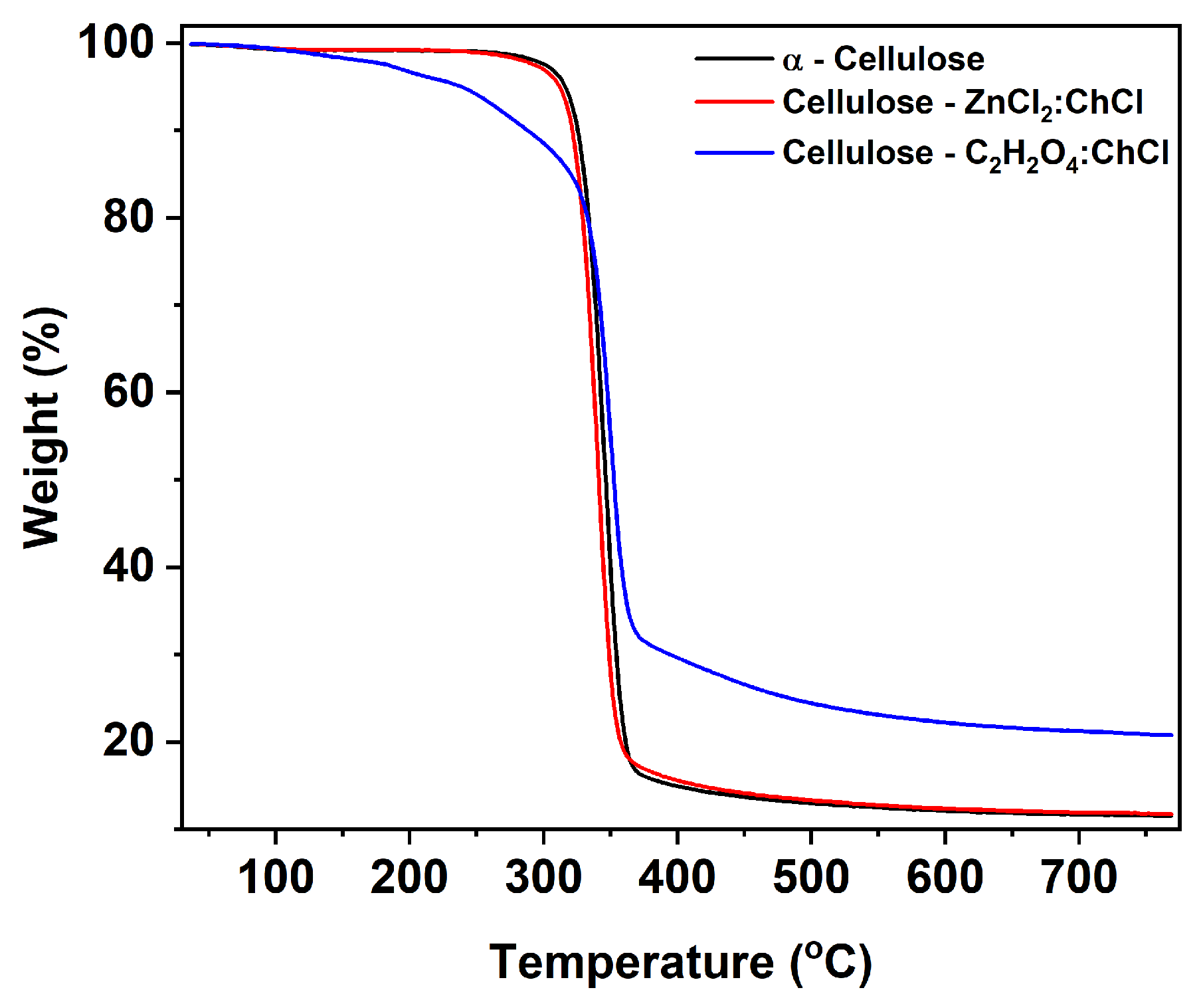
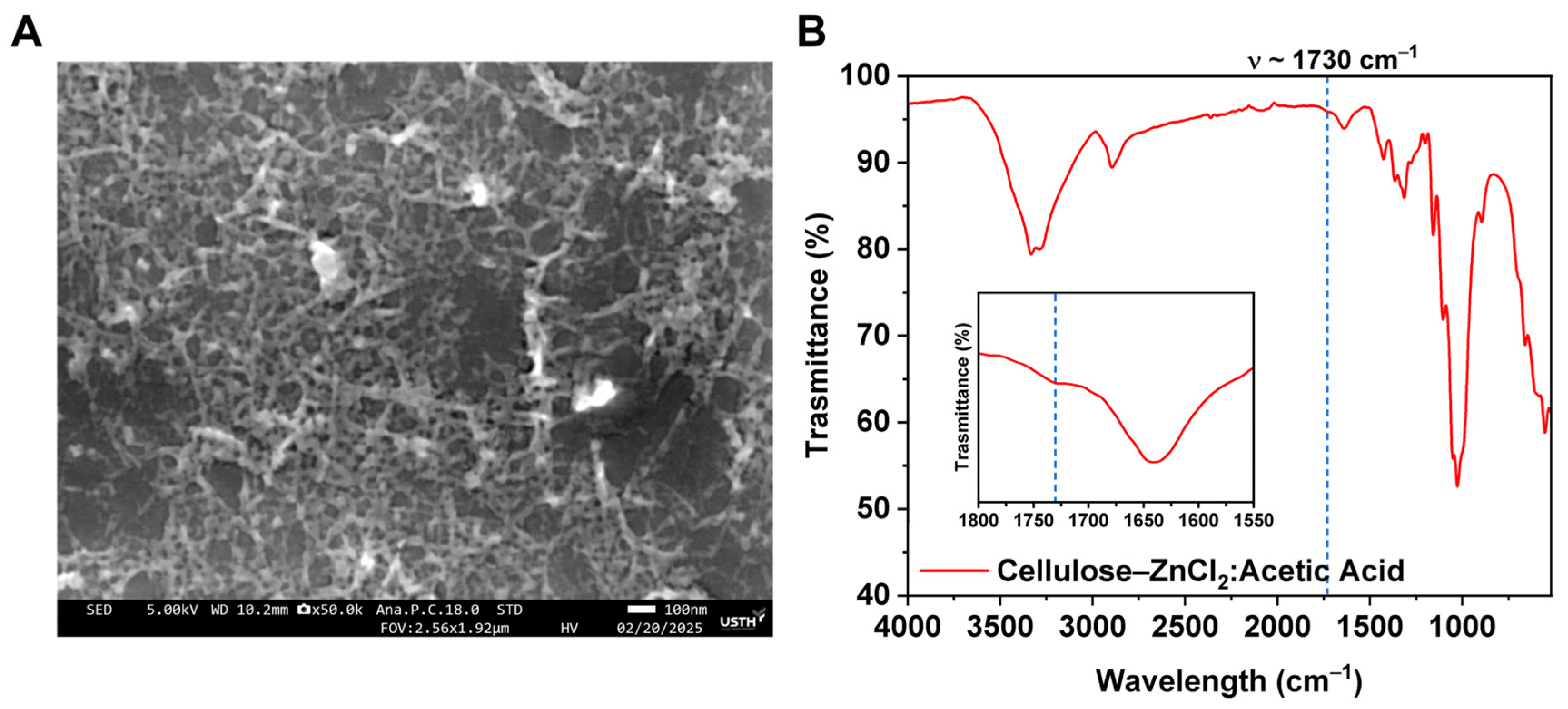
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MCC | Microcrystalline Cellulose |
| DES | Deep Eutectic Solvent |
| CNCs | Cellulose Nanocrystals |
| CNFs | Cellulose Nanofibrils |
References
- Dong, Q.; Zhang, X.; Qian, J.; He, S.; Mao, Y.; Brozena, A.H.; Zhang, Y.; Pollard, T.P.; Borodin, O.A.; Wang, Y.; et al. A cellulose-derived supramolecule for fast ion transport. Sci. Adv. 2022, 8, eadd2031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Chen, S.; Zhang, X.; Ma, J.; He, J. Oxidized cellulose-based hemostatic materials. Carbohydr. Polym. 2020, 230, 115585. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, Y.; Zeng, K.; Heinze, T.; Groth, T.; Zhang, K. Recent Progress on Cellulose-Based Ionic Compounds for Biomaterials. Adv. Mater. 2020, 33, e2000717. [Google Scholar] [CrossRef]
- Ye, Y.; Yu, L.; Lizundia, E.; Zhu, Y.; Chen, C.; Jiang, F. Cellulose-Based Ionic Conductor: An Emerging Material toward Sustainable Devices. Chem. Rev. 2023, 123, 9204–9264. [Google Scholar] [CrossRef]
- Biranje, S.S.; Sun, J.; Cheng, L.; Cheng, Y.; Shi, Y.; Yu, S.; Jiao, H.; Zhang, M.; Lu, X.; Han, W.; et al. Development of Cellulose Nanofibril/Casein-Based 3D Composite Hemostasis Scaffold for Potential Wound-Healing Application. ACS Appl. Mater. Interfaces 2022, 14, 3792–3808. [Google Scholar] [CrossRef]
- Montazerian, H.; Davoodi, E.; Baidya, A.; Baghdasarian, S.; Sarikhani, E.; Meyer, C.E.; Haghniaz, R.; Badv, M.; Annabi, N.; Khademhosseini, A.; et al. Engineered Hemostatic Biomaterials for Sealing Wounds. Chem. Rev. 2022, 122, 12864–12903. [Google Scholar] [CrossRef]
- Knutsen, M.F.; Agrenius, K.; Ugland, H.; Petronis, S.; Haglerod, C.; Håkansson, J.; Chinga-Carrasco, G. Oxygenated Nanocellulose—A Material Platform for Antibacterial Wound Dressing Devices. ACS Appl. Bio Mater. 2021, 4, 7554–7562. [Google Scholar] [CrossRef]
- Ahmad, H. Celluloses as support materials for antibacterial agents: A review. Cellulose 2021, 28, 2715–2761. [Google Scholar] [CrossRef]
- Hemraz, U.D.; Lam, E.; Sunasee, R. Recent advances in cellulose nanocrystals-based antimicrobial agents. Carbohydr. Polym. 2023, 315, 120987. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Lee, S.-Y.; Wei, T.; Li, J.; Fan, Z. Nanocellulose: A promising nanomaterial for advanced electrochemical energy storage. Chem. Soc. Rev. 2018, 47, 2837–2872. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, D.; Lee, Y.H.; Chen, W.; Lee, S.Y. Nanocellulose for Energy Storage Systems: Beyond the Limits of Synthetic Materials. Adv. Mater. 2019, 31, e1804826. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Du, H.; Liu, H.; Liu, W.; Zhang, X.; Si, C.; Liu, P.; Zhang, K. Advanced Nanocellulose-Based Composites for Flexible Functional Energy Storage Devices. Adv. Mater. 2021, 33, 2101368. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.; Winter, A.; Subbiahdoss, G.; Oberlerchner, J.T.; Beaumont, M.; Tamada, Y.; Rosenau, T. Partial Amorphization of Cellulose through Zinc Chloride Treatment: A Facile and Sustainable Pathway to Functional Cellulose Nanofibers with Flame-Retardant and Catalytic Properties. ACS Sustain. Chem. Eng. 2020, 8, 13576–13582. [Google Scholar] [CrossRef]
- Yang, W.; Mei, Z.; Feng, S.; Li, C.; Guo, J.; Bian, H.; Xiao, H.; Dai, H.; Hu, C.; Han, J. Cellulose Nanocrystal Preparation via Rapid Hydrolysis of Wood Cellulose Fibers Using Recyclable Molten Ferric Chloride Hexahydrate. ACS Sustain. Chem. Eng. 2023, 11, 10172–10182. [Google Scholar] [CrossRef]
- Erythropel, H.C.; Zimmerman, J.B.; de Winter, T.M.; Petitjean, L.; Melnikov, F.; Lam, C.H.; Lounsbury, A.W.; Mellor, K.E.; Janković, N.Z.; Tu, Q.; et al. The Green ChemisTREE: 20 years after taking root with the 12 principles. Green Chem. 2018, 20, 1929–1961. [Google Scholar] [CrossRef]
- Ma, G.; Yin, W.; Shi, Z.; Xu, Y.; Yang, G. Direct Carboxymethylation of Cellulose Fibers by Ternary Reactive Deep Eutectic Solvents for the Preparation of High-Yield Cellulose Nanofibrils. ACS Sustain. Chem. Eng. 2024, 12, 5871–5883. [Google Scholar] [CrossRef]
- Baheg, N.M.; Abdel-Hakim, A.; El-Wakil, A.E.A.A.; Mekewi, M.; Halim, S. Development of Novel Natural Deep Eutectic Solvent for Cellulose Nanofibrillation of Orange Peel via New Surface Functionalization. ACS Sustain. Chem. Eng. 2023, 11, 14793–14806. [Google Scholar] [CrossRef]
- Jiang, J.; Carrillo-Enríquez, N.C.; Oguzlu, H.; Han, X.; Bi, R.; Song, M.; Saddler, J.N.; Sun, R.-C.; Jiang, F. High Production Yield and More Thermally Stable Lignin-Containing Cellulose Nanocrystals Isolated Using a Ternary Acidic Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2020, 8, 7182–7191. [Google Scholar] [CrossRef]
- Panyamao, P.; Charumanee, S.; Ruangsuriya, J.; Saenjum, C. Efficient Isolation of Cellulosic Fibers from Coffee Parchment via Natural Acidic Deep Eutectic Solvent Pretreatment for Nanocellulose Production. ACS Sustain. Chem. Eng. 2023, 11, 13962–13973. [Google Scholar] [CrossRef]
- Ma, Y.; Xia, Q.; Liu, Y.; Chen, W.; Liu, S.; Wang, Q.; Liu, Y.; Li, J.; Yu, H. Production of Nanocellulose Using Hydrated Deep Eutectic Solvent Combined with Ultrasonic Treatment. ACS Omega 2019, 4, 8539–8547. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, Z.; Liu, S.; Xia, Q.; Liu, Y.; Chen, W.; Yu, H.; Zhang, K. Scalable production of carboxylated cellulose nanofibres using a green and recyclable solvent. Nat. Sustain. 2024, 7, 315–325. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Visanko, M. Anionic wood nanofibers produced from unbleached mechanical pulp by highly efficient chemical modification. J. Mater. Chem. A Mater. 2017, 5, 21828–21835. [Google Scholar] [CrossRef]
- Suopajärvi, T.; Sirviö, J.A.; Liimatainen, H. Nanofibrillation of deep eutectic solvent-treated paper and board cellulose pulps. Carbohydr. Polym. 2017, 169, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Sirviö, J.A.; Haapala, A.; Liimatainen, H. Cellulose nanofibrils from nonderivatizing urea-based deep eutectic solvent pretreatments. ACS Appl. Mater. Interfaces 2017, 9, 2846–2855. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Visanko, M.; Liimatainen, H. Acidic Deep Eutectic Solvents as Hydrolytic Media for Cellulose Nanocrystal Production. Biomacromolecules 2016, 17, 3025–3032. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Xia, Q.; Guo, B.; Wang, Q.; Liu, S.; Liu, Y.; Li, J.; Yu, H. Efficient Cleavage of Lignin–Carbohydrate Complexes and Ultrafast Extraction of Lignin Oligomers from Wood Biomass by Microwave-Assisted Treatment with Deep Eutectic Solvent. ChemSusChem 2017, 10, 1692–1700. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, B.; Xia, Q.; Meng, J.; Chen, W.; Liu, S.; Wang, Q.; Liu, Y.; Li, J.; Yu, H. Efficient Cleavage of Strong Hydrogen Bonds in Cotton by Deep Eutectic Solvents and Facile Fabrication of Cellulose Nanocrystals in High Yields. ACS Sustain. Chem. Eng. 2017, 5, 7623–7631. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef]
- Do, T.A.; Thi, A.T.P.; Le, T.H.; Van, D.D.; Kim, T.N.; Van Nguyen, Q. Cellulose Nanomaterials Functionalized with Carboxylic Group Extracted from Lignocellulosic Agricultural Waste: Isolation and Cu(II) Adsorption for Antimicrobial Application. ACS Omega 2025, 10, 6243. [Google Scholar] [CrossRef]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal crystallinity index revisited by the simulation of X-ray diffraction patterns of cotton cellulose Iβ and cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, N.; Yin, X.; Long, L.; Hou, X.; Zhao, J.; Yuan, X. Preparation of cellulose nanospheres via combining ZnCl2·3H2O pretreatment and p-toluenesulfonic hydrolysis as a two-step method. Int. J. Biol. Macromol. 2021, 181, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Deng, M.; Li, S.; Guan, Z.; Xia, Y.; Yang, J.; Lin, X. Room temperature preparation of cellulose nanocrystals with high yield via a new ZnCl2 solvent system. Carbohydr. Polym. 2022, 278, 118946. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Hu, C.; Zhang, W.; Lin, X.; Qi, W.; Zhang, Z.; Gu, J. Acidified ZnCl2 molten salt hydrate systems as hydrolytic media for cellulose I and II nanocrystal production: From rods to spheres. Cellulose 2022, 29, 7629–7647. [Google Scholar] [CrossRef]
- Pelosi, C.; Quaranta, A.; Rollo, M.; Martinelli, E.; Duce, C.; Ciancaleoni, G.; Bernazzani, L. Preparation and Characterization of Zinc(II)-Based Lewis/Brønsted Acidic Deep Eutectic Solvents. Molecules 2023, 28, 8054. [Google Scholar] [CrossRef]
- Tamaddon, F.; Rashidi, H. ZnCl2:2HOAc: A deep eutectic solvent for the Friedel–Crafts acetylation of poly-phenols and chemo-selective protection of alcohols. Res. Chem. Intermed. 2023, 49, 3589–3603. [Google Scholar] [CrossRef]
- Chen, M.; Li, R.-M.; Runge, T.; Feng, J.; Hu, S.; Shi, Q.-S. Solvent-Free Acetylation of Cellulose by 1-Ethyl-3-methylimidazolium Acetate-Catalyzed Transesterification. ACS Sustain. Chem. Eng. 2019, 7, 16971–16978. [Google Scholar] [CrossRef]
- Oberlintner, A.; Huš, M.; Likozar, B.; Novak, U. Multiscale Study of Functional Acetylation of Cellulose Nanomaterials by Design: Ab Initio Mechanisms and Chemical Reaction Microkinetics. ACS Sustain. Chem. Eng. 2022, 10, 15480–15489. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, H.A.; Le, Q.T.; Nguyen, V.Q. A Direct Preparation of Cellulose Nanocrystals by ZnCl2-Based Deep Eutectic Solvent. Polysaccharides 2025, 6, 61. https://doi.org/10.3390/polysaccharides6030061
Vu HA, Le QT, Nguyen VQ. A Direct Preparation of Cellulose Nanocrystals by ZnCl2-Based Deep Eutectic Solvent. Polysaccharides. 2025; 6(3):61. https://doi.org/10.3390/polysaccharides6030061
Chicago/Turabian StyleVu, Hoai An, Quang Tung Le, and Van Quyen Nguyen. 2025. "A Direct Preparation of Cellulose Nanocrystals by ZnCl2-Based Deep Eutectic Solvent" Polysaccharides 6, no. 3: 61. https://doi.org/10.3390/polysaccharides6030061
APA StyleVu, H. A., Le, Q. T., & Nguyen, V. Q. (2025). A Direct Preparation of Cellulose Nanocrystals by ZnCl2-Based Deep Eutectic Solvent. Polysaccharides, 6(3), 61. https://doi.org/10.3390/polysaccharides6030061





